DOI:10.32604/biocell.2022.019873

| BIOCELL DOI:10.32604/biocell.2022.019873 |  |
| Viewpoint |
Dancing to a somewhat different rhythm: Cell migration along the natural basement membrane
Department of Biological Sciences, Oakland University, Rochester, MI, 48309-4476, USA
*Address correspondence to: Sheldon R. Gordon, srgordon@oakland.edu
Received: 21 October 2021; Accepted: 20 January 2022
Abstract: Much of our understanding of the events which underlie cell migration has been derived from studies of cells in tissue culture. One of the components that mediates this process is the dynamic actin-based microfilament system that can reorganize itself into so-called stress fibers that are considered essential components for cell motility. In contrast, relatively few studies have investigated cell movement along an extracellular matrix (ECM) which is known to influence both cellular organization and behavior. This opinion/viewpoint article briefly reviews cell migration during corneal endothelial wound repair along the tissue’s natural basement membrane, Descemet’s membrane. Because the tissue exists as a cell monolayer it affords one an opportunity to readily explore the effect of cell/matrix influences on cell motility. As such, cell movement along this substrate differs somewhat from that found in vitro and migrating endothelial cells also demonstrate an ability to move along the ECM without the benefit of having an organized actin cytoskeleton.
Keywords: Corneal endothelium; Cell migration; Microfilaments; Basement membrane
A Perspective on Cell Migration Studies
In all animal systems, migration is a fundamental biological property of cells that occurs in both normal and diseased states. Thus, it is an area of cell biology that has received extensive attention. Since the advent of modern cell culture technology in the 1950’s, this phenomenon has been intensely explored by numerous investigators employing various approaches from the morphological, biochemical and molecular levels in a concerted effort to ascertain those mechanisms responsible for this phenomenon. Results obtained from this plethora of studies have revealed some very basic and fundamental insights into mechanisms that allow for cell movement in processes such as wound repair, tissue development, and cancer metastasis. In addition, in order to more closely mimic the in vivo environment, work on this subject has further expanded into understanding the so-called 3-D migratory processes as they relate to some cells that move within an extracellular connective tissue scaffolding (Stixt, 2012; Yamada and Sixt, 2012; Friedl et al., 2012; Friedl et al., 1998). Interestingly, under this condition, migration appears to involve ameboid-like movements within the matrix resulting in an intimate matrix/cell association that appears to eliminate the need for focal adhesions (Paluch et al., 2016).
Many observations on cell movement have come from experiments employing cultured cells. While findings using this approach have provided us with insights into the mechanisms and physiological processes that cells employ to migrate (Reig et al., 2014; Ridley et al., 2003; Wong and Gotlieb, 1984), these observations come mostly from systems in which cells exist on artificial surfaces (Pelhan and Wang, 1998), or surfaces that have been coated with an extracellular matrix (ECM) material such as fibronectin or laminin (Hartman et al., 2017; Perris and Perissinotto, 2000; Bailey et al., 1993; McCarthy et al., 1985; McCarthy and Furcht, 1984). Within the literature there are studies, though few compared to cultured cell usage, that investigate how a highly organized and complex natural ECM influences cell motility. These studies all describe a highly dynamic structure that provides mechanical and chemical cues to guide migrating cells (Sherwood, 2021), some of which provided by cellular-mediated proteolysis of the substrate to generate bioactive ECM fragments with unique signaling activities (Ricard-Blum and Vallet, 2019). Indeed, studies from the author’s laboratory have demonstrated that when injured organ cultured endothelium are exposed to protease inhibitors, cell movement into the wound area is retarded (Gordon and DeMoss, 1999). Thus, basement membranes have been shown to be dynamic structures capable of influencing those mechanisms that regulate cell movement.
As it turns out, although much of what has been learned in vitro can be applied to in vivo or in situ studies, there are differences which underlie how cells move along an ECM, either in 2D or 3D situations, relative to migrating in a tissue culture milieu (Yamada et al., 2019), especially in the latter case where migration appears to be mediated by the formation of lobopodia (Petrie et al., 2017). In addition, studies have shown that the dimensionality of the underlying matrix, either in a 1D, 2D or 3D organization will influence mechanisms that regulate cell migration (Doyle et al., 2013). Thus, as these recent investigations demonstrate, the organization of the matrix will influence and dictate mechanisms that cells undertake to migrate.
The Influence of the ECM and Descemet’s Membrane on the Corneal Endothelium
Because epithelial cells reside on basement membranes, these rather large, highly organized ECM complexes play significant roles in their life and function, from gene expression to virtually all physiological processes. As a result, they display a highly polarized cellular organization with distinct apical and basal ends (Chang et al., 2019; Cooperman and Djiane, 2016). In response to a stimulus such as an injury, these cells migrate while maintaining a close adherence with their underlying ECM. In the case of a wound, epithelial cells migrate along their basement membrane (so-called 2-D migration) and thus, are greatly influenced by their interactions with this natural underlying ECM. In the case of the corneal endothelium, it exists as a cell monolayer on Descemet’s membrane (Figs. 1A–1C), on the posterior aspect of the cornea and contains no direct nerve or blood supply, thus making the tissue amenable to organ culture. As such, it represents a model system for studying how the underlying ECM influences cell organization and regulates cell migration during wound repair.
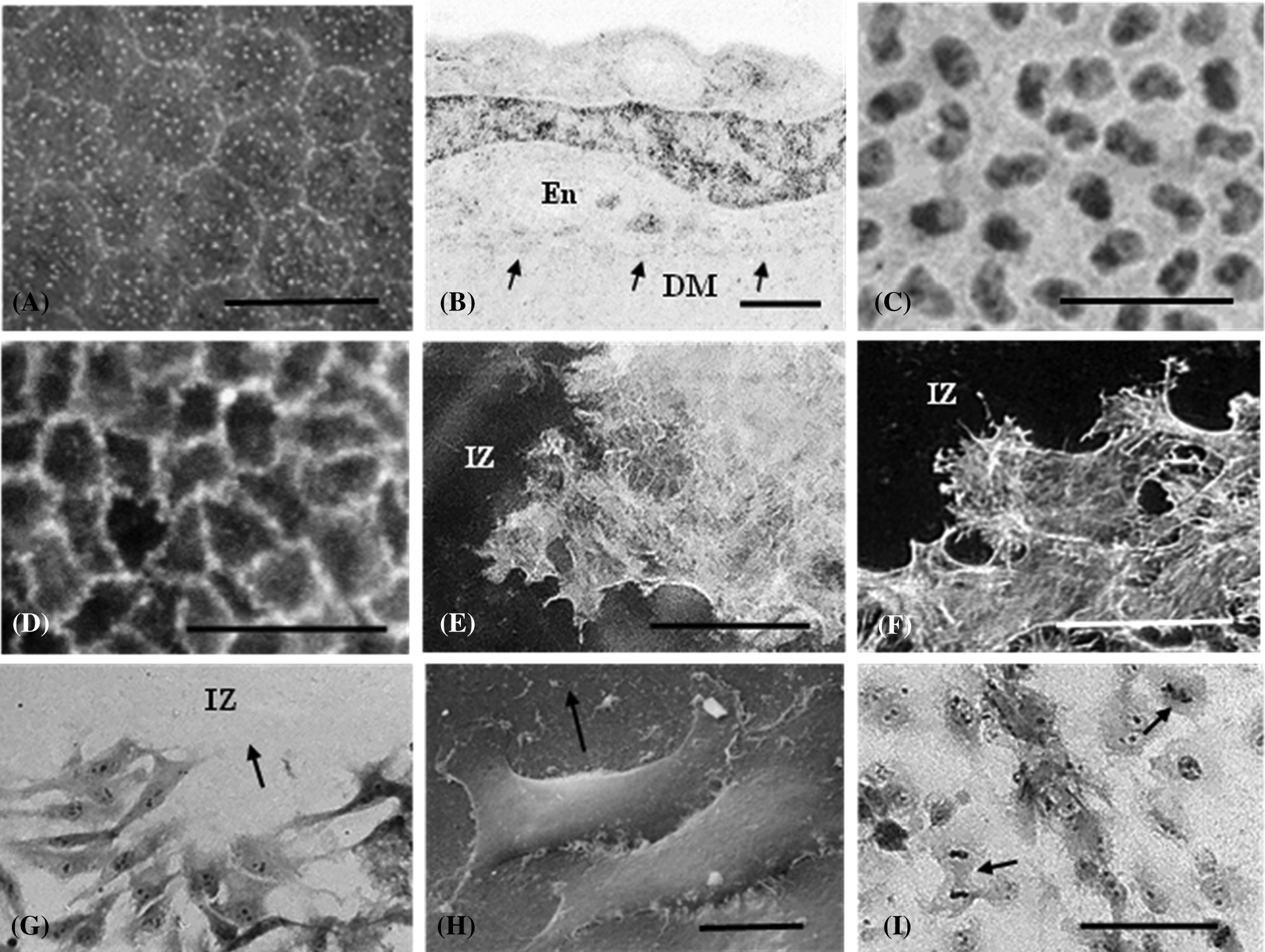
Figure 1: Various images of the rat corneal endothelium. Scanning electron micrograph of the normal non-injured tissue. A. Polyhedral cells form a monolayer that resides on the underlying Descemet’s membrane. B. Cross sectional transmission electron micrograph of an endothelial cell (En) resting upon Descemet’s membrane (DM). Arrows represent the cell/DM interface. The cell nucleus (N) is rather prominent in this perspective. C. Light micrograph of a tissue flat mount. Nuclei appear oval to kidney-shaped in their appearance. D. Fluorescence micrograph of circumferential microfilament bands in a normal tissue stained with TRITC conjugated phalloidin. E. 6 h post-injury, peripheral microfilament bands are not observed in cells adjacent to the injury zone (IZ), although actin fibers, but not stress fibers are detected. F. 24 h after a circular freeze injury, cells surrounding the injury zone (IZ), now display prominent stress fibers as they move into the wound. G. Endothelial cells migrating into the injury zone (IZ) at 36 h after wounding. H. Scanning electron micrograph of endothelial cells migrating into the wound region (IZ) along the basement membrane. Arrow points in the direction of cell movement. I. By 48 h post-injury cells have repopulated the wound area. Mitotic figures are noted (arrows). Scale bars: A = 25 µ, B = 2 µ, C = 50 µ, D = 50 µ, E = 50 µ, F = 40 µ, G = 100 µ, H = 100 µ, I = 10 µ.
The endothelium, as all epithelial systems on ECM, displays a very polarized organization with distinct apical and basal surfaces. Apically, actin is organized into distinct peripheral microfilament bands (Fig. 1D) that intersect with the zonula adherens (Gordon and Wood, 2009) as part of the junctional complex that contributes to the tissue’s barrier function and integrity (Barry et al., 1995). In contrast, when endothelial cells are grown in vitro to confluency, although they assume a classical cobblestone appearance, they display a much more extensive actin cytoskeletal framework around all the cell membranes (apical, basal and lateral) (Gordon et al., 1982), akin to a cage-like appearance, possibly as a response to being grown on an artificial foreign surface.
Effect of Descemet’s Membrane on Endothelial Wound Repair
An in situ circular freeze injury results in a denuded region of ECM that cells adjacent to the wound migrate along in order to repopulate and restore the monolayer. An early response to the injury is the loss of the peripheral microfilament bands with actin becoming reorganized into stress fibers (Fig. 1F), (Gordon et al., 2005). In addition, alterations in cell morphology concomitantly occur as the polygonal cells become more akin in their appearance to migrating cultured cells. Subsequently, these cells migrate into the wound region (Figs. 1G–1I). As the monolayer reforms, migration ceases, stress fibers disappear, and peripheral microfilament bands become reestablished as the integrity of the tissue monolayer is restored.
Existing on an ECM modifies some characteristics of endothelial cell movement during wound repair relative to what has been observed in cultured cells. For example, these cells move into the wound area, maintaining a very close association between their basal cell membrane and the ECM, almost as if they are sliding along the matrix surface. These migrating cells display a tapered leading edge that occasionally displays a slender filopodia. Though this is somewhat akin to what is observed in moving cultured cells, this morphological modification accompanied by a highly organized actin cytoskeleton are not absolute requirements for their movement along an ECM. Several studies from my laboratory, using agents that led to actin depolymerization, slowed, but did not prevent cell migration and subsequent wound closure. In addition, soybean agglutinin (SBA) exposure not only led to the loss of actin organization, but resulted in the leading edge becoming blunt and rounded with no evidence for the presence of filopodial extensions, yet wound closure still occurred (Gordon et al., 2020). This is suggestive that these cells compensate for the loss of an organized actin cytoskeleton. In addition, in contrast to reports of moving cultured cells forming “cytoplasmic tails” and leaving small amounts of the cell attached to the substrate, migrating corneal endothelial cells do not appear to do this, as the entire cell is translocated from point to point (Fig. 1H).
Recently, studies in my laboratory (Gordon et al., 2020) also showed that migration into a wound was prevented by interfering with the PI-3K signaling pathway, whereas, interfering with the cdc-42 pathway did not inhibit cell movement. In both conditions, the actin cytoskeleton was disrupted using either cytochalasin B or SBA, thus indicating that movement into the wound was dependent on PI-3K signaling more so than an organized actin framework. This result does not dismiss the contribution of microfilaments to efficient cell motility, but serves to indicate that cells on a natural basement membrane have the ability to “override” microfilament loss. This strongly contrasts to studies in vitro where inhibiting stress fibers leads to a cessation of cell movement (Schenk et al., 2015; Molinuevo et al., 2007) and only after they are allowed to reform is movement reinitiated.
Evidence that Other Cellular Mechanisms Probably Compensate for the Loss of Stress Fibers
Additional studies in my laboratory suggest cell migration along an ECM is not absolutely dependent on actin organization. Evidence shows cells employ a variety of interacting mechanisms to ensure cell movement occurs when required. For example, despite residing on an ECM, endothelial cells deposit fibronectin (Gordon, 1988; Sabet and Gordon, 1989; Gordon, 1994), and proteases (Gordon and DeMoss, 1999) to help facilitate their migration. In addition, disrupting microtubules slows down cell movement into a wound more than does cytochalasin B exposure (Gordon and Staley, 1990), possibly because not only do they appear to guide focal adhesion formation (Garcin and Strauben, 2019; Seetharaman and Etienne-Manneville, 2019), but also act as conduits for deposition of new matrix protein (Sabet and Gordon, 1989) (Figs. 2A and 2B). Furthermore, injured tissues treated with a short exposure to actinomycin D, fail to undergo wound closure by 72 h post-injury, despite the presence of stress fibers (Gordon and Staley, 1990). In conclusion, although general features of motility are seen in all migrating cell types, the fact is that cells living on an ECM employ several mechanisms, operating in a cooperative fashion, to allow them to compensate for the loss of actin organization to achieve cell migration.
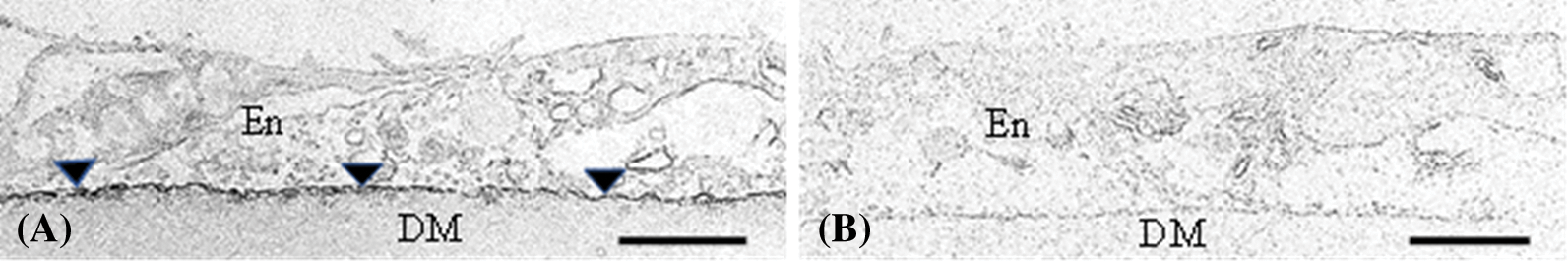
Figure 2: Electron micrographs from the author’s laboratory showing immunoperoxidase stained corneal endothelium (En) at 48 h post-injury. Tissues were not stained with lead prior to immunostaining. In (A), a control tissue stained to demonstrate fibronectin deposition (arrowheads) along the basement membrane (dark line). In (B), tissues treated with colchicine fail to deposit fibronectin along the basement membrane. Under this condition, migration into the wound area is suppressed. DM = Descemet’s membrane. Scale bars = 2 µ.
Availability of Data and Materials: Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Author Contribution: The author has the sole responsibility for the writing, editing, preparation and submission of this manuscript.
Ethics Approval: All experimental procedures that served as the basis for this opinion/viewpoint article were carried out and approved in accordance with the guidelines and regulations set forth by the Institutional Animal Care and Use Committee of Oakland University (Approval #18121; Date of approval: January 14, 2019).
Funding Statement: The funding for the author’s research has been supported by the National Eye Institute—NIH, The Michigan Eye Bank, Midwest Eye Bank, Eversight, the Research Excellence Fund of the Center for Biomedical Sciences, Oakland University and the Department of Biological Sciences.
Conflicts of Interest: The author declares that there are no conflicts of interest for this present manuscript.
Bailey SB, Eicher ME, Villadiego A, Rich KM (1993). The influence of fibronectin and laminin during Schwann cell migration and peripheral nerve regeneration through silicon chambers. Journal of Neurocytology 22: 176–184. DOI 10.1007/BF01246356. [Google Scholar] [CrossRef]
Barry PA, Petroll WM, Andrews PM, Cavanagh HD, Jester JV (1995). The spatial organization of corneal endothelial cytoskeletal proteins and their relationship to the apical junctional complex. Investigative Ophthalmology and Visual Science 36: 1115–1124. [Google Scholar]
Chang B, Svoboda KKH, Liu X (2019). Cell polarization: From epithelial cells to odontoblasts. European Journal of Cell Biology 98: 1–11. DOI 10.1016/j.ejcb.2018.11.003. [Google Scholar] [CrossRef]
Cooperman P, Djiane A (2016). Adherens junction and E-cadherin complex regulation by epithelial polarity. Cellular and Molecular Life Sciences 73: 3535–3553. DOI 10.1007/s00018-016-2260-8. [Google Scholar] [CrossRef]
Doyle AD, Petrie RJ, Kutys ML, Yamada KM (2013). Dimensions in cell migration. Current Opinion in Cell Biology 25: 642–649. DOI 10.1016/j.ceb.2013.06.004. [Google Scholar] [CrossRef]
Friedl P, Sahai E, Weiss S, Yamada KM (2012). New dimensions in cell migration. Nature Reviews in Molecular Cell Biology 13: 743–747. DOI 10.1038/nrm3459. [Google Scholar] [CrossRef]
Friedl P, Zänker KS, Bröcker EB (1998). Cell migration strategies in 3-D extracellular matrix: Differences in morphology, cell matrix interactions, and integrin function. Microscopy and Research Technique 43: 369–378. DOI 10.1002/(ISSN)1097-0029. [Google Scholar] [CrossRef]
Garcin C, Strauben A (2019). Microtubules in cell migration. Essays in Biochemistry 63: 509–520. DOI 10.1042/EBC20190016. [Google Scholar] [CrossRef]
Gordon SR (1988). Changes in distribution of extracellular matrix proteins during wound repair in corneal endothelium. Journal of Histochemistry and Cytochemistry 36: 409–416. DOI 10.1177/36.4.3279112. [Google Scholar] [CrossRef]
Gordon SR (1994). Cytological and immunocytochemical approaches to the study of corneal endothelial wound repair. Progress in Histochemistry and Cytochemistry 28: 1–66. DOI 10.1016/S0079-6336(11)80033-1. [Google Scholar] [CrossRef]
Gordon SR, Climie M, Hitt AL (2005). 5-Fluorouridine interferes with actin organization, stress fiber formation and cell migration in corneal endothelial cells during wound repair along the natural basement membrane. Cell Motility and the Cytoskeleton 62: 244–258. DOI 10.1002/(ISSN)1097-0169. [Google Scholar] [CrossRef]
Gordon SR, DeMoss J (1999). Exposure to lysosomotropic amines and protease inhibitors retard corneal endothelial cell migration along the natural basement membrane during wound repair. Experimental Cell Research 246: 233–242. DOI 10.1006/excr.1998.4298. [Google Scholar] [CrossRef]
Gordon SR, Gordon GH, Dimovski S (2020). Rat corneal endothelial cell migration during wound repair on the basement membrane depends more on the PI-3K pathway than the cdc-42 pathway or actin stress fibers. Cell and Tissue Research 382: 351–366. DOI 10.1007/s00441-020-03229-2. [Google Scholar] [CrossRef]
Gordon SR, Rothstein H, Essner E (1982). In situ demonstration of actin in normal and injured ocular tissues using 7-nitrobenz-2-oxa-1,3-diazole phallacidin. Cell Motility 2: 343–354. DOI 10.1002/cm.970020404. [Google Scholar] [CrossRef]
Gordon SR, Staley CA (1990). Role of the cytoskeleton during injury-induced migration in corneal endothelium. Cell Motility and the Cytoskeleton 16: 47–57. DOI 10.1002/(ISSN)1097-0169. [Google Scholar] [CrossRef]
Gordon SR, Wood M (2009). Soybean agglutinin binding to corneal endothelial cell surfaces disrupts in situ monolayer integrity and actin organization and interferes with wound repair. Cell and Tissue Research 335: 551–563. DOI 10.1007/s00441-008-0741-2. [Google Scholar] [CrossRef]
Hartman CD, Isenberg BC, Chua SG, Yong JY (2017). Extracellular matrix type modulates cell migration on mechanical gradients. Experimental Cell Research 359: 361–366. DOI 10.1016/j.yexcr.2017.08.018. [Google Scholar] [CrossRef]
McCarthy JB, Basara ML, Palm SL, Sas DF, Furcht LT (1985). The role of cell adhesion proteins—laminin and fibronectin—in the movement of malignant and metastatic cells. Cancer and Metastasis Reviews 4: 125–152. DOI 10.1007/BF00050692. [Google Scholar] [CrossRef]
McCarthy JB, Furcht LT (1984). Laminin and fibronectin support the haptotactic migration of B16 mouse melanoma cells in vitro. Journal of Cell Biology 98: 1474–1480. DOI 10.1083/jcb.98.4.1474. [Google Scholar] [CrossRef]
Molinuevo MS, Bruzzone L, Cortizo AM (2007). Alendronate induces anti-migratory effects and inhibition of neutral phosphatases in UMR106 osteosarcoma cells. European Journal of Pharmacology 562: 28–33. DOI 10.1016/j.ejphar.2007.01.054. [Google Scholar] [CrossRef]
Paluch EK, Alpalter IM, Stixt M (2016). Focal adhesion-independent cell migration. Annual Review of Cell and Developmental Biology 32: 469–490. DOI 10.1146/annurev-cellbio-111315-125341. [Google Scholar] [CrossRef]
Pelhan RJJr, Wang Y (1998). Cell locomotion and focal adhesions are regulated by substrate flexibility. PNAS 94: 13661–13665. DOI 10.1073/pnas.94.25.13661. [Google Scholar] [CrossRef]
Perris R, Perissinotto D (2000). Role of the extracellular matrix during neural crest cell migration. Mechanism of Development 95: 3–21. DOI 10.1016/S0925-4773(00)00365-8. [Google Scholar] [CrossRef]
Petrie RJ, Harlin HM, Korsak LIT, Yamada KM (2017). Activating the nuclear piston mechanism of 3D migration in tumor cells. Journal of Cell Biology 216: 93–100. DOI 10.1083/jcb.201605097. [Google Scholar] [CrossRef]
Reig G, Pulgar E, Concha ML (2014). Cell migration: From tissue culture to embryos. Development 141: 1999–2013. DOI 10.1242/dev.101451. [Google Scholar] [CrossRef]
Ricard-Blum S, Vallet SD (2019). Fragments generated upon extracellular matrix remodeling: Biological regulators and potential drugs. Matrix Biology 75–76: 170–189. DOI 10.1016/j.matbio.2017.11.005. [Google Scholar] [CrossRef]
Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR (2003). Cell migration: Integrating signals from front to back. Science 302: 1704–1709. DOI 10.1126/science.1092053. [Google Scholar] [CrossRef]
Sabet MD, Gordon SR (1989). Ultrastructural immunocytochemical localization of fibronectin deposition during corneal endothelial wound repair. Evidence for cytoskeletal involvement. Biology of the Cell 65: 171–179. DOI 10.1111/j.1768-322X.1989.tb00786.x. [Google Scholar] [CrossRef]
Schenk M, Aykut B, Teske C, Giese NA, Weitz J (2015). Salinomycin inhibits growth of pancreatic cancer cell migration by disruption of actin stress fibers. Cancer Letters 358: 161–169. DOI 10.1016/j.canlet.2014.12.037. [Google Scholar] [CrossRef]
Seetharaman S, Etienne-Manneville S (2019). Microtubules at focal adhesions-a double-edged sword. Journal of Cell Science 132: jcs232843. DOI 10.1242/jcs.232843. [Google Scholar] [CrossRef]
Sherwood DR (2021). Basement membrane remodeling guides cell migration and cell morphogenesis during development. Current Opinion in Cell Biology 72: 19–27. DOI 10.1016/j.ceb.2021.04.003. [Google Scholar] [CrossRef]
Stixt M (2012). Cell migration: Fibroblasts find a new way to get ahead. Journal of Cell Biology 197: 347349. [Google Scholar]
Wong MK, Gotlieb AI (1984). In vitro reendothelialization of a single-cell wound. Role of microfilament bundles in rapid lamellipodia-mediated wound closure. Laboratory Investigation 51: 75–81. [Google Scholar]
Yamada KM, Collins JW, Cruz Walma DA, Doyle AD, Gonzalez Morales S et al. (2019). Extracellular matrix dynamics in cell migration, invasion and tissue morphogenesis. International Journal of Experimental Pathology 100: 144–152. DOI 10.1111/iep.12329. [Google Scholar] [CrossRef]
Yamada KM, Sixt M (2012). Mechanisms of cell migration. Nature Reviews Molecular Cell Biology 20: 738–752. DOI 10.1038/s41580-019-0172-9. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |