

 | Congenital Heart Disease |  |
DOI: 10.32604/CHD.2020.011512
ARTICLE
Pulmonary Hemodynamics and Outcome in a Large Cohort of Patients with Sinus Venosus Septal Defect
1Faculty of Medicine, KU Leuven, Department of Internal Medicine, KU Leuven, Leuven, 3000, Belgium
2Division of Structural and Congenital Cardiology, University Hospitals Leuven, Leuven, 3000, Belgium
3Department of Cardiovascular Sciences, KU Leuven, Leuven, 3000, Belgium
4Division of Cardiac Surgery, University Hospitals Leuven, Leuven, 3000, Belgium
5Division of Pediatric Cardiology, University Hospitals Leuven, Leuven, 3000, Belgium
6Department of Public Health and Primary Care, KU Leuven, Leuven, 3000, Belgium
7Institute of Health and Care Science, University of Gothenburg, Göteborg, 41346, Sweden
8Department of Paediatrics and Child Health, University of Cape Town, Cape Town, 7700, South Africa
9Adult Congenital Heart Disease Program, University Heart Center, Zurich, Switzerland
*Corresponding Author: Alexander Van De Bruaene. Email: alexander.vandebruaene@uzleuven.be
Received: 13 May 2020; Accepted: 11 June 2020
Abstract: Background: Left-to-right shunt in sinus venosus septal defect (SVSD) may affect resistive (pulmonary vascular resistance–PVR) and elastic (pulmonary artery compliance-PAC) pulmonary artery properties. This study aimed at evaluating (1) impact of age, (2) pulmonary hemodynamics, and (3) outcome in a large cohort of SVSD patients. Methods: This study included 136 patients with SVSD (median age at diagnosis 14 (IQR 5–48) years, 47% male) of which 87 underwent catheterization. Pressures were measured and cardiac output was evaluated using the Fick principle at diagnosis. PVR, PAC and their product (RC time) were calculated. Results: Surgical repair was performed in 128 (94%) at a median age of 13 (IQR 5–43) years. During a median follow-up time of 31 (IQR 17–55) years, 12 (9%) patients died, 13 (10%) developed heart failure, 4 (3%) Eisenmenger syndrome, 19 (14%) atrial arrhythmia, 6 (4%) sick sinus syndrome and 7 (5%) required pacemaker implantation. In those who underwent catheterization, median shunt ratio was 2.5 (IQR 2.0–2.9). Thirty (34%) had mean PA pressure ≥25 mmHg. PVR indexed, PAC indexed, and RC time was 3.5 (IQR 2.4–7.5) WU.m², 1.8 (IQR 1.3–2.5) mL/mmHg.m² and 0.39 (0.26–0.53) sec with an inverse hyperbolic relationship between PVR and PAC. Mean PA pressure (P < 0.0001); wedge pressure (P = 0.001), PVR indexed (P = 0.002) and PAC indexed (P = 0.002) changed significantly with age at diagnosis, but shunt ratio did not. Conclusion: SVSD has good long-term outcome, albeit with late morbidities. Thirty-four percent has mean PA pressure ≥25 mmHg, but Eisenmenger syndrome is rare (3%). PVR and PAC are inversely related and change significantly with older age.
Keywords: Heart septal defects; atrial; pulmonary hypertension; heart failure; outcome
Sinus venosus septal defects (SVSD) represent 4–11% of atrial septal defects [1] and are associated with one or more abnormal pulmonary venous connections (APVC) in 80% of cases [2]. Most typical is a deficiency of the common wall between the superior caval vein and the right-sided pulmonary veins with a left-to-right shunt causing volume load to the right heart and pulmonary circulation. This increased volume load may cause progressive pulmonary vascular disease [3], and in some patients lead to the development of pulmonary arterial hypertension (PAH) with shunt reversal (Eisenmenger syndrome) [4]. Left-to-right shunt in patients with SVSD may therefore affect both resistive (pulmonary vascular resistance-PVR) and elastic (pulmonary artery compliance-PAC) properties of the pulmonary arteries. PAC has been reported as a predictor of adverse outcome in patient with PAH [5] and scleroderma [6] and even as a stronger predictor of adverse outcome than PVR in patients with heart failure [7].
In older patients with atrial septal defects, including patients with SVSDs, shunt ratio may increase when left ventricular (LV) compliance decreases. The increase in right ventricular (RV) volume load (due to left-to-right shunt) may contribute to right heart failure. Similarly, increases in pulmonary capillary wedge pressure (PCWP) (due to decreased LV compliance) may augment RV pulsatile load as previously observed [8] and further contribute to right heart failure in patients with SVSD. The product of PAC and PVR, or RC time, is relatively constant, but can be affected by PCWP. It represents the decay of pulmonary artery pressure in diastole (Fig. 1) [9]. Still, little is known about PVR, PAC and RC time in patients with SVSD. Therefore, this study aimed at evaluating (1) impact of age on pulmonary hemodynamics, (2) the relationship between PVR and PAC, and (3) outcome in a large cohort of patients with SVSD.
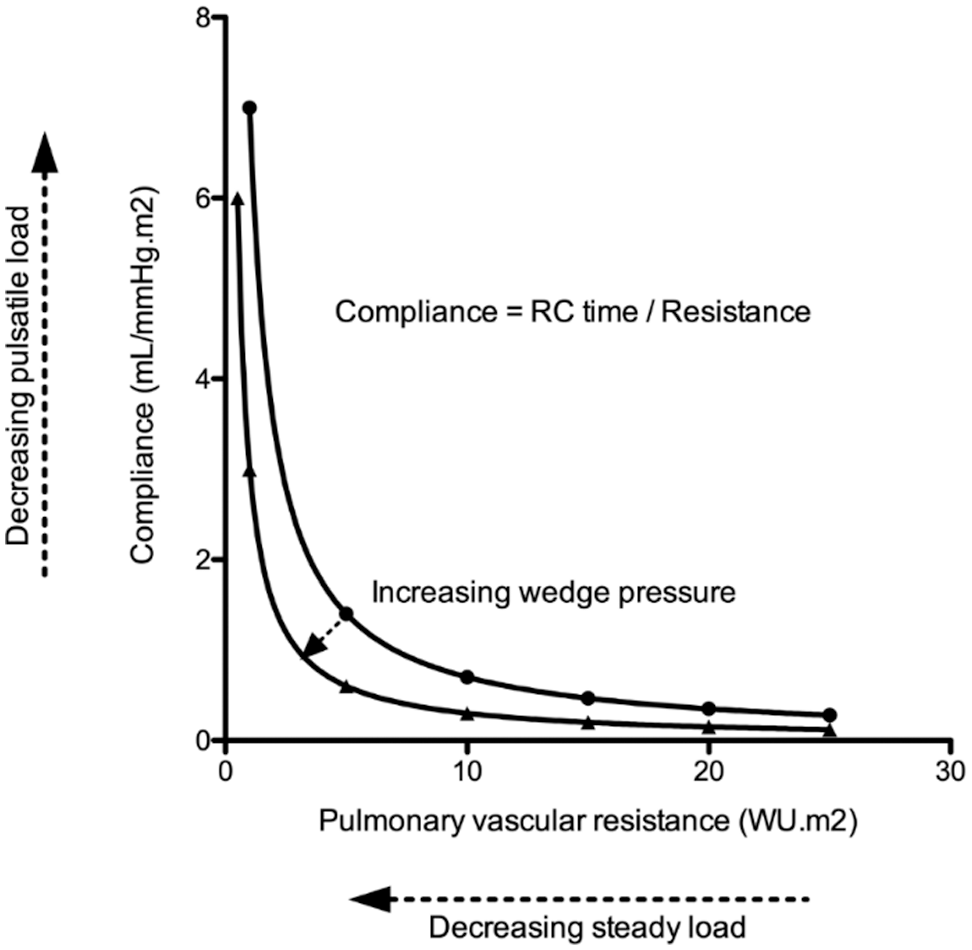
Figure 1: Illustrative figure indicating the relationship between pulmonary artery compliance (PAC-pulsatile load) and pulmonary vascular resistance (PVR-resistive load). The product of PAC and PVR or RC time has the unit of time and is relatively constant. Still, increased pulmonary capillary wedge pressure decreases RC time resulting in enhanced pulsatile load to the right ventricle (i.e., it lowers PAC for any given PVR)
All patients with SVSD followed at the Pediatric and Adult Congenital Heart Disease clinic of the University Hospitals Leuven were included in this retrospective study. Patients’ records were reviewed after pseudonymizing patient data conform the Global Data Protection Regulation (GDPR). The institutional ethics committee approved the study protocol (MP007972) and the study was conducted in compliance with the principles of the Declaration of Helsinki.
Presence and number of APVCs, demographic data, medical and surgical history were recorded. Clinical, electrocardiographic and invasive hemodynamic data at the time of diagnosis were noted. At latest follow-up, electrocardiographic data and New York Heart Association (NYHA) class was collected. Data were also collected if any of the following occurred: atrial arrhythmia, sick sinus syndrome, pacemaker implantation, heart failure, transplant, death. The presence of arrhythmia was assessed on ECG during an outpatient clinic visit or using Holter assessment when clinically indicated. Heart failure was defined as signs and/or symptoms of heart failure requiring medical therapy [10].
2.2 Right Heart Catheterization
Date and indication for catheterization, right atrial pressure, systolic and end-diastolic right ventricular (RV) pressure, systolic, diastolic and mean pulmonary artery (PA) pressure, pulmonary capillary wedge pressure (PCWP) and systolic and end-diastolic left ventricular (LV) pressure were collected at the time of diagnosis (prior to surgical repair). An estimated oxygen consumption (VO2) using the LaFarge-Miettinen formula was used for cardiac output (CO) calculations with the Fick principle [11] and indexed for body surface area (BSA). PVR indexed was calculated using the formula PVR indexed = (mean PA pressure – PCWP/cardiac index (CI)), and expressed in WU.m2. PAC was calculated as PAC = (stroke volume indexed/PA pulse pressure) and expressed in mL/mmHg.m². The RC time is the product of PVR and PAC (not indexed) and expressed in sec [12]. Shunt ratio was calculated using the Fick principle.
Data were analysed using SPSS® for Mac (version 23, SPSS, Chicago) and tested for normality with the Kolmogorov-Smirnov test. Descriptive data for continuous variables are presented as means ± SD in case of a normal distribution or as medians with interquartile ranges (IQR) in case of a non-normal distribution. Descriptive data for discrete variables are presented as frequencies and percentages. Follow-up duration was calculated from date of diagnosis until date of death or date of censure, which was September 1st, 2019. Kaplan-Meier survival curves were plotted to visualize outcome starting at birth. For between-group comparisons, the independent Student t-test, Chi-square or Fischer-exact test was used as appropriate. The relationship between PVR indexed and PAC indexed was performed using an inverse model, as described before [8]. All tests were two-sided and P < 0.05 was considered statistically significant.
There were 136 patients with SVSD included in the study between January 1976 and September 2019. Median age at diagnosis was 14 (IQR 5–48) years, 47% of patients were male. One hundred and twenty-eight patients (94%) underwent surgical repair at a median age of 13 (IQR 5–43) years, whereas 8 patients did not undergo repair because of elevated PA pressures (and PVR) precluding safe SVSD closure. One hundred twenty (94%) patients underwent intracardiac baffle repair using a patch to baffle blood from the orifices of the abnormal pulmonary veins to the left atrium. Eight (6%) patients underwent a Warden procedure. Of the 128 patients who underwent surgical repair, thirty-three patients (26%) were <5 years, 87 (68%) were 5–60 years old and 8 (6%) were >60 years (the oldest being 79 years). APVC was present in 91% of patients. Seventy-two (53%) had one APVC, 41 (30%) two, and eight (6%) ≥3 APVC present. Eight (6%) patients required re-intervention (1 due to pericardial effusion, 3 (2%) due to residual leak, 1 (1%) to close a patent ductus arteriosus and 3 unknown). There were 3 (2%) patients with some degree of caval vein obstruction. Patients who did not undergo repair were older (72 (IQR 52–79) vs. 12 (IQR 5–43) years; p < 0.0001), had higher mean PA pressure (40 (IQR 35–62) vs. 21 (IQR 17–25) mmHg; P < 0.0001), higher PVR indexed (15 (IQR 9–28) vs. 3 (IQR 2–6) WU.m²; P < 0.0001) and lower PAC indexed (0.51 (IQR 0.40–0.86) vs. 1.86 (IQR 1.41–2.61) mL/mmHg.m²; P < 0.0001) but similar PCWP (11 (IQR 3–6) vs. 11 (IQR 6–15); P = 0.665).
Catheterization data was available in 87 (64%) patients and is summarized in Tab. 1. Fifty (57%) patients had mean PA pressure >20 mmHg and 30 (34%) patients had mean PA pressure ≥25 mmHg and would therefore have pulmonary hypertension according to the new and old guidelines, respectively [13,14]. Patient and cardiac catheterization data of patients who did not undergo repair or who had significantly elevated PA pressures prior to repair are summarized in Tab. 2. Of 8 patients who did not undergo repair, 4 (50%) had Eisenmenger syndrome and 4 (50%) had elevated PA pressures in the setting of heart failure. The youngest patient with Eisenmenger syndrome was 36 years, whereas unrepaired patients with heart failure were all >70 years of age. In patients who underwent repair, there was a linear relationship between mean PA pressure (R² = 0.23; P < 0.0001), wedge pressure (R² = 0.24; P < 0.0001), PVR indexed (R² = 0.16; P = 0.0039), PAC indexed (R² = 0.07; P = 0.0226) and age (Fig. 2). There was an inverse hyperbolic relationship between PVR indexed and PAC indexed with an RC time of 0.39 (IQR 0.26–0.53) (Fig. 3) and a weak inverse correlation between RC time and wedge pressure (R²–0.08; P = 0.041). When comparing patients with a catheterization <10 years, 10–50 years and >50 years of age, systolic PA pressure, mean PA pressure, PCWP, PVR indexed, and PAC indexed all changed significantly, whereas transpulmonary gradient and diastolic PA pressure tended to change (Tab. 1).
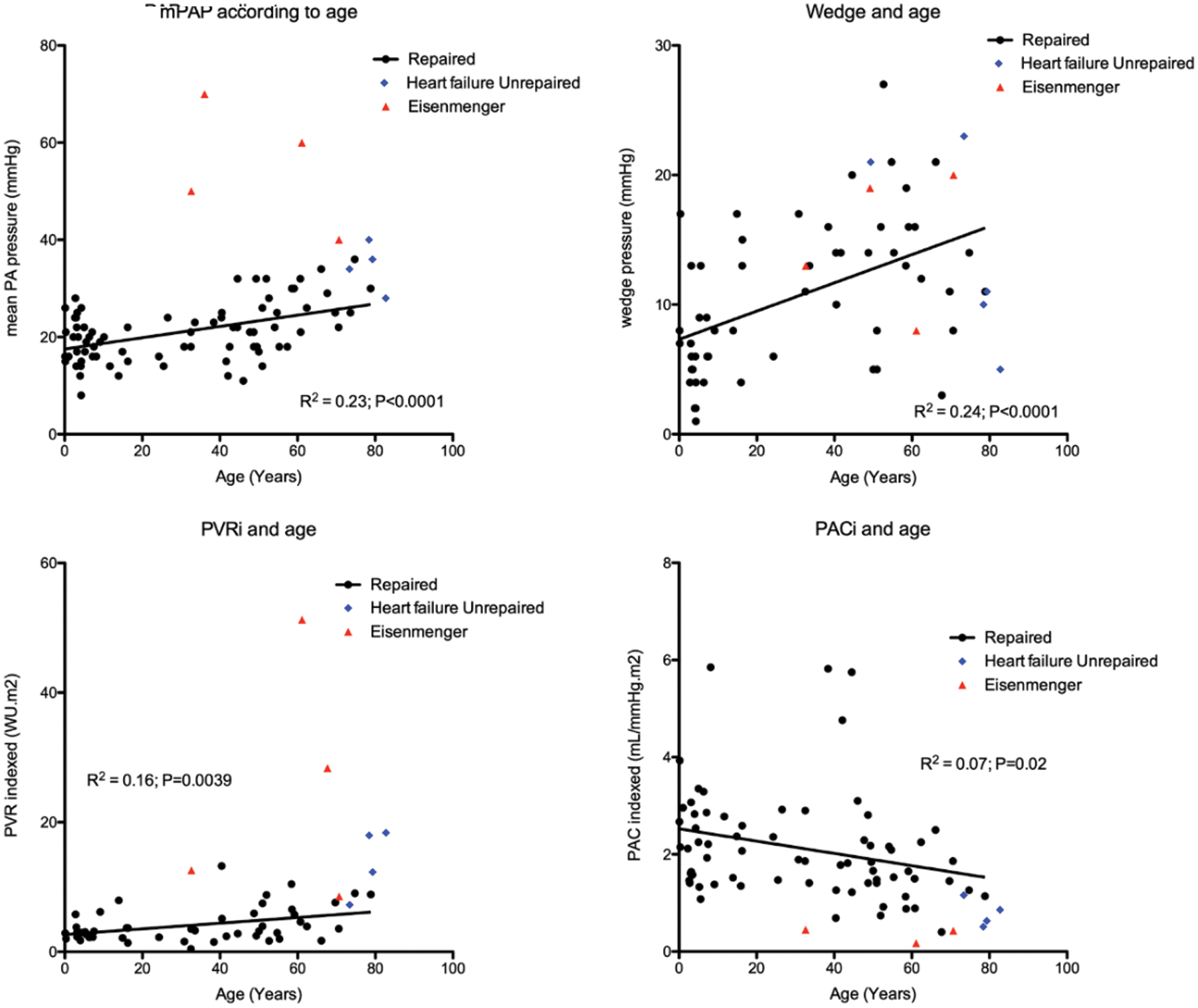
Figure 2: Relationship of mean PA pressure (mPAP), PCWP (wedge), PVR indexed (PVRi) and PAC indexed (PACi) to age at catheterization. The red triangles indicate patients with Eisenmenger syndrome, the blue dots indicate patients who did not undergo repair due to elevated PA pressures in the setting of heart failure
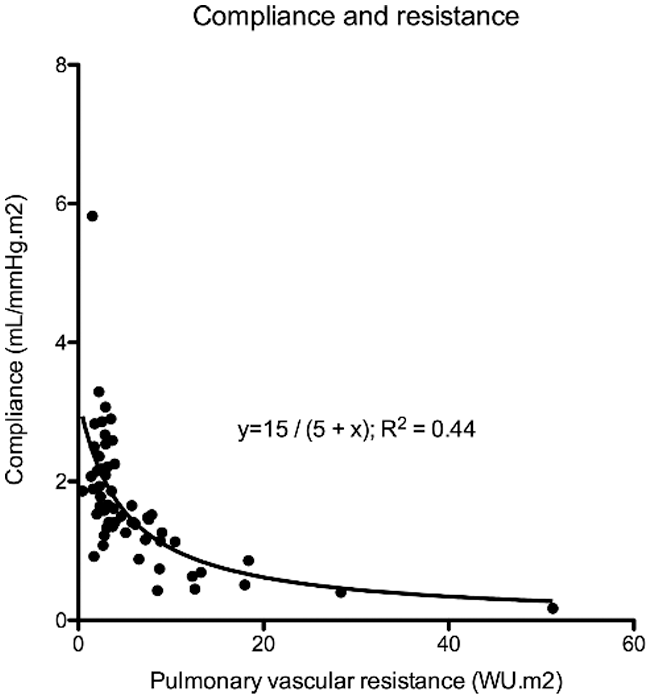
Figure 3: There is a hyperbolic relationship between PAC indexed and PVR indexed
During a median follow-up time of 31 (IQR 17–55) years, 12 (9%) patients died, 1 (1%) underwent heart-lung transplant, 13 (10%) developed heart failure, 19 (14%) had atrial arrhythmia, 6 (4%) sick sinus syndrome and 7 (5%) required pacemaker implantation. Survival was 100% at 30 years, 94% at 50 years and 78% at 70 years (Fig. 4). When comparing patients with age at diagnosis <10 years, 10–50 years and >50 years of age, the proportion of patients with mean PA pressure ≥25 mmHg, NYHA ≥2, atrial arrhythmia (atrial fibrillation and/or flutter) and heart failure increased with age, whereas the proportion of patients with sick sinus syndrome (SSS) did not (Tab. 3).
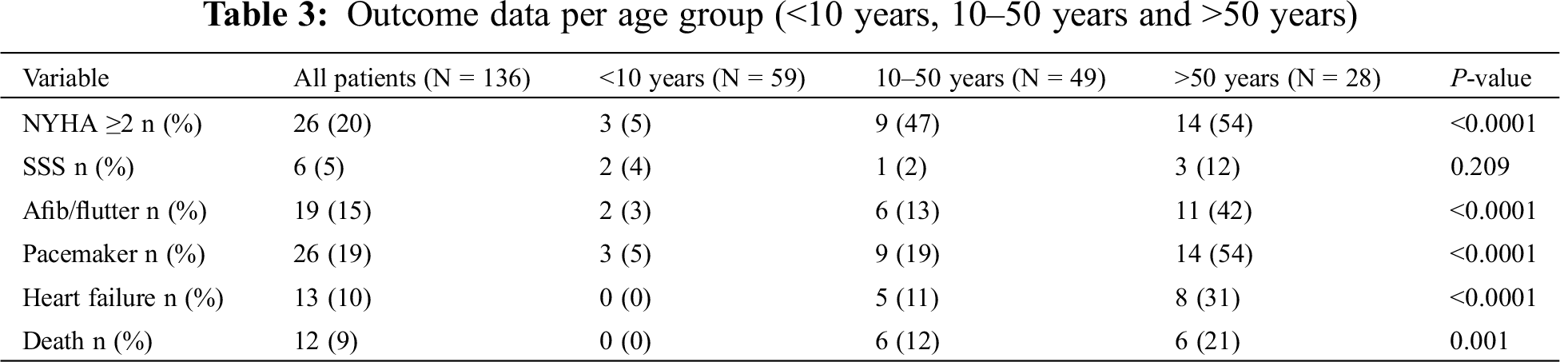
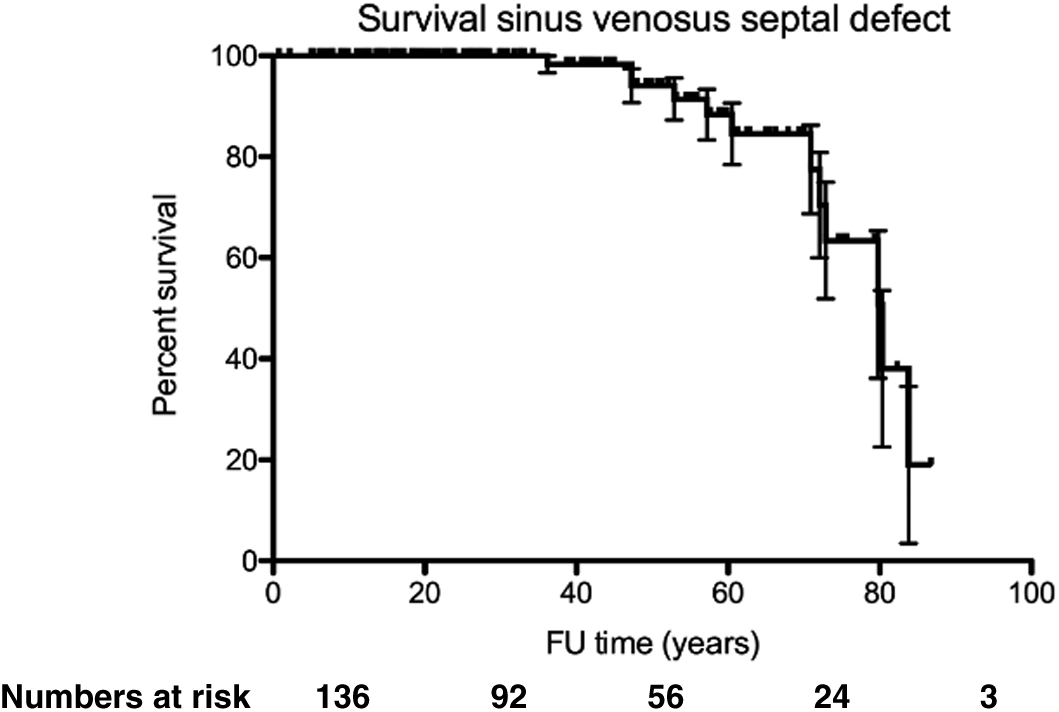
Figure 4: Kaplan–Meier graph indicating survival from birth in patients with SVSD
The present study including 136 patients with SVSD, of which 90% underwent surgical repair, indicated overall good survival (survival rate at 70 years 78%) with few perioperative morbidities. Late comorbidities such as atrial arrhythmias, pacemaker requirement and heart failure increased with age. Of those who underwent cardiac catheterization, a significant proportion of patients (34–57% depending if a cut-off of mean PA pressure ≥25 mmg or >20 mmHg was used) had pulmonary hypertension prior to repair. Nevertheless only 4 (3%) of patients had Eisenmenger syndrome at the time of diagnosis, the youngest being 36 years of age. The remainder of unrepaired patients had elevated PA pressures due to left heart failure. Mean PA pressure, PCWP, and PVR indexed increased with age, whereas PAC indexed decreased. There was an inverse hyperbolic relation between PVR and PAC.
4.1 Pulmonary Hypertension, Compliance and Resistance
With catheterization data available in 87 (64%) patients, PA pressures ≥25 mmHg were present in 34% of patients, and the prevalence of mean PA pressure ≥25 mmHg increased with age. For most cases an increased PA pressure was not a contra-indication for surgical repair of the SVSD since it related to increased pulmonary blood flow due to a left-to-right shunt with PVR still being low. Interestingly, although we could demonstrate that PA pressures increased with age, this rather represented an increase in PCWP and a mild increase in PVR indexed with increasing age. The presence of severe PAH with shunt reversal and arterial desaturation (Eisenmenger syndrome) in 4 (3%) patients occurred rather irrespective of age in a few patients, which would support an underlying susceptibility for developing pulmonary vascular disease in some patients [15]. One patient underwent heart-lung transplant (no pulmonary vasodilators prior to transplant) and 3 patients received pulmonary vasodilator therapy (bosentan monotherapy in 2, bosentan and sildenafil in 1). None of the patients with Eisenmenger syndrome were younger than 30 years of age. Therefore, we assume that a routine cardiac catheterization is not strictly indicated in young patients if anatomy is clear and there are no signs of pulmonary hypertension on non-invasive cardiac imaging. However, in adults, we believe threshold for cardiac catheterization should remain low in order to screen for the presence of pulmonary (arterial) hypertension. In older patients (age >50), cardiac catheterization (along with CT angiography and/or magnetic resonance imaging for anatomical evaluation) provides valuable information on both pulmonary artery pressures, left-sided filling pressures and magnitude of the left-to-right shunt. This is important since these patients often present with complex hemodynamics and a full assessment is needed to see whether surgical repair is feasible. Closure of the defect may not be indicated since this could increase LV filling pressures and worsen systemic heart failure [16,17]. Our data, with increasing pulmonary artery pressure, PVRi and PCWP, indicates the need for a cardiac catheterization is reflected in our data where the proportion of patients who underwent cardiac catheterization increased with age at diagnosis. Similarly, surgical repair was deferred in 4 patients >70 years of age because of elevated PA pressures in the setting of left heart failure.
Of interest, we report two cases where the SVSD was repaired despite the presence of a significantly elevated PA pressure and PVR. One patient underwent surgical repair with fenestration of the interatrial septum (in order to decrease shunt ratio) because of refractory cyanosis. The patient is doing reasonably well with persistent cyanosis (84% on room air), single pulmonary vasodilator and diuretic therapy. The second patient had elevated PA pressures in the presence of a large left-to-right shunt (confirmed on cardiac magnetic resonance imaging). After surgical repair the patient developed combined pre- and postcapillary pulmonary hypertension in the setting of heart failure and died a few years later. These patients reflect the limited prognosis observed in patients with persistent pulmonary hypertension after shunt closure [18].
Our data appears to be in line with data in patients with an atrial septal defect (ASD) type secundum who have a progressive increase in PA pressures with increasing age [19,20]. This study provides additional information by showing that there is also an age-related increase in PCWP. Although not unexpected, it underscores the importance of a full cardiac catheterization since a substantial portion of older SVSD patients will have post-capillary or combined pre- and post-capillary pulmonary hypertension in the setting of heart failure. In contrast, the occurrence of Eisenmenger in our dataset was rare, occurring in 3% of cases and absent <30 years of age, which appears in line with earlier reports [21].
Our data also supports an inverse hyperbolic relationship between PVR and PAC in patients with SVSD, indicating that substantial declines in PAC occur before increases in PVR are observed [8,22]. RC time does not change to a great extent with age, which is in line with previous reports [8]. Tedford et al. showed that the relationship between PAC and PVR is sensitive to changes in PCWP, as an elevation in PCWP lowers PAC for any given PVR (Fig. 1). We also observed a weak correlation between PCWP and RC time. This could be important in older patients where the presence of an atrial shunt will initially serve as a pop-off, leading to an increased left-to-right shunt and the accompanying increase in wedge pressure could cause increased pulsatile RV load further contributing to RV failure [8]. It is known that the RV is able to sustain volume load quite well, but poorly tolerates increases in pressure afterload. We have previously shown that patients with an unrepaired secundum type ASD have a steeper increase in PA pressure during exercise [3], which was related to a lower RV contractile reserve during exercise [23]. This has also been observed in PAH [24], chronic obstructive lung disease [25] and heart failure [26]. Muneuchi et al. [27] albeit in a different cohort of young infants (age 2–4 months) indicated a decrease in RC time following VSD repair (and therefore decrease in shunt ratio). It must be highlighted, however, that some authors question the relative impact of the changes observed in RC time [28].
Although we, in accordance to others [1], report good results after surgical repair of SVSDs, overall clinical status (NYHA) deteriorates and co-morbidities increase with advancing age. This finding and the inability to repair some older patients with heart failure underscore the importance of early diagnosis and intervention.
Sinus node dysfunction, although more common than after ASD repair [29] was rather infrequent occurring in 5% of patients and independent of age of diagnosis/repair. Notably, but not unexpected, was the occurrence of atrial fibrillation and flutter in 15% of patients, with a clear relationship with older age at diagnosis (42% >50 years of age) [19,30]. Surgical scar, atrial dilatation, and PA pressures probably all contribute to the occurrence of atrial arrhythmias [20,30]. Heart failure is common, occurring in 10% of patients, with a prevalence of 30% in those >50 years of age at diagnosis. Although it is reassuring that heart failure is rare at a younger age, it complicates management later in life and underscores the need for early diagnosis and treatment [31]. Nevertheless, it is worth noting that overall outcome is reasonable with 100% survival at 30 years and 78% survival at 70 years of age.
The current study is limited by its retrospective, single center design. Some information, such as ECG, echocardiography and CMR was not available in all patients. There was no standard ambulatory monitoring for atrial arrhythmias, which could therefore be underreported. PAC was measured using the ratio of stroke volume over pulse pressure, which correlates well with other methods to measure PAC22. Pulmonary hemodynamics were not available in all patients. For the purpose of this study, we evaluated PAC using the pulse pressure method since parameters were available at the time of right heart catheterization. Patients with isolated abnormal pulmonary venous connection (i.e., in the absence of a sinus venosus defect) were not included in the current analysis.
SVSD has good overall outcome with a survival rate at 70 years of 78%, although it is associated with late morbidities, such as atrial arrhythmias, need for pacemaker implantation and heart failure. Thirty percent has mean PA pressure ≥25 mmHg, but Eisenmenger syndrome is rare (3%). PVR and PAC are inversely related, with a higher shunt ratio impacting RV pulsatile loading, which may be clinically relevant in older patients with (concomitant) left heart failure.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Attenhofer Jost, C. H., Connolly, H. M., Danielson, G. K., Bailey, K. R., Schaff, H. V. et al. (2005). Sinus venosus atrial septal defect: long-term postoperative outcome for 115 patients. Circulation, 112(13), 1953–1958. DOI 10.1161/CIRCULATIONAHA.104.493775. [Google Scholar] [CrossRef]
2. Said, S. M., Burkhart, H. M., Dearani, J. A., Eidem, B., Stensrud, P. et al. (2011). Outcome of caval division techniques for partial anomalous pulmonary venous connections to the superior vena cava. Annnals of Thoracic Surgery, 92(3), 980–984. DOI 10.1016/j.athoracsur.2011.04.110. [Google Scholar] [CrossRef]
3. Van De Bruaene, A., La Gerche, A., Prior, D. L., Voigt, J. U., Delcroix, M. et al. (2011). Pulmonary vascular resistance as assessed by bicycle stress echocardiography in patients with atrial septal defect type secundum. Circulation: Cardiovasc Imaging, 4(3), 237–245. DOI 10.1161/CIRCIMAGING.110.962571. [Google Scholar] [CrossRef]
4. Brida, M., Gatzoulis, M. A. (2018). Pulmonary arterial hypertension in adult congenital heart disease. Heart, 104(19), 1568–1574. DOI 10.1136/heartjnl-2017-312106. [Google Scholar] [CrossRef]
5. Mahapatra, S., Nishimura, R. A., Sorajja, P., Cha, S., McGoon, M. D. (2006). Relationship of pulmonary arterial capacitance and mortality in idiopathic pulmonary arterial hypertension. Journal of American College of Cardiology, 47(4), 799–803. DOI 10.1016/j.jacc.2005.09.054. [Google Scholar] [CrossRef]
6. Campo, A., Mathai, S. C., Le Pavec, J., Zaiman, A. L., Hummers, L. K. et al. (2010). Hemodynamic predictors of survival in scleroderma-related pulmonary arterial hypertension. American Journal of Respiratory and Critical Care Medicine, 182(2), 252–260. DOI 10.1164/rccm.200912-1820OC. [Google Scholar] [CrossRef]
7. Al-Naamani, N., Preston, I. R., Paulus, J. K., Hill, N. S., Roberts, K. E. (2015). Pulmonary arterial capacitance is an important predictor of mortality in heart failure with a preserved ejection fraction. JACC Heart Fail, 3(6), 467–474. DOI 10.1016/j.jchf.2015.01.013. [Google Scholar] [CrossRef]
8. Tedford, R. J., Hassoun, P. M., Mathai, S. C., Girgis, R. E., Russell, S. D. et al. (2012). Pulmonary capillary wedge pressure augments right ventricular pulsatile loading. Circulation, 125(2), 289–297. DOI 10.1161/CIRCULATIONAHA.111.051540. [Google Scholar] [CrossRef]
9. Lankhaar, J. W., Westerhof, N., Faes, T. J., Gan, C. T., Marques, K. M. et al. (2008). Pulmonary vascular resistance and compliance stay inversely related during treatment of pulmonary hypertension. European Heart Journal, 29(13), 1688–1695. DOI 10.1093/eurheartj/ehn103. [Google Scholar] [CrossRef]
10. Budts, W., Roos-Hesselink, J., Rädle-Hurst, T., Eicken, A., McDonagh, T. A. et al. (2016). Treatment of heart failure in adult congenital heart disease: A position paper of the working group of grown-up congenital heart disease and the heart failure association of the European society of cardiology. European Heart Journal, 37(18), 1419–1427. DOI 10.1093/eurheartj/ehv741. [Google Scholar] [CrossRef]
11. LaFarge, C. G., Miettinen, O. S. (1970). The estimation of oxygen consumption. Cardiovasc Research, 4(1), 23–30. DOI 10.1093/cvr/4.1.23. [Google Scholar] [CrossRef]
12. Saouti, N., Westerhof, N., Postmus, P. E., Vonk-Noordegraaf, A. (2010). The arterial load in pulmonary hypertension. European Respiratory Review, 19(117), 197–203. DOI 10.1183/09059180.00002210. [Google Scholar] [CrossRef]
13. Galiè, N., Humbert, M., Vachiery, J. L., Gibbs, S., Lang, I. et al. (2016). 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The joint task force for the diagnosis and treatment of pulmonary hypertension of the European society of cardiology and the European respiratory society: Endorsed by: Association for European paediatric and congenital cardiology, International society for heart and lung transplantation. European Heart Journal, 37(1), 67–119. DOI 10.1093/eurheartj/ehv317. [Google Scholar] [CrossRef]
14. Simonneau, G., Montani, D., Celermajer, D. S., Denton, C. P., Gatzoulis, M. A. et al. (2019). Haemodynamic definitions and updated clinical classification of pulmonary hypertension. European Respiratory Journal, 53(1), 1801913. DOI 10.1183/13993003.01913-2018. [Google Scholar] [CrossRef]
15. Therrien, J., Rambihar, S., Newman, B., Siminovitch, K., Langleben, D. et al. (2006). Eisenmenger syndrome and atrial septal defect: nature or nurture? Canadian Journal of Cardiology, 22(13), 1133–1136. DOI 10.1016/s0828-282x (06)70950-3. [Google Scholar] [CrossRef]
16. Stout, K. K., Daniels, C. J., Aboulhosn, J. A., Bozkurt, B., Broberg, C. S. et al. (2019). 2018 AHA/ACC guideline for the management of adults with congenital heart disease: Executive summary: A report of the American college of cardiology/American heart association task force on clinical practice guidelines. Journal of the American College of Cardiology, 73(12), 1494–1563. DOI 10.1016/j.jacc.2018.08.1028. [Google Scholar] [CrossRef]
17. Nashat, H., Montanaro, C., Li, W., Kempny, A., Wort, S. J. et al. (2018). Atrial septal defects and pulmonary arterial hypertension. Journal of Thoracic Disease, 10(Suppl 24), S2953–S2965. DOI 10.21037/jtd.2018.08.92. [Google Scholar] [CrossRef]
18. Manes, A., Palazzini, M., Leci, E., Bacchi Reggiani, M. L. (2014). Current era survival of patients with pulmonary arterial hypertension associated with congenital heart disease: A comparison between clinical subgroups. European Heart Journal, 35(11), 716–724. DOI 10.1093/eurheartj/eht072. [Google Scholar] [CrossRef]
19. Gatzoulis, M. A., Freeman, M. A., Siu, S. C., Webb, G. D., Harris, L. (1999). Atrial arrhythmia after surgical closure of atrial septal defects in adults. The New England Journal of Medicine, 340(11), 839–846. DOI 10.1056/NEJM199903183401103. [Google Scholar] [CrossRef]
20. Van De Bruaene, A., Delcroix, M., Pasquet, A., De Backer, J., Paelinck, B. et al. (2011). The importance of pulmonary artery pressures on late atrial arrhythmia in transcatheter and surgically closed ASD type secundum. International Journal of Cardiology, 152(2), 192–195. DOI 10.1016/j.ijcard.2010.07.014. [Google Scholar] [CrossRef]
21. Engelfriet, P. M., Duffels, M. G., Möller, T., Boersma, E., Tijssen, J. G. et al. (2007). Pulmonary arterial hypertension in adults born with a heart septal defect: The Euro heart survey on adult congenital heart disease. Heart, 93, 682–687. DOI 10.1136/hrt.2006.098848. [Google Scholar] [CrossRef]
22. Lankhaar, J. W., Westerhof, N., Faes, T. J., Marques, K. M., Marcus, J. T. et al. (2006). Quantification of right ventricular afterload in patients with and without pulmonary hypertension. American Journal of Physiology-Heart and Circulatory Physiology, 291(4), H1731–1737. DOI 10.1152/ajpheart.00336.2006. [Google Scholar] [CrossRef]
23. Van De Bruaene, A., De Meester, P., Buys, R., Vanhees, L., Delcroix, M. et al. (2013). Right ventricular load and function during exercise in patients with open and closed atrial septal defect type secundum. European Journal of Preventive Cardiology, 20(4), 597–604. DOI 10.1177/2047487312444372. [Google Scholar] [CrossRef]
24. Holverda, S., Gan, C. T., Marcus, J. T., Postmus, P. E., Boonstra, A. et al. (2006). Impaired stroke volume response to exercise in pulmonary arterial hypertension. Journal of the American College of Cardiology, 47(8), 1732–1733. DOI 10.1016/j.jacc.2006.01.048. [Google Scholar] [CrossRef]
25. Holverda, S., Rietema, H., Westerhof, N., Marcus, J. T., Gan, C. T. et al. (2009). Stroke volume increase to exercise in chronic obstructive pulmonary disease is limited by increased pulmonary artery pressure. Heart, 95(2), 137–141. DOI 10.1136/hrt.2007.138172. [Google Scholar] [CrossRef]
26. Lewis, G. D., Murphy, R. M., Shah, R. V., Pappagianopoulos, P. P., Malhotra, R. et al. (2011). Pulmonary vascular response patterns during exercise in left ventricular systolic dysfunction predict exercise capacity and outcomes. Circulation: Heart Fail, 4, 276–285. DOI 10.1161/CIRCHEARTFAILURE.110.959437. [Google Scholar] [CrossRef]
27. Muneuchi, J., Nagatomo, Y., Watanabe, M., Joo, K., Onzuka, T. et al. (2016). Relationship between pulmonary arterial resistance and compliance among patients with pulmonary arterial hypertension and congenital heart disease. Journal of Thoracic and Cardiovascular Surgery, 152(2), 507–513. DOI 10.1016/j.jtcvs.2016.03.080. [Google Scholar] [CrossRef]
28. Naeije, R., Delcroix, M. (2014). Is the time constant of the pulmonary circulation truly constant? European Respiratory Journal, 43(5), 1541–1542. DOI 10.1183/09031936.00174213. [Google Scholar] [CrossRef]
29. Arensman, F. W., Boineau, J. P., Balfour, I. C., Flannery, D. B., Moore, H. V. (1986). Sinus venosus atrial septal defect and pacemaker requirement in a family. American Journal of Cardiology, 57(4), 368–369. DOI 10.1016/0002-9149(86)90933-1. [Google Scholar] [CrossRef]
30. Van De Bruaene, A., Moons, P., Belmans, A., Post, M. C., Luermans, J. G. et al. (2013). Predictive model for late atrial arrhythmia after closure of an atrial septal defect. International Journal of Cardiology, 164(3), 318–322. DOI 10.1016/j.ijcard.2011.07.010. [Google Scholar] [CrossRef]
31. Van De Bruaene, A., Hickey, E. J., Kovacs, A. H., Crean, A. M., Wald, R. M. et al. (2018). Phenotype, management and predictors of outcome in a large cohort of adult congenital heart disease patients with heart failure. International Journal of Cardiology, 252, 80–87. DOI 10.1016/j.ijcard.2017.10.086. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |