DOI:10.32604/biocell.2022.019277

| BIOCELL DOI:10.32604/biocell.2022.019277 |  |
| Article |
Astaxanthin delayed the pathogenesis of diabetic nephropathy in type 1 diabetic rats
1Department of Diagnostics, The Second School of Clinical Medicine, Binzhou Medical University, Yantai, 264003, China
2School of Public Health and Management, Binzhou Medical University, Yantai, 264003, China
3Department of Nephrology, Binzhou Medical University Hospital, Binzhou, 256603, China
*Address correspondence to: Xiaomin Zhang, zhangxiaomin7926@126.com
Received: 14 September 2021; Accepted: 13 December 2021
Abstract: This study was designed to investigate the protective effects of Astaxanthin (AST) in rats with diabetes mellitus (DM) induced by streptozotocin. SD rats were divided into control group (n = 5, only received normal saline), DM group (n = 8) and AST + DM group (n = 8; AST: 50 mg/kg/day). DM rats were induced by intraperitoneal injection of streptozocin (STZ, 65 mg/kg). Blood glucose level and body weight were determined at weeks 0, 2, 4, 6 and 8, respectively. At week 8, kidney function was determined, together with expression of P53 and dynamin-related protein-1 (Drp1) by Western blot analysis and immunofluorescence. AST led to increase of body weight in rats with DM. AST + DM group showed a significant decrease in blood glucose level at week 4 compared with DM group (P < 0.05). AST improved renal function and significantly reduced expression of P53 and Drp1 in DM rats. In addition, AST can effectively reduce the blood glucose in DM rats, and delayed the pathogenesis of diabetic nephropathy. Such delay mediated by AST may be associated with the downregulation of Drp1 and P53.
Keywords: Astaxanthin; Diabetic nephropathy; Dynamin-related protein-1; P53; Renal function
Abbreviations
| AST: | Astaxanthin |
| DM: | diabetes mellitus |
| DN: | diabetic nephropathy |
| Drp1: | dynamin-related protein-1 |
| ROS: | reducing oxidative stress |
| OS: | oxidative stress |
| SD: | Sprague–Dawley |
| PVDF: | polyvinylidene difluoride |
| SEM: | standard error of mean |
Diabetic nephropathy (DN), a severe complication of diabetes mellitus (DM), is currently the leading cause of end-stage renal disease. Despite optimal management, DN is still a major contributor to morbidity and mortality of diabetic patients worldwide. It is featured by thickening in glomerular basement membrane, accumulation of extracellular matrix, as well as destabilization of podocyte processes. Accumulating evidence suggests that hyperglycemia or high glucose mediates renal injury in DN via multiple molecular mechanisms such as induction of oxidative stress, upregulation of renal transforming growth factor beta-1 expression, production of proinflammatory cytokines, activation of fibroblasts and renin angiotensin system, and depletion of adenosine triphosphate. To date, the related therapies could only delay the disease progression rather than preventing its recurrence. In the presence of obvious proteinuria, the disease will enter an irreversible stage. Nowadays, the prevalence of DN is still rising worldwide, which has been considered a global burden. Therefore, there is urgent need to develop novel therapies for treating the disease. In the past decades, Astaxanthin (AST) has been commonly utilized for DM treatment as it is a powerful antioxidant agent (Kanwugu et al., 2021). Besides, it contributes to the attenuation of DN symptoms by reducing oxidative stress (ROS) and renal injuries (Naito et al., 2004). However, little is known about the exact mechanism of how AST modulates DN.
Mitochondria is the primary source of excessive production of ROS, which shows the capacity to exceed production of endogenous antioxidants. Persistent oxidative stress (OS) production leads to modifications of DNA capable of triggering damages to mitochondrial genetic material and the nuclear DNA. Dynamin-related protein 1 (Drp1) has been well acknowledged to be associated with the mitochondrial fission (Fonseca et al., 2019), which is considered to play a pivotal role in many human diseases (Serasinghe and Chipuk, 2017). P53 protein, closely related to the apoptosis, constitutes an axis with Drp1 in regulating mitochondrial fission and the consequent apoptosis. For instance, P53 can upregulate Drp1 expression in a transcription-dependent manner, while Drp1 can convey the apoptotic signal of P53 by triggering mitochondrial fission. In addition, P53/Drp1-dependent mitochondrial fission mediates aldosterone-induced podocyte injury and mitochondrial dysfunction (Yuan et al., 2018). As P53 signaling pathway and mitochondrial fission have been reported to be closely related to several diseases (Zhou et al., 2018), we hypothesized that AST may participate in the pathogenesis of DN and its complications by modulating the mitochondrial function. In this study, we aimed to investigate the efficiency of AST in the pathogenesis of DN through determining expression of P53 and Drp1 in rats.
Twenty-five male Sprague–Dawley (SD) rats (180–200 g) were provided by the Lukang Experimental Animal Center (Jining, China). Rats were housed under a 12 h light/12 h dark cycle at room temperature, under a humidity of 50%–80%. All the animals were free access to food and water. All experiments were performed in line with the relevant laws. The study protocols were approved by the Ethics Committee of Binzhou Medical University.
Type 1 DM was induced through single intraperitoneal injection of streptozocin (STZ, 65 mg/kg) according to the previous description (Al-Awar et al., 2016). After fasting for 10 h, the rats were injected with STZ solution once intraperitoneally at a rate of 50 mg/kg. The diagnostic standard for type 1 DM was as follows: blood glucose ≥ 16.8 mmol/L within 24 h, and stable for 5 days. Two days after STZ administration, the circulating glucose concentration was measured. DM was confirmed in presence of blood glucose levels of ≥17 mmol/L. SD rats were randomly divided into control group (n = 5, only received normal saline), DM group (n = 8) and AST + DM group (n = 8), respectively. In control group, animals were gavage of normal saline for 8 weeks, while in the DM group, DM rats were subject to gavage of normal saline for 8 weeks. In AST + DM group (n = 8), DM rats were treated by gavage of AST (50 mg/kg per day) for 8 weeks. All groups were gavaged once daily at the same time for 8 weeks. At week 8, renal function and renal morphometry were measured. Those with renal failure were diagnosed with DN according to the previous description (Korrapati et al., 2012).
The kidney tissue sections were fixed in 4% paraformaldehyde, rinsed with PBS, incubated in 0.5% Triton X-100 for 20 min. Upon washing with PBS three times, the renal tissues were then blocked with 1% BSA in PBS for 30 min. Primary antibodies including anti-P53 (EP155Y, 1:150, Santa Cruz, USA), anti-Drp1 (4F6, 1:150, Santa Cruz, USA) were incubated at 4°C overnight. Subsequently, the treated renal tissues were rinsed with PBS and then incubated with HRP conjugated secondary antibodies including goat anti-mouse anti-P53 (ZD2305, Cell Signaling Tech, USA) and goat anti-rabbit anti-Drip1 (ZD2301, Cell Signaling Tech, USA) for one hour at room temperature. DEPI was used for nuclear staining 5 min, and then washed with PBS. Renal tissues were mounted in glycerine and detected under a laser scanning confocal microscope (Leica, USA).
Protein was extracted from renal tissues as conventionally described. Protein concentration was quantified using BCA method. Protein sample (40 μg) was separated on 15% SDS-PAGE gel for 2 h, followed by transferring to polyvinylidene difluoride (PVDF) membrane. Afterwards, the mixture was blocked in 5% non-fat milk for 2 h at room temperature and was incubated with primary antibodies including anti-P53 (EP155Y, 1:2500, Santa Cruz, USA) and anti-Drp1 (4F6, 1:2000, Santa Cruz, USA) at 4°C overnight. Then the membrane was washed using tris-buffered saline for three times, followed by incubating with HRP conjugated secondary antibodies including goat anti-mouse P53 antibody (1:5000, ZD2305, Cell Signaling Tech, USA) and goat anti-rabbit Drip1 antibody (1:5000, ZD2301, Cell Signaling Tech, USA) for 1 h at room temperature. Finally, bands were visualized by enhanced chemiluminescence. GAPDH (1:800) was utilized as internal standard.
Body weight, physical activity and blood glucose were measured in each group at weeks 0, 2, 4, 6 and 8, respectively. Blood glucose was determined using commercial kits (Beyotime, China). Blood was taken from rat tail vein and detected by rapid blood glucose meter. The contents of serum creatinine (SCR) and urea nitrogen (BUN) were measured by automatic biochemical analyzer at week 8. Blood was taken from the abdominal aorta of rats, and according to the manufacturer’s instructions. All the tests were performed at least in triplicate.
Data were expressed as mean ± standard error of mean (SEM) from the tests that were conducted at least in triplicate. Statistical analysis was performed with SPSS19.0 software by one-way ANOVA and Student’s t-test. Correlation analysis was explored by Spearman’s correlation coefficient. A P value < 0.05 was considered to be statistically significant.
Effects of AST on physical activity
All rats in normal control group survived with normal physical appearance and behavior. The fur was smooth, and body weight showed increase. One rat died in DM group, and the surviving rats exhibited unresponsive behavior and rough fur, together with significant decline in body weight. Six rats (6/7) showed cataracts. The surviving rats in AST + DM group exhibited better behavior with less rough fur. Two rats (2/8) presented cataracts. Compared with control group, significant decline was noticed in body weight in rats of DM group (P < 0.05). Compared with DM group, rats in AST + DM group showed increase in body weight (P < 0.05, Fig. 1a).
Effects of AST on blood glucose
There was significant elevation in blood glucose concentration in DM group compared with that of control group at weeks 2, 4, 6 and 8, respectively (P < 0.01). AST + DM group showed a significant decrease in blood glucose level at weeks 4, 6 and 8 compared with DM group (P < 0.05, Fig. 1b).
AST delayed DN pathogenesis through improving renal function
According to our experiences, the majority of DM rats began to present DN symptoms at week 8 after DM induction (data not shown). In this part, we determined effects of AST on renal function at week 8 by determining concentrations of Scr and BUN in DM rats. The prevalence of DN in DM group was significantly higher than that of control group, while the prevalence of DN rats in AST + DM group showed significant decline compared with that of DM group (P < 0.05). The Scr level in DM group was significantly higher at week 8 compared with control group (P < 0.05). After treating with AST, significant decrease was noticed in Scr compared with DM group (P < 0.05, Fig. 1c). In addition, the BUN in DM group was significantly higher than that of control group at week 8 (P < 0.05). In AST + DM group, the BUN was significantly lower compared with DM group (P < 0.05, Fig. 1d). Taken together, AST could delay the progression of DN in DM rats.
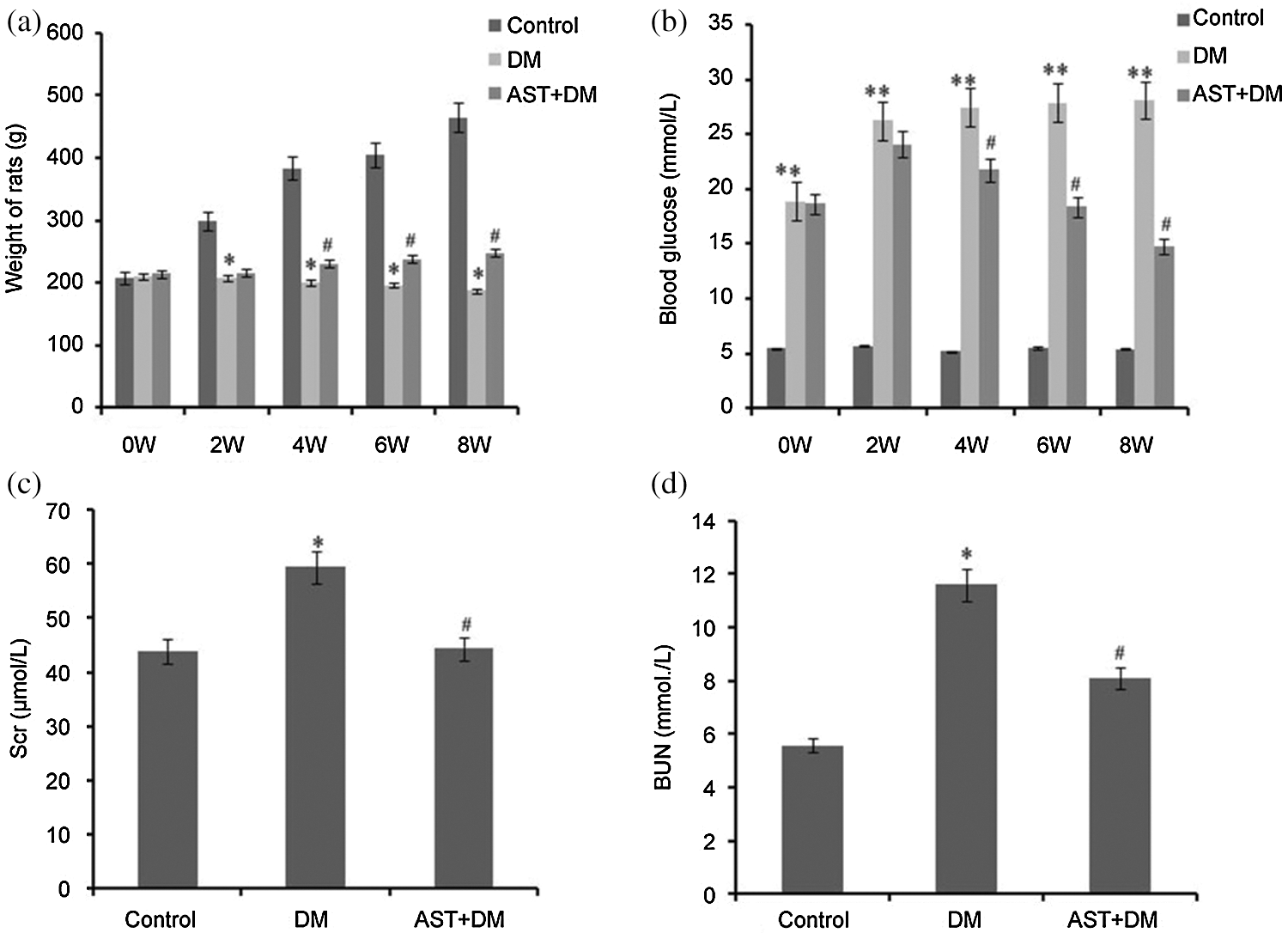
Figure 1: Body weight (a), blood glucose (b), Scr (c), and BUN (d) in each group. (a) *P < 0.05, compared with the control group. #P < 0.05, compared with the DM group. (b) **P < 0.01, compared with the control group. #P < 0.05, compared with the DM group. (c) *P < 0.05, compared with the control group. #P < 0.05, compared with the DM group. (d) *P < 0.05, compared with the control group. #P < 0.05, compared with the DM group. Statistical analysis was performed using ANOVA. Data were presented as mean ± standard deviation (SD).
AST decreased expression of P53 and Drp1 in renal tissues in DM rats
Expression of P53 showed increase in renal tissues in DM rats compared with control group (Fig. 2a). Compared with DM group, AST treatment led to significant decrease in P53 expression (P < 0.05). In AST + DM group, significant decline was noticed in the expression of P53 compared with DM group (P < 0.01, Fig. 2b). Compared with control group, the Drp1 expression in renal tissues in DM group was significantly higher (P < 0.05). Compared with DM group, there was significant decline in Drp1 expression after treating with AST (P < 0.01, Figs. 2c and 2d). Taken together, AST decreased expression of P53 and Drp1 in renal tissues in DM rats.
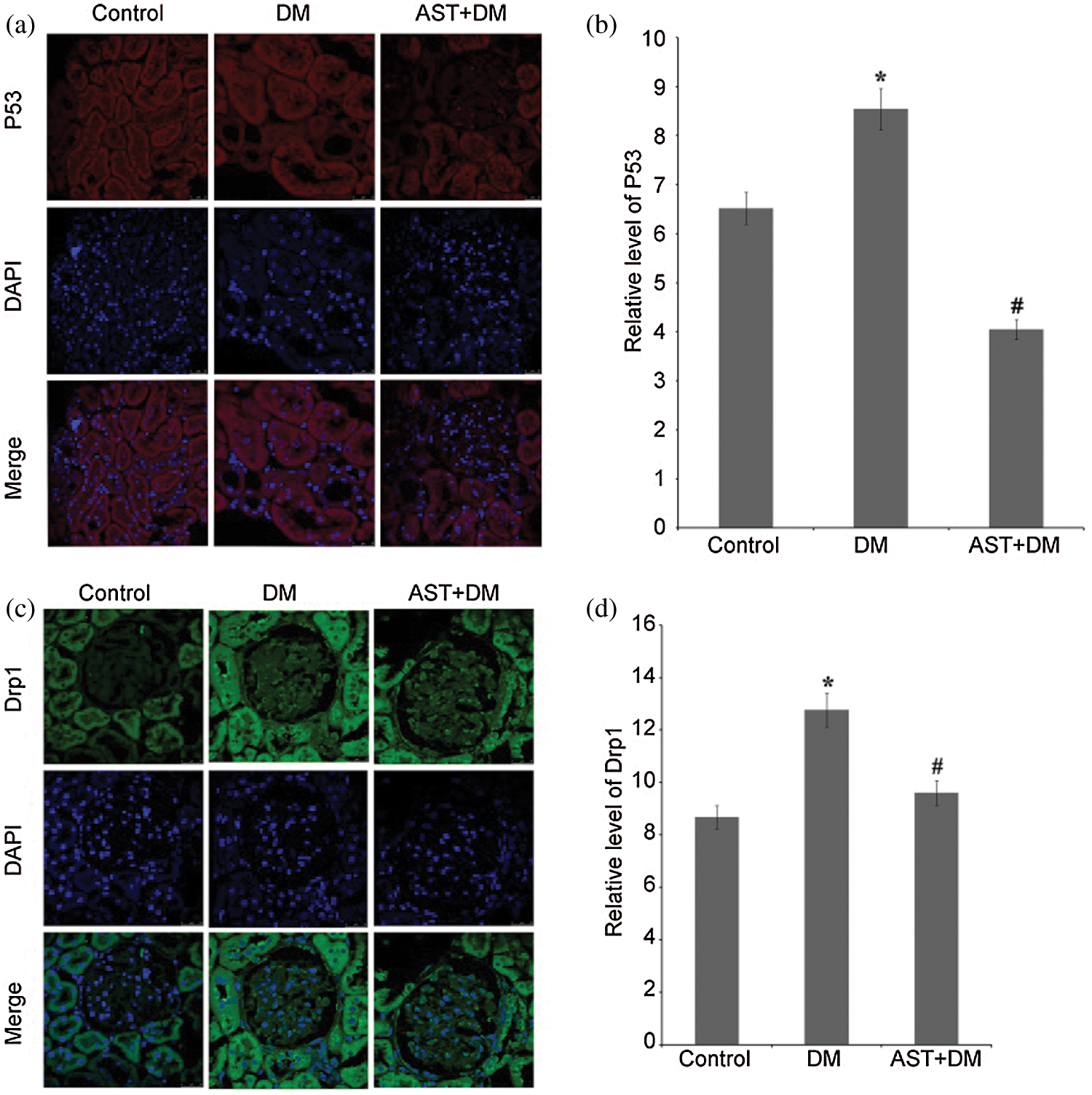
Figure 2: Immunofluorescence staining results and fluorescence intensity evaluation for P53 and Drp1. (a) P53 expression in renal tissues was stained in red, and nuclei was stained in blue. (b) The mean fluorescence intensity of P53 in each whole image was quantified. Data were presented as mean ± standard error of mean (SEM) in five animals per group. *P < 0.05, compared with the control group. #P < 0.01, compared with the DM group. (c) Drp1 expression in renal tissues was stained in green and nuclei was stained in blue. (d) Mean fluorescence intensity of Drp1 in five animals per group. *P < 0.05, compared with the control group. #P < 0.05, compared with the DM group.
Compared with control group, P53 expression showed increase in DM rats (P < 0.05, Fig. 3a). Compared with DM group, significant down-regulation of P53 was observed in AST + DM group (P < 0.01, Fig. 3b). Drp1 expression showed elevation in DM rats compared with that of control, while AST inhibited Drp1 expression in AST + DM group compared with DM (P < 0.05, Fig. 3c). We concluded that expression of P53 and Drp1 in renal tissues was up-regulated in DM rats. After AST treatment, there was down-regulation of Drp1 and P53 in renal tissues.
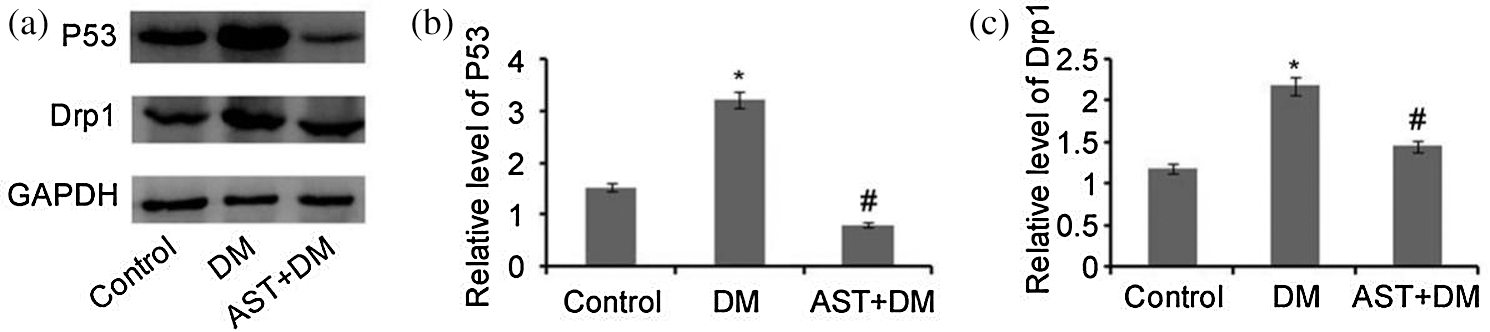
Figure 3: Western blot analysis of P53 and Drp1. (a) P53 and Drp1 were analyzed by Western blot. Levels of Drp1 and P53 were normalized to GAPDH. (b) The relative expression of P53, *P < 0.05, compared with the control group. #P < 0.05, compared with the DM group. (c) Relative expression of Drp1. *P < 0.05, compared with the control group. #P < 0.05, compared with the DM group.
DN affecting approximately 20%–40% of DM patients worldwide is the leading cause of end-stage renal failure (Kaikini et al., 2021). Inflammation and oxidative stress have been reported to play crucial roles in pathogenesis and progression of DM and DN. To date, AST is considered to be effective for treating DM through regulating the antioxidant process (Kanwugu et al., 2021). In this study, we aim to explore the effects of AST on hyperglycemia and kidney function. Our data showed that AST could decrease the blood glucose, protect the renal function and delay the progression of DN, which may be associated with the down-regulation of Drp1 and P53 in DM rats.
AST is a fat soluble compound with good biological activity (Yuan et al., 2011). It could significantly modulate immune function under in vitro and in vivo conditions (Ranga Rao et al., 2010). Besides, it could reduce the oxidative stress caused by hyperglycemia in pancreatic β-cells, and improve glucose and serum insulin levels (Uchiyama et al., 2002). Moreover, it also inhibited glycation and glycated protein induced cytotoxicity in human umbilical vein endothelial cells by preventing lipid/protein oxidation (Nishigaki et al., 2010). In a previous study, AST may play an important role in reducing oxidative damages and prevention of pathological changes in diabetic rats (Sila et al., 2015). Furthermore, AST could protect against kidney damage associated with diabetes and prevent DN by modulating oxidative stress and renal cell damages (Naito et al., 2004). AST has been used in treating cardiovascular diseases (Visioli and Artaria, 2017; Wang et al., 2021), ischemic brain damages (Kuo et al., 2019; Zhang et al., 2021) and DN (Chen et al., 2020b). Specifically, AST would involve in these diseases through modulating mitochondrial permeability transition, neuron loss and apoptosis (Song et al., 2014; Zhang et al., 2016). Our study suggested BUN showed significant increase in DM group compared with the control group at week 8. In contrast, after AST administration, the BUN showed significant decline compared with DM group. On this basis, we speculated that AST can delay renal injury in diabetic rats. In a previous study, AST acted as a protective factor in DN, which was associated its renoprotective and anti-diabetic effects (Qamar et al., 2020). Our data showed that AST induced down-regulation of P53 and Drp1 in DM rats, which presenting delay in the DN progression. In future, more studies are required to investigate the roles of P53 and Drp1 in the DN delay induced after AST.
Mitochondrial dysfunction has been reported to be closely related to the onset of DN. P53 promoted formation of highly interconnected and elongated mitochondria prior to onset of cellular senescence. Besides, P53-induced mitochondrial elongation resulted in mitochondrial dysfunction and subsequent increase in intracellular ROS (Kim et al., 2020). Previously, SIRT1/P53 signaling pathway was related to mitochondria-mediated apoptosis (Zhang et al., 2020). In addition, deletion of P53 decreased cardiac injury by protecting mitochondria through attenuating OS and calpain activation during ischemia-reperfusion (Chen et al., 2020a). Although several proapoptotic factors have been identified to be able to mediate the signals of P53, the exact molecular mechanism by which P53 activates the intrinsic apoptotic pathway remains to be fully elucidated. In this study, AST administration could induce the down-regulation of P53 in DN rats.
Drp1 is a GTPase that causes scission of the mitochondrial outer membrane, resulting in fission of mitochondrial tubules into fragments (Cho et al., 2010). It is also responsible for cytochrome C release and caspase activation (Estaquier and Arnoult, 2007). Meanwhile, binding of Drp1 to P53 induced mitochondria-related necrosis (Guo et al., 2014). In this study, our data showed that AST induced the down-regulation of Drp1 in DN rats. In future, we will focus on the silencing of Drp1 gene in DM rats, in order to further illustrate the potential roles in the pathogenesis of DN and investigate the mitochondrial morphology.
It has been well acknowledged that there is a mutual interaction between P53 and Drp1. To our best knowledge, P53 can upregulate Drp1 expression in a transcription-dependent manner, while Drp1 can convey the apoptotic signal of P53 by inducing mitochondrial fission. Meanwhile, P53 and Drp1 constituted an axis in regulating mitochondrial fission, which triggered apoptosis. Specifically, P53 mediated apoptotic pathways in neurons through inhibition of Drp1-dependent P53 mitochondrial translocation (Filichia et al., 2016). Additionally, P53/Drp1-dependent mitochondrial fission mediated aldosterone-induced podocyte injury and mitochondrial dysfunction (Yuan et al., 2018). In this study, the expression of P53 and Drp1 was measured by immunofluorescence and Western blot, which demonstrated up-regulation of P53 and Drp1 in DN rats. However, expression of P53 and Drp1 showed significant decrease in AST + DM group compared with the DM rats. According to the previous description, the expression pattern (i.e., up-regulation and down-regulation) of Drp1 was usually consistent with that of P53, and the effects of Drp1 may be mediated by P53 pathway (Tilokani et al., 2018; Wang et al., 2014). In summary, our study revealed the beneficial effects of AST on treatment of DN. We indicated the specific roles of AST in regulating P53 and Drp1, and there might be potential link between their expression and DN delay. In future, this requires more investigation and may provide a novel strategy for the treatment of DN.
There are some limitations in our study. First, the sample size is not large, there is really death for the modelling group in our study. Second, we could not figure out how P53 and Drp1 involved in attenuating DN after AST administration.
In summary, AST could effectively reduce the blood glucose in DM rats, and delay the progression of DN. The protective effects of AST in delaying DN progression may be related to the downregulation of Drp1 and P53. Thus, future works are required to examine the potential effects of AST in this process.
Availability of Data and Materials: All data generated or analyzed during this study are included in this published article.
Authors’ Contribution: The authors confirm contribution to the paper as follows: study conception and design: ZXM; data collection: MLH, LSP, ZXW; analysis and interpretation of results: QWW, MLN; draft manuscript preparation: MLH. All authors reviewed the results and approved the final version of the manuscript.
Ethics Approval: This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Binzhou Medical University (Approval No. 2021-241, March 2021).
Funding Statement: This study was supported by Yantai Science and Technology Plan Project [Grant No. 2018ZHGY088].
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Al-Awar A, Kupai K, Veszelka M, Szűcs G, Attieh Z, Murlasits Z, Török S, Pósa A, Varga C (2016). Experimental diabetes mellitus in different animal models. Journal of Diabetes Research 2016: 1–12. DOI 10.1155/2016/9051426. [Google Scholar] [CrossRef]
Chen Q, Thompson J, Hu Y, Lesnefsky EJ (2020a). Cardiomyocyte specific deletion of p53 decreases cell injury during ischemia-reperfusion: Role of Mitochondria. Free Radical Biology and Medicine 158: 162–170. DOI 10.1016/j.freeradbiomed.2020.06.006. [Google Scholar] [CrossRef]
Chen Z, Li W, Shi L, Jiang L, Li M, Zhang C, Peng H (2020b). Kidney-targeted astaxanthin natural antioxidant nanosystem for diabetic nephropathy therapy. European Journal of Pharmaceutics and Biopharmaceutics 156: 143–154. DOI 10.1016/j.ejpb.2020.09.005. [Google Scholar] [CrossRef]
Cho SG, Du Q, Huang S, Dong Z (2010). Drp1 dephosphorylation in ATP depletion-induced mitochondrial injury and tubular cell apoptosis. American Journal of Physiology—Renal Physiology 299: F199–F206. DOI 10.1152/ajprenal.00716.2009. [Google Scholar] [CrossRef]
Estaquier J, Arnoult D (2007). Inhibiting Drp1-mediated mitochondrial fission selectively prevents the release of cytochrome c during apoptosis. Cell Death and Differentiation 14: 1086–1094. DOI 10.1038/sj.cdd.4402107. [Google Scholar] [CrossRef]
Filichia E, Hoffer B, Qi X, Luo Y (2016). Inhibition of Drp1 mitochondrial translocation provides neural protection in dopaminergic system in a Parkinson’s disease model induced by MPTP. Scientific Reports 6: 32656. DOI 10.1038/srep32656. [Google Scholar] [CrossRef]
Fonseca TB, Sánchez-Guerrero Á, Milosevic I, Raimundo N (2019). Mitochondrial fission requires DRP1 but not dynamins. Nature 570: E34–E42. DOI 10.1038/s41586-019-1296-y. [Google Scholar] [CrossRef]
Guo X, Sesaki H, Qi X (2014). Drp1 stabilizes p53 on the mitochondria to trigger necrosis under oxidative stress conditions in vitro and in vivo. Biochemical Journal 461: 137–146. DOI 10.1042/BJ20131438. [Google Scholar] [CrossRef]
Kaikini AA, Muke S, Peshattiwar V, Bagle S, Dighe V, Sathaye S (2021). Ethyl ferulate, a lipophilic phenylpropanoid, prevents diabetes-associated renal injury in rats by amelioration of hyperglycemia-induced oxidative stress via activation of nuclear factor erythroid 2-related factor 2. Journal of Food Biochemistry 45: 1445. DOI 10.1111/jfbc.13607. [Google Scholar] [CrossRef]
Kanwugu ON, Glukhareva TV, Danilova IG, Kovaleva EG (2021). Natural antioxidants in diabetes treatment and management: Prospects of astaxanthin. Critical Reviews in Food Science and Nutrition 12: 1–24. DOI 10.1080/10408398.2021.1881434. [Google Scholar] [CrossRef]
Kim YY, Um JH, Yoon JH, Lee DY, Lee YJ, Kim DH, Park JI, Yun J (2020). p53 regulates mitochondrial dynamics by inhibiting Drp1 translocation into mitochondria during cellular senescence. FASEB Journal 34: 2451–2464. DOI 10.1096/fj.201901747RR. [Google Scholar] [CrossRef]
Korrapati MC, Shaner BE, Neely BA, Alge JL, Arthur JM, Schnellmann RG (2012). Diabetes-induced renal injury in rats is attenuated by Suramin. Journal of Pharmacology and Experimental Therapeutics 343: 34–43. DOI 10.1124/jpet.112.196964. [Google Scholar] [CrossRef]
Kuo MH, Lee HF, Tu YF, Lin LH, Cheng YY, Lee HT (2019). Astaxanthin ameliorates ischemic-hypoxic-induced neurotrophin receptor p75 upregulation in the endothelial cells of neonatal mouse brains. International Journal of Molecular Sciences 20: 6168. DOI 10.3390/ijms20246168. [Google Scholar] [CrossRef]
Naito Y, Uchiyama K, Aoi W, Hasegawa G, Nakamura N, Yoshida N, Maoka T, Takahashi J, Yoshikawa T (2004). Prevention of diabetic nephropathy by treatment with astaxanthin in diabetic db/db mice. BioFactors 20: 49–59. DOI 10.1002/biof.5520200105. [Google Scholar] [CrossRef]
Nishigaki I, Rajendran P, Venugopal R, Ekambaram G, Sakthisekaran D, Nishigaki Y (2010). Cytoprotective role of astaxanthin against glycated protein/iron chelate-induced toxicity in human umbilical vein endothelial cells. Phytotherapy Research 24: 54–59. DOI 10.1002/ptr.2867. [Google Scholar] [CrossRef]
Qamar AY, Fang X, Bang S, Shin ST, Cho J (2020). The effect of astaxanthin supplementation on the post-thaw quality of dog semen. Reproduction in Domestic Animals 55: 1163–1171. DOI 10.1111/rda.13758. [Google Scholar] [CrossRef]
Ranga Rao A, Raghunath Reddy RL, Baskaran V, Sarada R, Ravishankar GA (2010). Characterization of microalgal carotenoids by mass spectrometry and their bioavailability and antioxidant properties elucidated in rat model. Journal of Agricultural and Food Chemistry 58: 8553–8559. DOI 10.1021/jf101187k. [Google Scholar] [CrossRef]
Serasinghe MN, Chipuk JE (2017). Mitochondrial fission in human diseases. In: Handbook of Experimental Pharmacology, vol. 240, pp. 159–188. DOI 10.1007/978-3-319-57313-7. [Google Scholar] [CrossRef]
Sila A, Ghlissi Z, Kamoun Z, Makni M, Nasri M, Bougatef A, Sahnoun Z (2015). Astaxanthin from shrimp by-products ameliorates nephropathy in diabetic rats. European Journal of Nutrition 54: 301–307. DOI 10.1007/s00394-014-0711-2. [Google Scholar] [CrossRef]
Song X, Wang B, Lin S, Jing L, Mao C, Xu P, Lv C, Liu W, Zuo J (2014). Astaxanthin inhibits apoptosis in alveolar epithelial cells type II in vivo and in vitro through the ROS-dependent mitochondrial signalling pathway. Journal of Cellular and Molecular Medicine 18: 2198–2212. DOI 10.1111/jcmm.12347. [Google Scholar] [CrossRef]
Tilokani L, Nagashima S, Paupe V, Prudent J (2018). Mitochondrial dynamics: Overview of molecular mechanisms. Essays in Biochemistry 62: 341–360. DOI 10.1042/EBC20170104. [Google Scholar] [CrossRef]
Uchiyama K, Naito Y, Hasegawa G, Nakamura N, Takahashi J, Yoshikawa T (2002). Astaxanthin protects beta-cells against glucose toxicity in diabetic db/db mice. Redox Report 7: 290–293. DOI 10.1179/135100002125000811. [Google Scholar] [CrossRef]
Visioli F, Artaria C (2017). Astaxanthin in cardiovascular health and disease: Mechanisms of action, therapeutic merits, and knowledge gaps. Food & Function 8: 39–63. DOI 10.1039/C6FO01721E. [Google Scholar] [CrossRef]
Wang DB, Kinoshita C, Kinoshita Y, Morrison RS (2014). p53 and mitochondrial function in neurons. Acta Biochimica et Biophysica Sinica 1842: 1186–1197. DOI 10.1016/j.bbadis.2013.12.015. [Google Scholar] [CrossRef]
Wang W, Liu T, Liu Y, Yu L, Yan X, Weng W, Lu X, Zhang C (2021). Astaxanthin attenuates alcoholic cardiomyopathy via inhibition of endoplasmic reticulum stress-mediated cardiac apoptosis. Toxicology and Applied Pharmacology 412: 115378. DOI 10.1016/j.taap.2020.115378. [Google Scholar] [CrossRef]
Yuan JP, Peng J, Yin K, Wang JH (2011). Potential health-promoting effects of astaxanthin: A high-value carotenoid mostly from microalgae. Molecular Nutrition & Food Research 55: 150–165. DOI 10.1002/mnfr.201000414. [Google Scholar] [CrossRef]
Yuan Y, Zhang A, Qi J, Wang H, Liu X et al. (2018). p53/Drp1-dependent mitochondrial fission mediates aldosterone-induced podocyte injury and mitochondrial dysfunction. American Journal of Physiology-Renal Physiology 314: F798–F808. DOI 10.1152/ajprenal.00055.2017. [Google Scholar] [CrossRef]
Zhang J, Zou Y, Cheng-Jing Y, Xiang-Heng L, Wang XP, Yu XJ, Li GS, Wang J (2020). Pioglitazone alleviates cisplatin nephrotoxicity by suppressing mitochondria-mediated apoptosis via SIRT1/p53 signalling. Journal of Cellular and Molecular Medicine 24: 11718–11728. DOI 10.1111/jcmm.15782. [Google Scholar] [CrossRef]
Zhang XS, Lu Y, Li W, Tao T, Peng L et al. (2021). Astaxanthin ameliorates oxidative stress and neuronal apoptosis via SIRT1/NRF2/Prx2/ASK1/p38 after traumatic brain injury in mice. British Journal of Pharmacology 178: 1114–1132. DOI 10.1111/bph.15346. [Google Scholar] [CrossRef]
Zhang ZW, Xu XC, Liu T, Yuan S (2016). Mitochondrion-permeable antioxidants to treat ROS-burst-mediated acute diseases. Oxidative Medicine and Cellular Longevity 2016: 1–10. DOI 10.1155/2016/6859523. [Google Scholar] [CrossRef]
Zhou H, Du W, Li Y, Shi C, Hu N, Ma S, Wang W, Ren J (2018). Effects of melatonin on fatty liver disease: The role of NR4A1/DNA-PKcs/p53 pathway, mitochondrial fission, and mitophagy. Journal of Pineal Research 64: e12450. DOI 10.1111/jpi.12450. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |