DOI:10.32604/biocell.2022.019969

| BIOCELL DOI:10.32604/biocell.2022.019969 |  |
| Viewpoint |
Mechanobiology of the cell surface: Probing its remodeling dynamics using membrane tether pulling assays with optical tweezers
1Centro Nacional de Biologia Estrutural e Bioimagem-CENABIO, Universidade Federal do Rio de Janeiro, Rio de Janeiro, 21941-902, Brazil
2Programa de Pós-graduação em Ciências Biológicas Biofísica, Instituto de Biofísica Carlos Chagas Filho, Universidade Federal do Rio de Janeiro, Rio de Janeiro, 21941-902, Brazil
3Programa de Pós-graduação Multidisciplinar em Física Aplicada, Instituto de Física, Universidade Federal do Rio de Janeiro, Rio de Janeiro, 21941-972, Brazil
4Instituto de Ciências Biomédicas, Universidade Federal do Rio de Janeiro, Rio de Janeiro, 21941-902, Brazil
5Programa de Pós-graduação em Biotecnologia, Instituto de Ciências Biológicas, Universidade Federal do Amazonas, Manaus, 69067-005, Brazil
*Address correspondence to: Bruno Pontes, bpontes@icb.ufrj.br
#These authors contributed equally
Received: 28 October 2021; Accepted: 27 January 2022
Abstract: Mammalian cell surfaces consist of the plasma membrane supported by an underneath cortical cytoskeleton. Together, these structures can control not only the shape of cells but also a series of cellular functions ranging from migration and division to exocytosis, endocytosis and differentiation. Furthermore, the cell surface is capable of exerting and reacting to mechanical forces. Its viscoelastic properties, especially membrane tension and bending modulus, are fundamental parameters involved in these responses. This viewpoint summarizes our current knowledge on how to measure the viscoelastic properties of cell surfaces employing optical tweezers-based tether assays, paving the way for a better understanding of how cells react to external mechanical forces, with a glance on their remodeling dynamics and possible consequences on downstream cellular processes.
Keywords: Cell surface architecture; Actin cortex; Membrane-cytoskeleton complex; Optical tweezers; Tether extraction
Mammalian cell surfaces are extremely dynamic and complex structures, mainly composed of a lipid bilayer membrane supported by a handful of proteins and other accessory molecules. A set of polymerizing proteins make up the underneath cell cortex, involved in maintaining cell shape and integrity, and allowing cell movement, division, and tissue morphogenesis (Chugh and Paluch, 2018). In most eukaryotic cells, the cortex is a well-conserved actin-based network composed of F-actin filaments, myosin, and actin-binding proteins, and thus is called the actomyosin cortex (Chugh and Paluch, 2018; Svitkina, 2020).
The cell membrane and its associated actomyosin cortex, also known as the membrane-cytoskeleton complex (MCC), are important regulators of cell functions, from migration and shape/size determination to molecule-presenting and signaling (Salbreux et al., 2012). Besides interacting with plenty of biochemical stimuli, the MCC exerts and reacts to mechanical forces from its environment (Salbreux et al., 2012).
In this context, MCC’s viscoelastic properties, especially membrane tension and bending modulus, are fundamental parameters involved in their interaction with the intra- and extracellular spaces (Pontes et al., 2017a). In this viewpoint manuscript, we present how these properties are measured, their implications on cell functions, as well as a detailed description of the membrane tether extraction experiment using optical tweezers (OT), the gold-standard tool to perform these measurements (Pompeu et al., 2021). Finally, we also discuss how membrane tether-pulling assays can be used to probe cell surface remodeling dynamics together with possible consequences on downstream cellular processes.
OT are described as single-beam gradient force optical traps that explore the property of photons being able to transfer momentum to small particles in focused laser beams. Reflection and refraction of light by a transparent particle near the laser beam with a Gaussian intensity profile causes a change in the photons’ momentum that is translated as force, attracting it to the focus point. There, the particle experiences a balance of forces that maintains it trapped. These conditions describe a successfully employed OT (Ashkin et al., 1986).
Several biological transparent objects can be trapped with OT, including viruses, bacteria or even suspended cells (Ashkin and Dziedzic, 1987). However, the most suitable way to interact with biological systems using OT is to capture dielectric transparent microspheres with the focused laser beam (Ashkin et al., 1986; Neuman and Block, 2004). It is possible to use these trapped microspheres as handles, attaching them to cell surfaces, and then displacing the microscope stage. This procedure will produce forces (in the piconewton range, pN) on the membrane and can also be used to measure its reaction, given that any displacement in the microsphere position relative to its focal point (
Over the years, OT and other micromanipulation tools, such as atomic force microscopy (AFM), traction force microscopy, magnetic twisting cytometry and micropipette aspiration have been employed to exert forces on MCCs to characterize their mechanical responses (Moeendarbary and Harris, 2014). Membrane tether-pulling is one of the most common assays in this regard. Tether extractions were first performed in red blood cells, using a flow channel experiment where cells previously attached to a coverslip were subjected to fluid shear stress until they began to form membrane tubes connected to the substrate (Hochmuth et al., 1973). An improvement was later introduced using micropipettes. On one side of the red blood cell a portion of its surface was aspirated, and on the opposite side a microsphere (held by another micropipette) was attached to the cell surface and subsequently removed to generate a membrane tether (Hochmuth and Evans, 1982; Hochmuth et al., 1982). However, this assay could not be easily applied to adherent cells. Thus, an OT-based membrane tether pulling method was created (Dai and Sheetz, 1995) and has been widely applied to extract tethers from cells to determine their membrane tension and bending modulus (Ayala et al., 2017; Hissa et al., 2017; Hissa et al., 2013; Pontes et al., 2013; Pontes et al., 2017a; Pontes et al., 2017b; Pontes et al., 2011; Soares et al., 2020; Farias et al., 2020; Gomez et al., 2020).
Briefly, in this assay, an optically trapped microsphere is attached to the MCC and then withdrawn when the microscope stage is set to move (in the xy direction). The trapped microsphere position (
Several studies have demonstrated that these physical parameters, measured with tether extraction experiments, are not only cell-type specific (for more information, see Table 1 in Pontes et al., 2017a), but also depends on the MCC, more specifically on the lipid composition (Hissa et al., 2017; Hissa et al., 2013; Khatibzadeh et al., 2012), the actomyosin cortex organization (Pontes et al., 2011; Masters et al., 2013; Diz-Muñoz et al., 2016; Ayala et al., 2017) and, more strikingly, the membrane-cortex attachment (Nambiar et al., 2009; Diz-Muñoz et al., 2010; Bergert et al., 2021). The membrane tension and bending rigidity of a cell is thus a combination which includes the tension and bending rigidity of the plasma membrane itself plus the membrane-cortex attachment (Dai and Sheetz, 1999; Pontes et al., 2013).
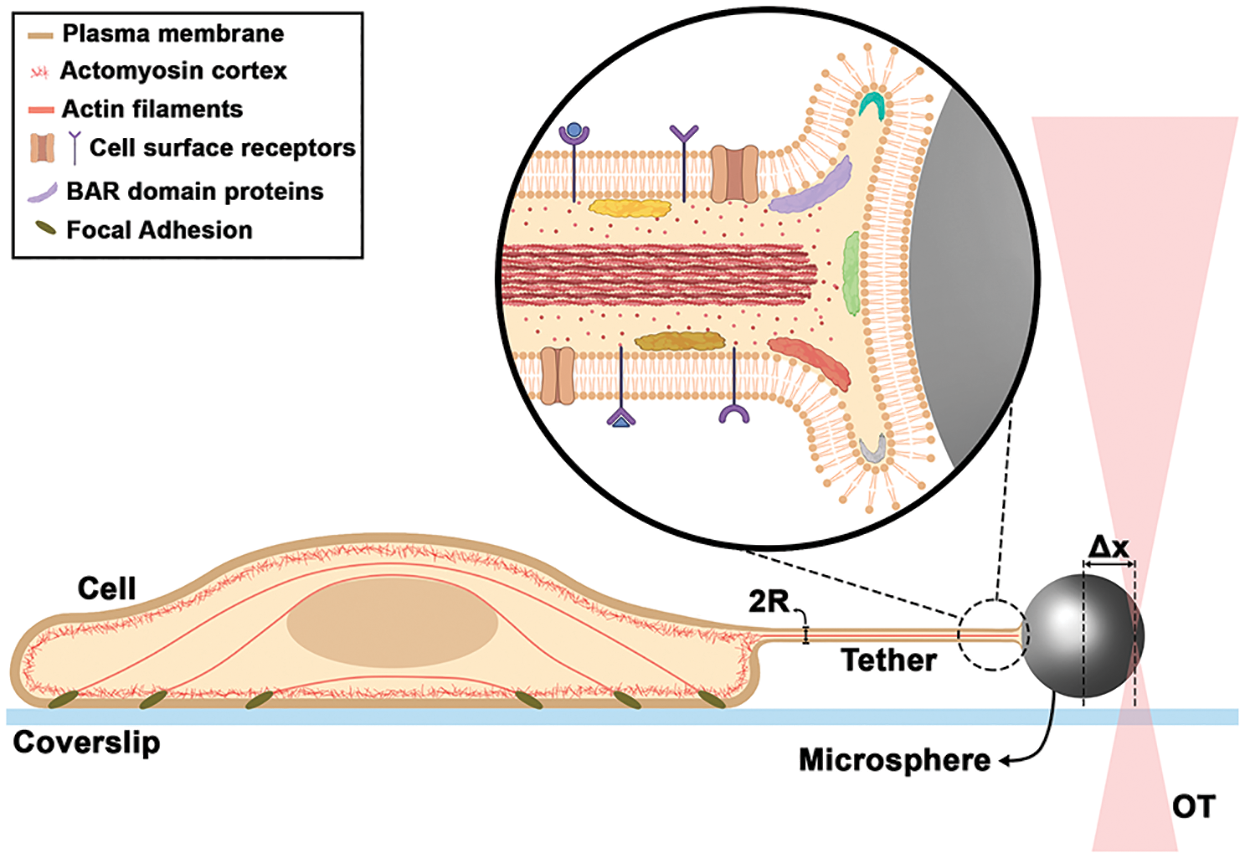
Figure 1: Schematic of a tether extraction experiment with OT, highlighting the nanotube internal organization. Δx is the trapped microsphere position and R is the tether radius.
Moreover, other studies have shown the importance of these parameters, particularly membrane tension, as capable of orchestrating a series of cellular functions ranging from endocytosis (Boulant et al., 2011; Bucher et al., 2018; Sinha et al., 2011; Del Pozo et al., 2021; Djakbarova et al., 2021), exocytosis (Gauthier et al., 2011; Bretou et al., 2014; Masedunskas et al., 2011) and phagocytosis (Masters et al., 2013) to migration (Pontes et al., 2017b; Hetmanski et al., 2019), polarity (Houk et al., 2012; Graziano et al., 2019) and differentiation (Bergert et al., 2021; de Belly et al., 2021). High tension impairs endocytosis, exocytosis, phagocytosis and the overall migration but maintains cell polarity by confining signals to the leading edge of cells. In addition, tension decrease was correlated with a decrease in membrane-cytoskeleton attachment, increased endocytosis and enhanced ERK signaling, which allows exit from naïve to primed pluripotency in embryonic stem cells (Bergert et al., 2021; de Belly et al., 2021). A more detailed description of how membrane tension controls these and other cellular processes are better reviewed in Pontes et al. (2017a) and Sitarska and Diz-Muñoz (2020). In contrast, little is known about how bending rigidity can alter cellular events.
In order to determine these two physical properties, it is necessary to measure both the tether force and radius (Pontes et al., 2017a; Derenyi et al., 2002; Powers et al., 2002). Measuring the tether force and/or radius individually does not allow an absolute estimation of mechanical parameters, although most studies consider indirect measurements with the tether force alone.
Tether force can be measured during OT-tether extraction experiments, as already mentioned. However, measuring the tether radius (50–150 nm) is a bigger challenge, as its size is typically below the resolving limit of conventional optical microscopes (~250 nm) (Fig. 1). Therefore, a correlative microscopy-based method was established (Pontes et al., 2013; Pontes et al., 2011; Pompeu et al., 2021). In this method, a tether is extracted via OT and the force required to perform tether extraction is obtained during the experiment, while the tether radius is later measured by scanning electron microscopy (SEM). The most challenging step is to perform the SEM images of tethers. Regardless of the difficulties inherent to correlative experiments, this is currently the most reliable method to determine the mechanical properties of cell membranes (for a step-by-step procedure, see Pompeu et al., 2021). New optical microscopy methods integrated with OT are needed in order to measure tether radius concomitant with its generation and without other steps, such as fixation for SEM. A proposed method based on quantitative phase imaging, known as spatial light interference microscopy (SLIM) (Wang et al., 2011) combined with OT appears to be very promising in this regard (Lu and Anvari, 2020; Sarshar et al., 2016). Tether radii between 55 and 110 nm were measured for ovarian cancer cells using this method (Lu and Anvari, 2020). Also, stimulated emission depletion (STED) microscopy and AFM have been used; however, for AFM the tether needs to be adhered to the substrate and, as a result of this adhesion, the tube morphology gets slightly deformed (Lamour et al., 2020). And for STED combined with OT, the initial study was carried out in giant unilamellar vesicles (Roy et al., 2020) and no application in cells has been performed so far.
In addition to the mechanical characterization of cell surfaces, membrane tether-pulling can also probe how a cell is able to dynamically remodel its surface in response to an external force. Contrary to some observations (Raucher et al., 2000; Gabella et al., 2014) tethers from adherent cells have been shown to present F-actin inside (Pontes et al., 2011; Pontes et al., 2013; Bornschlögl et al., 2013; Leijnse et al., 2020), which is probably coming from the actomyosin cortex. Moreover, studies of tether-pulling from mast cells (Farrell et al., 2013) and neuronal axons (Datar et al., 2015) found evidence of dynamic saw-tooth-shaped force peaks, with slow rises and sharp decays, arising beyond the tether force plateau region when tethers were kept stretched. Possible explanations for such observations are based on actin polymerization/depolymerization dynamics, together with the action of molecular motors. While the slow rises in force were attributed to the polymerization of F-actin, the decays were associated with depolymerization and/or active rearward movement due to molecular motors such as myosin II (Farrell et al., 2013; Datar et al., 2015). In addition, recently, in a manuscript yet to be published (Leijnse et al., 2020), the presence of actin inside tethers was confirmed not only from the initial moments of extraction, but also increasing after a few minutes post-extraction. The authors also demonstrated that the dynamics of force peaks may be associated with twists and buckles of the F-actin inside the tether, such as those happening in filopodia (Leijnse et al., 2020; Leijnse et al., 2015).
All the experimental evidences described above point to membrane tethers-pulling not only as a strategy to measure the mechanical properties and their variations according to different situations to which cells are exposed, but also as a tool to follow the dynamic rearrangement of cell surfaces. Important consequences of such method could be the elucidation of several molecular mechanisms of protein-membrane interactions and particularly how proteins are able to shape membranes. Also important is the activation/deactivation of local membrane proteins, such as ion channels or other cell receptors after an external pulling force is applied, together with their effects when membrane curvature increases. All proposed observations would be influenced by bending rigidity. A combination of OT and fluorescence microscopy, as previously highlighted (Arbore et al., 2019), can help the field to advance. A schematic summarizing some of the findings described in this viewpoint together with future implications is presented in Fig. 1. Further studies exploring such possibilities could greatly improve our understanding of the role of forces acting on cell surfaces together with their consequences in several downstream cellular processes.
Author Contribution: The authors confirm contribution to the paper as follows: study conception and design: BP. All authors contributed to the writing and approved the final version of the manuscript.
Funding Statement: This work was supported by the Brazilian agencies Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES)–Financial Code 001, Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), Fundação de Amparo à Pesquisa do Estado do Amazonas (FAPEAM), and Instituto Nacional de Ciência e Tecnologia de Fluidos Complexos (INCT-FCx) together with Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP). BP was supported by a JCNE grant from FAPERJ.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Arbore C, Perego L, Sergides M, Capitanio M (2019). Probing force in living cells with optical tweezers: From single-molecule mechanics to cell mechanotransduction. Biophysical Reviews 11: 765–782. DOI 10.1007/s12551-019-00599-y. [Google Scholar] [CrossRef]
Ashkin A, Dziedzic JM, Bjorkholm JE, Chu S (1986). Observation of a single-beam gradient force optical trap for dielectric particles. Optics Letters 11: 288. DOI 10.1364/OL.11.000288. [Google Scholar] [CrossRef]
Ashkin A, Dziedzic JM (1987). Optical trapping and manipulation of viruses and bacteria. Science 235: 1517–1520. DOI 10.1126/science.3547653. [Google Scholar] [CrossRef]
Ayala YA, Pontes B, Hissa B, Monteiro AC, Farina M, Moura-Neto V, Viana NB, Nussenzveig HM (2017). Effects of cytoskeletal drugs on actin cortex elasticity. Experimental Cell Research 351: 173–181. DOI 10.1016/j.yexcr.2016.12.016. [Google Scholar] [CrossRef]
Bergert M, Lembo S, Sharma S, Russo L, Milovanović D et al. (2021). Cell surface mechanics gate embryonic stem cell differentiation. Cell Stem Cell 28: 209–216. DOI 10.1016/j.stem.2020.10.017. [Google Scholar] [CrossRef]
Bornschlögl T, Romero S, Vestergaard CL, Joanny JF, Nhieu GTV, Bassereau P (2013). Filopodial retraction force is generated by cortical actin dynamics and controlled by reversible tethering at the tip. Proceedings of the National Academy of Sciences 110: 18928–18933. DOI 10.1073/pnas.1316572110. [Google Scholar] [CrossRef]
Boulant S, Kural C, Zeeh JC, Ubelmann F, Kirchhausen T (2011). Actin dynamics counteract membrane tension during clathrin-mediated endocytosis. Nature Cell Biology 13: 1124–1131. DOI 10.1038/ncb2307. [Google Scholar] [CrossRef]
Bretou M, Jouannot O, Fanget I, Pierobon P, Larochette N et al. (2014). Cdc42 controls the dilation of the exocytotic fusion pore by regulating membrane tension. Molecular Biology of the Cell 25: 3195–3209. DOI 10.1091/mbc.e14-07-1229. [Google Scholar] [CrossRef]
Bucher D, Frey F, Sochacki KA, Kummer S, Bergeest JP et al. (2018). Clathrin-adaptor ratio and membrane tension regulate the flat-to-curved transition of the clathrin coat during endocytosis. Nature Communications 9: 1109. DOI 10.1038/s41467-018-03533-0. [Google Scholar] [CrossRef]
Chugh P, Paluch EK (2018). The actin cortex at a glance. Journal of Cell Science 131: jcs186254. DOI 10.1242/jcs.186254. [Google Scholar] [CrossRef]
Dai J, Sheetz MP (1995). Mechanical properties of neuronal growth cone membranes studied by tether formation with laser optical tweezers. Biophysical Journal 68: 988–996. DOI 10.1016/S0006-3495(95)80274-2. [Google Scholar] [CrossRef]
Dai J, Sheetz MP (1999). Membrane tether formation from blebbing cells. Biophysical Journal 77: 3363–3370. DOI 10.1016/S0006-3495(99)77168-7. [Google Scholar] [CrossRef]
Datar A, Bornschlögl T, Bassereau P, Prost J, Pullarkat PA (2015). Dynamics of membrane tethers reveal novel aspects of cytoskeleton-membrane interactions in axons. Biophysical Journal 108: 288–497. DOI 10.1016/j.bpj.2014.11.3480. [Google Scholar] [CrossRef]
de Belly H, Stubb A, Yanagida A, Labouesse C, Jones PH, Paluch EK, Chalut KJ (2021). Membrane tension gates ERK-mediated regulation of pluripotent cell fate. Cell Stem Cell 28: 273–284. DOI 10.1016/j.stem.2020.10.018. [Google Scholar] [CrossRef]
Del Pozo MA, Lolo FN, Echarri A (2021). Caveolae: Mechanosensing and mechanotransduction devices linking membrane trafficking to mechanoadaptation. Current Opinion in Cell Biology 68: 113–123. DOI 10.1016/j.ceb.2020.10.008. [Google Scholar] [CrossRef]
Derenyi I, Julicher F, Prost J (2002). Formation and interaction of membrane tubes. Physical Review Letters 88: 238101. DOI 10.1103/PhysRevLett.88.238101. [Google Scholar] [CrossRef]
Diz-Muñoz A, Krieg M, Bergert M, Ibarlucea-Benitez I, Muller DJ, Paluch E, Heisenberg CP (2010). Control of directed cell migration in vivo by membrane-to-cortex attachment. PLoS Biology 8: e1000544. DOI 10.1371/journal.pbio.1000544. [Google Scholar] [CrossRef]
Diz-Muñoz A, Thurley K, Chintamen S, Altschuler SJ, Wu LF, Fletcher DA, Weiner OD (2016). Membrane tension acts through PLD2 and mTORC2 to limit actin network assembly during neutrophil migration. PLoS Biology 14: e1002474. DOI 10.1371/journal.pbio.1002474. [Google Scholar] [CrossRef]
Djakbarova U, Madraki Y, Chan ET, Kural C (2021). Dynamic interplay between cell membrane tension and clathrin-mediated endocytosis. Biology of the Cell 113: 344–373. DOI 10.1111/boc.202000110. [Google Scholar] [CrossRef]
Dutra RS, Viana NB, Maia Neto PA, Nussenzveig HM (2014). Absolute calibration of forces in optical tweezers. Physical Review A 90: 013825. DOI 10.1103/PhysRevA.90.013825. [Google Scholar] [CrossRef]
Farias J, Pompeu P, Pontes B (2020). Exploring stem cell differentiation from a mechanobiological perspective: Insights from neural precursor cells and beyond. Journal of Stem Cells Research Development & Therapy 6: 053. [Google Scholar]
Farrell B, Qian F, Kolomeisky A, Anvari B, Brownell W (2013). Measuring forces at the leading edge: A force assay for cell motility. Integrative Biology 5: 204–214. DOI 10.1039/c2ib20097j. [Google Scholar] [CrossRef]
Gabella C, Bertseva E, Bottier C, Piacentini N, Bornert A, Jeney S, Forró L, Sbalzarini IF, Meister JJ, Verkhovsky AB (2014). Contact angle at the leading edge controls cell protrusion rate. Current Biology 24: 1126–1132. DOI 10.1016/j.cub.2014.03.050. [Google Scholar] [CrossRef]
Gauthier NC, Fardin MA, Roca-Cusachs P, Sheetz MP (2011). Temporary increase in plasma membrane tension coordinates the activation of exocytosis and contraction during cell spreading. PNAS 108: 14467–14472. DOI 10.1073/pnas.1105845108. [Google Scholar] [CrossRef]
Gomez F, Silva LS, Araujo GRS, Frases S, Pinheiro AAS, Agero U, Pontes B, Viana NB (2020). Effect of cell geometry in the evaluation of erythrocyte viscoelastic properties. Physical Review E 101: 062403. DOI 10.1103/PhysRevE.101.062403. [Google Scholar] [CrossRef]
Graziano BR, Town JP, Sitarska E, Nagy TL, Fošnarič M et al. (2019). Cell confinement reveals a branched-actin independent circuit for neutrophil polarity. PLoS Biology 17: e3000457. DOI 10.1371/journal.pbio.3000457. [Google Scholar] [CrossRef]
Hetmanski JHR, de Belly H, Busnelli I, Waring T, Nair RV et al. (2019). Membrane tension orchestrates rear retraction in matrix-directed cell migration. Developmental Cell 51: 460–475. DOI 10.1016/j.devcel.2019.09.006. [Google Scholar] [CrossRef]
Hissa B, Oakes PW, Pontes B, Ramirez-San Juan G, Gardel ML (2017). Cholesterol depletion impairs contractile machinery in neonatal rat cardiomyocytes. Scientific Reports 7: 43764. DOI 10.1038/srep43764. [Google Scholar] [CrossRef]
Hissa B, Pontes B, Roma PM, Alves AP, Rocha CD et al. (2013). Membrane cholesterol removal changes mechanical properties of cells and induces secretion of a specific pool of lysosomes. PLoS One 8: e82988. DOI 10.1371/journal.pone.0082988. [Google Scholar] [CrossRef]
Hochmuth RM, Evans EA (1982). Extensional flow of erythrocyte membrane from cell body to elastic tether I. Analysis. Biophysical Journal 39: 71–81. DOI 10.1016/S0006-3495(82)84492-5. [Google Scholar] [CrossRef]
Hochmuth RM, Mohandas N, Blackshear PLJr (1973). Measurement of the elastic modulus for red cell membrane using a fluid mechanical technique. Biophysical Journal 13: 747–762. DOI 10.1016/S0006-3495(73)86021-7. [Google Scholar] [CrossRef]
Hochmuth RM, Wiles HC, Evans EA, McCown JT (1982). Extensional flow of erythrocyte membrane from cell body to elastic tether II. Experiment. Biophysical Journal 39: 83–89. DOI 10.1016/S0006-3495(82)84493-7. [Google Scholar] [CrossRef]
Houk AR, Jilkine A, Mejean CO, Boltyanskiy R, Dufresne ER, Angenent SB, Altschuler SJ, Wu LF, Weiner OD (2012). Membrane tension maintains cell polarity by confining signals to the leading edge during neutrophil migration. Cell 148: 175–188. DOI 10.1016/j.cell.2011.10.050. [Google Scholar] [CrossRef]
Khatibzadeh N, Gupta S, Farrell B, Brownell WE, Anvari B (2012). Effects of cholesterol on nano-mechanical properties of the living cell plasma membrane. Soft Matter 8: 8350–8360. DOI 10.1039/c2sm25263e. [Google Scholar] [CrossRef]
Lamour G, Allard A, Pelta J, Labdi S, Lenz M, Campillo C (2020). Mapping and modeling the nanomechanics of bare and protein-coated lipid nanotubes. Physical Review X 10: 011031. DOI 10.1103/PhysRevX.10.011031. [Google Scholar] [CrossRef]
Leijnse N, Barooji YF, Verhagen B, Wullkopf L, Erler JT et al. (2020). Filopodia rotate and coil by actively generating twist in their actin shaft. Nature Communications 13: 1636. DOI 10.1038/s41467-022-28961-x. [Google Scholar] [CrossRef]
Leijnse N, Oddershede LB, Bendix PM (2015). Helical buckling of actin inside filopodia generates traction. PNAS 112: 136–141. DOI 10.1073/pnas.1411761112. [Google Scholar] [CrossRef]
Lu T, Anvari B (2020). Characterization of the viscoelastic properties of ovarian cancer cells membranes by optical tweezers and quantitative phase imaging. Frontiers in Physics 8: 582956. DOI 10.3389/fphy.2020.582956. [Google Scholar] [CrossRef]
Masedunskas A, Porat-Shliom N, Weigert R (2011). Linking differences in membrane tension with the requirement for a contractile actomyosin scaffold during exocytosis in salivary glands. Communicative and Integrative Biology 5: 84–87. DOI 10.4161/cib.18258. [Google Scholar] [CrossRef]
Masters TA, Pontes B, Viasnoff V, Li Y, Gauthier NC (2013). Plasma membrane tension orchestrates membrane trafficking, cytoskeletal remodeling, and biochemical signaling during phagocytosis. PNAS 110: 11875–11880. DOI 10.1073/pnas.1301766110. [Google Scholar] [CrossRef]
Moeendarbary E, Harris AR (2014). Cell mechanics: Principles, practices, and prospects. Wiley Interdisciplinary Reviews: Systems Biology and Medicine 6: 371–388. DOI 10.1002/wsbm.1275. [Google Scholar] [CrossRef]
Nambiar R, McConnell RE, Tyska MJ (2009). Control of cell membrane tension by myosin-I. PNAS 106: 11972–11977. DOI 10.1073/pnas.0901641106. [Google Scholar] [CrossRef]
Neuman KC, Block SM (2004). Optical trapping. The Review of Scientific Instruments 75: 2787–2809. DOI 10.1063/1.1785844. [Google Scholar] [CrossRef]
Pompeu P, Lourenço PS, Ether DS, Soares J, Farias J, Maciel G, Viana NB, Nussenzveig HM, Pontes B (2021). Protocol to measure the membrane tension and bending modulus of cells using optical tweezers and scanning electron microscopy. Star Protocols 2: 100283. DOI 10.1016/j.xpro.2020.100283. [Google Scholar] [CrossRef]
Pontes B, Ayala Y, Fonseca ACC, Romão LF, Amaral RF et al. (2013). Membrane elastic properties and cell function. PLoS One 8: e67708. DOI 10.1371/journal.pone.0067708. [Google Scholar] [CrossRef]
Pontes B, Monzo P, Gauthier NC (2017a). Membrane tension: A challenging but universal physical parameter in cell biology. Seminars in Cell & Developmental Biology 71: 30–41. DOI 10.1016/j.semcdb.2017.08.030. [Google Scholar] [CrossRef]
Pontes B, Monzo P, Gole L, Le Roux AL, Kosmalska AJ et al. (2017b). Membrane tension controls adhesion positioning at the leading edge of cells. Journal of Cell Biology 216: 2959–2977. DOI 10.1083/jcb.201611117. [Google Scholar] [CrossRef]
Pontes B, Viana NB, Salgado LT, Farina M, Moura-Neto V, Nussenzveig HM (2011). Cell cytoskeleton and tether extraction. Biophysical Journal 101: 43–52. DOI 10.1016/j.bpj.2011.05.044. [Google Scholar] [CrossRef]
Powers TR, Huber G, Goldstein RE (2002). Fluid-membrane tethers: Minimal surfaces and elastic boundary layers. Physical Review E 65: 041901. DOI 10.1103/PhysRevE.65.041901. [Google Scholar] [CrossRef]
Raucher D, Stauffer T, Chen W, Shen K, Guo S, York JD, Sheetz MP, Meyer T (2000). Phosphatidylinositol 4,5-bisphosphate functions as a second messenger that regulates cytoskeleton-plasma membrane adhesion. Cell 100: 221–228. DOI 10.1016/S0092-8674(00)81560-3. [Google Scholar] [CrossRef]
Roy D, Steinkühler J, Zhao Z, Lipowsky R, Dimova R (2020). Mechanical tension of biomembranes can be measured by super resolution (STED) microscopy of force-induced nanotubes. Nano Letters 20: 3185–3191. DOI 10.1021/acs.nanolett.9b05232. [Google Scholar] [CrossRef]
Salbreux G, Charras G, Paluch EK (2012). Actin cortex mechanics and cellular morphogenesis. Trends in Cell Biology 22: 536–545. DOI 10.1016/j.tcb.2012.07.001. [Google Scholar] [CrossRef]
Sarshar M, Lu T, Anvari B (2016). Combined optical micromanipulation and interferometric topography (COMMIT). Biomedical Optics Express 7: 1365–1374. DOI 10.1364/BOE.7.001365. [Google Scholar] [CrossRef]
Sinha B, Köster D, Ruez R, Gonnord P, Bastiani M et al. (2011). Cells respond to mechanical stress by rapid disassembly of caveolae. Cell 144: 402–413. DOI 10.1016/j.cell.2010.12.031. [Google Scholar] [CrossRef]
Sitarska E, Diz-Muñoz A (2020). Pay attention to membrane tension: Mechanobiology of the cell surface. Current Opinion in Cell Biology 66: 11–18. DOI 10.1016/j.ceb.2020.04.001. [Google Scholar] [CrossRef]
Soares J, Araujo GRS, Santana C, Matias D, Moura-Neto V et al. (2020). Membrane elastic properties during neural precursor cell differentiation. Cells 9: 1323. DOI 10.3390/cells9061323. [Google Scholar] [CrossRef]
Svitkina TM (2020). Actin cell cortex: Structure and molecular organization. Trends in Cell Biology 30: 556–565. DOI 10.1016/j.tcb.2020.03.005. [Google Scholar] [CrossRef]
Wang Z, Millet L, Mir M, Ding H, Unarunotai S, Rogers J, Gillette MU, Popescu G (2011). Spatial light interference microscopy (SLIM). Optics Express 19: 1016–1026. DOI 10.1364/OE.19.001016. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |