DOI:10.32604/biocell.2022.020069

| BIOCELL DOI:10.32604/biocell.2022.020069 |  |
| Article |
Wnt3a-induced ST2 decellularized matrix ornamented PCL scaffold for bone tissue engineering
1Laboratory of Skeletal Development and Regeneration, The Institute of Life Science, Chongqing Medical University, Chongqing, 400016, China
2Department of Anatomy and Cell Biology, Indiana University School of Medicine, Indianapolis, IN46202, USA
3Bioengineering Collage, Chongqing University, Chongqing, 400044, China
*Address correspondence to: Xiaolin Tu, xtu@cqmu.edu.cn
Received: 18 November 2021; Accepted: 03 January 2022
Abstract: The limited bioactivity of scaffold materials is an important factor that restricts the development of bone tissue engineering. Wnt3a activates the classic Wnt/β-catenin signaling pathway which effects bone growth and development by the accumulation of β-catenin in the nucleus. In this study, we fabricated 3D printed PCL scaffold with Wnt3a-induced murine bone marrow-derived stromal cell line ST2 decellularized matrix (Wnt3a-ST2-dCM-PCL) and ST2 decellularized matrix (ST2-dCM-PCL) by freeze-thaw cycle and DNase decellularization treatment which efficiently decellularized >90% DNA while preserved most protein. Compared to ST2-dCM-PCL, Wnt3a-ST2-dCM-PCL significantly enhanced newly-seeded ST2 proliferation, osteogenic differentiation and upregulated osteogenic marker genes alkaline phosphatase (Alp), Runx2, type I collagen (Col 1) and osteocalcin (Ocn) mRNA expression. After 14 days of osteogenic induction, Wnt3a-ST2-dCM-PCL promoted ST2 mineralization. These results demonstrated that Wnt3a-induced ST2 decellularized matrix improve scaffold materials’ osteoinductivity and osteoconductivity.
Keywords: Wnt3a; Decellularized matrix; Bone marrow stromal cells; Osteogenic differentiation; Bone tissue engineering
The regeneration of large bone defects is a major challenge in the field of orthopedics (Schmidt, 2021). Autologous bone is the gold standard for repairing such defects. However, the morbidity of the donor site and the size of the graft limit its clinical application (Al-Abedalla et al., 2015). Bone grafts of the same species and xenotypes have been used clinically. But they can cause immunological rejection which lead to migration failure. What’s more, they have differences in bone regeneration for the gender, age, race, and living habits of the donors, which restricted their clinical practices (Tournier et al., 2021; Visscher et al., 2016). With the continuous development of tissue engineering technology, artificial bones can be produced in large quantities (Almubarak et al., 2016). 3D printing technology is favored for personalized manufacturing scaffolds (Battafarano et al., 2021; Wu et al., 2019). And scaffold materials play an important role in the construction of artificial bones. The ideal scaffold material should have similar components to natural bone, provide similar mechanical strength and biological microenvironment, which promote adhesion, growth, proliferation, migration and differentiation of cells on the scaffold (Chae et al., 2021; Nikolova and Chavali, 2019). However, the current scaffold materials cannot provide complex cell signals in natural bone microenvironment, and bone repair effect needs further enhanced (Long et al., 2015; Shang et al., 2021). Living cells are printed to fabricate bone repair scaffolds in bone tissue engineering to improve scaffold materials’ bioactivity, which will cause ethical issues, and the transformation to clinical trials is still challenging (Bose et al., 2013). Therefore, the development of scaffold materials with good osteogenesis and mechanical properties has become a research hotspot in bone tissue engineering.
Decellularized matrix (dCM) removes immunogenic substances such as DNA from cells, while retains the precise and orderly network structure composed of proteins and polysaccharides (Bracaglia and Fisher, 2015), and its three-dimensional microstructure can provide the body closest to cell growth. The dCM is rich in various active molecules to provide the basis for various cell activities (Aamodt and Grainger, 2016). Decellularized matrix (dCM) has been widely used as scaffold materials for regenerative medicine due to its natural properties, low immune repulsion and excellent biocompatibility. Demineralized bone matrix (DBM), a kind of allograft material, has been used to enhance joint fusion and spinal fusion in clinic orthopaedic surgery for its similar composition, structure and function to autologous bone (Drosos et al., 2007). However, the osteogenic activity of commercially available DBM depends on specific donor characteristics, such as age, sex and lifestyle and its preparation method. In addition, several commercial decellularized matrix have received FDA approval for use in humans, including dermal tissue, pig heart valve and porcine bladder. Decellularized scaffolds made from porcine small intestinal submucosa (SIS) have been used in orthopedic surgery to repair rotator cuff in preclinical trials. Although tissue-derived biological scaffolds are commonly used to repair non-homologous anatomical sites, studies of tissue engineering in skeletal muscle suggested that biological scaffolds from homologous tissues at specific sites may be more suitable for constructive tissue remodeling than non-site-specific scaffolds (Zhang et al., 2022). Decellularized bone effectively promotes bone regeneration, but its source is limited and cannot meet clinical needs (Cheng et al., 2014). Cell-derived dCM has attracted much attention due to its wide range of sources (Parmaksiz et al., 2020). Studies have found that dCM of fibroblasts and endothelial cells promoted angiogenesis (Fu et al., 2018). Chondrocyte dCM promotes cartilage repair (Pei and He, 2012). Mesenchymal stem cells (marrow mesenchymal stem cells, MSCs) dCM-modified titanium scaffold upregulated the expression of osteogenic genes of mesenchymal stem cells (Benders et al., 2013). Osteoblast (MC3T3-E1) dCM-modified calcined bone accelerated the repair of rabbit radius defects (Ma et al., 2017). A large number of studies have proved that the cell-derived decellularized matrix has great potential as a tissue engineering repair material.
The Wnt/β-catenin signaling pathway plays an important role in the growth and development of bone (Benoit, 2014). The activated classic Wnt signaling pathway promotes the osteogenic differentiation of bone marrow mesenchymal stem cells (BMSCs) by upregulating osteogenic differentiation related genes (Zhong et al., 2012). As the protein of classic Wnt signaling pathway, Wnt3a protein is widely involved in regulating cell biological behavior (Boland et al., 2004). The previous study of our group found that Wnt3a protein promoted osteogenic differentiation of BMSCs (Tu et al., 2007), but Wnt protein is unstable in vivo (Boland et al., 2004). Therefore, in this study, we modified 3D printed PCL scaffold with decellularized matrix from Wnt3a-induced murine bone marrow-derived stromal cell line ST2 (Minetaro Ogawa et al., 1988) which is widely used in osteogenic differentiation for its osteogenic potency, easy to obtain and culture in vitro (Li et al., 2019a; Yuan et al., 2021). We evaluated Wnt3a-induced ST2 dCM ornamented PCL scaffold on ST2 activity, proliferation, osteogenesis and mineralization. The results proved Wnt3a-induced ST2 dCM endow the scaffold material with biological activity and is a new potential bone repair scaffold materials for bone tissue engineering.
Polycaprolactone (PCL) and cetylpyridinium chloride were purchased from Aladdin Reagent Co., Ltd., Shanghai, China. Fetal Bovine Serum and α-Minimum Essential Medium were purchased from Biological Industries Israel Beit Haemek Ltd, Beit Haemek, Israel. Reverse transcription kit, real-time fluorescent quantitative PCR kit and Trizol were purchased from Accurate Biotechnology Co., Ltd., Hunan, China. Pancreatin, penicillin-streptomycin, RIPA Lysis Solution, BCIP-NBT Alkaline Phosphatase Color Development Kit, Alkaline Phosphatase Assay Kit, BCA Protein Assay Kit and hemotoxylin-eosin staining were purchased from Beyotime Institute of Biotechnology, Shanghai, China. β-catenin antibody was purchased from Wanlei Institute of Biotechnology, Shenyang, China. Hochest 33258 nuclear staining solution and DNaseI were purchased from Solarbio Institute of Biotechnology, Beijing, China. Quant-iT™ PicoGreen® dsDNA Reagent Kit and LIVE-DEAD™ cell viability Kit were purchased from Invitrogen, USA. Cell Counting Kit-8 was purchased from Dojindo Laboratories (Japan).
Cell culture and collection of Wnt3a conditioned medium
Wnt3a condition medium was collected as previous study (Tu et al., 2007). In brief, murine bone marrow-derived stromal cell line ST2 and Wnt3a cells were cultured in basal medium (α-MEM medium containing 10% FBS and 1% penicillin-streptomycin). Then ST2 was cultured in Wnt3a conditioned medium to basal medium at ratio of 0, 1:2, 1:4, and 1:8 for 3 days to detect the appropriate ratio.
RT-qPCR was performed as previous study (Tu et al., 2007). Osteogenic marker genes expression of Alp, Runx2, Osx, Ocn and β-catenin and Wnt target genes Smad6, Axin2, BMP4, Lef1 mRNA was detected. The relative gene expression level was analyzed by 2−ΔCt method. The genes and corresponding primers are listed in Table 1. Alkaline phosphatase staining was used to detect the level of osteogenic differentiation. β-catenin immunofluorescence staining was used to detect β-catenin entering the nucleus.
Scaffolds were printed by polycaprolactone (PCL) using a 3D bioprinter according to a predesigned 3D structure model and then cut into 5 × 5 × 1 mm scaffolds. The scaffolds were immersed in 75% ethanol for 1 h, then immersed in sterile PBS 3 times under UV light before cell seeding.
Preparation of PCL scaffolds ornamented with decellularized matrix
Decellularized matrix ornamented PCL scaffolds were prepared as a previous study (Pati et al., 2015). In short, PCL scaffolds were seeded with 1 × 105 ST2 and incubated in incubator 2 h allow cells attach to scaffolds. And then basal medium and Wnt3a conditioned medium to α-MEM medium 1:4 were added respectively. After 3 days, two group scaffolds were decellularized by three freeze-thaw (F-T) cycles in liquid nitrogen and 37°C water bath (10 min each) respectively. Then the scaffolds were incubated in 1 mL DNase I solution (0.2 mg/mL) in an incubator for an hour. DNase is an enzymatic decellularization agent, used for breaking down DNA fragments and removal of the nucleotides lysis. There are no reported adverse effects of DNase on matrix (Pati et al., 2015). The goal of the decellularization process was to maximize removal of cellular debris while minimizing the disruption of CM components.
Evaluation of decellularization effect
Before and after decellularization, scaffolds were fixed with 2.5% glutaraldehyde in PBS for 1 h, then washed with PBS and dehydrated successively in 70%, 90%, 95%, and 100% ethanol for 15 min each. The fixed samples were vacuum dried, sputter-coated with platinum, and examined under a scanning electron microscope operated at 10 kV.
To verify the decellularized effect, DNA and total protein were quantified before and after decellularization. The total DNA was quantified by Quant-iT™ PicoGreen® dsDNA Reagent Kits. Cell nuclei stained with Hoechst 33258 was also used to determine whether the decellularization process was completed. Two group scaffolds were extracted with 300 µL RIPA lysis buffer in respectively. The protein content accumulating on scaffolds was tested by BCA Protein Assay Kit as the direction. Three samples were tested for each condition.
Effect of dCM ornamented PCL scaffolds on ST2 viability and proliferation
Cell viability and proliferation were detected as a previous study (Yu et al., 2020). ST2 were seeded on PCL and dCM ornamented PCL scaffolds to study the effect of dCM on cell viability, proliferation, and differentiation. Cell-seeded scaffolds were incubated 2 h to allow cells attach to scaffolds, then basal medium was added. Cell viability was assessed by live-dead cell viability assay 24 h later and live-dead rate was calculated by ImageJ. Cell proliferation was performed on days 1, 4, and 7 by CCK-8 assay. 10% CCK-8 solution was added to each sample and incubated at 37°C for 1 h, then measured absorbance at a wavelength of 450 nm using a microplate reader (Biotek Instruments, USA).
Effect of dCM ornamented PCL scaffolds on ST2 osteogenesis
For osteogenic differentiation, 1 × 105 ST2 were seeded on dCM ornamented PCL scaffolds for 7 and 14 days respectively. Then two group scaffolds were stained for alkaline phosphatase (ALP) by BCIP-NBT Alkaline Phosphatase Color Development Kit. Quantitative real-time PCR (qRT-PCR) was performed to detect four osteogenic marker genes expression. The study was performed in triplicate.
For assessing mineralization, the cell proliferating medium was replaced by osteogenic medium after 7 days and then cultured for 14 days. Alizarin red S staining was performed to evaluate the calcium deposition of different groups. The scaffolds were stained by alizarin red stain at room temperature for 10 min and the calcium nodules were examined under microscope after washing five times with deionized water. For quantitative analysis, the stained samples were air-dried and immersed in 10% cetylpyridinium chloride for 20 min to elute the stain which was prepared according to a previous report (Chen et al., 2020). The absorbance of the elution solution was measured at 562 nm.
The appropriate ratio of Wnt3a conditioned medium
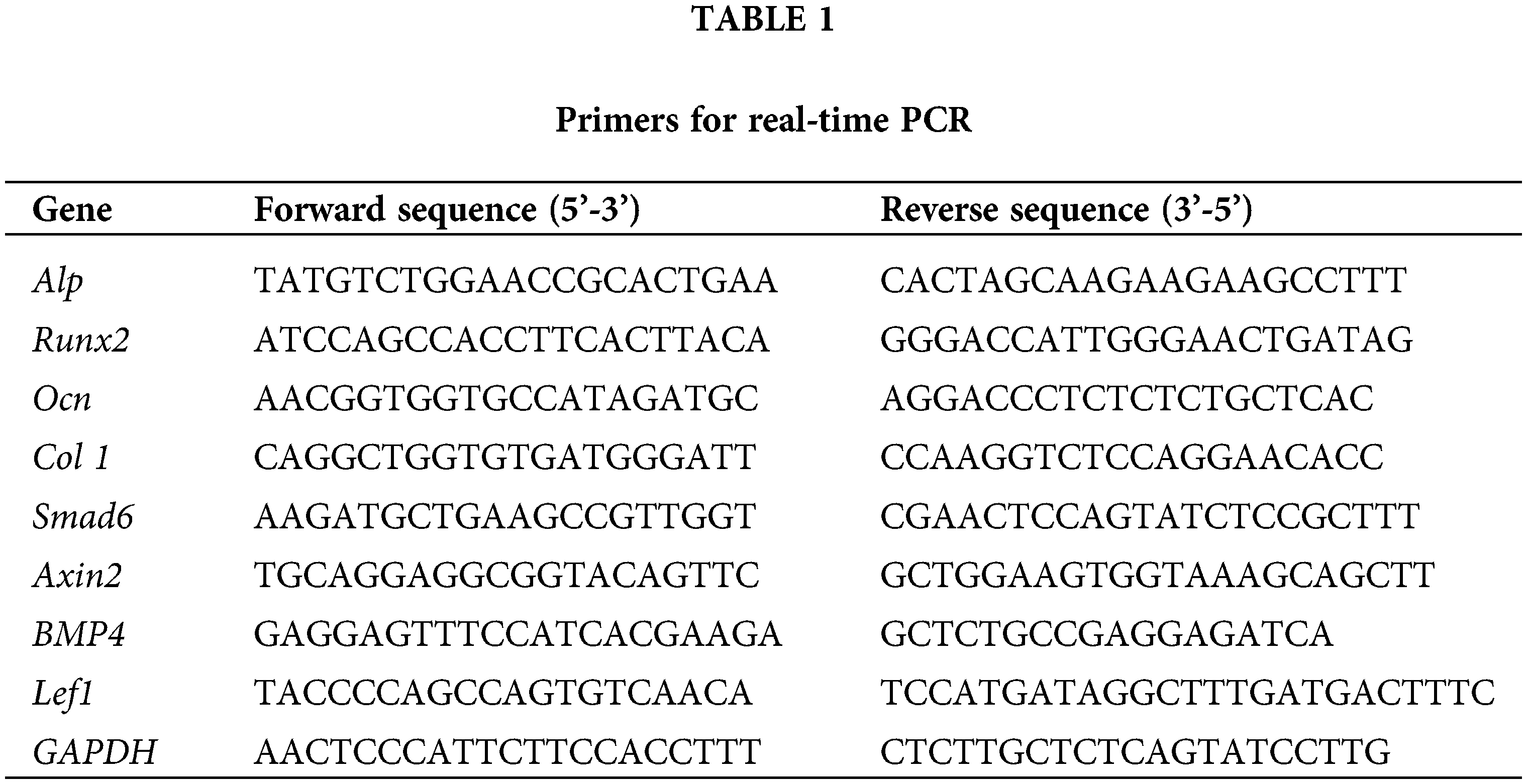
ST2 cells were cultured in Wnt3a conditioned medium to basal medium at ratio of 0, 1:2, 1:4, 1:8 for 3 days. Alkaline phosphatase staining, Alp activity and qPCR results showed that ST2 osteogenic differentiation and osteogenic marker genes Alp, Runx2, Ocn, and Col 1 mRNA expression were significantly strongest in Wnt3a conditioned medium to basal medium at the ratio of 1:4 group among the four groups (P < 0.05) (Figs. 1A–1C). In the Wnt3a conditioned medium to basal medium at ratio of 1:4 group, a large amount of β-catenin can be seen into the nucleus, and there were >50% β-catenin positive cells (Figs. 2A and 2B). QPCR results showed that β-catenin and Wnt signaling target genes Smad6, Axin2, BMP4, Lef1 mRNA expression were significantly higher than control (Fig. 2c). These results indicated that Wnt3a conditioned medium to basal medium at the ratio of 1:4 has best osteogenic effect, so the following experiments use this ratio of medium to induce ST2.
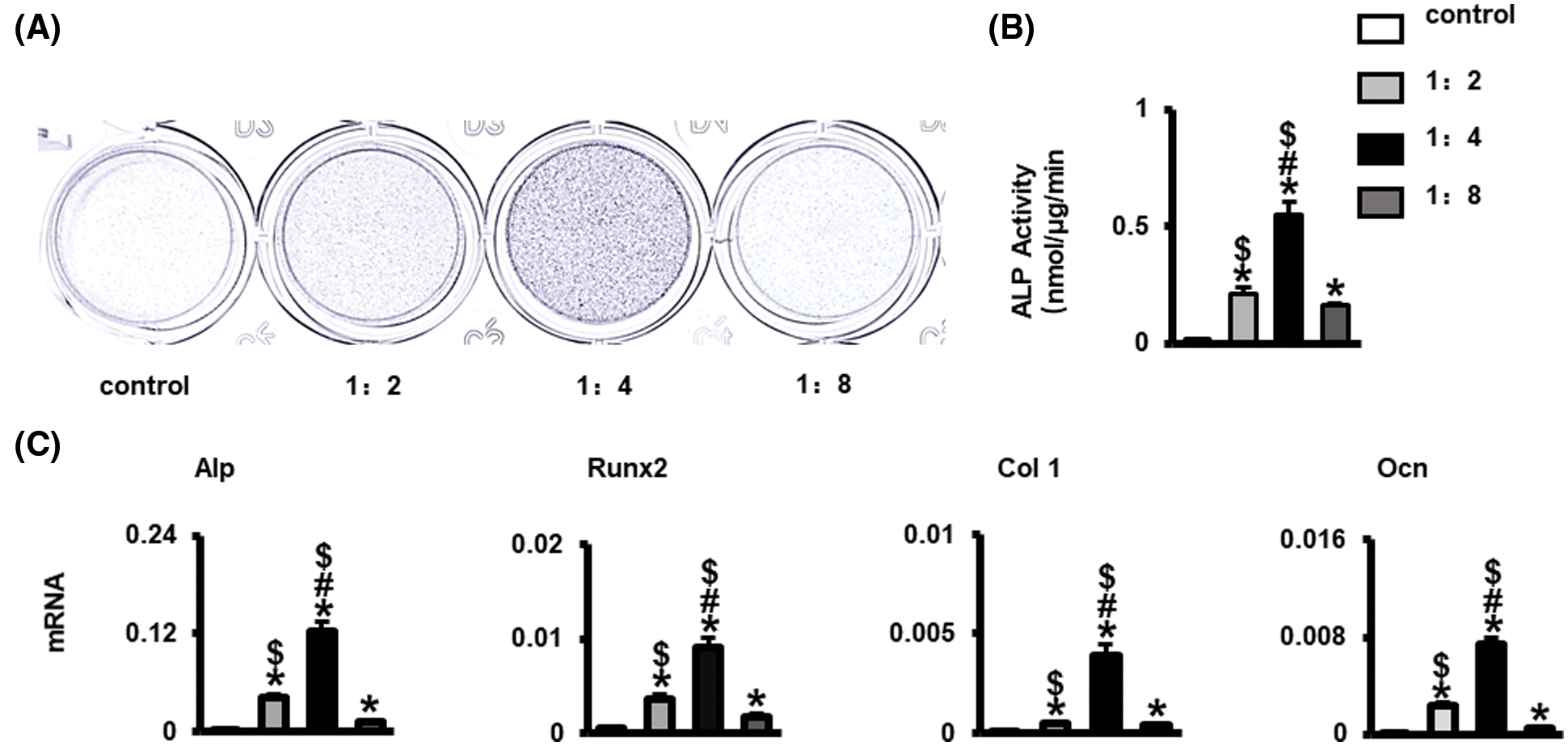
Figure 1: Wnt3a conditioned medium to basal medium at the ratio of 1:4 promoted ST2 osteogenesis. (A, B) AP staining and AP activity of ST2 osteogenic differentiation after culturing in Wnt3a conditioned medium to basal medium at the ratio of 0,1:2,1:4,1:8, respectively. (C) Gene expressions of Alp, Runx2, Col 1 and Ocn after 3 days culturing. Data were expressed as mean ± SD. *P < 0.05 vs. control; #P < 0.05 vs. 1:2 group; $P < 0.05 vs. 1:8 group by one way ANOVA. Control means the group without Wnt3a conditioned medium. 1:2, 1:4 and 1:8 mean the ratio of Wnt3a conditioned medium to basal medium is 1:2, 1:4 and 1:8 group, respectively.
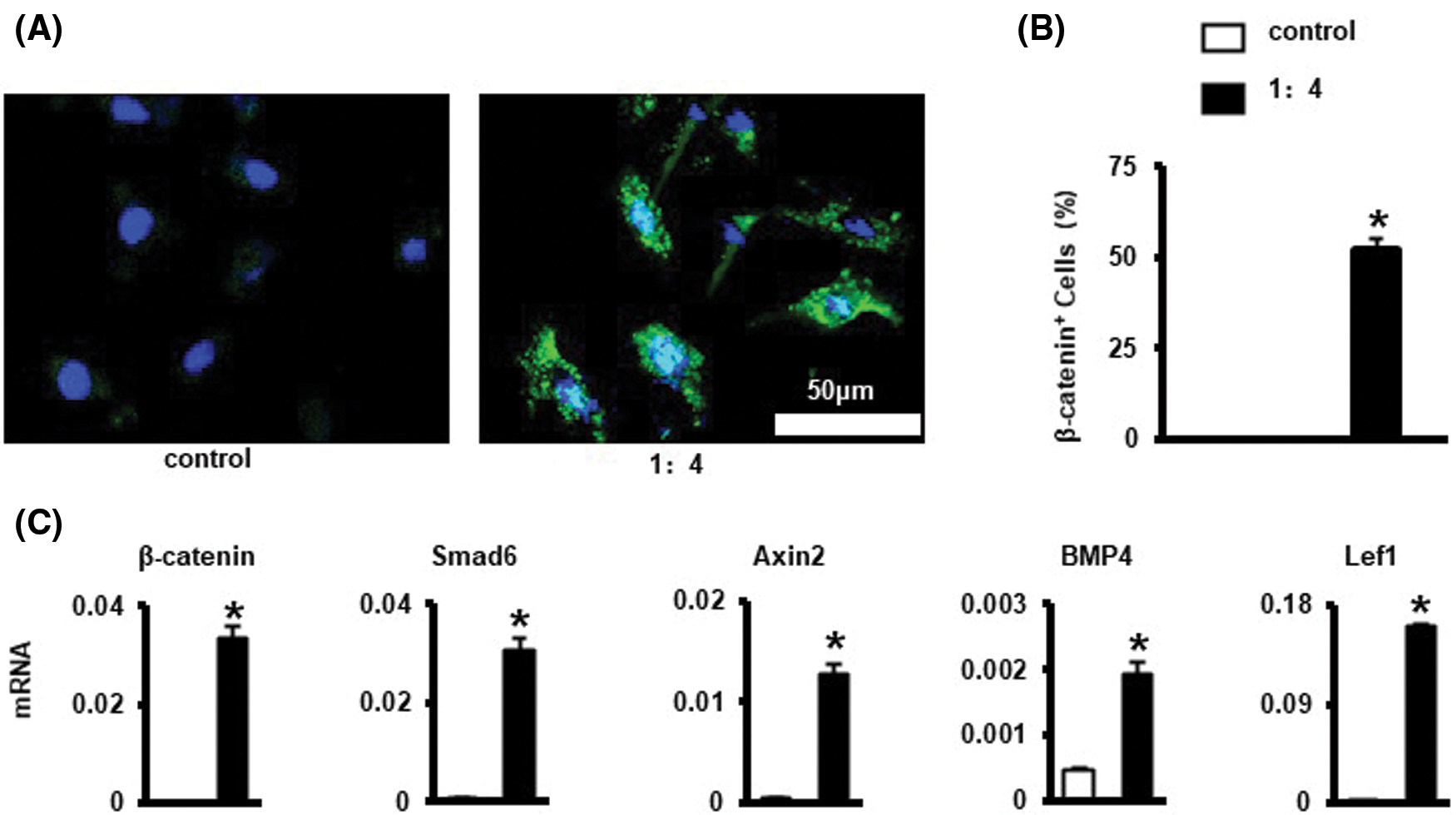
Figure 2: Wnt3a conditioned medium to basal medium at the ratio of 1:4 promoted β-catenin into the nucleus of ST2. (A) Immunofluorescence staining of β-catenin into the nucleus, the green fluorescence is β-catenin, and the blue fluorescence DAPI is the nucleus. Scale bars 50 μm. (B) The number of β-catenin staining positive cells. (C) Gene expressions of β-catenin and Wnt signaling target genes Smad6, Axin2, BMP4, and Lef1. Data were expressed as mean ± SD. *P < 0.05 vs. control by one way ANOVA. Control means the group without Wnt3a conditioned medium; 1:4 means the ratio of Wnt3a conditioned medium to basal medium is 1:4.
Characterization of dCM ornamented PCL scaffold
SEM micrographs showed the surface morphology of the scaffolds before and after decellularization. Cells proliferated well on the scaffolds. After 3 days incubation, the scaffold surface was almost entirely covered by proliferating cells and their synthesized matrix (Fig. 3B). After decellularization, residual aggregations and layers of the cellular matrix could be observed on the scaffolds (Fig. 3e). Even after decellularization, the CM was well attached to the polymer surface (Fig. 3C). The CM attached to the surface of the scaffolds can provide an anchoring site for the cells and can influence cellular attachment and proliferation.
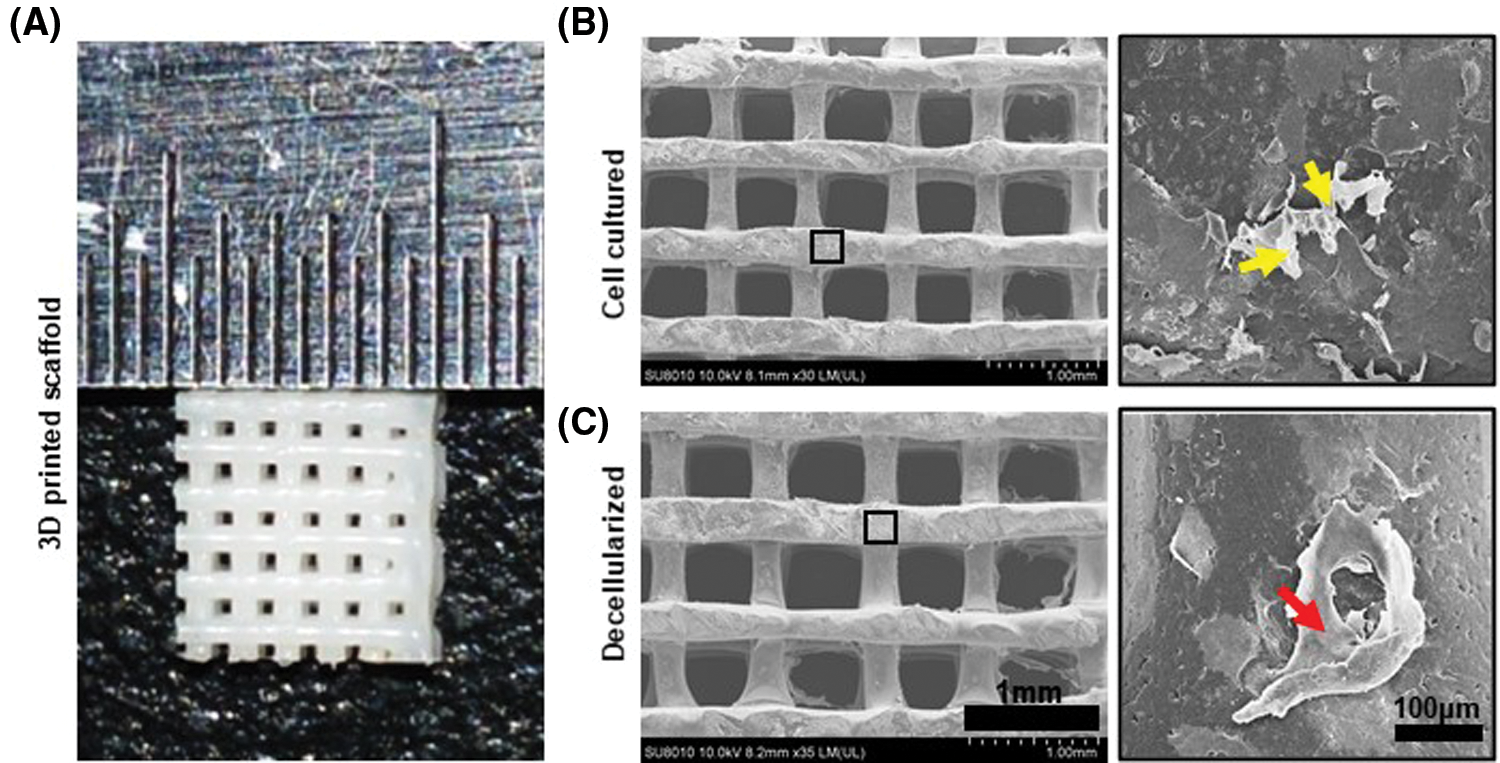
Figure 3: Characterization of dCM ornamented 3D printed PCL scaffold. (A) The scaffold is a 5 × 5 × 1 mm cube. (B) SEM images of the scaffolds before decellularization. Cells were marked with yellow arrow. The scaffold has a line width of 300 μm and pore size of 400 μm. Scale bars 1 mm. (C) SEM images of the scaffolds after decellularization. DCM was marked with red arrow. There was a large amount of cellular matrix attached to the scaffold, and almost no cell morphology and cell debris.
Evaluation of the effect of decellularization
To obtain dCM ornamented PCL scaffolds, cell-seeded 3D printed PCL scaffolds were cultured for 3 d, then decellularized. Hochest 33258 staining showed only a small amount of blue fluorescent staining of cell nuclei can be seen on the decellularized scaffold (Fig. 4A). The DNA and decellularized matrix proteins of Wnt3a-ST2-PCL group before and after decellularization were higher than those of ST2-PCL group (Figs. 4A–4C). DNA quantification showed that more than 90% of DNA components were removed in both groups (Fig. 4B), and the total protein quantification results showed that more than 60% of cellular matrix proteins were retained on the decellularized matrix modified scaffold (Fig. 4C).
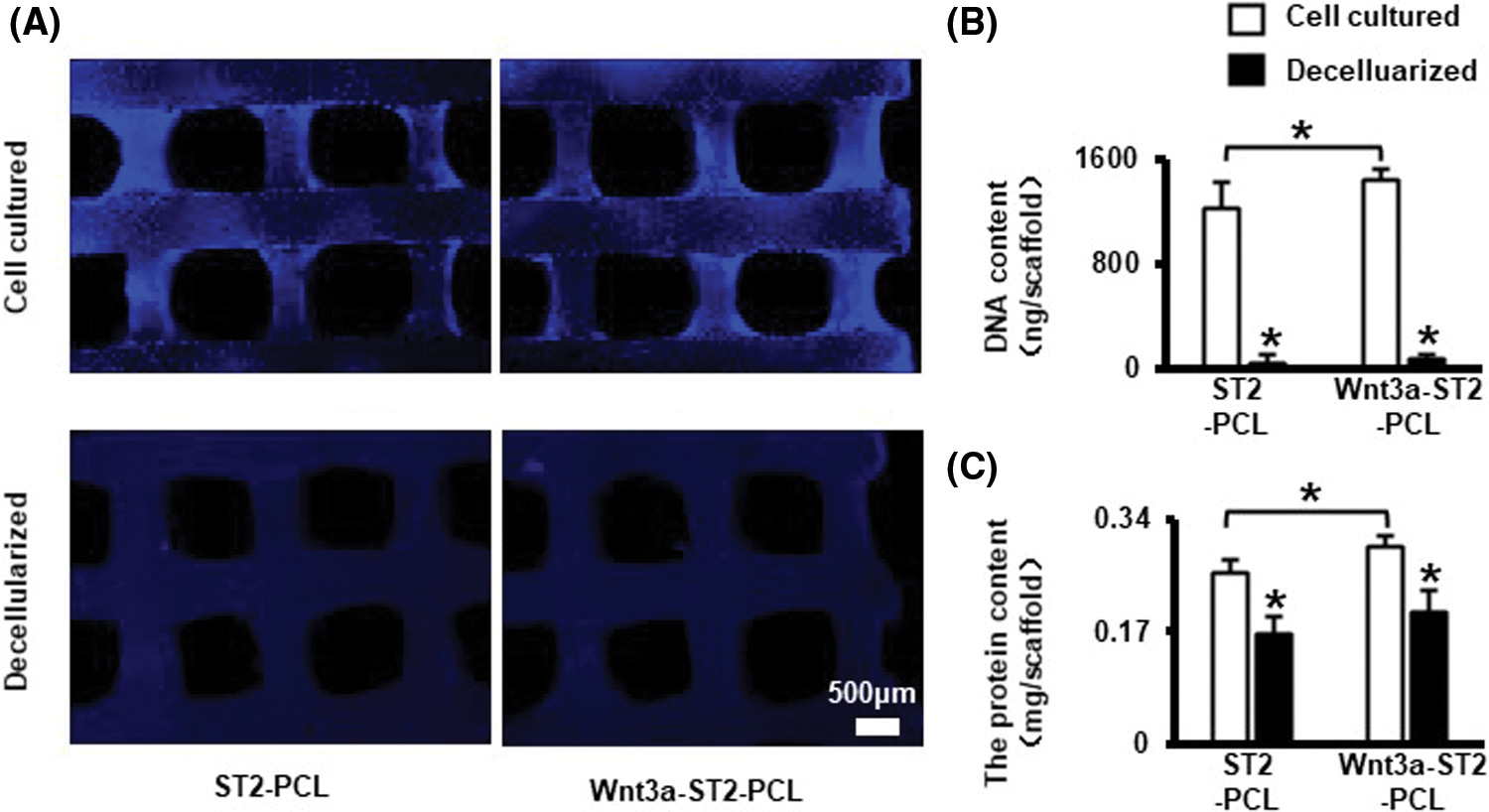
Figure 4: Freeze/thaw cycle and DNase decellularization removed >90% DNA and preserved >60% matrix. (A) Hochest 33258 staining for cell nucleus before and after decellularization. Scale bars 500 μm. (B, C) DNA and total protein quantitation of the scaffolds before and after decellularization. Data were expressed as mean ± SD. *P < 0.05 vs. cell cultured by one way ANOVA. ST2-PCL means PCL scaffold inoculated with ST2 cultured in basal medium; Wnt3a-ST2-PCL means the PCL scaffold inoculated with ST2 cultured in Wnt3a conditioned medium and basal medium at ratio of 1:4.
Effect of dCM ornamented PCL scaffolds on cell viability and proliferation
To evaluate the effects of dCM ornamented PCL scaffolds on cell viability, ST2 were cultured on PCL, ST2-dCM-PCL and Wnt3a-ST2-dCM-PCL scaffolds for 24 h and characterized by live-dead staining (Fig. 5A). <10% dead cells were seen in all three groups by ImageJ analysis (Fig. 5B), indicating decent biocompatibility of these scaffolds. CCK-8 tests at 1, 4, and 7 d showed that ST2 proliferated with the extension of the culture time in 3 groups (Fig. 5C), but proliferated faster on the scaffold modified with decellularized matrix, and the Wnt3a-ST2-dCM-PCL scaffold promoted ST2 proliferation significantly higher than the other two groups (P < 0.05) (Fig. 6B).
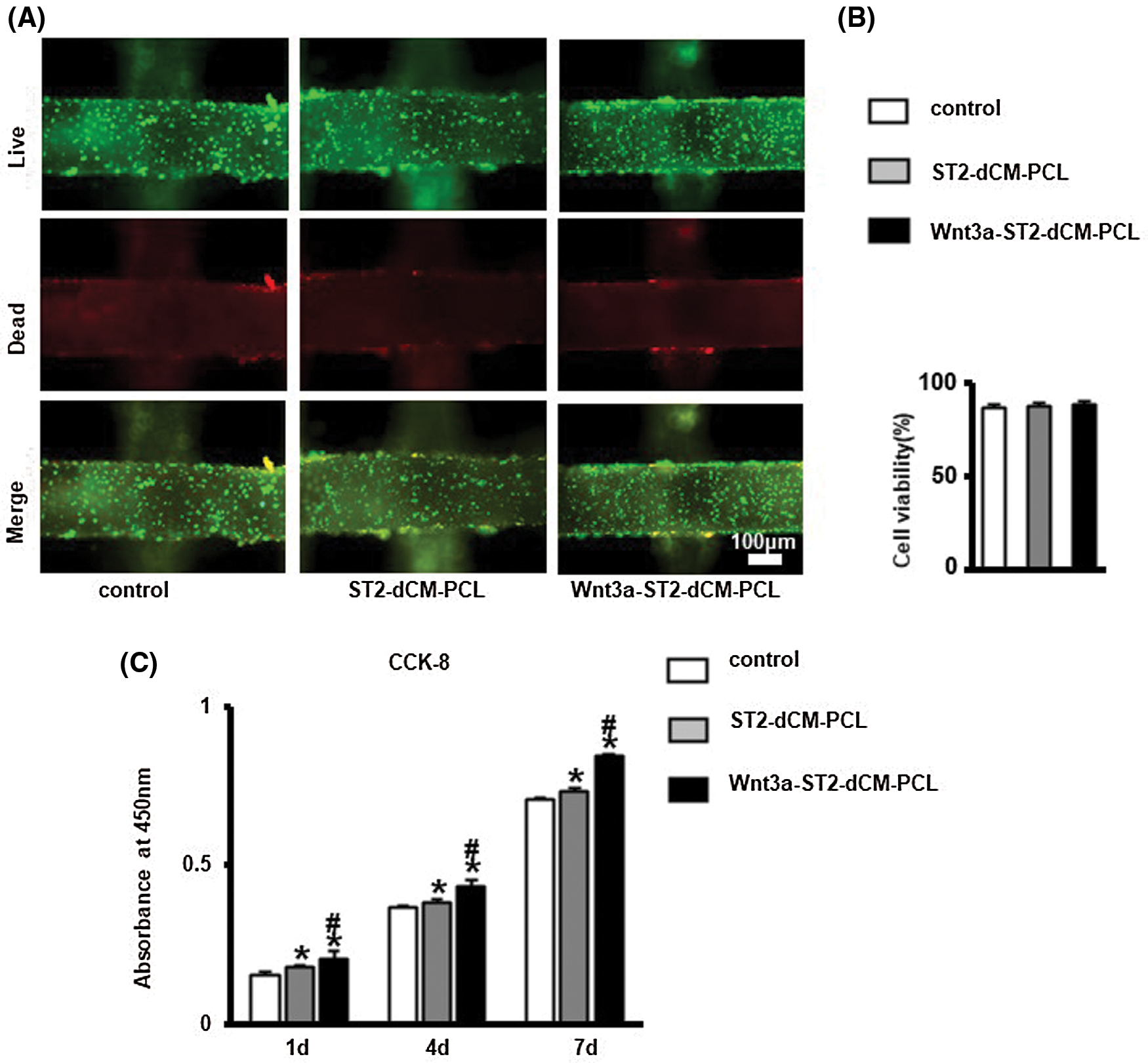
Figure 5: DCM ornamented scaffolds were biocompatible and Wnt3a-introduced ST2 dCM ornamented scaffolds promoted ST2 proliferation. (A) Live-Dead staining to analyze the viability of ST2 on the dCM ornamented scaffolds for 24 h, live cell (green) and dead cells (red). Scale bars 100 µm. (B) ImageJ analysis of the vitality of ST2 on dCM ornamented scaffolds. (C) CCK-8 analysis the proliferation of ST2 on dCM and PCL scaffolds at day 1, 4, 7. dCM, decellularized cellular matrix; Control, PCL scaffold; ST2-dCM-PCL means ST2 decellularized matrix ornamented PCL scaffold; Wnt3a-ST2-dCM-PCL means Wnt3a-induced ST2 decellularized matrix ornamented PCL scaffold.
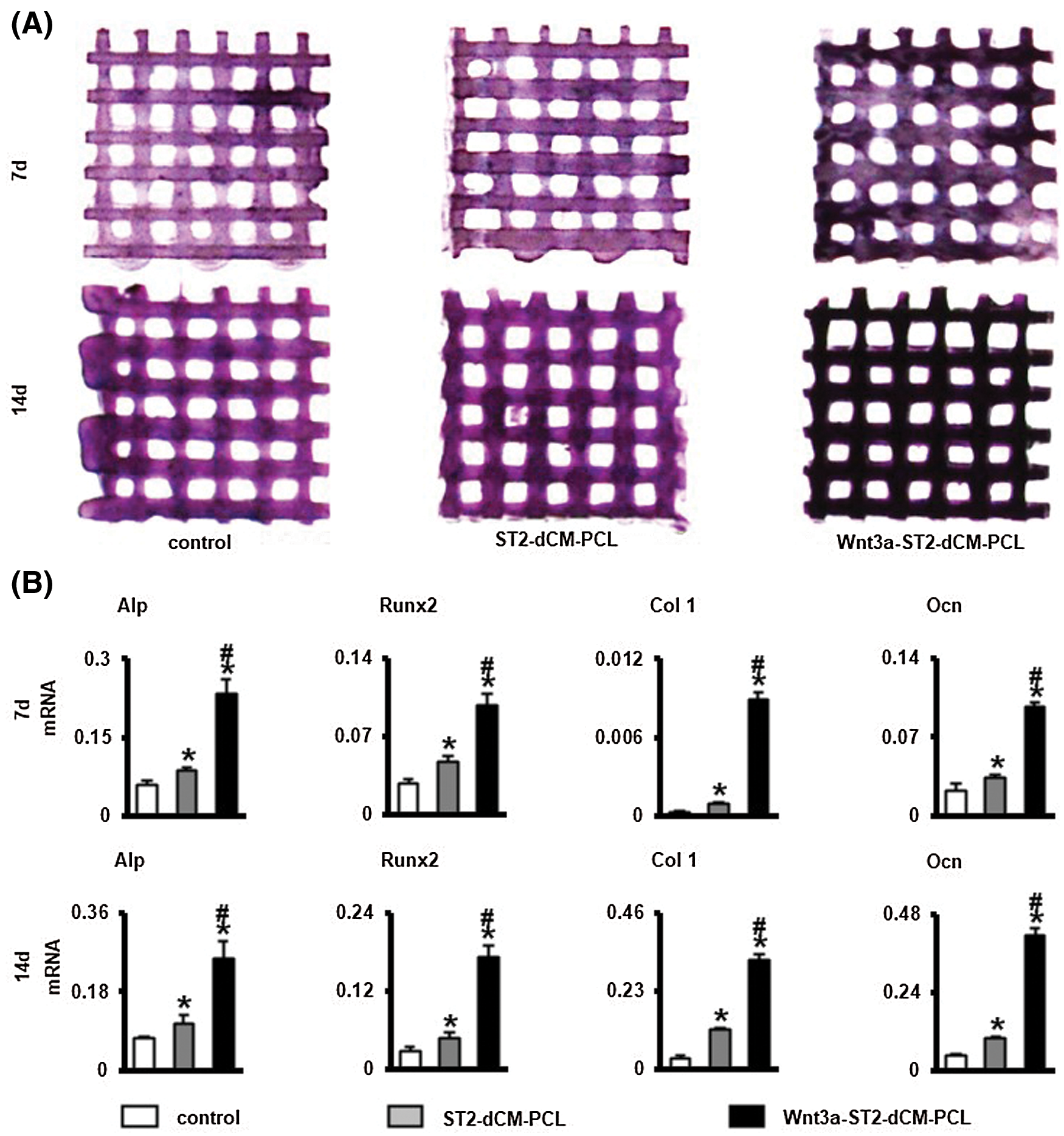
Figure 6: Wnt3a-introduced ST2 dCM ornamented scaffolds promoted ST2 osteogenic differentiation. (A, B) AP staining and AP activity assays of ST2 on dCM ornamented scaffolds after culturing in basal medium 7 and 14 d, respectively. (c) Gene expressions of Alp, Runx2, Col 1 and Ocn after 7 and 14 d of culturing. dCM, decellularized matrix. Control, PCL scaffold; ST2-dCM-PCL means ST2 decellularized matrix ornamented PCL scaffold; Wnt3a-ST2-dCM-PCL means Wnt3a-induced ST2 decellularized matrix ornamented PCL scaffold. Data were expressed as mean± SD. *P < 0.05 vs. control; . #P < 0.05 vs. ST2-dCM-PCL by one way ANOVA.
In vitro osteogenic differentiation of ST2 on dCM ornamented PCL scaffolds
After ST2 cultured on scaffolds 7 and 14 days in basal medium, AP staining presented highest on the Wnt3a-ST2-dCM-PCL scaffolds, ST2-dCM-PCL followed, and the pure PCL scaffold stained the lightest (Fig. 6A). In vitro osteogenic gene expression analysis clearly indicated that ST2 cultured in Wnt3a-ST2-dCM-PCL scaffolds showed significantly higher expression of Alp, Runx2, Col 1, and Ocn genes after 7 and 14 d than ST2-dCM-PCL and pure PCL scaffolds (Fig. 6B). These results implied that Wnt3a-ST2-dCM-PCL promoted early osteogenic differentiation of ST2 in vitro, possibly because Wnt3a-ST2-dCM produced several bioactive factors that stimulated osteogenic differentiation.
The effect of decellularized matrix ornamented PCL scaffolds on mineralization
In order to further evaluate the effect of dCM on ST2 mineralization, alizarin red staining was performed 14 days after osteogenic induction (Fig. 7A). Wnt3a-ST2-dCM-PCL had the most mineralized nodules. Quantitative analysis also confirmed the result of Alizarin Red staining (Fig. 7B). These results indicate that the Wnt3a-ST2-dCM-PCL scaffold has a better ability to induce ST2 mineralization in vitro than the other two groups.
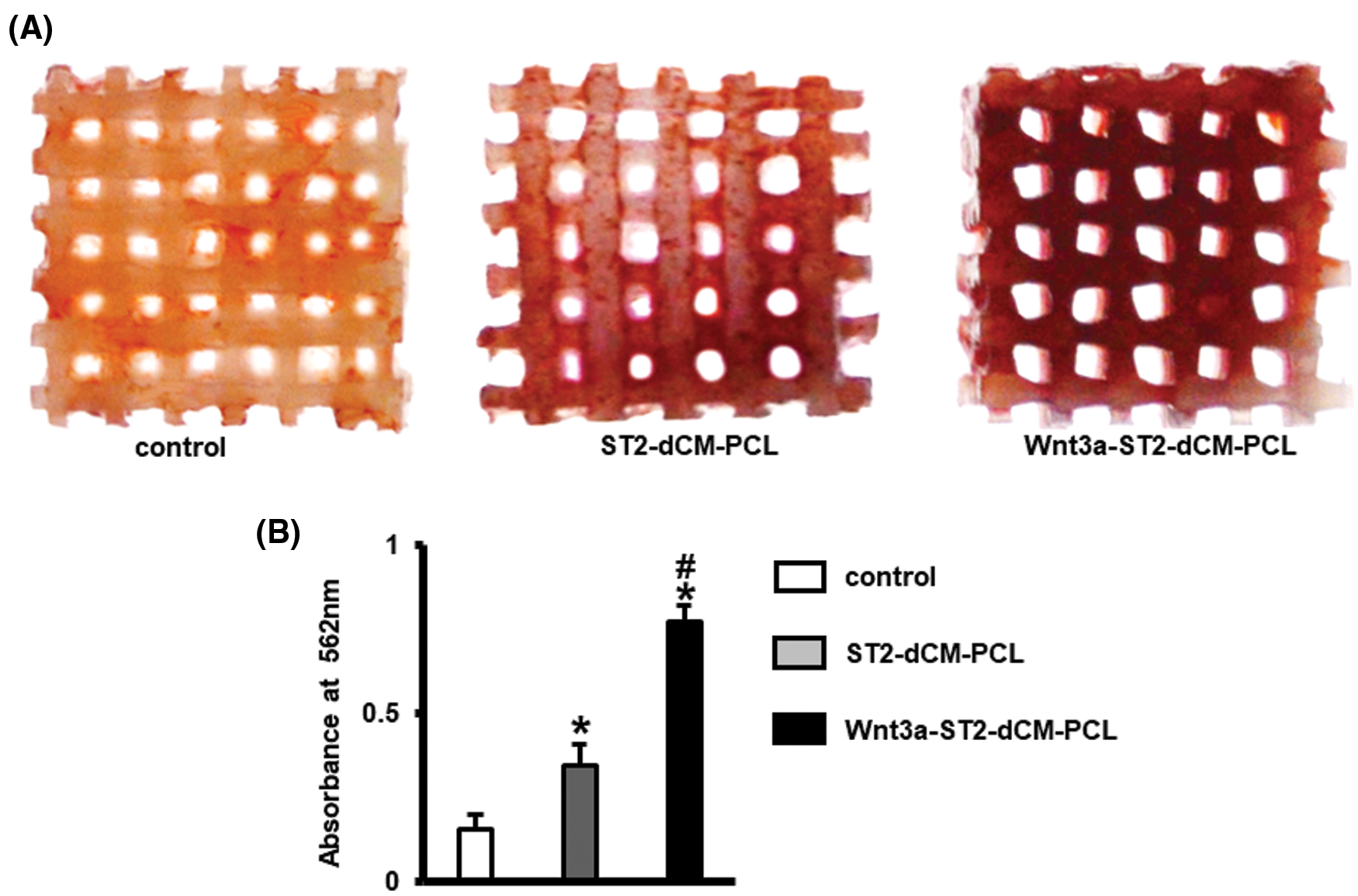
Figure 7: Wnt3a-introduced ST2 dCM ornamented scaffolds promoted ST2 mineralization. (A) Alizarin red S stained calcium mineral nodules deposited by ST2 after incubation for 14 d in osteogenesis induction medium on control, ST2-PCL, Wnt3a-ST2-PCL scaffolds respectively. (B) Alizarin Red activity were measured at day 21. dCM, decellularized matrix; Control, PCL scaffold ST2-dCM-PCL means ST2 decellularized matrix ornamented PCL scaffold; Wnt3a-ST2-dCM-PCL means Wnt3a-induced ST2 decellularized matrix ornamented PCL scaffold. Data were expressed as mean± SD. *P < 0.05 vs. control; . #P < 0.05 vs. ST2-dCM-PCL by one way ANOVA.
In recent decades, while the research of bone tissue engineering has made rapid progress, the comprehensive repair of large segment bone defects is still challenging, and effective therapies are constantly being explored (Al-Abedalla et al., 2015). Decellularized matrix (dCM) composed of a complex network of proteins, proteoglycans, growth factors and other constituents (Mansour et al., 2017). It is an ideal candidate for tissue engineering and regenerative medicine because most cells are in close contact with neighboring dCM environment that provides both biophysical and biochemical signals essential for cell adhesion, migration, and differentiation (Garcia and Martin, 2019). In addition, decellularized matrix can influence cell fate by directly binding to cell surface receptors. For example, decellularized matrix components such as type I collagen, fibronectin, and laminin bind to integrin receptors to activate pathways associated with osteogenic differentiation (Lin et al., 2020). What’s more, scaffold geometry, mechanical properties and surface morphology can affect cell behavior (Ballini et al., 2017). We have ornamented scaffold with Wnt3a induced ST2-dCM to improve scaffold’s bioactivity in our study. And dCM from human adipose stem cells significantly promoted the proliferation of bone marrow stromal cells (BMSCs), alkaline phosphatase (ALP) activity, calcium salt deposition, osteogenic gene markers and protein expression (Wei et al., 2017). Decellularized matrix ornamented scaffold with BMP-2 can significantly promote BMSCs osteogenic differentiation and bone regeneration (Kim et al., 2015). Pati’ group constructed a dCM modified material scaffold from human inferior turbinate derived mesenchymal stem cells to simulate the bone microenvironment. The scaffold promoted osteoblast differentiation of newly implanted hTMSC and promoted bone formation at ectopic and in situ defects in rats by upregulating osteogenic marker gene expression and increasing calcium deposition (Pati et al., 2015). Therefore, decellularized matrix will improve the properties of material scaffolds and has great potential in regenerative therapy. Recently, decellularization of tissue or organ has been widely used to obtain native enviromental mimicking scaffolds to modulate stem cell function (Silva et al., 2020). In addition, combining dCM with biomaterials not only produced constructs with structural components and sustainable mechanical properties, but also growth factors and regulatory proteins that were conducive to tissue regeneration (Pei and He, 2012; Wang et al., 2020). Furthermore, the combination of cell-derived dCM cultured in vitro with biomaterials allows the use of patient-specific cells to form a hybrid scaffold to eliminate donor site morbidity and anti-host immune response (Garcia and Martin, 2019). Cell-derived dCM generated by in vitro culturing has been considered a promising method among many kinds of decellularized matrix.
Wnt/β-catenin is one of the five major signal pathways of bone development. It participates in the entire osteogenic differentiation process. The activation of the classic Wnt/β-catenin signal promotes bone regeneration by promoting stem cell proliferation, osteogenic differentiation, and self-renewal (Zhong et al., 2012). Wnt3a is a member of the Wnt protein family and is the main agonist protein of classic Wnt/β-catenin signaling. A large number of studies have found that Wnt3a can promote osteogenic differentiation (Tu et al., 2007), but there are studies found that high-dose Wnt3a slows down the occurrence of osteogenesis differentiated stem cell matrix calcification progresses and inhibits osteogenesis. It can be seen that the regulation effect of Wnt3a on the osteogenic differentiation of stem cells is related to the dose of Wnt3a (Tu et al., 2007). Studies have confirmed that Wnt3a promotes the osteogenesis of bone grafts (Boland et al., 2004), but Wnt3a protein is not stable in the body and can cause adverse reactions in the body. Studies have found that reprogramming cells by small molecule compounds, exogenous transcription factors and biophysical cues regulated cell fate (Song et al., 2021). Therefore, we constructed a Wnt3a-induced ST2 decellularized matrix modified scaffold for bone defect repair. Our previous studies have confirmed that Wnt3a cells secrete biologically active Wnt3a protein, which is a great cell source for Wnt3a conditioned medium (Tu et al., 2007). Here, we screened the best ratio of Wnt3a conditioned medium to basal medium to promote ST2 osteogenic differentiation. Cellular matrix contains a variety of biologically active growth factors, which are increasingly used in tissue engineering (Mansour et al., 2017). However, its poor mechanical properties limited its application. Scaffold materials can be designed to provide mechanical cues and regulate the epigenetic state and function of cells (Song et al., 2020). Polycaprolactone (PCL) is a biocompatible and biodegradable material approved by the FDA and has been widely used in biomedicine (Aldemir Dikici et al., 2020). PCL has advantages in repairing the defects of hard and slowly regenerating tissues (such as bone), but it lacks biological activity (Jirofti et al., 2020). In this study, a 3D printed PCL scaffold with a pore size of 400 μm and a line width of 300 μm was used, which is conducive to bone and blood vessel formation (Hsieh et al., 2005; Otsuki et al., 2006). After ST2 seeded on the PCL scaffold in basal medium and 1:4 ratio of Wnt3a conditioned medium to basal medium for 3 days, the decellularized matrix modified PCL scaffold was obtained through the freeze-thaw cycle and DNase decellularization method. Then new seeded ST2 was cultured on these scaffolds in vitro to evaluate osteogenic performance. It was found that Wnt3a-ST2-dCM-PCL has good osteogenic and mineralization effects. This may be due to the activation of the classic Wnt signaling pathway that confers the scaffold biological activity, but the key components are still unclear. Bone regeneration is a complex process involving multiple factors. DCM ornamented scaffolds retain immunomodulatory cytokines, such as transforming growth factor-β, bone morphogenetic protein, and βFGF, which modulate pro-inflammatory responses (Zhang et al., 2022). 3D-printed scaffold modified with decellularized matrix of Human umbilical cord mesenchymal stem cells (hUCMSCs) has been reported not only attenuate host rejection, but also increase the accumulation of M2 macrophages to initiate tissue regeneration and combat inflammation (Deng et al., 2020). And studies have found appropriate inflammatory responses are necessary for bone regeneration and can alter tissue reconstruction after trauma (Ma et al., 2017; Tatullo et al., 2019). In addition, transforming growth factor beta-3 (TGF-β) and insulin-like growth factor 1 (IGF-1) have been discovered to promote meniscus-derived decellularized matrix (dCM) to induce the differentiation of human knee synovial fluid resident mesenchymal stem cells (SF-MSCs) towards a meniscus phenotype (Liang et al., 2018). So future research should focus on the use of proteomics analysis to identify the composition of various cell matrix proteins and biological factors.
Studies have shown that the number of cell debris and DNA residues is the main source of adverse immune responses and may interfere with the reconstruction of biological scaffolds (Garcia and Martin, 2019; Yang et al., 2020). Pati’s group prepared cell decellularized matrix modified 3D printed scaffolds by freeze-thaw cycles in the hypertonic solution, which effective removed DNA as well as retained most cell matrix components and matrix structure. The scaffolds promoted cell adhesion, proliferation, osteogenesis in vitro and promote in situ and ectopic bone formation in rats (Pati et al., 2015). Carvalho discovered freeze-thaw cycles combined with DNA enzymes removed more DNA than freeze-thaw cycles alone, but there was no difference in cell matrix components. The dCM can promote blood vessel and bone formation in vivo (Carvalho et al., 2019). Other studies have also proved that the freeze-thaw cycle-DNase decellularization method effectively removes DNA while retaining the cell matrix (Li et al., 2019b; Londono et al., 2017). In this study, the quantitation of DNA and total protein showed that freeze-thaw cycles and DNase removed cellular DNA while retaining most of the cellular matrix components. Scanning electron microscopy images of the PCL scaffold ornamented with decellularized matrix before and after decellularization also clearly showed that the cells on the scaffold grew well before decellularization, and a large amount of cellular matrix was attached to the scaffold after decellularization, with almost no cell morphology. This also further showed that the decellularization method used in this study ensures the accuracy of the experiment.
ST2 was seeded on the dCM ornamented PCL scaffolds and live and dead staining showed that the cells survived about 90% after 24 h. The proliferation of ST2 on the Wnt3a-ST2-dCM-PCL scaffold was significantly stronger than that of the other two groups. We speculate that the ST2 decellularized matrix induced by Wnt3a promotes the faster proliferation of ST2. At the same time, ST2 cultured on the Wnt3a-ST2-dCM-PCL scaffold for 7 d and 14 d osteogenic marker genes expression was significantly increased compared with the other two groups. After 14 days of osteogenic induction, the calcium deposition also increased significantly. These results all prove that the Wnt3a-ST2-dCM-PCL scaffold has good biocompatibility and promotes cell proliferation, early osteogenic differentiation, late mineralization.
The results of this study showed that Wnt3a-induced ST2 decellularized matrix ornamented 3D printed PCL scaffold promoted ST2 proliferation, osteogenic differentiation, and mineralization. And there are sufficient sources of ST2 and Wnt3a. Therefore, the ST2 decellularized matrix induced by Wnt3a can be mass-produced in vitro and is a potential bioactive bone repair material. However, there are some factors that hinder the transformation of dCM to clinical practice. Firstly, the most suitable cell type for bone regeneration therapy is still unclear. Although mesenchymal stem cells (MSCs) have great potential for cell-based tissue repair and regeneration therapy and are widely used in clinical and experiment for their proliferative, multidirectional potential, immunomodulatory and anti-inflammatory effects, the risks still exist (Bejleri and Davis, 2019). Autologous human periapical cyst enchymal stem cells (HPcy-Mscs) have been found to enhanced proliferation, cell viability and gene expression of osteogenic and odontogenic differentiation of scaffold materials (Tatullo et al., 2019). Therefore, autologous mesenchymal stem cells will be a good cell type of dCM for bone tissue engineering and regenerative medicine, which will be the focus of our future research. What’s more, the decellularization protocol should be optimized to minimize immunogenic substances and toxicity and to maximize the preservation of cellular matrix components. The difficulty in standardizing decellularized methods also limits clinical transformation (Zhang et al., 2022). The control of scaffold degradation requires precise modification. If the scaffold degrades rapidly, mechanical failure may occur. On the contrary, if the scaffold degrades slowly, it may activate the inflammatory response and hinder tissue repair (Haugen et al., 2019). Therefore, the balance between seasonal degradation and new bone formation is imperative and has proven to be a major challenge.
In summary, despite the shortcomings mentioned above, dCM is acknowledged as an ideal choice for bone tissue engineering. Besides, Wnt3a-induced ST2 dCM could provide a biomimetic microenvironment for integrating with stem cells and other bioactive materials, which is a promising inspiration for the design of future bioscaffolds as well as an optimized solution to problems in regenerative therapies. However, further research is needed to study the dCM ornamented scaffolds’ bone regeneration in vivo and the host's response, optimize the amount of decellularized matrix on the scaffold, and analyze the specific components of the dCM for clinical practices.
We successfully constructed Wnt3a induced ST2-dCM ornamented porous PCL scaffolds with a defined structure and enhanced biological performance which induced osteogenic differentiation and mineralization of stem cells. Many studies have induced specific cell functions by decellularized matrices of various cultured cells, and attempted to develop preparation methods that maintain the matrix microstructure and improve the ability of the matrix to induce specific cell functions. There are still many issues to be resolved regarding matrix preparation methods, cell sources, mechanisms and standardization of clinical use. However, cultured cell-derived decellularized matrix has many advantages in inducing specific cell functions. It is hopeful that the development of decellularized matrices derived from cultured cells will be further developed in the future. All contributors who do not meet the criteria for authorship should be listed in this section.
Acknowledgement: All contributors who do not meet the criteria for authorship should be listed in this section.
Availability of Data and Materials: All the datasets used in this study are available from the corresponding author upon reasonable request.
Author Contribution: The authors confirm contribution to the paper as follows: study conception and design: Xiaolin Tu; data collection: Xiaofang Wang, Yufei Ma, Jie Chen, Yang Song, Guangliang Liu; analysis and interpretation of results: Xiaofang Wang, Yufei Ma, Jie Chen; draft manuscript preparation: Xiaofang Wang, Yufei Ma, Jie Chen, Xiaolin Tu. All authors reviewed the results and approved the final version of the manuscript.
Funding Statement: This work was supported by the National Natural Science Foundation of China (Grant No. U1601220), the National Natural Science Foundation of China (Grant No. 82002310), the Chongqing Postgraduate Research and Innovation Project (Grant No. CYB20167), the Chongqing Postdoctoral Science Foundation (Grant No. csts2019jcyj-bsh0068).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Aamodt JM, Grainger DW (2016). Extracellular matrix-based biomaterial scaffolds and the host response. Biomaterials 86: 68–82. DOI 10.1016/j.biomaterials.2016.02.003. [Google Scholar] [CrossRef]
Al-Abedalla K, Torres J, Cortes AR, Wu X, Nader SA et al. (2015). Bone augmented with allograft onlays for implant placement could be comparable with native bone. Journal of Oral & Maxillofacial Surgery 73: 2108–2122. DOI 10.1016/j.joms.2015.06.151. [Google Scholar] [CrossRef]
Aldemir Dikici B, Reilly GC, Claeyssens F (2020). Boosting the osteogenic and angiogenic performance of multiscale porous polycaprolactone scaffolds by in vitro generated extracellular matrix decoration. ACS Applied Materials & Interfaces 12: 12510–12524. DOI 10.1021/acsami.9b23100. [Google Scholar] [CrossRef]
Almubarak S, Nethercott H, Freeberg M, Beaudon C, Jha A et al. (2016). Tissue engineering strategies for promoting vascularized bone regeneration. Bone 83: 197–209. DOI 10.1016/j.bone.2015.11.011. [Google Scholar] [CrossRef]
Ballini A, Boccaccio A, Saini R, van Pham P, Tatullo M (2017). Dental-derived stem cells and their secretome and interactions with bioscaffolds/biomaterials in regenerative medicine: From the in vitro research to translational applications. Stem Cells International 2017: 6975251–6975253. DOI 10.1155/2017/6975251. [Google Scholar] [CrossRef]
Battafarano G, Rossi M, de Martino V, Marampon F, Borro L et al. (2021). Strategies for bone regeneration: From graft to tissue engineering. International Journal of Molecular Sciences 22: 1128–1150. DOI 10.3390/ijms22031128. [Google Scholar] [CrossRef]
Bejleri D, Davis ME (2019). Decellularized extracellular matrix materials for cardiac repair and regeneration. Advanced Healthcare Materials 8: 1801217. DOI 10.1002/adhm.201801217. [Google Scholar] [CrossRef]
Benders KE, van Weeren PR, Badylak SF, Saris DB, Dhert WJ et al. (2013). Extracellular matrix scaffolds for cartilage and bone regeneration. Trends in Biotechnology 31: 169–176. DOI 10.1016/j.tibtech.2012.12.004. [Google Scholar] [CrossRef]
Benoit H (2014). Agonism of Wnt/β-catenin signaling promotes mesenchymal stem cell (MSC) expansion. Journal of Tissue Engineering and Regenerative 9: E13–26. DOI 10.1002/term.1736. [Google Scholar] [CrossRef]
Boland GM, Perkins G, Hall DJ, Tuan RS (2004). Wnt3a promotes proliferation and suppresses osteogenic differentiation of adult human mesenchymal stem cells. Journal of Cellular Biochemistry 93: 1210–1230. DOI 10.1002/(ISSN)1097-4644. [Google Scholar] [CrossRef]
Bose S, Vahabzadeh S, Bandyopadhyay A (2013). Bone tissue engineering using 3D printing. Materials Today 16: 496–504. DOI 10.1016/j.mattod.2013.11.017. [Google Scholar] [CrossRef]
Bracaglia LG, Fisher JP (2015). Extracellular matrix-based biohybrid materials for engineering compliant, matrix-dense tissues. Advanced Healthcare Materials 4: 2475–2487. DOI 10.1002/adhm.201500236. [Google Scholar] [CrossRef]
Carvalho MS, Silva JC, Cabral JMS, da Silva CL, Vashishth D (2019). Cultured cell-derived extracellular matrices to enhance the osteogenic differentiation and angiogenic properties of human mesenchymal stem/stromal cells. Journal of Tissue Engineering and Regenerative 13: 1544–1558. DOI 10.1002/term.2907. [Google Scholar] [CrossRef]
Chae S, Lee SS, Choi YJ, Hong DH, Gao G et al. (2021). 3D cell-printing of biocompatible and functional meniscus constructs using meniscus-derived bioink. Biomaterials 267: 120466. DOI 10.1016/j.biomaterials.2020.120466. [Google Scholar] [CrossRef]
Chen Y, Lee K, Yang Y, Kawazoe N, Chen G (2020). PLGA-collagen-ECM hybrid meshes mimicking stepwise osteogenesis and their influence on the osteogenic differentiation of hMSCs. Biofabrication 12: 025027. DOI 10.1088/1758-5090/ab782b. [Google Scholar] [CrossRef]
Cheng CW, Solorio LD, Alsberg E (2014). Decellularized tissue and cell-derived extracellular matrices as scaffolds for orthopaedic tissue engineering. Biotechnology Advances 32: 462–484. DOI 10.1016/j.biotechadv.2013.12.012. [Google Scholar] [CrossRef]
Deng M, Tan J, Hu C, Hou T, Peng W et al. (2020). Modification of PLGA scaffold by MSC-derived extracellular matrix combats macrophage inflammation to initiate bone regeneration via TGF-beta-induced protein. Advanced Healthcare Materials 9: e2000353. DOI 10.1002/adhm.202000353. [Google Scholar] [CrossRef]
Drosos G, Kazakos K, Kouzoumpasis P, Verettas D (2007). Safety and efficacy of commercially available demineralised bone matrix preparations: A critical review of clinical studies. Injury 38 Suppl 4: S13–S21. DOI 10.1016/s0020-1383(08)70005-6. [Google Scholar] [CrossRef]
Fu Y, Liu L, Cheng R, Cui W (2018). ECM decorated electrospun nanofiber for improving bone tissue regeneration. Polymers 10: 272. DOI 10.3390/polym10030272. [Google Scholar] [CrossRef]
Garcia A, Martin I (2019). Extracellular matrices to modulate the innate immune response and enhance bone healing. Frontiers in Immunology 10: 2256. DOI 10.3389/fimmu.2019.02256. [Google Scholar] [CrossRef]
Haugen HJ, Lyngstadaas SP, Rossi F, Perale G (2019). Bone grafts: Which is the ideal biomaterial? Journal of Clinical Periodontology 46 Suppl 21: 92–102. [Google Scholar]
Hsieh CY, Tsai SP, Wang DM, Chang YN, Hsieh HJ (2005). Preparation of gamma-PGA/chitosan composite tissue engineering matrices. Biomaterials 26: 5617–5623. DOI 10.1016/j.biomaterials.2005.02.012. [Google Scholar] [CrossRef]
Jirofti N, Mohebbi-Kalhori D, Samimi A, Hadjizadeh A, Kazemzadeh GH (2020). Fabrication and characterization of a novel compliant small-diameter PET/PU/PCL triad-hybrid vascular graft. Biomedical Materials 15: 055004. DOI 10.1088/1748-605X/ab8743. [Google Scholar] [CrossRef]
Kim IG, Hwang MP, Du P, Ko J, Ha CW et al. (2015). Bioactive cell-derived matrices combined with polymer mesh scaffold for osteogenesis and bone healing. Biomaterials 50: 75–86. DOI 10.1016/j.biomaterials.2015.01.054. [Google Scholar] [CrossRef]
Li K, Xiu C, Zhou Q, Ni L, Du J et al. (2019a). A dual role of cholesterol in osteogenic differentiation of bone marrow stromal cells. Journal of Cellular Physiology 234: 2058–2066. DOI 10.1002/jcp.27635. [Google Scholar] [CrossRef]
Li M, Zhang T, Jiang J, Mao Y, Zhang A et al. (2019b). ECM coating modification generated by optimized decellularization process improves functional behavior of BMSCs. Materials Science and Engineering 105: 110039. DOI 10.1016/j.msec.2019.110039. [Google Scholar] [CrossRef]
Liang Y, Idrees E, Szojka A, Andrews S, Kunze M et al. (2018). Chondrogenic differentiation of synovial fluid mesenchymal stem cells on human meniscus-derived decellularized matrix requires exogenous growth factors. Acta Biomaterialia 80: 131–143. DOI 10.1016/j.actbio.2018.09.038. [Google Scholar] [CrossRef]
Lin X, Patil S, Gao YG, Qian A (2020). The bone extracellular matrix in bone formation and regeneration. Frontiers in Pharmacology 11: 757. DOI 10.3389/fphar.2020.00757. [Google Scholar] [CrossRef]
Londono R, Dziki JL, Haljasmaa E, Turner NJ, Leifer CA et al. (2017). The effect of cell debris within biologic scaffolds upon the macrophage response. Journal of Biomedical Materials Research Part A 105: 2109–2118. DOI 10.1002/jbm.a.36055. [Google Scholar] [CrossRef]
Long T, Yang J, Shi SS, Guo YP, Ke QF et al. (2015). Fabrication of three-dimensional porous scaffold based on collagen fiber and bioglass for bone tissue engineering. Journal of Biomedical Materials Research Part B: Applied Biomaterials 103: 1455–1464. DOI 10.1002/jbm.b.33328. [Google Scholar] [CrossRef]
Ma J, Guo W, Gao M, Huang B, Qi Q et al. (2017). Biomimetic matrix fabricated by LMP-1 gene-transduced MC3T3-E1 cells for bone regeneration. Biofabrication 9: 045010. DOI 10.1088/1758-5090/aa8dd1. [Google Scholar] [CrossRef]
Mansour A, Mezour MA, Badran Z, Tamimi F (2017). Extracellular matrices for bone regeneration: A literature review. Tissue Engineering Part A 23: 1436–1451. DOI 10.1089/ten.tea.2017.0026. [Google Scholar] [CrossRef]
Ogawa M, Nishikawa S, Ikuta K, Yamamura F, Naito M et al. (1988). B cell ontogeny in murine embryo studied by a culture system with the monolayer of a stromal cell clone, ST2: B cell progenitor develops first in the embryonal body rather than in the yolk sac. EMBO Journal 7: 1337–1343. DOI 10.1002/j.1460-2075.1988.tb02949.x. [Google Scholar] [CrossRef]
Nikolova MP, Chavali MS (2019). Recent advances in biomaterials for 3D scaffolds: A review. Bioactive Materials 4: 271–292. DOI 10.1016/j.bioactmat.2019.10.005. [Google Scholar] [CrossRef]
Otsuki B, Takemoto M, Fujibayashi S, Neo M, Kokubo T et al. (2006). Pore throat size and connectivity determine bone and tissue ingrowth into porous implants: Three-dimensional micro-CT based structural analyses of porous bioactive titanium implants. Biomaterials 27: 5892–5900. DOI 10.1016/j.biomaterials.2006.08.013. [Google Scholar] [CrossRef]
Parmaksiz M, Elcin AE, Elcin YM (2020). Decellularized cell culture ECMs act as cell differentiation inducers. Stem Cell Reviews and Reports 16: 569–584. DOI 10.1007/s12015-020-09963-y. [Google Scholar] [CrossRef]
Pati F, Song TH, Rijal G, Jang J, Kim SW et al. (2015). Ornamenting 3D printed scaffolds with cell-laid extracellular matrix for bone tissue regeneration. Biomaterials 37: 230–241. DOI 10.1016/j.biomaterials.2014.10.012. [Google Scholar] [CrossRef]
Pei M, He F (2012). Extracellular matrix deposited by synovium-derived stem cells delays replicative senescent chondrocyte dedifferentiation and enhances redifferentiation. Journal of Cellular Physiology 227: 2163–2174. DOI 10.1002/jcp.22950. [Google Scholar] [CrossRef]
Schmidt AH (2021). Autologous bone graft: Is it still the gold standard? Injury 52: 18–22. DOI 10.1016/j.injury.2021.01.043. [Google Scholar] [CrossRef]
Shang F, Yu Y, Liu S, Ming L, Zhang Y et al. (2021). Advancing application of mesenchymal stem cell-based bone tissue regeneration. Bioactive Materials 6: 666–683. DOI 10.1016/j.bioactmat.2020.08.014. [Google Scholar] [CrossRef]
Silva JC, Carvalho MS, Udangawa RN, Moura CS, Cabral JMS et al. (2020). Extracellular matrix decorated polycaprolactone scaffolds for improved mesenchymal stem/stromal cell osteogenesis towards a patient-tailored bone tissue engineering approach. Journal of Biomedical Materials Research Part B: Applied Biomaterials 108: 2153–2166. DOI 10.1002/jbm.b.34554. [Google Scholar] [CrossRef]
Song Y, Soto J, Li S (2020). Mechanical regulation of histone modifications and cell plasticity. Current Opinion in Solid State and Materials Science 24: 100872. DOI 10.1016/j.cossms.2020.100872. [Google Scholar] [CrossRef]
Song Y, Soto J, Wang P, An Q, Zhang X et al. (2021). Asymmetric cell division of fibroblasts is an early deterministic step to generate elite cells during cell reprogramming. Advanced Science 8: 2003516. DOI 10.1002/advs.202003516. [Google Scholar] [CrossRef]
Tatullo M, Spagnuolo G, Codispoti B, Zamparini F, Zhang A et al. (2019). PLA-based mineral-doped scaffolds seeded with human periapical cyst-derived MSCs: A promising tool for regenerative healing in dentistry. Materials 12: 597. DOI 10.3390/ma12040597. [Google Scholar] [CrossRef]
Tournier P, Guicheux J, Pare A, Veziers J, Barbeito A et al. (2021). An extrudable partially demineralized allogeneic bone paste exhibits a similar bone healing capacity as the “Gold Standard” bone graft. Frontiers in Bioengineering and Biotechnology 9: 658853. DOI 10.3389/fbioe.2021.658853. [Google Scholar] [CrossRef]
Tu X, Joeng KS, Nakayama KI, Nakayama K, Rajagopal J et al. (2007). Noncanonical Wnt signaling through G protein-linked PKCdelta activation promotes bone formation. Developmental Cell 12: 113–127. DOI 10.1016/j.devcel.2006.11.003. [Google Scholar] [CrossRef]
Visscher DO, Farre-Guasch E, Helder MN, Gibbs S, Forouzanfar T et al. (2016). Advances in bioprinting technologies for craniofacial reconstruction. Trends in Biotechnology 34: 700–710. DOI 10.1016/j.tibtech.2016.04.001. [Google Scholar] [CrossRef]
Wang Z, Han L, Sun T, Ma J, Sun S et al. (2020). Extracellular matrix derived from allogenic decellularized bone marrow mesenchymal stem cell sheets for the reconstruction of osteochondral defects in rabbits. Acta Biomaterialia 118: 54–68. DOI 10.1016/j.actbio.2020.10.022. [Google Scholar] [CrossRef]
Wei W, Li J, Chen S, Chen M, Xie Q et al. (2017). In vitro osteogenic induction of bone marrow mesenchymal stem cells with a decellularized matrix derived from human adipose stem cells and in vivo implantation for bone regeneration. Journal of Materials Chemistry B 5: 2468–2482. DOI 10.1039/C6TB03150A. [Google Scholar] [CrossRef]
Wu YA, Chiu YC, Lin YH, Ho CC, Shie MY et al. (2019). 3D-Printed bioactive calcium silicate/poly-epsilon-caprolactone bioscaffolds modified with biomimetic extracellular matrices for bone regeneration. International Journal of Molecular Sciences 20: 942. DOI 10.3390/ijms20040942. [Google Scholar] [CrossRef]
Yang L, Ge L, van Rijn P (2020). Synergistic effect of cell-derived extracellular matrices and topography on osteogenesis of mesenchymal stem cells. ACS Applied Materials & Interfaces 12: 25591–25603. DOI 10.1021/acsami.0c05012. [Google Scholar] [CrossRef]
Yu Y, Wang Y, Zhang W, Wang H, Li J et al. (2020). Biomimetic periosteum-bone substitute composed of preosteoblast-derived matrix and hydrogel for large segmental bone defect repair. Acta Biomaterialia 113: 317–327. DOI 10.1016/j.actbio.2020.06.030. [Google Scholar] [CrossRef]
Yuan H, Li M, Feng X, Zhu E, Wang B (2021). miR-142a-5p promoted osteoblast differentiation via targeting nuclear factor IA. Journal of Cellular Physiology 236: 1810–1821. DOI 10.1002/jcp.29963. [Google Scholar] [CrossRef]
Zhang X, Chen X, Hong H, Hu R, Liu J et al. (2022). Decellularized extracellular matrix scaffolds: Recent trends and emerging strategies in tissue engineering. Bioactive Materials 10: 15–31. DOI 10.1016/j.bioactmat.2021.09.014. [Google Scholar] [CrossRef]
Zhong Z, Zylstra-Diegel CR, Schumacher CA, Baker JJ, Carpenter AC et al. (2012). Wntless functions in mature osteoblasts to regulate bone mass. PNAS 109: E2197–E2204. DOI 10.1073/pnas.1120407109. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |