

 | Congenital Heart Disease |  |
DOI: 10.32604/CHD.2020.011712
ARTICLE
QRS Duration and Outcome Late after Repair of Tetralogy of Fallot: Neurohormonal Activation Differentiates between Mechanical and Electrical Dyssynchrony
Department of Internal Medicine II, Medical University of Vienna, Vienna, 1090, Austria
*Corresponding Author: Matthias Schneider. Email: matthias.schneiderkard@gmail.com
Received: 26 May 2020; Accepted: 11 June 2020
Abstract: Background: Predicting the probability for sudden cardiac death (SCD) and thus evaluation of patients for electrical device therapy and/or ablation is one of the main tasks in clinics for adults with congenital heart disease (ACHD) following repaired tetralogy of Fallot (rTOF) patients. Previous data suggests that QRS complex analysis can help identifying those patients who subsequently suffer from SCD. We hypothesized that a long QRS duration is associated with adverse rhythm events if caused by conduction abnormalities but not if caused by right ventricular remodeling. Methods: A retrospective analysis was performed entailing all rTOF patients who were seen at our ACHD clinic between 01/01/2000 and 12/31/2018. Duration of QRS in the earliest available ECG as well as the earliest available NT-proBNP value were documented. A combined endpoint of mortality and adverse rhythmogenic events during 10-year follow-up was analyzed. Results: A total of 155 patients were included in the analysis, median age was 21 years (interquartile range 18–33), 60 (38.5%) were female. During 10-year follow-up, four (2.6%) patients died, and 7 (4.5%) experienced an adverse rhythmogenic event. The cohort was divided into patients with NT-proBNP < 250 pg/ml (n = 133 patients) and ≥ 250 pg/ml (n = 22 patients). In multivariable Cox regression analysis, duration of QRS was significantly predictive for the combined endpoint in the low NT-proBNP group but not in the high NT-proBNP group. Conclusion: Wide QRS duration accompanied by low NT-proBNP values should receive particular attention in rTOF, as these patients might benefit from close rhythm monitoring and early prevention measures.
Keywords: TOF; ECG; mortality; NT-proBNP
Tetralogy of Fallot is the most common cyanotic congenital heart defect. Early surgery and procedures avoiding RV outflow patches can result in a long-time survival rate of 96% [1]. Reasons for late mortality are heart failure and ventricular arrhythmia [2,3]. Predicting the probability for sudden cardiac death and thus selection of patients for preventive electrical device therapy is one of the main tasks in the care for adults with congenital heart disease (ACHD) following surgically repaired TOF (rTOF) patients.
Right bundle branch block (RBBB) morphology and QRS complex duration in the context of rTOF has been discussed in great detail before. Previous work suggested an association of both ventricular tachycardia (VT) and enlarged right heart dimensions with a wide QRS complex [4]. In rTOF patients, QRS duration correlates with right ventricular (RV) end-diastolic volume and RV mass [5]. A QRS width < 150 ms has been discussed an indicator of positive remodeling after pulmonary valve replacement [6], a wide QRS complex and a change of QRS duration during long-time follow-up were discussed to be predictive for rhythm events [7,8]. Recently, the concept of QRS fragmentation (QRS-f) rather than absolute QRS duration was introduced as a prognostic marker for rTOF patients [9,10].
Findings from left ventricular dyssynchrony studies have led to the concept of electrical vs. mechanical dyssynchrony. Here, morphological changes of the heart are included in the analysis beyond simple documentation of QRS duration [11]. Width of the QRS complex is an unspecific parameter. On the one hand it can be associated with either isolated or combined morphologic changes of the RV, e.g., dilatation or hypertrophy due to volume or pressure overload (referred to as mechanical dyssynchrony in this manuscript). On the other hand, it can be caused by electrical alterations (referred to as electrical dyssynchrony in this manuscript), e.g., an arrhythmogenic anatomical isthmus, which is the most common substrate for VT in rTOF [12] and has been shown to be associated with wide QRS complexes [13]. Considering the usually small cohorts evaluated in previous studies, it remains unknown if the conclusions drawn from QRS morphology were made on the grounds of mechanical or electrical dyssynchrony.
Several studies have focused on brain natriuretic peptide (BNP) in rTOF patients. NT-pro BNP is a surrogate of wall stress imposed on the ventricular and atrial myocardium. Elevated plasma levels are present in rTOF patients regardless of the presence of symptoms, and they are associated with RV dimension, severity of pulmonary regurgitation, and exercise capacity [14–18]. Significant elevation of BNP is associated with an increased risk of death [19].
We hypothesize that a long QRS duration is associated with adverse rhythm events if caused by electrical dyssynchrony but not if caused by mechanical dyssynchrony. We sought to analyze the predictive power of QRS duration in a large contemporary rTOF cohort divided into a low NT-proBNP group (long QRS duration not due to severe RV volume/pressure overload) and a high NT-proBNP group (long QRS duration due to RV volume/pressure overload).
A retrospective analysis was performed entailing all rTOF patients who were seen at our outpatient ACHD clinic between 01/01/2000 and 12/31/2018. The study protocol conformed to the ethical guidelines of the Declaration of Helsinki and was approved by the local ethics committee (EK #1178/2019).
At our clinic, most rTOF patients are followed once a year with comprehensive visits including clinical examination, ECG, and echocardiography, and laboratory parameters. Measurement of NT-proBNP is performed regularly in consecutive follow-up.
Stored ECGs were evaluated and QRS duration was obtained from the earliest ECG available. Age, sex, and New York heart association (NYHA) functional class were collected from the patient records. Furthermore, the earliest NT-proBNP was retrieved. All patients with already implanted cardiac implantable electronic devices at first presentation and all patients without NT-proBNPs during follow-up were excluded from final analysis.
2.2 Clinical Follow-up and Study Endpoints
We considered a combined end-point including all-cause death and significant atrial or ventricular arrhythmia during a 10-year follow-up.
Mortality was analyzed by a retrieval query of the Austrian Death Registry. Record linkage indicated date of death and cause of death encoded according to the International Code of Diseases, Ver. 10 (ICD10). Austrian law demands that all deaths of Austrian citizens (also in foreign countries, if reported to Austrian officials) have to be recorded in the central Austrian death registry, which allows an almost complete follow up of all patients [20].
An adverse arrhythmogenic event was defined as complete heart block requiring pacemaker implantation, unexplained syncope, or an acute ventricular arrhythmia leading to hospitalization and/or defibrillator implantation.
Continuous variables are given as median and interquartile range (25th–75th percentile). Categorical parameters are presented as absolute numbers and percentages.
The QRS duration was analyzed as a continuous variable as well as a categorical variable with the cut-off 180 ms. The patients were divided into two groups (low and high NT-proBNP group). A twofold upper limit of normal (125 pg/ml) was chosen as the cut-off value for NTproBNP by descriptive statistical weighting of the whole study cohort.
Differences between groups were analyzed by student’s t-test analysis. Kaplan Meier Curves are presented for survival analysis. Cox proportional hazard regression analysis was applied to assess the effect QRS duration on survival and significant arrhythmias during a 10-year follow-up. To account for potential confounding effects, we formed a confounder cluster encompassing age and sex. A two-sided p-value < 0.05 was used to indicate statistical significance. SPSS Version 24 (IBM SPSS, USA) was used for all analyses.
A total of 234 adult patients with rTOF were seen at our ACHD clinic during the observed time period, 22 were excluded due to missing baseline data, five were excluded because they already had a cardiac implantable electronic device at first presentation. Of the remaining 207 patients, 155 had an NT-proBNP value documented at one time point during follow-up. Median age of this final cohort was 21 years (IQR 18–33), 60 (39%) were female. Fifty-three (34%) patients had received a transannular patch in the initial repair surgery. A total of 135 (86.5%) patients had complete right bundle branch block, median QRS duration was 148 ms (IQR 120–160). One (0.6%) patient was in NYHA functional class IV, 16 (10%) in NYHA III, 25 (16%) in NYHA II, and 111 (72%) in NYHA I (Tab. 1).
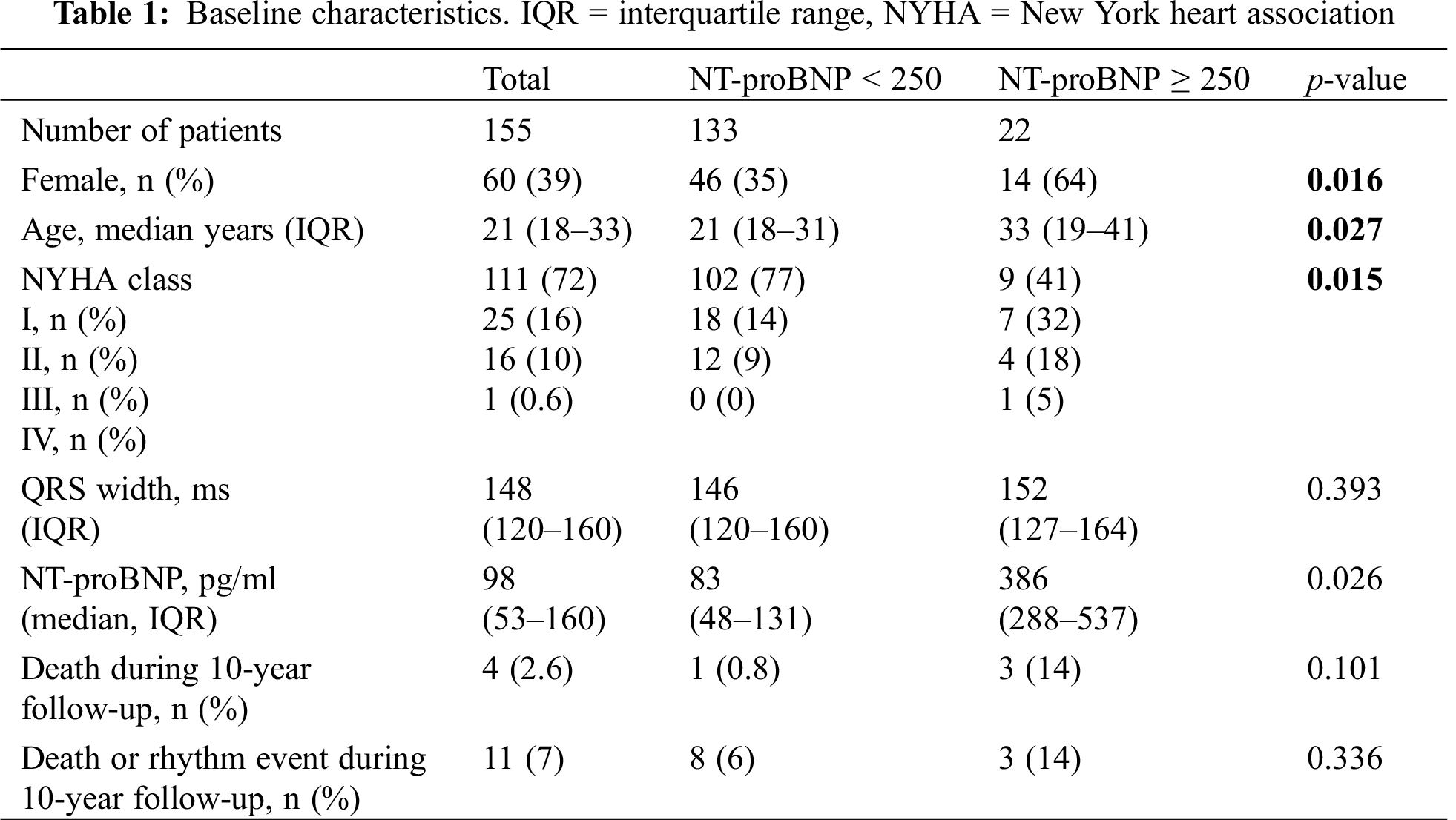
During 10-year follow-up (median 163 months, IQR 96-218), four (2.6%) patients died, and seven (4.5%) experienced a significant arrhythmia. Significant arrhythmias included patients with sustained VT (n = 3, 1.9%), complete heart block (n = 2, 1.3%) requiring pacemaker implantation, unexplained syncope (n = 1, 0.64%), and one patient (0.64%) who was resuscitated from ventricular fibrillation. Cause of death was retrieved from the central Austrian database. It was “cardiocirculatory failure” for one patient, “heart failure” for one patient, and “not further specified” for two patients. Between the patients with and without events, there was no difference regarding pulmonary stenosis or regurgitation, and right and left ventricular size and function (Tab. 2).
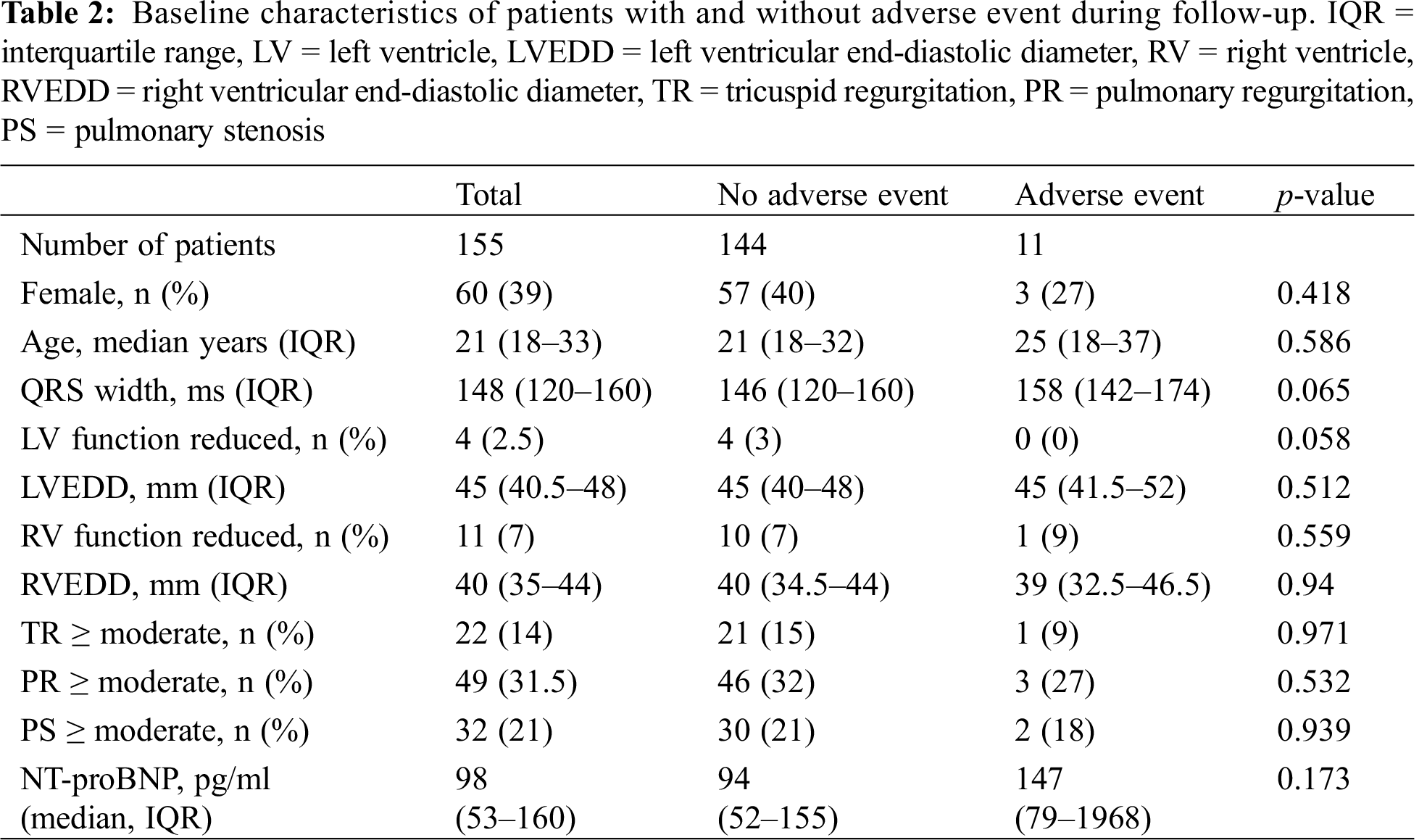
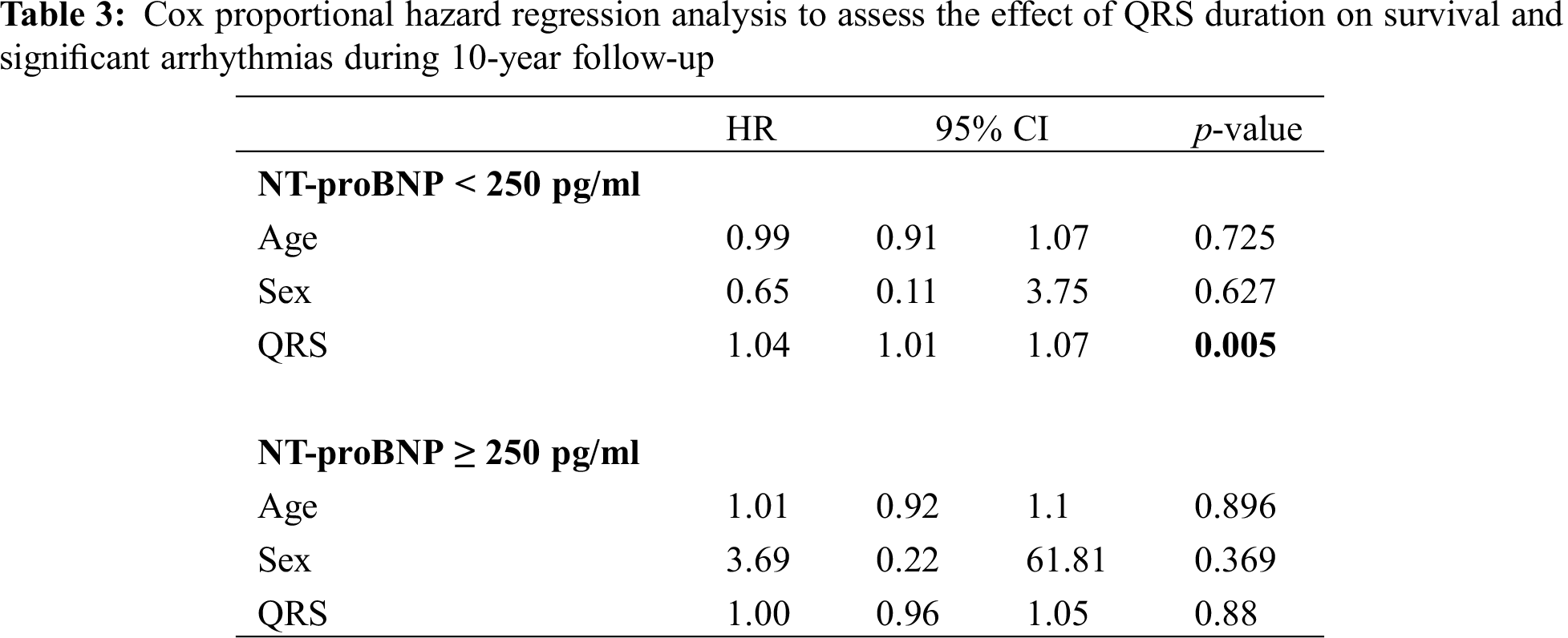
Median NT-proBNP was 98 pg/ml (IQR 53–160). The cohort was divided into patients with NT-proBNP < 250 pg/ml (n = 133 patients) and ≥ 250 pg/ml (n = 22 patients). The two groups differed significantly in age, sex, and NYHA functional class. The QRS duration was not different between the two groups (146 ms vs. 152 ms, p = 0.444). In multivariable Cox hazards regression analysis, duration of QRS as a continuous variable was significantly predictive for the combined endpoint in the low NT-proBNP group (HR 1.04, 95%CI 1.01–1.07, p = 0.005) but not in the high NT-proBNP group (HR 1.00, 95%CI 0.96–1.05, p = 0.88) (Tab. 3).
In the low NT-proBNP group, there was one death during 10-year follow-up (mortality 0.8%), while there were three deaths in the high NT-proBNP group (mortality 14%) (Fig. 1).
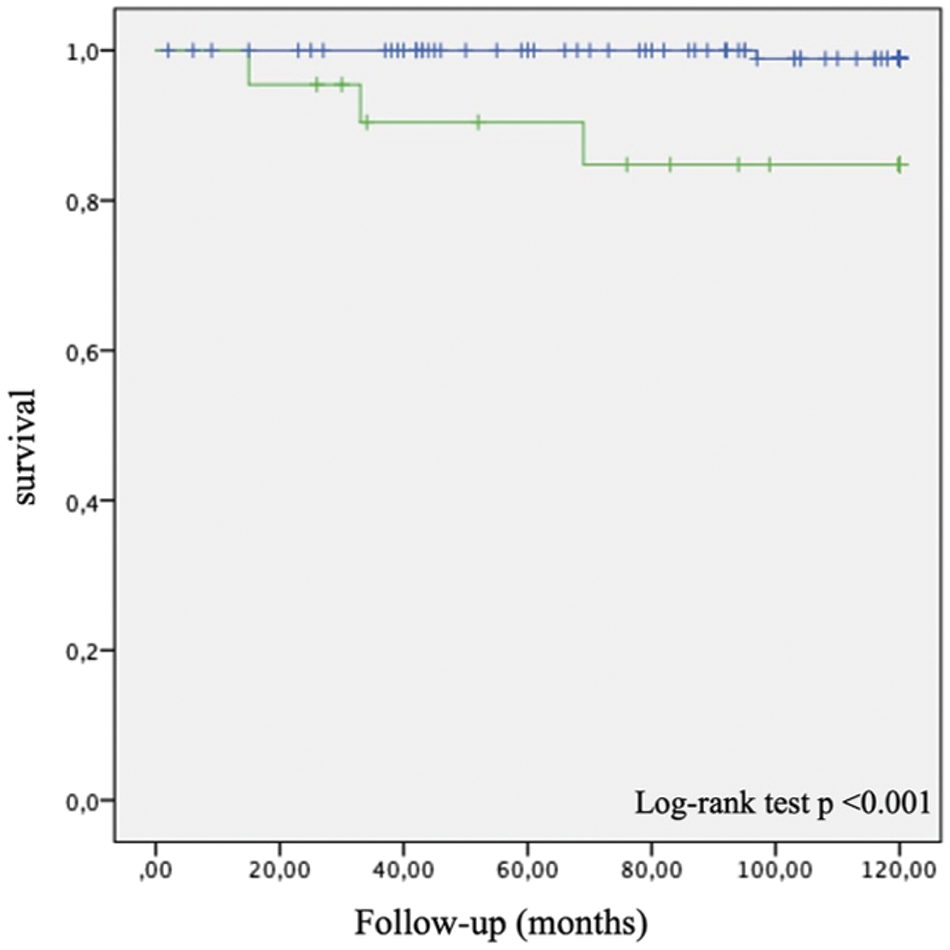
Figure 1: Kaplan Meier 10-year survival curves for patients with NT-proBNP < 250 pg/ml (blue) and with ≥ 250 pg/ml (green)
This study provides evidence that QRS duration should be interpreted not as a single parameter but in the context of morphological changes of the right heart in rTOF patients. A broad QRS complex despite absence of significant RV volume or pressure overload should be considered a surrogate for future arrhythmogenic events.
Mortality (2.6%) was low in this long-term follow-up of a contemporary rTOF cohort, despite a rather high number of patients with transannular patch surgery (34%). This high percentage can be explained with some of the initial surgeries being performed at our institution in the 1980s due to inclusion of adult patients starting in the year 2000. Median QRS duration in the overall group was 148 ms.
In agreement with previous studies, we found significant predictive power of NT-proBNP in rTOF patients. Plasma NT-proBNP < 250 pg/ml was associated with a 99.2% 10-year survival in our cohort.
RV dilatation is common in rTOF patients [21]. Nevertheless, stable courses with excellent exercise capacity are frequently observed as shown by our data with 88% of patients in NYHA functional class I or II. Heart failure in rTOF is usually caused by long-standing RV volume or pressure overload due to pulmonary regurgitation or pulmonary stenosis, or due to myocardial damage related to initial or consequent surgeries. Being independent of intra- and interobserver variability, the parameter NT-proBNP adds a robust variable to the follow-up of these patients. This is of particular clinical meaning, as evaluation of RV size and function can be challenging if only 2D echocardiography is applied [22]. Enlargement of the RV can be associated with a wide QRS complex in rTOF patients [4].
Most common substrates for VT in rTOF are arrhythmogenic anatomical isthmuses (AI). These are associated with RBBB and wide QRS complexes [12,13]. Identification of those patients with AI can have significant prophylactic therapeutic consequences, e.g., ablation therapy and/or ICD implantation [23].
Thus, mechanical and electrical dyssynchrony cannot be distinguished by QRS complex analysis alone. QRS duration is a surrogate parameter with low sensitivity and low specificity. By the addition of NT-proBNP, rTOF patients with poor prognosis towards adverse arrhythmogenic events can be identified. We hypothesize that low NT-proBNP excludes significant mechanical dyssynchrony, thus a wide QRS complex in low NT-proBNP patients is highly suspicious for electrical dyssynchrony, e.g., an arrhythmogenic anatomical isthmus.
The prognostic value of brain natriuretic peptides has been shown previously. In this study, NT-proBNP was shown to differentiate between those patients with significant RV wall stress and those without –independent of the cost-intensive methodology of cardiac magnetic resonance imaging (CMR), and independent of the interobserver variability that comes with transthoracic echocardiography. The novelty of the present findings is not the parameter itself but the “rediscovery” of the QRS width as a prognostic parameter–if applied in the right patients. While non-invasive screening for arrhythmias is mandatory in all rTOF patients, particular and additional caution should be applied in those patients with broad QRS complexes and low NT-proBNP values.
Despite the shown advantages of NT-proBNP identifying those patients who suffer from RV dilatation and dysfunction, echocardiography and CMR rather than soluble markers is the tool to follow morphologic alterations in rTOF patients. Therefore, the findings of this study are only hypothesis generating, future prospective studies should focus on CMR and 3D echo RV and left ventricular volumes and function and their (non)association with QRS duration and arrhythmic events.
This study has several limitations which must be addressed.
The is a retrospective study. Still, at our institution since the 1990s ACHD patients have been followed systematically by a highly trained and specialized team of physicians applying the same standardized routine care, including echocardiography and ECGs. Therefore, data of the present study is of high and consistent quality.
Due to the nature of a rare disease, the size of the study population was limited.
Wide QRS duration accompanied by low NT-proBNP values should receive particular attention in rTOF, as these patients might benefit from close rhythm monitoring and early prevention measures.
ACHD = adults with congenital heart disease
BNP = brain natriuretic peptide
CMR = cardiac magnetic resonance imaging
ECG = electrocardiogram
IQR = interquartile range
LV = left ventricle
NYHA = New York heart association.
QRS-f = QRS fragmentation
RV = right ventricle
RVF = right ventricular function
SCD = Sudden cardiac death
RBBB = Right bundle branch block
rTOF = repaired tetralogy of Fallot
TTE = transthoracic echocardiography
VT = ventricular tachycardia
Data Sharing: The individual participant data will not be shared.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Nollert, G., Fischlein, T., Bouterwek, S., Böhmer, C., Klinner, W. et al. (1997). Long-term survival in patients with repair of tetralogy of Fallot: 36-year follow-up of 490 survivors of the first year after surgical repair. Journal of the American College of Cardiology, 30(5), 1374–1383. [Google Scholar]
2. Cuypers, J. A., Menting, M. E., Konings, E. E., Opić, P., Utens, E. M. et al. (2014). Unnatural history of tetralogy of Fallot: prospective follow-up of 40 years after surgical correction. Circulation, 130(22), 1944–1953. [Google Scholar]
3. Villafañe, J., Feinstein, J. A., Jenkins, K. J., Vincent, R. N., Walsh, E. P. et al. (2013). Hot topics in tetralogy of Fallot. Journal of the American College of Cardiology, 62(23), 2155–2166. [Google Scholar]
4. Balaji, S., Lau, Y. R., Case, C. L., Gillette, P. C. (1997). QRS prolongation is associated with inducible ventricular tachycardia after repair of tetralogy of Fallot. American Journal of Cardiology, 80(2), 160–163. [Google Scholar]
5. Neffke, J. G., Tulevski, I. I., van der Wall, E. E., Wilde, A. A., van Veldhuisen, D. J. et al. (2002). ECG determinants in adult patients with chronic right ventricular pressure overload caused by congenital heart disease: relation with plasma neurohormones and MRI parameters. Heart, 88(3), 266–270. [Google Scholar]
6. Paech, C., Dähnert, I., Riede, F. T., Wagner, R., Kister, T. et al. (2017). QRS width as a predictor of right ventricular remodeling after percutaneous pulmonary valve implantation. Pediatric Cardiology, 38(6), 1277–1281. [Google Scholar]
7. Gatzoulis, M. A., Balaji, S., Webber, S. A., Siu, S. C., Hokanson, J. S. et al. (2000). Risk factors for arrhythmia and sudden cardiac death late after repair of tetralogy of Fallot: a multicentre study. Lancet, 356(9234), 975–981. [Google Scholar]
8. Gatzoulis, M. A., Till, J. A., Somerville, J., Redington, A. N. (1995). Mechanoelectrical interaction in tetralogy of Fallot. QRS prolongation relates to right ventricular size and predicts malignant ventricular arrhythmias and sudden death. Circulation, 92(2), 231–237. [Google Scholar]
9. Egbe, A. C., Miranda, W. R., Mehra, N., Ammash, N. M., Missula, V. R. et al. (2018). Role of QRS fragmentation for risk stratification in adults with tetralogy of fallot. Journal of the American Heart Association, 7(24), e010274. [Google Scholar]
10. Bokma, J. P., Winter, M. M., Vehmeijer, J. T., Vliegen, H. W., van Dijk, A. P. et al. (2017). QRS fragmentation is superior to QRS duration in predicting mortality in adults with tetralogy of Fallot. Heart, 103(9), 666–671. [Google Scholar]
11. Donal, E., Galli, E., Cosyns, B. (2019). Twenty years after starting cardiac resynchronization therapy, do we understand the electromechanical coupling? European Heart Journal Cardiovascular Imaging, 20(3), 257–259. [Google Scholar]
12. Brouwer, C., Kapel, G. F. L., Jongbloed, M. R. M., Schalij, M. J., de Riva Silva, M. et al. (2018). Noninvasive identification of ventricular tachycardia-related anatomical isthmuses in repaired tetralogy of Fallot: what is the role of the 12-lead ventricular tachycardia electrocardiogram. JACC Clin Electrophysiol, 4(10), 1308–1318. [Google Scholar]
13. Kapel, G. F. L., Brouwer, C., Jalal, Z., Sacher, F., Venlet, J. et al. (2018). Slow conducting electroanatomic isthmuses: an important link between QRS duration and ventricular tachycardia in tetralogy of fallot. Jacc Clinical Electrophysiology, 4(6), 781–793. [Google Scholar]
14. Koch, A. M., Zink, S., Glöckler, M., Seeliger, T., Dittrich, S. (2010). Plasma levels of B-type natriuretic peptide in patients with tetralogy of Fallot after surgical repair. International Journal of Cardiology, 143(2), 130–134. [Google Scholar]
15. Cheung, E. W., Lam, W. W., Chiu, C. S., Chau, A. K., Cheung, S. C. et al. (2007). Plasma brain natriuretic peptide levels, right ventricular volume overload and exercise capacity in adolescents after surgical repair of tetralogy of Fallot. International Journal of Cardiology, 121(2), 155–162. [Google Scholar]
16. Dodge-Khatami, A., Büchel, E. V., Knirsch, W., Kadner, A., Rousson, V. et al. (2006). Brain natriuretic peptide and magnetic resonance imaging in tetralogy with right ventricular dilatation. Annals of Thoracic Surgery, 82(3), 983–988. [Google Scholar]
17. Kitagawa, A., Oka, N., Kimura, S., Ando, H., Honda, T. et al. (2015). Clinical utility of the plasma brain natriuretic peptide level in monitoring tetralogy of fallot patients over the long term after initial intracardiac repair: considerations for pulmonary valve replacement. Pediatric Cardiology, 36(4), 752–758. [Google Scholar]
18. Hirono, K., Sekine, M., Shiba, N., Hayashi, S., Nakaoka, H. et al. (2014). N-terminal pro-brain natriuretic peptide as a predictor of reoperation in children with surgically corrected tetralogy of fallot. Circulation Journal, 78(3), 693–700. [Google Scholar]
19. Heng, E. L., Bolger, A. P., Kempny, A., Davlouros, P. A., Davidson, S. et al. (2015). Neurohormonal activation and its relation to outcomes late after repair of tetralogy of Fallot. Heart, 101(6), 447–454. [Google Scholar]
20. Winter, M. P., Wiesbauer, F., Blessberger, H., Pavo, N., Sulzgruber, P. et al. (2018). Lipid profile and long-term outcome in premature myocardial infarction. European Journal of Clinical Investigation, 48(10), e13008. [Google Scholar]
21. Chaowalit, N., Durongpisitkul, K., Krittayaphong, R., Komoltri, C., Jakrapanichakul, D. et al. (2012). Echocardiography as a simple initial tool to assess right ventricular dimensions in patients with repaired tetralogy of Fallot before undergoing pulmonary valve replacement: comparison with cardiovascular magnetic resonance imaging. Echocardiography, 29(10), 1239–1246. [Google Scholar]
22. Schneider, M., Ran, H., Aschauer, S., Binder, C., Mascherbauer, J. et al. (2019). Visual assessment of right ventricular function by echocardiography: how good are we? The International Journal of Cardiovascular Imaging, 35(11), 2001–2008. [Google Scholar]
23. Kapel, G. F., Sacher, F., Dekkers, O. M., Watanabe, M., Blom, N. A. et al. (2017). Arrhythmogenic anatomical isthmuses identified by electroanatomical mapping are the substrate for ventricular tachycardia in repaired Tetralogy of Fallot. European Heart Journal, 38(4), 268–276. [Google Scholar]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |