DOI:10.32604/biocell.2022.020391

| BIOCELL DOI:10.32604/biocell.2022.020391 |  |
| Article |
Mycorrhiza improves cold tolerance of Satsuma orange by inducing antioxidant enzyme gene expression
1College of Horticulture and Gardening, Yangtze University, Jingzhou, 434025, China
2College of Biology and Agricultural Resources, Huanggang Normal University, Huanggang, 438000, China
3Plant Production Department, College of Food and Agricultural Sciences, King Saud University, Riyadh, 11451, Saudi Arabia
*Address correspondence to: Qiang-Sheng Wu, wuqiangsh@163.com
Received: 21 November 2021; Accepted: 06 January 2022
Abstract: A potted experiment was carried out to study the effect of an arbuscular mycorrhizal fungus (Diversispora versiformis) and arbuscular mycorrhizal like fungus (Piriformospora indica) on antioxidant enzyme defense system of Satsuma orange (Citrus sinensis cv. Oita 4) grafted on Poncirus trifoliata under favourable temperature (25°C) and cold temperature (0°C) for 12 h. Such short-term treatment of cold temperature did not cause any significant change in root fungal colonization and spore density in soil. Under cold stress, D. versiformis inoculation did not change the activity of superoxide dismutase (SOD), catalase (CAT), and peroxidase (POD) in leaves and roots, whereas P. indica inoculation significantly increased the activity of CAT in roots and POD in leaves only. In addition, inoculation of two mycorrhizal fungi under cold stress significantly increased the relative expression levels of PtPOD and PtF-SOD in leaves, P. indica up-regulated the expression levels of PtCu/Zn-SOD in leaves, and D. versiformis also induced the expression levels of PtMn-SOD and PtCAT1 in leaves. In addition, inoculated Oita 4 trees maintained significantly lower hydrogen peroxide levels and malondialdehyde contents in leaves and roots under cold temperature, suggesting lower oxidative damage. Therefore, we concluded that arbuscular mycorrhizal fungi (especially P. indica) mainly induced the expression of antioxidant enzyme genes, depending on the fungal species, and thus mitigated oxidative damage for higher cold resistance in inoculated plants.
Keywords: Antioxidant defense system; Citrus; Cold stress; Mycorrhiza; Oxidative burst
Cold stress is an environmental factor that inhibits plant growth and development, limits plant geographic distribution, and reduces crop yield (Liu et al., 2019; Goharrizi et al., 2021). Under cold stress conditions, reactive oxygen species (ROS) including hydrogen peroxide (H2O2), superoxide anion radicals (O2•−) and hydroxyl radicals, are excessively accumulated, due to the disruption of the electron transport chain (Wang et al., 2020). Excessive production of ROS leads to oxidative damage in nucleic acids, proteins and lipids, disrupting cellular functions (Barrero-Sicilia et al., 2017; Ding et al., 2021). Plants also possess antioxidant defense systems to maintain the balance between the production and scavenging of ROS under stress conditions (Ermakov et al., 2019; Araújo et al., 2020). In the chloroplasts of plant cells, enzymes and non-enzymatic antioxidants are able to prevent oxidative damage. Among them, catalase (CAT), superoxide dismutase (SOD), and peroxidase (POD) can mitigate the deleterious effects of ROS on plant cells and protect them from oxidative damage (Caser et al., 2016; Ghanbarzadeh et al., 2019). SODs including Cu/Zn-SOD, Fe-SOD, Mn-SOD are a metalloenzyme that eliminates the conversion of O2•− into O2 and H2O2 (Garg and Manchanda, 2009); CAT is a heme-containing tetrameric enzyme with the potential to break down H2O2 directly into H2O and O2; POD also eliminates excess H2O2 (He et al., 2020b; Kong et al., 2020). As a result, antioxidant enzymes play a crucial role in the scavenging of ROS in plants during cold stress.
Arbuscular mycorrhizal fungi (AMF) are beneficial soil microorganisms in plant rhizosphere, which can form symbiotic associations with roots of 80% of terrestrial plants (Wu et al., 2013; He et al., 2020a). AMF obtain carbohydrates from their hosts to sustain their growth needs, and in return, the fungi also provide host plants with a variety of benefits, including enhanced mineral nutrition and tolerance to abiotic and biotic stresses (Parniske, 2008; Cheng et al., 2021a, 2021b). It has been shown that under drought conditions, AMF reduced the accumulation of ROS in host plants by enhancing antioxidant enzyme activities and associated gene expression (He et al., 2020b; Cheng et al., 2021b; Zou et al., 2021). Similarly, Pasbani et al. (2020) inoculated eggplants (Solanum melongena) with AMF at three different temperatures, and found that AMF activated antioxidant defense systems, accumulated protective molecules, and reduced membrane damage. Ma et al. (2015) reported that AMF improved the uptake of phosphorus and alleviated low temperature stress. Chu et al. (2016) observed less oxidative damage in mycorrhizal vs. non-mycorrhizal phyllanthus (Elymus nutans) under cold stress conditions, which may be related to higher antioxidant enzyme activities. These studies have shown that AMF enhanced cold tolerance in plants, while the regulation of AMF on antioxidant enzyme activities and their gene expression in citrus under cold stress conditions is not clear, especially for short-term cold stress.
The purpose of this study was to explore the effects of AMF (Diversispora versiformis) and arbuscular mycorrhizal like fungus (Piriformospora indica) on antioxidant enzyme activities and relevant gene expression of Satsuma orange under cold stress, in order to evaluate the function of AMF on tolerating cold stress of the host. Such results will provide a theoretical basis for the application of AMF in tolerating cold stress of citrus trees.
The experiment was arranged in the completely randomized block design: (i) fungal treatments with D. versiformis (DV), P. indica (PI), and non-AMF; (2) temperature stress at 25°C (favourable temperature, FT) and 0°C (cold temperature, CT) for 12 h. A total of six treatments was achieved, and each treatment had four replicates, for a total of 24 pots.
The tested strain of arbuscular mycorrhizal fungus, D. versiformis (P. Karst.) Oehl, G.A. Silva & Sieverd, was provided by the Institute of Root Biology, Yangtze University, and propagated by the host plant, white clover (Trifolium repens L.), for three months under potted conditions. The collected inoculum of D. versiformis included spores, hyphae, and colonized root segments. P. indica was provided by Prof. Zhihong Tian (Yangtze University, Jingzhou, China). The proliferation of PI was carried out by Yang et al. (2021). The spore suspension of PI was used as the inoculum, in which the concentration is 3.16 × 108 CFU/mL (OD600).
The tested plant was Citrus sinensis cv. Oita 4, an early-ripening Satsuma mandarin selected from Oita (Japan), with trifoliate orange (Poncirus trifoliata L. Raf.) as the rootstock. It was budded in autumn 2018. The Satsuma mandarin trees were provided by the Institute of Fruit and Tea Research, Hubei Academy of Agricultural Sciences (Wuhan, China). Before transplanting, the mycorrhizal colonization rate of the trees was 26.7 ± 5.2%, based on the protocol of Phillips and Hayman (1970).
On May 17, 2019, we transplanted the Oita 4 trees with similar growth vigor into a 5-gallon plastic pot that was supplied with 11 kg of growth substrate. The potted soil was collected from a citrus orchard on the West Campus of Yangtze University (Jingzhou, China), removed impurities, and mixed with the substrate consisted of peat, vermiculite, and perlite (69:25:6, v/v/v) in the ratio of 2:1 (v/v) as the growth substrate. Inoculation of fungi was carried out at the time of transplantation of Oita 4. For the DV treatment, a total of 600 g mycorrhizal inoculums of D. versiformis was applied to a pot. The 1.0-L spore suspension of P. indica was applied into a pot as the PI treatment. The non-fungi treatment also received the same amount of autoclaved mycorrhizal inoculums as the control. After inoculated with the fungi, the treated Oita 4 trees were grown in a greenhouse without any heating equipment.
On 20 November 2020 at 6:00 am, inoculated and uninoculated Oita 4 trees were divided into two parts: half of the trees was exposed to 25°C for 12 h, and the other half was subjected to 0°C for 12 h, along with 900 μmol/m2/s photo flux density and 68% relatively air humidity in an illumination incubator. Then, the experiment was ended.
After 12 h of cold stress (0°C) and favourable temperature (25°C), we immediately collected some of fine roots from all the potted trees and gently shook off the soil attached to the roots as rhizosphere soil. The top 4–5 leaves of treated trees were collected, immediately frozen in liquid nitrogen, and stored at –80°C for measurement of later physiological and molecular indicators.
Root fungal colonization was stained as per the protocol described by Phillips and Hayman (1970). The degree of root fungal colonization was calculated as the percentage of fungi-colonized root segment number per observed total root segment number. Spore number in soil was assayed by Wang et al. (2016).
Malondialdehyde (MDA) concentration in leaves was measured according to the method described by Sudhakar et al. (2001), based on the reaction with thiobarbituric acid. H2O2 concentration was determined by the method of Velikova et al. (2000), accompanied with the KI reaction. SOD activity was determined by the colorimetry described by Wu (2018). POD activity was assayed by the method of He et al. (2020b). CAT activity was determined by the colorimetry (240 nm) of He et al. (2020b). Soluble protein concentration was analyzed as per the coomassie blue G-250 method outlined by Wu (2018).
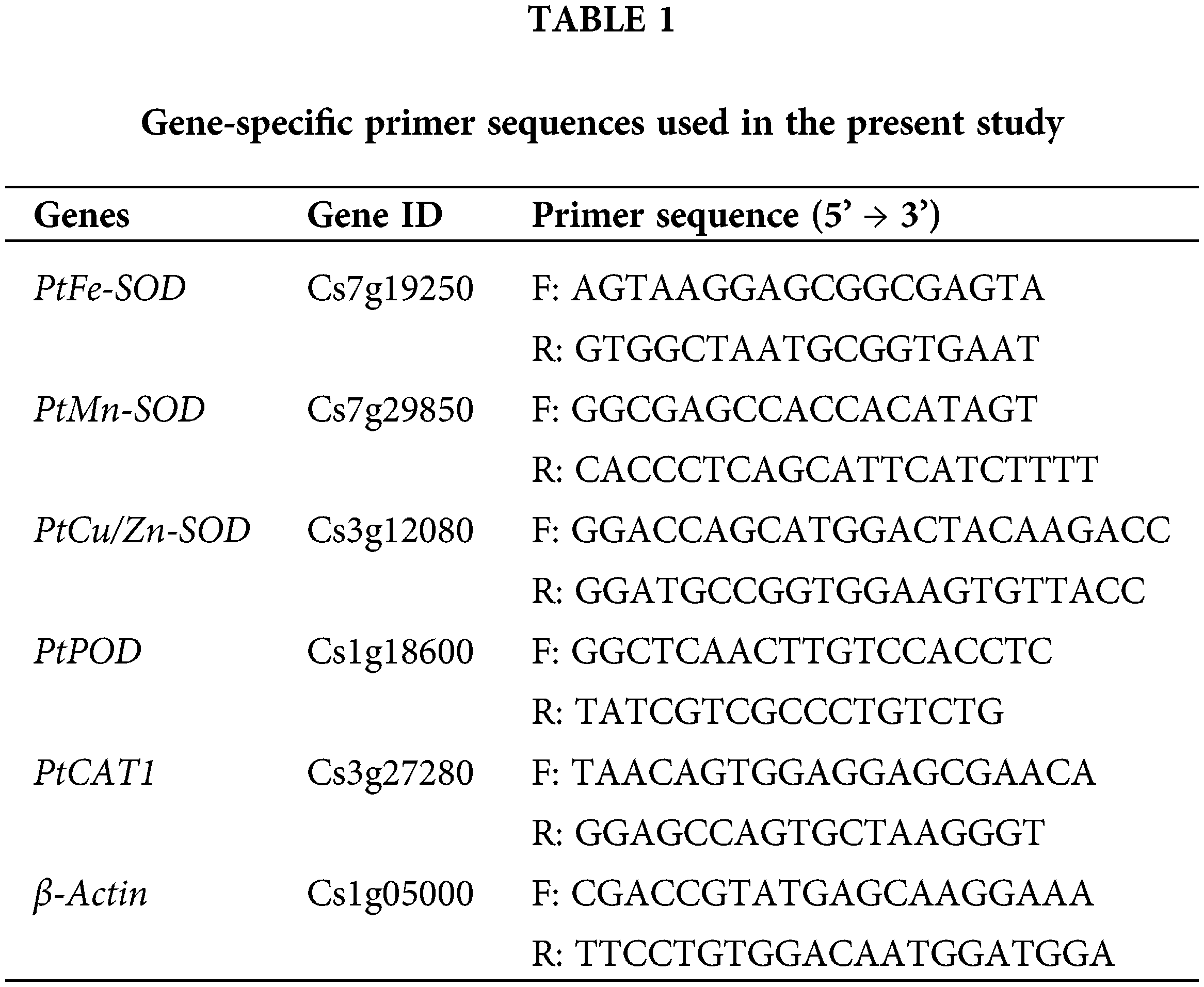
Frozen leaf samples were ground in liquid nitrogen. According to the manufacturer’s protocol, total RNA was extracted from powdered leaf tissue (50 mg) using RN38 EasySpin plus plant RNA Kit (Aidlab, Beijing, China). RNA integrity was examined in 1% agarose gel, and quantitative analysis was performed by spectrophotometric analysis using Bio Photometer Plus 6132 (Eppendorf, Hamburg, Germany). RNA purity was estimated by calculating the A260/A280 ratio. The RNA was reversely transcribed into the first strand cDNA using the transcript first strand cDNA synthesis kit with gDNA Eraser (PC5402, Aidlab, Beijing, China). Five antioxidant enzyme genes (PtFe-SOD, PtMn-SOD, PtCu/Zn-SOD, PtCAT1, and PtPOD) were identified from the genome-wide data of Citrus sinensis (http://citrus.hzau.edu.cn/) and the data obtained from our early transcriptome data. The Prime Premier 5 was used to design the primer sequence (Table 1). The qRT-PCR reaction contained 10 μL 2 × AceQ qPCR SYBR Green Master Mix kit, 0.4 μL of each primer, 2 μL diluted cDNA template, and 7.2 μL ddH2O. qRT-PCR reaction was carried out using CFX96 real-time PCR detection system (Biorad Laboratories, Hercules, CA, USA). The amplification procedure was as follows: initial denaturation at 95°C for 5 min; 40 cycles at 95°C for 10 s, 60°C for 30 s and 95°C for 15 s; 60°C for 60 s; and 95°C for 15 s. The experiment was repeated three times for each gene. Relative quantitative of genes was calculated by 2–ΔΔCT method (Livak and Schmittgen, 2001). The β-actin gene was selected as an internal reference gene. Measured transcripts were normalized to the values of non-AMF plants under FT conditions.
Data were statistically analyzed by analysis of variance (ANOVA) in SAS (8.1v), and the significance between treatments was compared by the Duncan’s multi-range test at the level of 0.05.
Effects of cold stress on mycorrhizal development of roots and soil
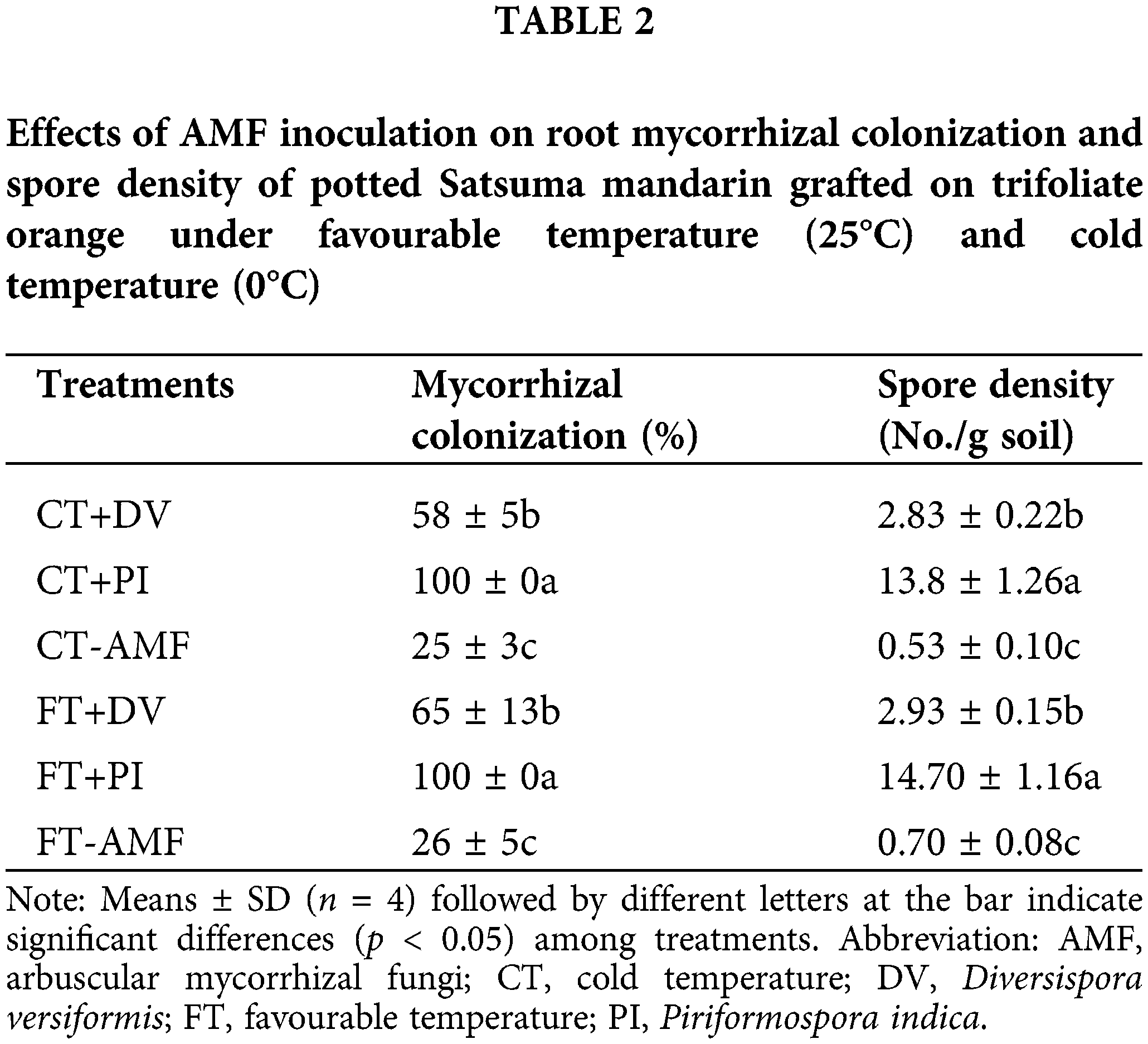
In this study, un-inoculated Oita 4 trees with AMF had a small amount of root mycorrhizal colonization and soil spores, while DV and PI inoculation significantly promoted the root fungal colonization and spore density in soil (Fig. 1; Table 2). Under FT conditions, compared with non-inoculated treatment, inoculation with DV and PI significantly increased root fungal colonization degree by 39% and 74% and spore density in soil by 319% and 2000%, respectively (Table 2). However, under CT conditions, mycorrhizal colonization distinctly increased respectively by 34% and 75% under DV and PI inoculation versus un-inoculated treatment, and spore density in soil considerably increased by 434% and 2503%, respectively (Table 2). This suggests that PI recorded better colonization in roots and soil than DV. Cold stress did not alter root mycorrhizal colonization degree and spore density in soil in DV- and PI-inoculated trees, which is inconsistent with the results of Pasbani et al. (2020) that cold temperature conditions reduced AMF colonization by the difference in the duration of cold temperature treatment. Wu (2011) also reported dramatical reduction of root mycorrhizal colonization by Glomus mosseae in trifoliate orange exposed to 15°C for 2 months, relative to 25°C. It concluded that short-term cold temperature treatment (only 12 h) had no significant effect on mycorrhizal formation in the rhizosphere.
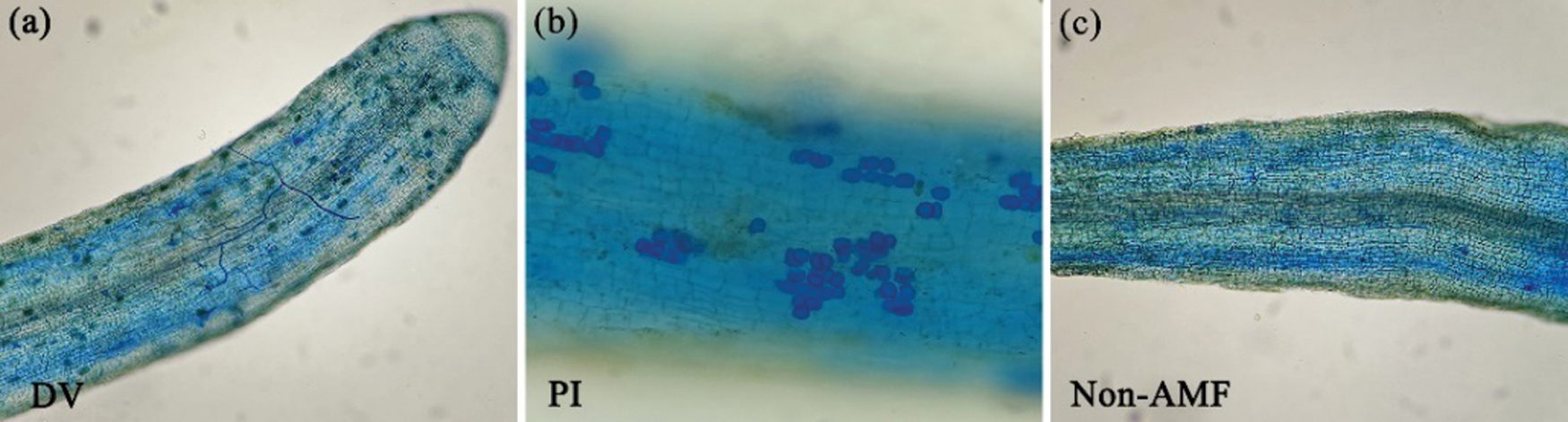
Figure 1: Root fungal colonization of Citrus sinensis cv. Oita 4 inoculated with Diversispora versiformis (a), Piriformospora indica (b), and non-AMF (c), respectively. Abbreviation: DV, Diversispora versiformis; non-AMF, non-arbuscular mycorrhizal fungi; PI, Piriformospora indica.
Effects of fungal inoculation on H2O2 concentrations under cold stress
Reactive oxygen species (ROS) are by-products of metabolism, and their production is increased under stress conditions through disruption of the electron transport system and oxidative metabolic activities occurring in chloroplasts, mitochondria and microsomes (Adriano et al., 2015). H2O2 is an important and relatively stable ROS, accumulated excessively under cold temperature (Pinheiro and Chaves, 2011; Adriano et al., 2015). In the present study, cold temperature caused a rapid accumulation of H2O2 in leaves and roots, independent of inoculated status (Fig. 2), which is in agreement with the findings of Wang et al. (2020) in tomato. Compared to FT-AMF treatment, H2O2 content was reduced by 51.2% and 16.7% in roots of FT+DV and FT+PI, and by 19.5% and 17.1% in leaves (Fig. 2), respectively; compared to CT-AMF treatment, H2O2 content was decreased in roots by 38.9% and 41.7% in CT+DV and CT+PI, and by 25.1% and 31.5% in leaves, respectively. The result implies that AMF reduced the accumulation of H2O2 in roots under cold stress, thus alleviating the damage of cold stress on citrus roots. This may be due to the fact that AMF promoted the activity of antioxidant enzymes to scavenge more ROS, thus, reducing the oxidative damage of cold stress on plant cell membrane (Zou et al., 2021). In addition, the formation of mycorrhizas promoted uptake of mineral nutrients (e.g., phosphorus) in host plants, which also facilitated resistance to cold stress (Ma et al., 2015)
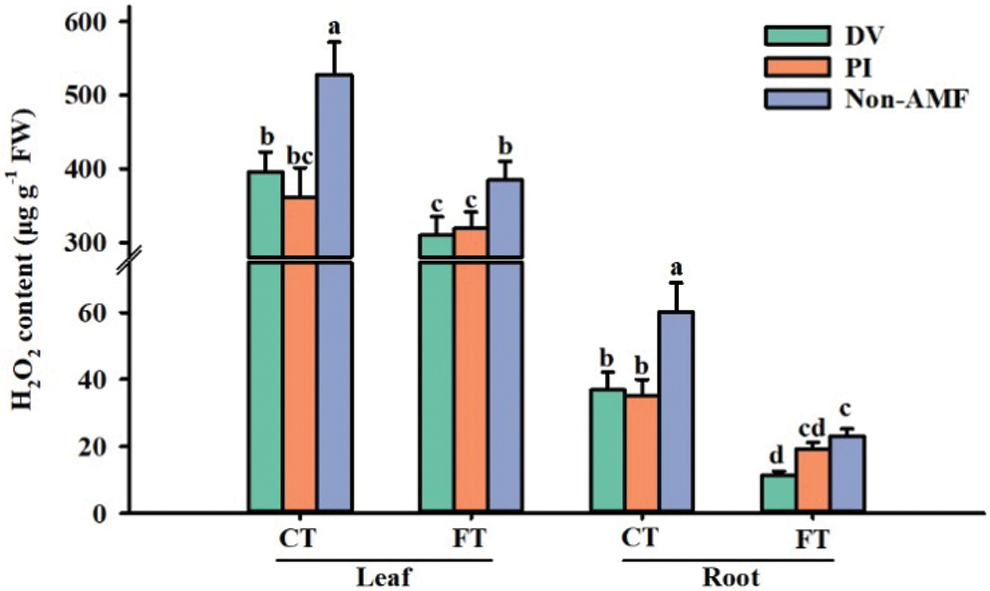
Figure 2: The effect of Diversispora versiformis and Piriformospora indica on H2O2 content of leaf and root of Satsuma mandarin under favourable temperature (25°C) and cold temperature (0°C). Data (means ± SD, n = 4) are significantly different (p < 0.05) if followed by different letters above the bars. Abbreviation: CT, cold temperature; DV, Diversispora versiformis; FT, favourable temperature; Non-AMF, non-arbuscular mycorrhizal fungi; PI, Piriformospora indica.
Effects of fungal inoculation on MDA concentrations under cold stress
Excessive production of ROS under cold stress can lead to severe oxidative damage in plants, resulting in membrane lipid peroxidation, which is manifested by elevated MDA content (Chu et al., 2016; Rezaie et al., 2020). In the present study, cold stress caused an increase in leaf MDA content of inoculated and uninoculated plants, implying that the 12-h cold temperature treatment had caused oxidative damage in plants with disrupted cell membranes, which is consistent with the findings of Wang et al. (2020). Compared to FT-AMF, the MDA content of leaves was reduced by 40.0% and 56.4% in roots of FT+DV and FT+PI, and no significant difference in leaves, respectively (Fig. 3); compared to CT-AMF, the MDA content was significantly reduced by 38.3% and 55.4% in roots of CT+DV and CT+YD, respectively, and by 42.5% and 23.3% in leaves (Fig. 3), which may be related to the reduction of H2O2 content and the enhancement of antioxidant enzyme activities by fungal inoculation. In addition, Wu et al. (2019) also reported higher unsaturation index of fatty acids of trifoliate orange seedlings after inoculated with AMF under soil water deficit conditions, which is another reason for maintaining a low membrane lipid peroxidation.
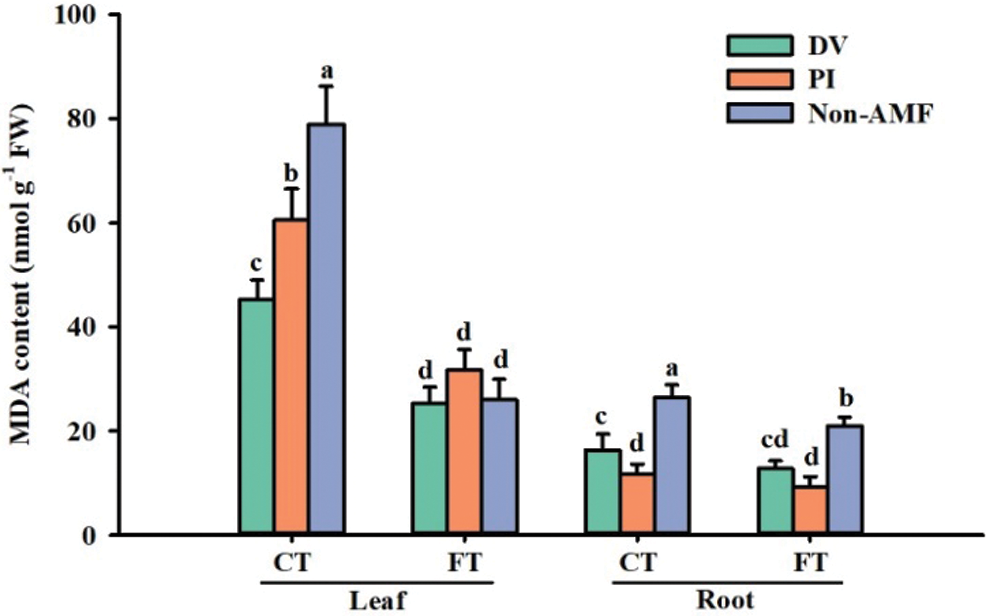
Figure 3: The effect of Diversispora versiformis and Piriformospora indica on MDA content of leaf and root of Satsuma mandarin under favourable temperature (25°C) and cold temperature (0°C). Data (means ± SD, n = 4) are significantly different (p < 0.05) if followed by different letters above the bars. Abbreviation: CT, cold temperature; DV, Diversispora versiformis; FT, favourable temperature; Non-AMF, non-arbuscular mycorrhizal fungi; PI, Piriformospora indica.
Effects of fungal inoculation on SOD activity under cold stress
SOD can catalyze O2•– into H2O2 (Garg and Manchanda, 2009). Chu et al. (2016) treated Elymus nutans with 5°C for 5 days and found that low temperature enhanced SOD activity, but there was no significant difference between AMF-inoculated and uninoculated plants. Nevertheless, Pasbani et al. (2020) reported the increase of SOD activity in Solanum melongena at 5°C for 1 h after AMF inoculation. This implies that SOD activity may be influenced by the duration of stress as well as the host plant and mycorrhizal species. In our study, cold stress only significantly induced the increase of SOD activity in leaves and roots of PI-inoculated plants, but had no effect on SOD activity in uninoculated and DV-inoculated plants. Under FT conditions, only DV inoculation significantly increased SOD activity in leaves; under CT conditions, both mycorrhizal fungi treatments did not significantly change SOD activity in leaves and roots. It suggested that the induction of SOD activity by mycorrhizas depended on the fungal species. It seems that SODs were hardly induced by mycorrhizal fungi under cold stress. However, the expression of SODs isoenzyme gene revealed that the expression of these enzyme genes preceded the enzyme activity in response to temperature treatment (Fig. 8), suggesting that the expression of antioxidant enzyme genes could be used as a good indicator of resistance to cold stress.
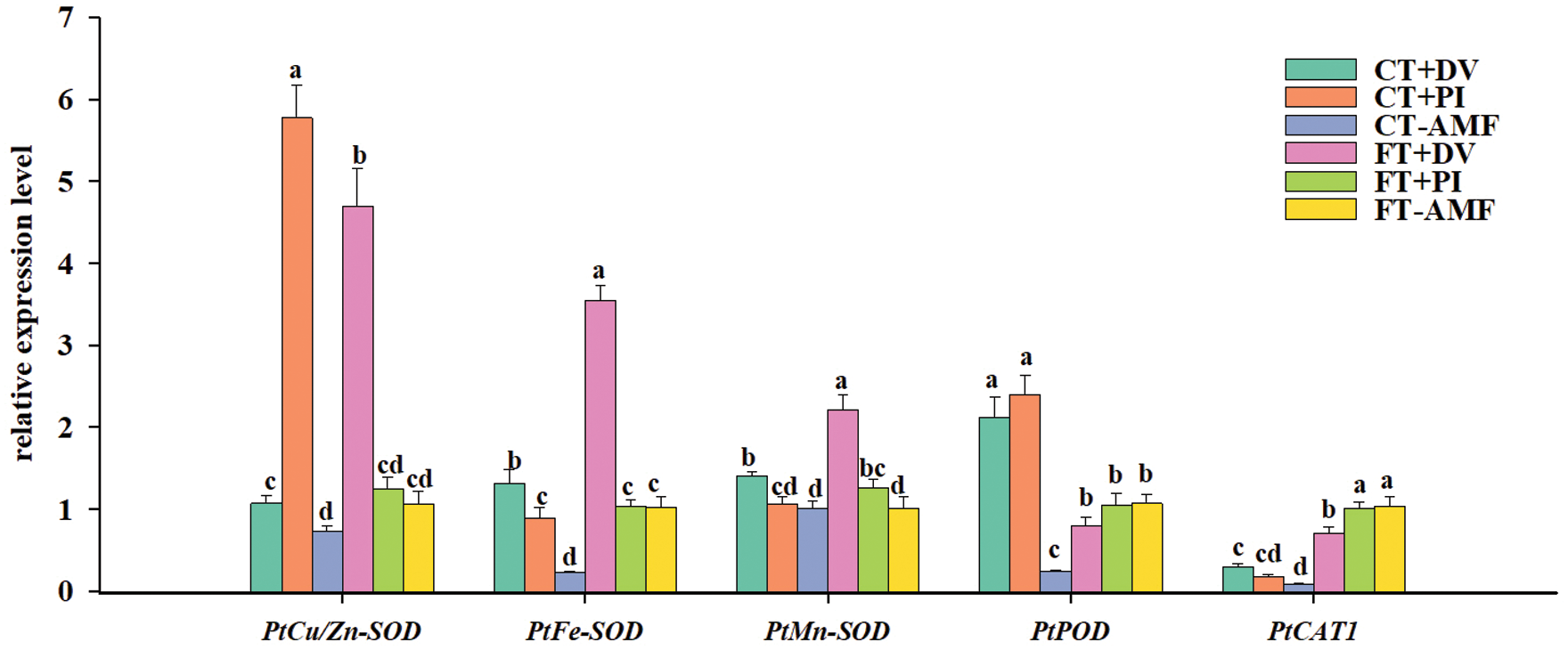
Figure 8: Effect of inoculation with Diversispora versiformis and Piriformospora indica on the relative expression level of leaf PtSODs, PtPOD, and PtCAT1 genes in Satsuma mandarin under favourable temperature (25°C) and cold temperature (0°C). Data (means ± SD, n = 3) are significantly different (p < 0.05) if followed by different letters above the bars. Abbreviation: AMF, arbuscular mycorrhizal fungi; CT, cold temperature; DV, Diversispora versiformis; FT, favourable temperature; PI, Piriformospora indica.
Effects of fungal inoculation on CAT activity under cold stress
CAT is a tetrametric heme enzyme that catalyzes the breakdown of H2O2 to H2O and O2, which is required for ROS detoxification during stress (Willekens et al., 1997). Our results showed that cold stress inhibited CAT activity in roots and leaves of Oita 4, which is consistent with the findings of Chen et al. (2014). Both DV and PI inoculations induced an increase in CAT activity in leaves and roots under FT conditions, whereas cold treatment only significantly induced an increase in CAT activity in leaves of PI-inoculated plants and a decrease in CAT activity in leaves and roots of uninoculated and DV-inoculated plants, as well as in roots of PI-inoculated plants. Compared to FT-AMF treatment, leaf CAT activity increased by 29.6% and 10.2% for FT+DV and FT+PI, respectively, and root CAT activity increased by 27.1% and 102.4% for FT+DV and FT+PI, respectively (Fig. 5). Zhu et al. (2010) found that cold treatment at 5°C induced a decrease in CAT activity in maize roots and leaves, which is consistent with the results of our study, while they also found that the inoculation treatment was effective in promoting CAT activity in roots and leaves compared to the non-inoculation treatment. Higher CAT activity of mycorrhizal plants exposed to cold stress would eliminate more H2O2, which thus causes low oxidative damage.
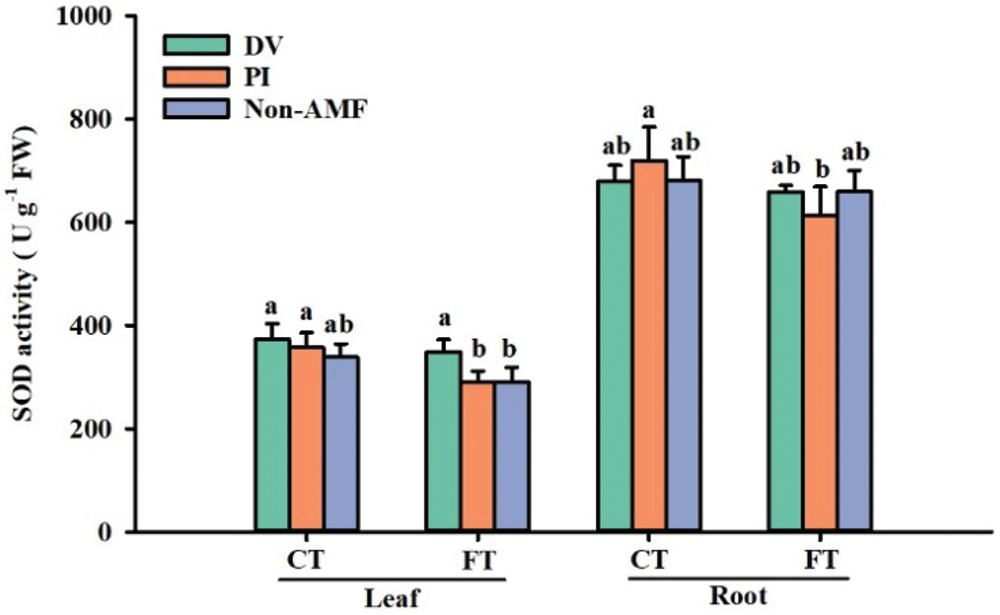
Figure 4: The effect of inoculation with Diversispora versiformis and Piriformospora indica on SOD activity of leaf and root of Satsuma mandarin under favourable temperature (25°C) and cold temperature (0°C). Data (means ± SD, n = 4) are significantly different (p < 0.05) if followed by different letters above the bars. Abbreviation: CT, cold temperature; DV, Diversispora versiformis; FT, favourable temperature; Non-AMF, non-arbuscular mycorrhizal fungi; PI, Piriformospora indica.
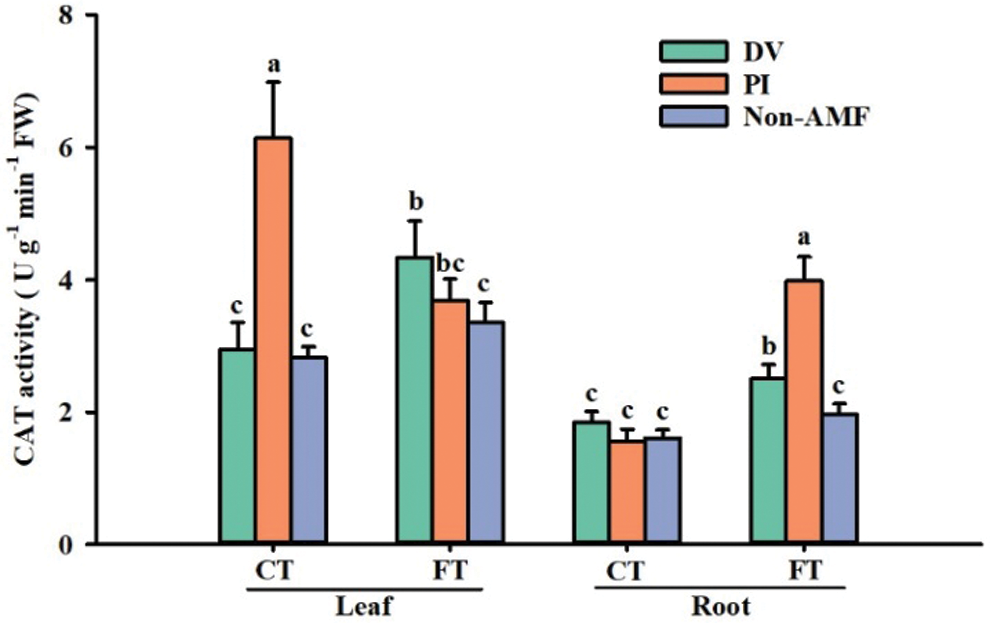
Figure 5: The effect of inoculation with Diversispora versiformis and Piriformospora indica on CAT activity of leaf and root of Satsuma mandarin under favourable temperature (25°C) and cold temperature (0°C). Data (means ± SD, n = 4) are significantly different (p < 0.05) if followed by different letters above the bars. Abbreviation: CT, cold temperature; DV, Diversispora versiformis; FT, favourable temperature; Non-AMF, non-arbuscular mycorrhizal fungi; PI, Piriformospora indica.
Effects of fungal inoculation on POD activity under cold stress
POD is an oxidoreductase enzyme commonly found in most plant tissues that can catalyze oxidative reactions using H2O2 as an electron acceptor (Garg and Manchanda, 2009). The results of this study showed that cold stress significantly promoted root POD activity, but inhibited leaf POD activity. In addition, compared to FT-AMF treatment, root POD activity of plants with FT+DV and FT+PI increased by 12.5% and 33%; under CT conditions, root POD activity increased by 18.2% and 52.3% in DV and PI inoculation, respectively, compared to non-AMF treatment (Fig. 6), implying that PI enhanced root POD activity under cold stress. This is in agreement with the results of Zhu et al. (2010), who reported that leaf POD activity was similar between inoculated and non-inoculated leaves under all temperature treatments, while root POD activity of mycorrhizal plants was higher than that of non-mycorrhizal plants. As a result, PI inoculation caused the increase of POD activity in roots under FT and CT conditions, indicating superior functioning of PI in eliminating ROS (e.g., H2O2) than DV.
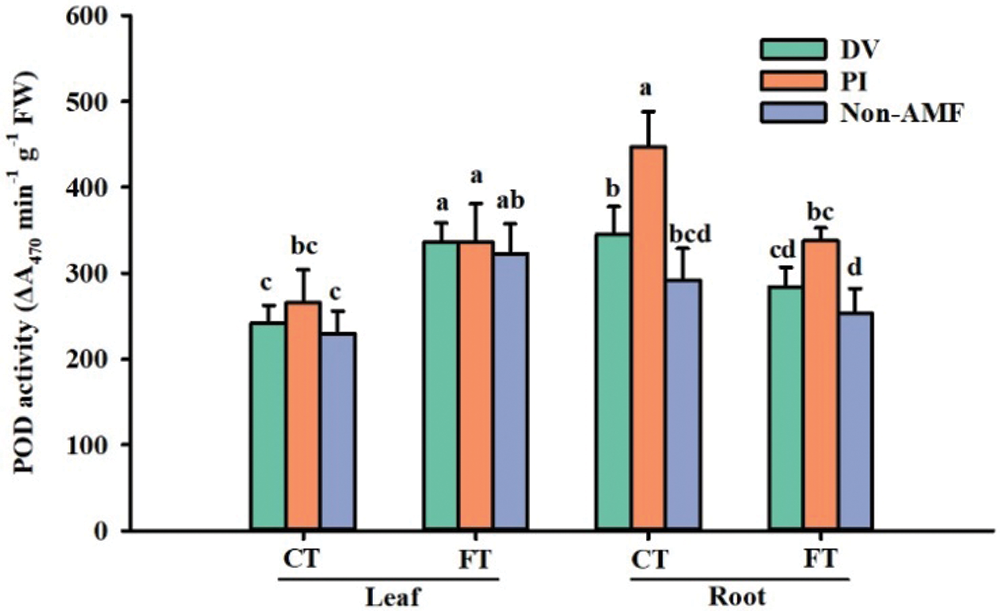
Figure 6: The effect of inoculation with Diversispora versiformis and Piriformospora indica on POD activity of leaf and root of Satsuma mandarin under favourable temperature (25°C) and cold temperature (0°C). Data (means ± SD, n = 4) are significantly different (p < 0.05) if followed by different letters above the bars. Abbreviation: CT, cold temperature; DV, Diversispora versiformis; FT, favourable temperature; Non-AMF, non-arbuscular mycorrhizal fungi; PI, Piriformospora indica.
Effects of fungal inoculation on total soluble protein concentration under cold stress
Genes induced under stress conditions are thought to protect cells from cold stress by producing several gene products, such as aquaporin proteins, protective proteins, and detoxification proteins (Shinozaki and Yamaguchishinozaki, 1996). The present study showed that cold stress induced an increase in total soluble protein in leaves and roots of PI-inoculated and uninoculated plants (Fig. 7). Compared with LT-AMF treatment, LT+PI treatment significantly increased total soluble protein in leaves and roots of citrus by 17.8% and 20.2%, respectively. In addition, DV inoculation significantly increased root soluble protein content under FT conditions by 71.6%, compared with non-AMF. Hence, DV accelerated soluble protein production in leaves and roots of citrus under cold stress conditions, indicating important roles in ROS detoxification.
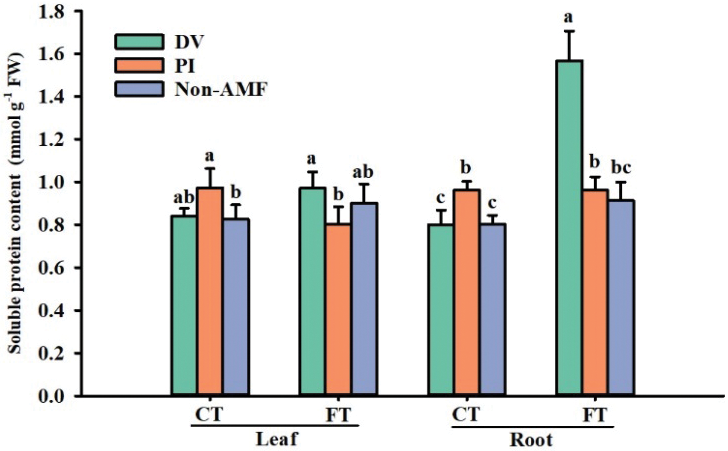
Figure 7: The effect of inoculation with Diversispora versiformis and Piriformospora indica on soluble protein content of leaf and root of Satsuma mandarin under favourable temperature (25°C) and cold temperature (0°C). Data (means ± SD, n = 4) are significantly different (p < 0.05) if followed by different letters above the bars. Abbreviation: CT, cold temperature; DV, Diversispora versiformis; FT, favourable temperature; Non-AMF, non-arbuscular mycorrhizal fungi; PI, Piriformospora indica.
Effects of fungal inoculation on relative expression levels of antioxidant enzyme genes in leaves under cold stress
Ji et al. (2017) found that genes involved in biosynthesis of unsaturated fatty acids, pathogen defense and phenylalanine metabolism were up-regulated at cold temperature, while genes encoding antioxidant enzymes were down-regulated for enhancing the transcription level of functional genes in resistance to cold damage. In the present study, we analyzed the relative expression level of five antioxidant enzyme genes in response to fungal inoculation and cold stress (Fig. 8). These antioxidant enzyme gene expression was down-regulated by cold stress, which is in agreement with Li et al. (2018). Compared with CT-AMF treatment, PtCu/Zn-SOD gene expression in leaves was up-regulated by 1.46 times and 7.90 times in CT+DV and CT+PI, respectively. The expression level of PtMn-SOD gene in leaves was up-regulated by 1.40 times and 1.05 times in CT+DV and CT+PI, respectively, and the expression level of PtFe-SOD gene in leaves was up-regulated by 5.88 times and 3.99 times in CT+DV and CT+PI, respectively, PtPOD gene expression was up-regulated by 8.67 times and 9.83 times respectively in CT+DV and CT+PI, and PtCAT1 gene expression was up-regulated by 3.33 and 2.05 times in CT+DV and CT+PI, respectively (Fig. 8). Nevertheless, under CT conditions, SOD and POD activity was not significantly influenced by DV and PI inoculation, compared with non-AMF inoculation. mRNA and protein expression of antioxidant enzymes were not directly related to antioxidant enzyme activity (He et al., 2020b). The transformation of mRNA into protein is regulated by microRNA, which is affected by ubiquitination and phosphorylation. Therefore, further research on the phosphorylation of antioxidant enzyme protein genes will be conducted. In addition, DV application also significantly up-regulated PtCu/Zn-SOD, PtFe-SOD and PtMn-SOD gene expressions in leaves under FT conditions, with 4.41-fold, 3,45-fold and 2.19-fold, respectively, compared with non-AMF treatment, suggesting that DV promotes the expression of SOD genes under FT conditions. Such induced expression of SODs in inoculated Oita 4 trees would remove more O2•–, thus maintaining relatively low ROS levels in inoculated plants.
Our results suggested that AMF induced the expression of antioxidase enzyme genes in citrus leaves at cold temperature, thus, mitigating oxidative burst of ROS. This means that AMF improves the cold tolerance of citrus by regulating the gene expression of PtCu/Zn-SOD, PtMn-SOD, PtFe-SOD, PtPOD and PtCAT1. ROS in root cells of the host plant is suddenly and temporarily increased during the initial stage of AMF colonization, and is reduced by increased antioxidant enzyme activity and carotenoid content (Zou et al., 2021), which may cause reprogramming involving transcriptomes, proteomes, and metabolites to establish a new metabolic homeostasis (Fürtauer et al., 2019; He et al., 2020b; Liang et al., 2021), thus improving the tolerance of abiotic stresses in plants.
In our study, short-term cold treatment (e.g., at 0°C for 12 h) did not cause any changes in mycorrhizal colonization and spore density in soil. However, mycorrhizal fungi inoculation significantly reduced the content of H2O2 and MDA in leaves and roots under CT conditions, and also significantly promoted the relative expression of PtCu/Zn-SOD, PtFe-SOD and PtMn-SOD in leaves under FT and CT conditions, coupled with the induced expression of PtPOD and PtCAT1 in leaves under CT conditions. Mycorrhizal fungal colonization almostly did not influence antioxiadnt enzyme activity in leaves and roots under cold stress, with the exception of increased CAT activity in roots and POD activity in leaves by PI inoculation, suggesting that antioxidant enzyme genes early responded to cold stress under inoculated versus un-inoculated conditions. This response pattern resulted in less oxidative damage to the inoculated Oita 4 trees, and thus showed higher resistance to cold stress. Among them, PI had stronger cold tolerance than DV, which is expected to be used in field, because PI can be cultured in vitro.
Acknowledgement: The authors would like to extend their sincere appreciation to the Researchers Supporting Project No. (RSP-2021/134), King Saud University, Riyadh, Saudi Arabia.
Availability of Data and Material: All data generated or analyzed in this study are included in this published article.
Authors’ Contribution: The authors confirm contribution of the paper as follows: study conception and design: Qiang-Sheng Wu; data collection: Ming-Ao Cao and Fei Zhang; analysis and interpretation of results: Ming-Ao Cao and Qiang-Sheng Wu; draft manuscript: Ming-Ao Cao, Elsayed Fathi Abd_Allah and Qiang-Sheng Wu. All authors reviewed the results and approved the final version of manuscript.
Ethics Approval: Not applicable.
Funding Statement: This study was supported by the Plan in Scientific and Technological Innovation Team of Outstanding Young Scientists, Hubei Provincial Department of Education (T201604) and the Hubei Agricultural Science and Technology Innovation Action Project (Hubei Nongfa [2018] No. 1). The authors would like to extend their sincere appreciation to the Researchers Supporting Project Number (RSP-2021/134), King Saud University, Riyadh, Saudi Arabia.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Adriano S, Antonio S, Maria N, Antonella V (2015). Ascorbate peroxidase and catalase activities and their genetic regulation in plants subjected to drought and salinity stresses. International Journal of Molecular Sciences 16: 13561–13578. [Google Scholar]
Araújo NO, Santos MNS, Araújo FF, Veras MLM, Tello TPJ, Arruda RS, Fugate KK, Finger FL (2020). Balance between oxidative stress and the antioxidant system is associated with the level of cold tolerance in sweet potato roots. Postharvest Biology and Technology 172: 111359. [Google Scholar]
Barrero-Sicilia C, Silvestre S, Haslam RP, Michaelson LV (2017). Lipid remodelling: Unravelling the response to cold stress in Arabidopsis and its extremophile relative Eutrema salsugineum. Plant Science 263: 194–200. [Google Scholar]
Caser M, D’Angiolillo F, Chitarra W, Lovisolo C, Ruffoni B, Pistelli L, Pistelli L, Scariot V (2016). Water deficit regimes trigger changes in valuable physiological and phytochemical parameters in Helichrysum petiolare Hilliard & B.L. Burtt. Industrial Crops and Products 83: 680–692. [Google Scholar]
Chen XY, Song FB, Liu FL, Tian CJ, Liu SQ et al. (2014). Effect of different arbuscular mycorrhizal fungi on growth and physiology of maize at ambient and low temperature regimes. Science World Journal 2014: 956141. [Google Scholar]
Cheng HQ, Zou YN, Wu QS, Kuča K (2021a). Arbuscular mycorrhizal fungi alleviate drought stress in trifoliate orange by regulating H+-ATPase activity and gene expression. Frontiers in Plant Science 12: 659694. [Google Scholar]
Cheng S, Zou YN, Kuča K, Hashem A, Abd_Allah EF, Wu QS (2021b). Elucidating the mechanisms underlying enhanced drought tolerance in plants mediated by arbuscular mycorrhizal fungi. Frontiers in Plant Science 12: 809473. [Google Scholar]
Chu XT, Fu JJ, Sun YF, Xu YM, Miao YJ, Xu YF, Hu TM (2016). Effect of arbuscular mycorrhizal fungi inoculation on cold stress-induced oxidative damage in leaves of Elymus nutans Griseb. South African Journal of Botany 104: 21–29. [Google Scholar]
Ding F, Wang C, Xu N, Wang ML, Zhang SX (2021). Jasmonic acid-regulated putrescine biosynthesis attenuates cold-induced oxidative stress in tomato plants. Scientia Horticulturae 288: 110373. [Google Scholar]
Ermakov A, Bobrovskikh A, Zubairova U, Konstantinov D, Doroshkov A (2019). Stress-induced changes in the expression of antioxidant system genes for rice (Oryza sativa L.) and bread wheat (Triticum aestivum L.). PeerJ 7: 7791. [Google Scholar]
Fürtauer L, Weiszmann J, Weckwerth W, Nägele T (2019). Dynamics of plant metabolism during cold acclimation. International Journal of Molecular Sciences 20: 5411. [Google Scholar]
Garg N, Manchanda G (2009). ROS generation in plants: Boon or bane? Plant Biosystems 143: 81–96. [Google Scholar]
Ghanbarzadeh Z, Mohsenzadeh S, Rowshan V, Moradshahi A (2019). Evaluation of the growth, essential oil composition and antioxidant activity of Dracocephalum moldavica under water deficit stress and symbiosis with Claroideoglomus etunicatum and Micrococcus yunnanensis. Scientia Horticulturae 256: 108652. [Google Scholar]
Goharrizi KG, Meru C, Kermani SG, Heidarinezhad A, Salehi F (2021). Short-term cold stress affects physiological and biochemical traits of pistachio rootstocks. South African Journal of Botany 141: 90–98. [Google Scholar]
He JD, Chi GG, Zou YN, Shu B, Wu QS, Srivastava AK, Kuča K (2020a). Contribution of glomalin-related soil proteins to soil organic carbon in trifoliate orange. Applied Soil Ecology 154: 103592. [Google Scholar]
He JD, Zou YN, Wu QS, Kua K (2020b). Mycorrhizas enhance drought tolerance of trifoliate orange by enhancing activities and gene expression of antioxidant enzymes. Scientia Horticulturae 262: 108745. [Google Scholar]
Ji CY, Chung WH, Kim HS, Jung WY, Kang L, Jeong JC, Kwak SS (2017). Transcriptome profiling of sweetpotato tuberous roots during low temperature storage. Plant Physiology and Biochemistry 112: 97–108. [Google Scholar]
Kong XM, Ge WY, Wei BD, Zhou Q, Zhou X, Zhao YB, Ji SJ (2020). Melatonin ameliorates chilling injury in green bell peppers during storage by regulating membrane lipid metabolism and antioxidant capacity. Postharvest Biology and Technology 170: 111315. [Google Scholar]
Li X, Yang HQ, Lu GQ (2018). Low-temperature conditioning combined with cold storage inducing rapid sweetening of sweetpotato tuberous roots (Ipomoea batatas (L.) Lam) while inhibiting chilling injury. Postharvest Biology and Technology 142: 1–9. [Google Scholar]
Liang SM, Zhang F, Zou YN, Kuča K, Wu QS (2021). Metabolomics analysis reveals drought responses of trifoliate orange by arbuscular mycorrhizal fungi with a focus on terpenoid profile. Frontiers in Plant Science 12: 740524. [Google Scholar]
Liu T, Ye XL, Li M, Li JM, Qi HY, Hu XH (2019). H2O2 and no are involved in trehalose-regulated oxidative stress tolerance in cold-stressed tomato plants. Environmental and Experimental Botany 171: 103961. [Google Scholar]
Livak KJ, Schmittgen TD (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCT method. Methods 25: 402–408. [Google Scholar]
Ma J, Janouková M, Li YS, Yu XC, Yan Y, Zou ZR, He CX (2015). Impact of arbuscular mycorrhizal fungi (AMF) on cucumber growth and phosphorus uptake under cold stress. Functional Plant Biology 42: 1158–1167. [Google Scholar]
Parniske M (2008). Arbuscular mycorrhiza: The mother of plant root endosymbioses. Nature Reviews Microbiology 6: 763–775. [Google Scholar]
Pasbani B, Salimi A, Aliasgharzad N, Hajiboland R (2020). Colonization with arbuscular mycorrhizal fungi mitigates cold stress through improvement of antioxidant defense and accumulation of protecting molecules in eggplants. Scientia Horticulturae 272: 109575. [Google Scholar]
Phillips JM, Hayman DS (1970). Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Transactions of the British Mycological Society 55: 158–161. [Google Scholar]
Pinheiro C, Chaves MM (2011). Photosynthesis and drought: Can we make metabolic connections from available data? Journal of Experimental Botany 63: 869–882. [Google Scholar]
Rezaie R, Mandoulakani BA, Fattahi M (2020). Cold stress changes antioxidant defense system, phenylpropanoid contents and expression of genes involved in their biosynthesis in Ocimum basilicum L. Scientific Reports 10: 5290. [Google Scholar]
Shinozaki K, Yamaguchishinozaki K (1996). Molecular responses to drought and cold stress. Current Opinion in Biotechnology 7: 161–167. [Google Scholar]
Sudhakar C, Lakshmi A, Giridarakumar S (2001). Changes in the antioxidant enzyme efficacy in two high yielding genotypes of mulberry (Morus alba L.) under NaCl salinity. Plant Science 161: 613–619. [Google Scholar]
Velikova V, Yordanov I, Edreva A (2000). Oxidative stress and some antioxidant systems in acid rain-treated bean plants. Plant Science 151: 59–66. [Google Scholar]
Wang ML, Zhang SX, Ding F (2020). Melatonin mitigates chilling-induced oxidative stress and photosynthesis inhibition in tomato plants. Antioxidants 9: 218. [Google Scholar]
Wang P, Wu SH, Wen MX, Wang Y, Wu QS (2016). Effects of combined inoculation with Rhizophagus intraradices and Paenibacillus mucilaginosus on plant growth, root morphology, and physiological status of trifoliate orange (Poncirus trifoliata L. Raf.) seedlings under different levels of phosphorus. Scientia Horticulturae 205: 97–105. [Google Scholar]
Willekens H, Chamnongpol S, Davey M, Schraudner M, Langebartels C, Montagu MV, Camp WV (1997). Catalase is a sink for H2O2 and is indispensable for stress defence in C3 plants. EMBO Journal 16: 4806–4816. [Google Scholar]
Wu QS (2011). Mycorrhizal efficacy of trifoliate orange seedlings on alleviating temperature stress. Plant Soil and Environment 57: 459–464. [Google Scholar]
Wu QS (2018). Experimental Guidelines in Plant Physiology. Beijing, China: China Agriculture Press. [Google Scholar]
Wu QS, He JD, Srivastava AK, Zou YN, Kuča K (2019). Mycorrhizas enhance drought tolerance of citrus by altering root fatty acid compositions and their saturation levels. Tree Physiology 39: 1149–1158. [Google Scholar]
Wu QS, Srivastava AK, Zou YN (2013). AMF-induced tolerance to drought stress in citrus: A review. Scientia Horticulturae 164: 77–87. [Google Scholar]
Yang L, Zou YN, Tian ZH, Wu QS, Kuča K (2021). Effects of beneficial endophytic fungal inoculants on plant growth and nutrient absorption of trifoliate orange seedlings. Scientia Horticulturae 277: 109815. [Google Scholar]
Zhu X, Song F, Xu H (2010). Influence of arbuscular mycorrhiza on lipid peroxidation and antioxidant enzyme activity of maize plants under temperature stress. Mycorrhiza 20: 325–332. [Google Scholar]
Zou YN, Wu QS, Kuča K (2021). Unravelling the role of arbuscular mycorrhizal fungi in mitigating the oxidative burst of plants under drought stress. Plant Biology 23: 50–57. [Google Scholar]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |