DOI:10.32604/biocell.2022.019363

| BIOCELL DOI:10.32604/biocell.2022.019363 |  |
| Review |
Mesenchymal stem cell secretome and nanotechnology: Combining therapeutic strategies
Laboratory of Stem Cell Research, Roberto Alcantara Gomes Biology Institute, Rio de Janeiro State University, Rio de Janeiro, 20540170, Brazil
*Address correspondence to: Alessandra A. Thole, alethole@uol.com.br
Received: 19 September 2021; Accepted: 13 December 2021
Abstract: Mesenchymal stem cells (MSC) have pushed the field of stem cell-based therapies by inducing tissue regeneration, immunosuppression, and angiogenesis mainly through vesicles and soluble factors release (paracrine signaling). MSC-extracellular vesicles (MSC-EV) adaptable secretome and homing to injured sites allowed researchers to unlock a new era of cell-free based therapy. In parallel, nanoparticles (NP) have been explored in contributing to transport and drug delivery systems, giving drugs desired physical-chemical properties to exploit cell behavior. However, NPs can be quickly recognized by immune cells and cleared from circulation. In this viewpoint, we explore how combining both therapeutic strategies can improve efficacy and circumvent limitations of both therapies. MSC-EV benefit from the potent MSC membrane composition, guiding chemotaxis to tumor sites, a very restricted microenvironment. MSC-EV has low immunogenicity, high stability, long half-life and can explore tissue targeting ligands as a precise drug carry, even across biological barriers. Those properties promote enhanced targeted drug delivery that can be combined with NP, exploring biological membrane production through: 1. direct cell therapy with NP-infused MSC; 2. NP-containing MSC-EV generated by NP-infused MSC; 3. by coating NP in MSC membrane (“MSC NanoGhosts”), allowing precise cargo definition without losing targeting. Therefore, nanotechnology combined with cell-based therapeutic resources can greatly improve targeted drug delivery, improving efficacy and opening a new venue of therapeutic possibilities.
Keywords: Mesenchymal stem cells; Nanoparticles; Secretome; Exosome; Targeted drug delivery
Mesenchymal stem cells (MSC) have quickly become pivotal in stem cell-based therapies during the last years. This phenomenon is due to their ability to induce regeneration, improve tissue homeostasis, and the immunosuppressive ability to repair damaged tissues releasing a range of factors (Gao et al., 2016). Important research has proven that MSC exerts therapeutic effects not only through the physical proximity of transplanted cells to damaged tissue but mainly by the paracrine effect (Harrell et al., 2019). MSCs are known to release a myriad of bioactive molecules, including growth factors, cytokines, miRNA, mRNA, microvesicles, and exosomes (Rani et al., 2015). The ability of MSCs homing to injured sites and release their secretome allowed researchers to unlock a new era of cell-free-based therapy.
Even though MSC displays local homing and differentiation into mesenchymal trilineage, limitations remained such as comprehension of long-term tissue interaction and MSCs (Ding et al., 2011; Gomez-Salazar et al., 2020). The MSC secretome showed similar benefits to those observed after transplantation of MSC. The main advantage which makes the secretome very attractive is the possibility to set a specific cocktail of molecules best fitted to each therapy. In the secretome, we find MSC-EV and soluble factors produced in vitro, during MSC preconditioning with specific cytokines to induce the desired response according to specific disease models (Ferreira et al., 2018). MSC-EV is a powerful tool in cell-free therapy, alleviating immune response, inducing angiogenesis, and anti-apoptotic effects, providing tissue regeneration and repair (Vishnubhatla et al., 2014). Also, they avoid invasive procedures, mitigate the risk of unwanted cellular differentiation, embolism formation with relative uncomplicated production and storage, thus an efficient approach compared to cellular methods (Ghafouri-Fard et al., 2021; Sun et al., 2019; Vizoso et al., 2017).
MSC-EVs are a sophisticated and complex way of cellular communication, which are categorized according to their origin and size (Ferreira et al., 2018; Vizoso et al., 2017; Yáñez-Mó et al., 2015). Microvesicles (100–1,000 nm) are the larger class of MSC-EV size that interacts through microtubules and SNARE proteins to deliver their contents to neighboring or distant sites. Their formation occurs by outward budding or protrusion of the plasma membrane (Doyle and Wang, 2019; Eleuteri and Fierabracci, 2019; Ståhl et al., 2019). Conversely, exosomes are smaller membrane-bound vesicles (30 nm–100 nm) protected by lipid bilayer membrane and released through exocytosis. They are formed in the intracellular compartment, through endosomes that fuse with endocytic vesicles, known as multivesicular bodies (MVB). Then, MVB containing intraluminal vesicles merge with the plasma membrane and empty their content into extracellular space (Doyle and Wang, 2019; Eleuteri and Fierabracci, 2019). The MSC-EVs internalization in target cells can occur through ligands, allowing precise binding, cell entrance, and release of the substance into the cytoplasm, exerting effects in recipient cells. Also, MSC-EV display a long-circulating half-life (Doyle and Wang, 2019; Lei et al., 2021; Vizoso et al., 2017). Particularly, both MSC-EV types contain miRNA, mRNA, lipids, and proteins that differ according to MSC tissue source and preconditioning (Ferreira et al., 2018; Eleuteri and Fierabracci, 2019; Lei et al., 2021; Shin et al., 2021).
The soluble factors are also one of the cornerstones by which MSC exert their therapeutic effects. Also referred to as trophic factors, they are part of MSC’s repair machinery composed of cytokines, growth factors, hormones, and pro-angiogenic factors (Ferreira et al., 2018; Zhao et al., 2020).
For a given therapeutic proposal, preconditioning MSC using proinflammatory cytokines, such as IFN-γ, TNF-α, or IL1-β, downregulated both inflammation and immune response mainly mediated by the release of IDO, PGE2, IL-1RA, and IL-10, as well several cytokines and chemokines, regulating innate/adaptive immune system (Ferreira et al., 2018; Aggarwal and Pittenger, 2005; Harrell et al., 2020; Jiang and Xu, 2020). Preconditioning with hypoxia or growth factors protocols using BDNF, GDNF, HIF1-α, IGF-1 are described as inducing VEGF production (Ge et al., 2018; Haider et al., 2008; Tögel et al., 2009) and reducing fibrosis in vivo (Ferreira et al., 2018) (Fig. 1).
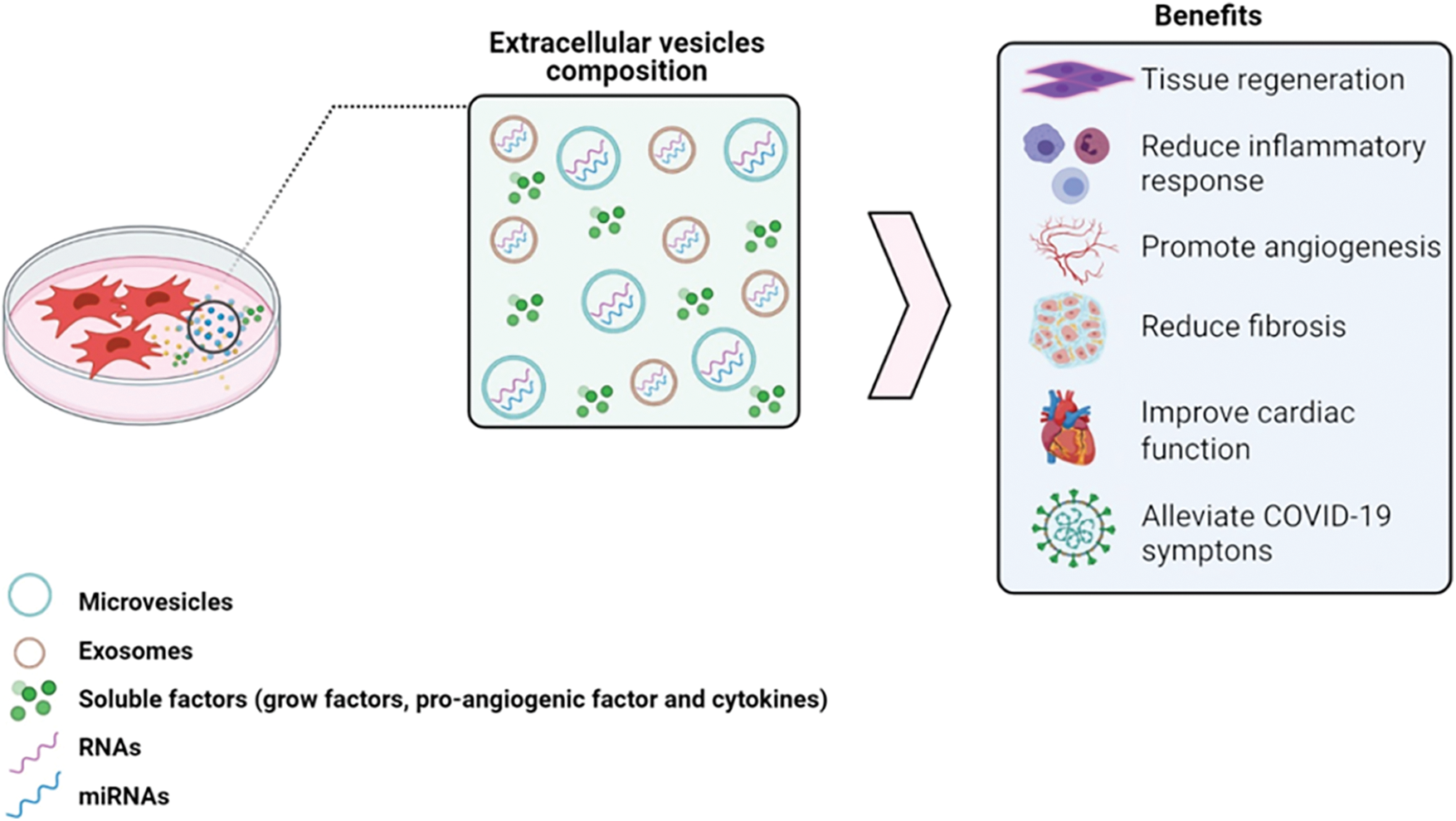
Figure 1: Composition of secretome responsible for the therapeutic effects of MSCs. The secretome is rich in soluble factors and MSC-EV that act to control inflammation and stimulate tissue regeneration, which represents potential strategies to treat several diseases. Mesenchymal stem cells-extracellular vesicles (MSC-EV). Image made with BioRender.com.
The damaged tissue is often exposed to conditions of enhanced reactive oxygen species production by aerobic metabolism, which causes damage to cellular protein, DNA, and lipids, leading to increased apoptosis, and reduced cell proliferation. MSCs secretome has enzymes with antioxidant activity that help bypass the microenvironment with oxidative stress and promote repair (Chaudhari et al., 2014). MSC-EVs modulate the apoptotic response through anti-apoptotic proteins, in addition to high levels of pro-angiogenic factors, such as VEGF, contributing to the restructuring of the injured tissue (Kachgal and Putnam, 2011).
Through selectively packing miRNA and cytokines, MSC-EV can promote tissue remodeling, inhibiting fibrotic responses by resident fibroblasts (Shentu et al., 2017; Lira et al., 2017; Almeida et al., 2021). They also have cardioprotective capacity, reducing infarction extent and increasing myocardial viability (Lai et al., 2010; Arslan et al., 2013; Yu et al., 2015). Additionally, MSC secretome reduced inflammation in inflammatory bowel diseases (Liu et al., 2019). Recently, the world has been affected by the COVID-19 pandemic and the use of cell-based or secretory-based therapies has been shown to contribute to disease resolution. MSC or MSC-EV treatments normalized cell counts (Liang et al., 2020), reduced pro-inflammatory cytokines and serum chemokines, whereas increased the anti-inflammatory IL-10, reducing the cytokine storm, allowing lung tissue regeneration. (Leng et al., 2020; Shi et al., 2021; Paris et al., 2021).
Combining promising therapeutic strategies–Cell and NanoTechnology
As the therapeutic capacity of MSC’s secretome is continually validated in pre-clinical and clinical trials, with robust safety for patients, we can devise its use as a tool, prospecting future therapies that aggregate multiple technologies. Besides the cellular technology used in MSC identification, isolation, and therapeutic potential validation, nanotechnology has been emerging as a field of study with great integrative potential in medicine, contributing to transport and drug delivery systems, biocompatible nanomaterials, gene therapies, and even mRNA vaccines effectiveness, as recently demonstrated in COVID-19 pandemic (Keech et al., 2020). Nanotechnology development allows for precise modification in high-interest biomolecules, manipulating molecule’s size, structure, conductivity, reactivity, functional conformation, and melting temperature, which ultimately dictates its physical, chemical, and biological properties (Mazzeo and Santos, 2018).
Nanoparticles (NP) are small-sized (1 nm–100 nm (Vert et al., 2012)) lipid-based, polymeric, quantum dots, inorganic or metal-derived particles. NPs can be linked to various known drugs, thereby modifying their properties, improving drug accessibility to the target tissue and cellular drug uptake while reducing toxicity, cellular drug resistance, and efflux (Markman et al., 2013). Also, their intrinsic conditional activation by variations in pH, heat, ultrasound, light, and magnetic field enhances effectiveness, as seen in gold NPs use in radiotherapy (Mura et al., 2013; Laprise-Pelletier et al., 2018). Although NPs provide beneficial features to drugs, several limiting factors still prevail. NPs are quickly recognized by immune cells and cleared from the circulation by the mononuclear phagocyte system, and may present poor biocompatibility or biodegradability, restricting their utility as drug carriers (Gao et al., 2013). Therefore, nanotechnology combined with cell-based therapeutic resources has drawn interest due to low immunogenicity and targeted drug delivery (Wu et al., 2019).
A cell-based targeted delivery system has been developed granting low immunogenicity, low intrinsic mutation rate (Sotiropoulou et al., 2006), long circulation time, no neurotoxicity or tumorigenicity, and integration of receptors (Thanuja et al., 2018). MSC therapy holds those qualities as it has been proved safe in multiple physiological contexts and routes of administration. More importantly, transplanted MSC migrate towards injured tissue, inflammatory, and tumor sites (Hu et al., 2010; Kidd et al., 2008). Tumor tropism has been observed for MSCs either administered via intravenous (Yang et al., 2009), intraarterial (Nakamizo et al., 2005), or peritumoral routes (Hong et al., 2009; Wu et al., 2019).
NPs incorporation in biological systems such as in cells, vesicles, proteins, and genetic material allows for imaging/diagnostic detection and can improve targeted therapy, especially for tumors. Usually, the immunomodulated tumor microenvironment hampers drugs or cells entry. Drug-loaded NPs can be incorporated by cells through clathrin-mediated endocytosis pathways (Saulite et al., 2017) as intracellular drug depots and achieve sustained release, thereby decreasing anticancer-drug cytotoxicity and chemotherapeutic loss by rapid drug efflux, since drug delivery would be targeted to the tumor/inflamed area. Therefore MSC’s tropism for tumor sites is an extremely desired quality since the tumor microenvironment is very restrictive to new immunological cells entry as seen in CAR-T cell studies (Dapkute et al., 2017; de Boeck et al., 2013; Kidd et al., 2009; Moradian Tehrani et al., 2018; Sadhukha et al., 2014; Usha et al., 2013; Vegh et al., 2013).
And just as MSC, MSC-EV maintain chemotaxis towards tissue injury, inflammation, and tumor (Kriebel et al., 2018). The low immunogenicity, stability, long half-life, and tissue targeting ligands potentiate MSC-EV as an appropriate candidate for precise drug carriers even across different biological barriers (Heidarzadeh et al., 2021). Exosomes can cross the blood-brain barrier, a much-desired characteristic for drug delivery, and can readily access nearly all types of human biofluids, which make them promising biomarkers for gliomas (Cheng et al., 2020). In addition, MSC-EV membranes might also regulate the microenvironment as their receptors can act as decoy receptors, binding to ligands without generating the final response, diminishing continuous adverse signaling (Madsen et al., 2017; Harrell et al., 2020). Many of those properties arise from the MSC-derived membrane present in MSC-EV, an intrinsic benefit from using MSC’s secretome.
Furthermore, exosomes are selectively packaged by ESCRT-dependent or independent mechanisms associated with the plasma membrane (Colombo et al., 2013) and are highly variable depending upon the source of the parental cells, age, and pathophysiological conditions (Pegtel and Gould, 2019). It has been shown that the ESCRT-independent mechanism selects precise exosomal cargo via raft-based microdomains enriched in sphingomyelinases, controlling the lipid composition of exosomes during biogenesis (Zhang et al., 2019). After secretion, exosomes interact with neighboring or distant specific recipient cells through several ways, including ligand/receptor interaction, direct membrane fusion, and endocytosis, to modulate the activities of recipient cells (Heidarzadeh et al., 2021). MSC membrane is a crucial asset as it improves EV uptake by receiving cells (Shentu et al., 2017). Therefore, parental plasma membrane plays a role in MSC-EV cargo sorting and targeting, making MSC membrane of high interest.
Hence, synthetic technology has tried to emulate those desired qualities, but the highly complex composition and organization of MSC’s membrane makes it unable to precisely reproduce it with today’s technology, even though progress is being made as synthetic vesicles are now able to migrate towards a gradient (Pan et al., 2019).
Three protocols to explore this tool resorting to biological membrane production are:
1. Inserting NP-drug complex in MSC and using direct cell therapy, achieving condition-specific EV production, sustained release and targeted drug delivery;
2. Inserting NP-drug complex in MSC and letting them produce NP-drug-containing MSC-EV;
3. Striping MSC’s membranes to form engineered NP-drug-containing vesicles (MSC-EV-NPs), also called “MSC NanoGhosts” allowing precise control over EV cargo (Toledano Furman et al., 2013) (Fig. 2).
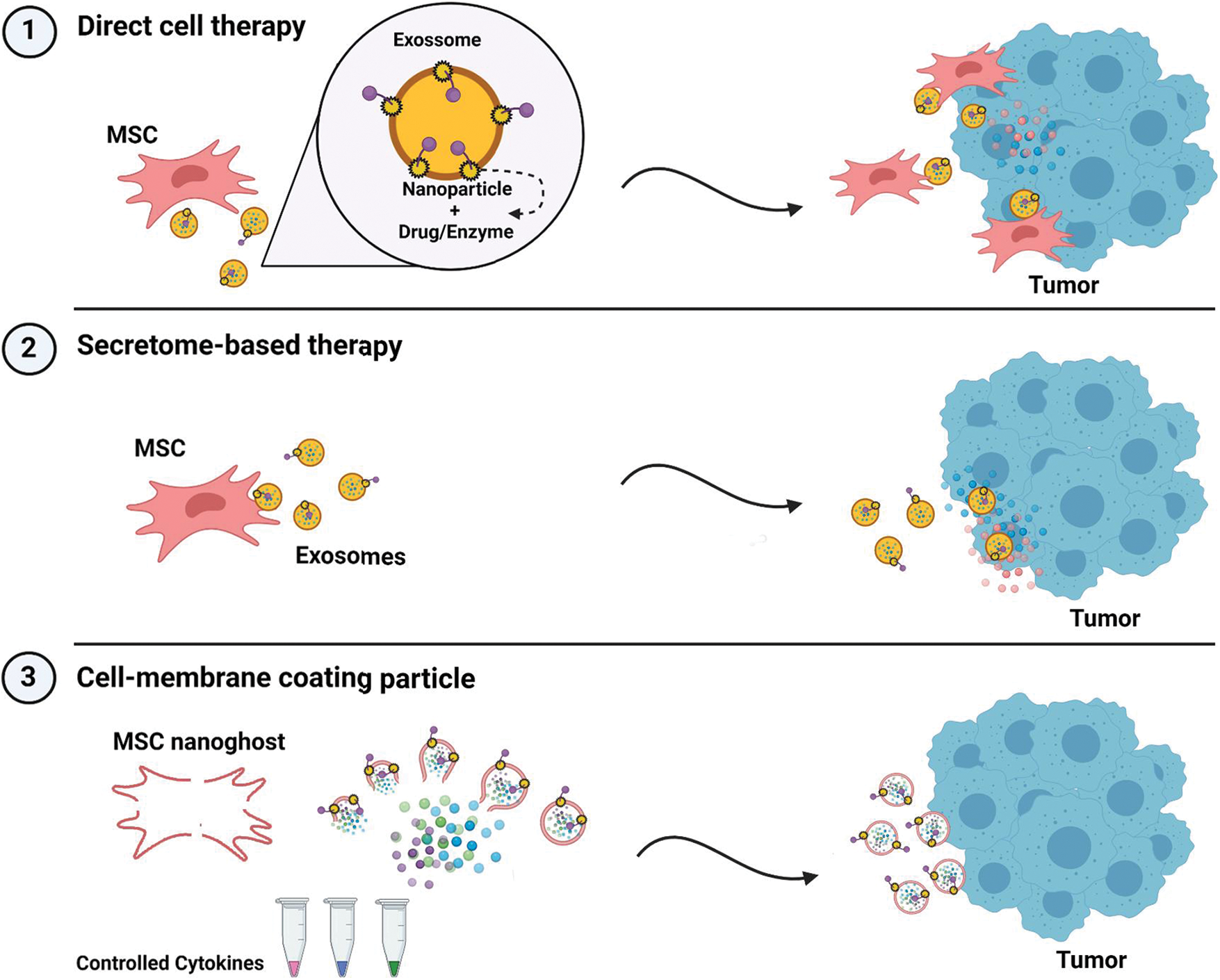
Figure 2: Protocols for combining MSC-EV and nanotechnology into an improved targeted drug delivery system. Nanoparticles can be inserted into the plasma membrane by selected affinity while carrying a drug or enzyme of interest. The complex NP + drug can vary in orientation. Thanks to MSC and MSC-EV homing ability, the complex can be carried to the tumor site while directly inserted in the membrane of a migrating cell (1) or inserted in an EV (2). As these qualities are membrane-specific, MSC membrane can be ruptured and used to make synthetic EV, precisely controlling cargo content (3). Image made with https://BioRender.com.
Therefore, this technique reflects a transfer of technology, upgrading targeted drug delivery and allowing complex interactions with the microenvironment that will lead to specific secretome production, improving efficacy. Nanotechnology combined with cell-based therapeutic resources has promising therapeutic potential as a targeted drug delivery platform with low immunogenicity.
Author Contributions: The authors jointly wrote the manuscript. A.L.F. and G.P.C. made the figures. E.A.C.C., S.N.C., L.C., A.A.T. revised the paper. All authors reviewed the results and approved the final version of the manuscript.
Funding Statement: This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-Brasil (CAPES)-Finance Code 001, the “Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq)”, and the “Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ)”.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Aggarwal S, Pittenger MF (2005). Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 105: 1815–1822. DOI 10.1182/blood-2004-04-1559. [Google Scholar] [CrossRef]
Almeida A, Lira R, Oliveira M, Martins M, Azevedo Y et al. (2021). Bone marrow-derived mesenchymal stem cells transplantation ameliorates renal injury through anti-fibrotic and anti-inflammatory effects in chronic experimental renovascular disease. Biomedical Journal 29: S2319–4170. DOI 10.1016/j.bj.2021.07.009. [Google Scholar] [CrossRef]
Arslan F, Lai RC, Smeets MB, Akeroyd L, Choo A et al. (2013). Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Research 10: 301–312. DOI 10.1016/j.scr.2013.01.002. [Google Scholar] [CrossRef]
Chaudhari N, Talwar P, Parimisetty A, Lefebvre d’Hellencourt C, Ravanan P (2014). A molecular web: Endoplasmic reticulum stress, inflammation, and oxidative stress. Frontiers in Cellular Neuroscience 8: 213. DOI 10.3389/fncel.2014.00213. [Google Scholar] [CrossRef]
Cheng J, Meng J, Zhu L, Peng Y (2020). Exosomal noncoding RNAs in Glioma: Biological functions and potential clinical applications. Molecular Cancer 19: 66. DOI 10.1186/s12943-020-01189-3. [Google Scholar] [CrossRef]
Colombo M, Moita C, van Niel G, Kowal J, Vigneron J et al. (2013). Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. Journal of Cell Science 126: 5553–5565. DOI 10.1242/jcs.128868. [Google Scholar] [CrossRef]
Dapkute D, Steponkiene S, Bulotiene D, Saulite L, Riekstina U et al. (2017). Skin-derived mesenchymal stem cells as quantum dot vehicles to tumors. International Journal of Nanomedicine 12: 8129–8142. DOI 10.2147/IJN. [Google Scholar] [CrossRef]
De Boeck A, Pauwels P, Hensen K, Rummens JL, Westbroek W et al. (2013). Bone marrow-derived mesenchymal stem cells promote colorectal cancer progression through paracrine neuregulin 1/HER3 signalling. Gut 62: 550–560. DOI 10.1136/gutjnl-2011-301393. [Google Scholar] [CrossRef]
Doyle LM, Wang MZ (2019). Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells 8: 727–728. DOI 10.3390/cells8070727. [Google Scholar] [CrossRef]
Ding DC, Shyu WC, Lin SZ (2011). Mesenchymal stem cells. Cell Transplantation 20: 5–14. DOI 10.3727/096368910X. [Google Scholar] [CrossRef]
Eleuteri S, Fierabracci A (2019). Insights into the secretome of mesenchymal stem cells and its potential applications. International Journal of Molecular Sciences 20: 4597. DOI 10.3390/ijms20184597. [Google Scholar] [CrossRef]
Ferreira JR, Teixeira GQ, Santos SG, Barbosa MA, Almeida-Porada G et al. (2018). Mesenchymal stromal cell secretome: Influencing therapeutic potential by cellular pre-conditioning. Frontiers in Immunology 9: 2837. DOI 10.3389/fimmu.2018.02837. [Google Scholar] [CrossRef]
Gao Z, Zhang L, Hu J, Sun Y (2013). Mesenchymal stem cells: A potential targeted-delivery vehicle for anti-cancer drug, loaded nanoparticles. Nanomedicine: Nanotechnology, Biology, and Medicine 9: 174–184. DOI 10.1016/j.nano.2012.06.003. [Google Scholar] [CrossRef]
Gao F, Chiu SM, Motan DAL, Zhang Z, Chen L et al. (2016). Mesenchymal stem cells and immunomodulation: Current status and future prospects. Cell Death and Disease 7: e2062. DOI 10.1038/cddis.2015.327. [Google Scholar] [CrossRef]
Ge Q, Zhang H, Hou J, Wan L, Cheng W et al. (2018). VEGF secreted by mesenchymal stem cells mediates the differentiation of endothelial progenitor cells into endothelial cells via paracrine mechanisms. Molecular Medicine Reports 17: 1667–1675. DOI 10.3892/mmr.2017.8059. [Google Scholar] [CrossRef]
Ghafouri-Fard S, Niazi V, Hussen BM, Omrani MD, Taheri M et al. (2021). The emerging role of exosomes in the treatment of human disorders with a special focus on mesenchymal stem cells-derived exosomes. Frontiers in Cell and Developmental Biology 9: 653296. DOI 10.3389/fcell.2021.653296. [Google Scholar] [CrossRef]
Gomez-Salazar M, Gonzalez-Galofre ZN, Casamitjana J, Crisan M, James AW et al. (2020). Five decades later, are mesenchymal stem cells still relevant? Frontiers in Bioengineering and Biotechnology 8: 148. DOI 10.3389/fbioe.2020.00148. [Google Scholar] [CrossRef]
Haider HK, Jiang S, Idris NM, Ashraf M (2008). IGF-1-Overexpressing mesenchymal stem cells accelerate bone marrow stem cell mobilization via paracrine activation of SDF-1α/CXCR4 signaling to promote myocardial repair. Circulation Research 21: 1300–1308. DOI 10.1161/CIRCRESAHA.108.186742. [Google Scholar] [CrossRef]
Harrell C, Fellabaum C, Jovicic N, Djonov V, Arsenijevic N et al. (2019). Molecular mechanisms responsible for therapeutic potential of mesenchymal stem cell-derived secretome. Cells 8: 467. DOI 10.3390/cells8050467. [Google Scholar] [CrossRef]
Harrell CR, Markovic BS, Fellabaum C, Arsenijevic N, Djonov V et al. (2020). The role of Interleukin 1 receptor antagonist in mesenchymal stem cell-based tissue repair and regeneration. BioFactors 46: 263–275. DOI 10.1002/biof.1587. [Google Scholar] [CrossRef]
Heidarzadeh M, Gürsoy-Özdemir Y, Kaya M, Eslami Abriz A, Zarebkohan A et al. (2021). Exosomal delivery of therapeutic modulators through the blood-brain barrier; promise and pitfalls. Cell & Bioscience 11. DOI 10.1186/s13578-021-00650-0. [Google Scholar] [CrossRef]
Hong X, Miller C, Savant-Bhonsale S, Kalkanis SN (2009). Antitumor treatment using interleukin- 12-secreting marrow stromal cells in an invasive glioma model. Neurosurgery 64: 1137–1139. DOI 10.1227/01.NEU.0000345646.85472.EA. [Google Scholar] [CrossRef]
Hu YL, Fu YH, Tabata Y, Gao JQ (2010). Mesenchymal stem cells: A promising targeted-delivery vehicle in cancer gene therapy. Journal of Controlled Release 147: 154–162. DOI 10.1016/j.jconrel.2010.05.015. [Google Scholar] [CrossRef]
Jiang W, Xu J (2020). Immune modulation by mesenchymal stem cells. Cell Proliferation 53: e12712. DOI 10.1111/cpr.12712. [Google Scholar] [CrossRef]
Kachgal S, Putnam AJ (2011). Mesenchymal stem cells from adipose and bone marrow promote angiogenesis via distinct cytokine and protease expression mechanisms. Angiogenesis 14: 47–59. DOI 10.1007/s10456-010-9194-9. [Google Scholar] [CrossRef]
Keech C, Albert G, Cho I, Robertson A, Reed P et al. (2020). Phase 1-2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. New England Journal of Medicine 383: 2320–2332. DOI 10.1056/NEJMoa2026920. [Google Scholar] [CrossRef]
Kidd S, Spaeth E, Dembinski JL, Dietrich M, Watson K et al. (2009). Direct evidence of mesenchymal stem cell tropism for tumor and wounding microenvironments using in vivo bioluminescent imaging. Stem Cells 27: 2614–2623. DOI 10.1002/stem.187. [Google Scholar] [CrossRef]
Kidd S, Spaeth E, Klopp A, Andreeff M, Hall B et al. (2008). The (in) auspicious role of mesenchymal stromal cells in cancer: Be it friend or foe. Cytotherapy 10: 657–667. DOI 10.1080/14653240802486517. [Google Scholar] [CrossRef]
Kriebel PW, Majumdar R, Jenkins LM, Senoo H, Wang W et al. (2018). Extracellular vesicles direct migration by synthesizing and releasing chemotactic signals. Journal of Cell Biology 217: 2891–2910. DOI 10.1083/jcb.201710170. [Google Scholar] [CrossRef]
Lai RC, Arslan F, Lee MM, Sze NS, Choo A et al. (2010). Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Research 4: 214–222. DOI 10.1016/j.scr.2009.12.003. [Google Scholar] [CrossRef]
Laprise-Pelletier M, Simão T, Fortin MA (2018). Gold nanoparticles in radiotherapy and recent progress in nanobrachytherapy. Advanced Healthcare Materials 7: 1701460. DOI 10.1002/adhm.201701460. [Google Scholar] [CrossRef]
Lei LM, Lin X, Xu F, Shan SK, Guo B et al. (2021). Exosomes and obesity-related insulin resistance. Frontiers in Cell and Developmental Biology 9: 651996. DOI 10.3389/fcell.2021.651996. [Google Scholar] [CrossRef]
Leng Z, Zhu R, Hou W, Feng Y, Yang Y et al. (2020). Transplantation of ACE2- mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging and Disease 11: 216–228. DOI 10.14336/AD.2020.0228. [Google Scholar] [CrossRef]
Liang B, Chen J, Li T, Wu H, Yang W et al. (2020). Clinical remission of a critically ill COVID-19 patient treated by human umbilical cord mesenchymal stem cells: A case report. Medicine 99: e21429. DOI 10.1097/MD.0000000000021429. [Google Scholar] [CrossRef]
Lira R, Oliveira M, Martins M, Silva C, Carvalho S et al. (2017). Transplantation of bone marrow-derived MSCs improves renal function and Na++K+-ATPase activity in rats with renovascular hypertension. Cell and Tissue Research 369: 287–301. DOI 10.1007/s00441-017-2602-3. [Google Scholar] [CrossRef]
Liu H, Liang Z, Wang F, Zhou C, Zheng X et al. (2019). Exosomes from mesenchymal stromal cells reduce murine colonic inflammation via a macrophage-dependent mechanism. JCI Insight 4: e131273. DOI 10.1172/jci.insight.131273. [Google Scholar] [CrossRef]
Madsen SD, Russell KC, Tucker HA, Glowacki J, Bunnell BA et al. (2017). Decoy TRAIL receptor CD264: A cell surface marker of cellular aging for human bone marrow-derived mesenchymal stem cells. Stem Cell Research & Therapy 8: 201. DOI 10.1186/s13287-017-0649-4. [Google Scholar] [CrossRef]
Yáñez-Mó M, Siljander PRM, Andreu Z, Bedina Zavec A, Borràs FE et al. (2015). Biological properties of extracellular vesicles and their physiological functions. Journal of Extracellular Vesicles 4: 1. DOI 10.3402/jev.v4.27066. [Google Scholar] [CrossRef]
Markman JL, Rekechenetskiy A, Holler E, Ljubimova JY (2013). Nanomedicine therapeutic approaches to overcome cancer drug resistance. Advanced Drug Delivery Reviews 65: 1866–1879. DOI 10.1016/j.addr.2013.09.019. [Google Scholar] [CrossRef]
Mazzeo A, Santos EJC (2018). Nanotechnology and multipotent adult progenitor cells in reparative medicine: Therapeutic perspectives. Einstein (Sao Paulo) 16: eRB4587. DOI 10.31744/einstein_journal/2018RB4587t. [Google Scholar] [CrossRef]
Moradian Tehrani R, Verdi J, Noureddini M, Salehi R, Salarinia R et al. (2018). Mesenchymal stem cells: A new platform for targeting suicide genes in cancer. Journal of Cellular Physiology 233: 3831–3845. DOI 10.1002/jcp.26094. [Google Scholar] [CrossRef]
Mura S, Nicolas J, Couvreur P (2013). Stimuli-responsive nanocarriers for drug delivery. Nature Materials 12: 991–1003. DOI 10.1038/nmat3776. [Google Scholar] [CrossRef]
Nakamizo A, Marini F, Amano T, Khan A, Studeny M et al. (2005). Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Research 65: 3307–3318. DOI 10.1158/0008-5472.CAN-04-1874. [Google Scholar] [CrossRef]
Pan J, Du Y, Qiu H, Upton LR, Li F et al. (2019). Mimicking chemotactic cell migration with DNA programmable synthetic vesicles. Nano Letters 19: 9138–9144. DOI 10.1021/acs.nanolett.9b04428. [Google Scholar] [CrossRef]
Paris GC, Azevedo AA, Ferreira AL, Azevedo Y, Rainho MA, Oliveira GP, Silva KR, Cortez E, Stumbo AC, Carvalho SN, de Carvalho L, Thole AA (2021). Therapeutic potential of mesenchymal stem cells in multiple organs affected by COVID-19. Life Sciences 278: 119510. DOI 10.1016/j.lfs.2021.119510. [Google Scholar] [CrossRef]
Pegtel DM, Gould SJ (2019). Exosomes. Annual Review of Biochemistry 88: 487–514. DOI 10.1146/annurev-biochem-013118-111902. [Google Scholar] [CrossRef]
Rani S, Ryan AE, Griffin MD, Ritter T (2015). Mesenchymal stem cell-derived extracellular vesicles: Toward cell-free therapeutic applications. Molecular therapy: The Journal of the American Society of Gene Therapy 23: 812–823. DOI 10.1038/mt.2015.44. [Google Scholar] [CrossRef]
Sadhukha T, O’Brien TD, Prabha S (2014). Nano-engineered mesenchymal stem cells as targeted therapeutic carriers. Journal of Controlled Release: Official Journal of the Controlled Release Society 196: 243–251. DOI 10.1016/j.jconrel.2014.10.015. [Google Scholar] [CrossRef]
Saulite L, Dapkute D, Pleiko K, Popena I, Steponkiene S et al. (2017). Nano-engineered skin mesenchymal stem cells: Potential vehicles for tumour-targeted quantum-dot delivery. Beilstein Journal of Nanotechnology 8: 1218–1230. DOI 10.3762/bjnano.8.123. [Google Scholar] [CrossRef]
Shentu TP, Huang TS, Cernelc-Kohan M, Chan J, Wong SS et al. (2017). Thy-1 dependent uptake of mesenchymal stem cell-derived extracellular vesicles blocks myofibroblastic differentiation. Scientific Reports 7: 18052. DOI 10.1038/s41598-017-18288-9. [Google Scholar] [CrossRef]
Shi L, Wang L, Xu R, Zhang C, Xie Y et al. (2021). Mesenchymal stem cell therapy for severe COVID-19. Signal Transduction and Targeted Therapy 6: 339. DOI 10.1038/s41392-021-00754-6. [Google Scholar] [CrossRef]
Shin S, Lee J, Kwon Y, Park KS, Jeong JH et al. (2021). Comparative proteomic analysis of the mesenchymal stem cells secretome from adipose, bone marrow, placenta and wharton’s jelly. International Journal of Molecular Sciences 22: 845. DOI 10.3390/ijms22020845. [Google Scholar] [CrossRef]
Sotiropoulou PA, Perez SA, Salagianni M, Baxevanis CN, Papamichail M (2006). Characterization of the optimal culture conditions for clinical scale production of human mesenchymal stem cells. Stem Cells 24: 462–471. DOI 10.1634/stemcells.2004-0331. [Google Scholar] [CrossRef]
Ståhl AL, Johansson K, Mossberg M, Kahn R, Karpman D (2019). Exosomes and microvesicles in normal physiology, pathophysiology, and renal diseases. Pediatric Nephrology 34: 11–30. DOI 10.1007/s00467-017-3816-z. [Google Scholar] [CrossRef]
Sun DZ, Abelson B, Babbar P, Damaser MS (2019). Harnessing the mesenchymal stem cell secretome for regenerative urology. Nature Reviews Urology 166: 363–375. DOI 10.1038/s41585-019-0169-3. [Google Scholar] [CrossRef]
Thanuja MY, Anupama C, Ranganath SH (2018). Bioengineered cellular and cell membrane-derived vehicles for actively targeted drug delivery: So near and yet so far. Advanced Drug Delivery Reviews 132: 57–80. DOI 10.1016/j.addr.2018.06.012. [Google Scholar] [CrossRef]
Tögel F, Zhang P, Hu Z, Westenfelder C (2009). VEGF is a mediator of the renoprotective effects of multipotent marrow stromal cells in acute kidney injury. Journal of Cellular and Molecular Medicine 13: 2109–2114. DOI 10.1111/j.1582-4934.2008.00641.x. [Google Scholar] [CrossRef]
Toledano Furman NE, Lupu-Haber Y, Bronshtein T, Kaneti L, Letko N et al. (2013). Reconstructed stem cell nanoghosts: A natural tumor targeting platform. Nano Letters 13: 3248–3255. DOI 10.1021/nl401376w. [Google Scholar] [CrossRef]
Usha L, Rao G, Kent C, Li K, Xu X (2013). Mesenchymal stem cells develop tumor tropism but do not accelerate breast cancer tumorigenesis in a somatic mouse breast cancer model. PLoS One 8: e67895. DOI 10.1371/journal.pone.0067895. [Google Scholar] [CrossRef]
Vegh I, Grau M, Gracia M, Grande J, de la Torre P et al. (2013). Decidua mesenchymal stem cells migrated toward mammary tumors in vitro and in vivo affecting tumor growth and tumor development. Cancer Gene Therapy 20: 8–16. DOI 10.1038/cgt.2012.71. [Google Scholar] [CrossRef]
Vert M, Doi Y, Hellwich KH, Hess M, Hodge P et al. (2012). Terminology for biorelated polymers and applications (IUPAC Recommendations 2012). Pure and Applied Chemistry 84: 377–410. DOI 10.1351/PAC-REC-10-12-04. [Google Scholar] [CrossRef]
Vishnubhatla I, Corteling R, Stevanato L, Hicks C, Sinden J (2014). The development of stem cell-derived exosomes as a cell-free regenerative medicine. Journal of Circulating Biomarkers 3: 2. DOI 10.5772/58597. [Google Scholar] [CrossRef]
Vizoso F, Eiro N, Cid S, Schneider J, Perez-Fernandez R (2017). Mesenchymal stem cell secretome: Toward cell-free therapeutic strategies in regenerative medicine. International Journal of Molecular Sciences 18: 1852. DOI 10.3390/ijms18091852. [Google Scholar] [CrossRef]
Wu HH, Zhou Y, Tabata Y, Gao JQ (2019). Mesenchymal stem cell-based drug delivery strategy: From cells to biomimetic. Journal of Controlled Release 294: 102–113. DOI 10.1016/j.jconrel.2018.12.019. [Google Scholar] [CrossRef]
Yang B, Wu X, Mao Y, Bao W, Gao L et al. (2009). Dual-targeted antitumor effects against brainstem glioma by intravenous delivery of tumor necrosis factor-related, apoptosis-inducing, ligand-engineered human mesenchymal stem cells. Neurosurgery 65: 610–624. DOI 10.1227/01.NEU.0000350227.61132.A7. [Google Scholar] [CrossRef]
Yu B, Kim HW, Gong M, Wang J, Millard RW et al. (2015). Exosomes secreted from GATA-4 overexpressing mesenchymal stem cells serve as a reservoir of anti-apoptotic microRNAs for cardioprotection. International Journal of Cardiology 182: 349–360. DOI 10.1016/j.ijcard.2014.12.043. [Google Scholar] [CrossRef]
Zhang Y, Liu Y, Liu H, Tang WH (2019). Exosomes: Biogenesis, biologic function and clinical potential. Cell & Bioscience 9: 19. DOI 10.1186/s13578-019-0282-2. [Google Scholar] [CrossRef]
Zhao L, Hu C, Han F, Cai F, Wang J et al. (2020). Preconditioning is an effective strategy for improving the efficiency of mesenchymal stem cells in kidney transplantation. Stem Cell Research & Therapy 11: 197. DOI 10.1186/s13287-020-01721-8. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |