

 | Congenital Heart Disease |  |
DOI: 10.32604/CHD.2020.011376
ARTICLE
Assessing Univentricular Function in Adult Fontan Using 3D Echocardiography
1University Hospitals Birmingham NHS Foundation Trust, Birmingham, UK
2Institute of Cardiovascular Sciences, University of Birmingham, Birmingham, UK
3Worcestershire Acute Hospitals NHS Trust, Worcester, UK
*Corresponding Author: Karina V. Bunting. Email: k.v.bunting@bham.ac.uk
Received: 05 May 2020; Accepted: 17 June 2020
Abstract: Objective: To determine the accuracy of assessing univentricular function in adult Fontan patients using three-dimensional (3D) volumetric echocardiography. Design: A prospective observational study in an adult Fontan patient cohort. Setting: University Hospitals Birmingham, NHS Foundation Trust. Patients: 26 patients were enrolled in the study all aged over 18 years, possessing the Fontan anatomy, with no contraindications to Cardiac Magnetic Resonance (CMR) imaging and in sinus rhythm. Intervention: All patients underwent transthoracic echocardiography using a Philips EPIQ 7 and X5-1 transducer. End diastolic volume (EDV), end systolic volume (ESV), stroke volume (SV) and ejection fraction (EF) were obtained using two dimensional (2D) and 3D acquisitions. CMR was performed within 3 months according to standard protocols. Outcome Measures: The agreement and correlation between 2D, 3D and CMR derived parameters were determined by Bland and Altman analysis and Pearson’s correlation coefficient method. The inter-observer variability was also assessed for all three modalities. Results: 3D volumetric acquisitions of the single ventricle were feasible in 18/26 (69%) patients. 3D volumes strongly correlated with CMR but with a systematic bias to under-estimation: EDV r = 0.66, bias = –47.1 (–109.6 to 15.2); ESV r = 0.82, bias = –19.4 (–59.9 to 21.1); EF r = 0.73, –1.56 (–18.8 to 15.7) and SV r = 0.32, –27.7 (–70.2 to 14.7). Inter-observer variability was lowest with CMR, when compared to echocardiographic techniques. The inter-observer variability for 3D when compared with 2D echocardiography was lower across all parameters except EDV. Conclusions: 3D volumes correlate strongly with CMR and may be used for serial assessment of univentricular function. However, 3D volumes on echo are not interchangeable with CMR due to systematic underestimation of volume.
Keywords: Adult Fontan; univentricular function; 3D echocardiography; cardiovascular magnetic resonance; 2D echocardiography
The Fontan operation is a palliative procedure carried out on patients with a single functioning ventricle. Without surgery, survival to adult life is rare. The operation involves ‘bypassing’ the right heart, connecting the superior and inferior vena cava directly to the pulmonary arteries, thereby committing the single ventricle to the systemic circulation. This alleviates cyanosis, reduces the volume load on the single ventricle and prolongs life [1,2]. Increasing number of patients are surviving to adulthood, however ventricular dysfunction is one of the main long-term complications in adult Fontan patients and so it is essential that reliable surveillance of ventricular function is performed [3,4]. Cardiovascular magnetic resonance (CMR) imaging is considered the gold-standard method for quantification of ventricular volumes and ejection fraction, as well as providing additional detailed functional and anatomical information [5]. Alternative modalities must be available however, for use in those with contra-indications, including incompatible intra-cardiac devices, in those in whom CMR imaging is poor quality, and those who are intolerant to the procedure. Although computed tomography (CT) has superior spatial resolution when compared to transthoracic echocardiography (TTE), TTE does not expose young patients to ionising radiation, is less expensive and more freely available. 2D echocardiography however has its limitations and current recommendations suggest the use of 3D where possible [6–8].
3D echocardiography has proven accuracy in the assessment of ventricular volumes in anatomically normal hearts [6,9–11] and has greater agreement with CMR than 2D echocardiography [12]. Due to the often distorted shape of the single ventricle, most markedly in the systemic right ventricle, 2D Simpson’s biplane may be unreliable as the formula is based on the assumption that the ventricle is cylindrical in shape. Therefore, 3D should provide an accurate and safe alternative that can be used to detect changes in the size and function of the single ventricle in both systemic right and left ventricles [13]. The aim of our study was to assess the accuracy of 3D echocardiography in obtaining ventricular volumes in the adult Fontan patient, compared to standard 2D echocardiography and CMR.
This was a prospective cohort observational study approved by research ethics committee (REC:15/WS/0046). All adult Fontan patients in the tertiary referral service for adult congenital heart disease at the University Hospital Birmingham, NHS Foundation trust, Birmingham were screened from the trust’s Fontan database for inclusion. Inclusion criteria were: age over 18 years, Fontan anatomy, in sinus rhythm and able to provide informed consent. Exclusion criteria were: contraindications for CMR, pregnancy, inability to give informed consent. Eligible subjects were consecutively approached at their annual echocardiogram over a period of eight months. Informed consent was obtained from all individual participants included in the study.
Transthoracic echocardiography (TTE) was performed using a Phillips EPIQ 7 with an X5-1 transducer. All images and measurements were obtained by a single operator with over ten years’ experience and accredited in both TTE through the British Society of Echocardiography and accredited in echocardiography of congenital heart disease through the European Society of Cardiovascular Imaging (EACVI). To assess ventricular function, end-diastolic volume (ml), end-systolic volume (ml), stroke volume (ml) and ejection fraction (%) were obtained using the 2D Simpson’s biplane method and 3D full volumetric method. The image quality in the included patients was graded according to the visualisation of the endocardial borders of the single ventricle. If all endocardial segments were clearly seen this was graded as “good”. If one or two segments (not adjacent to each other) were not well seen this was graded as “limited”. If more than two endocardial borders or two adjacent endocardial borders were not clearly seen, this was graded as “poor”. Images in which two or more wall segments of the endocardial borders were not seen were excluded from the analysis. In the case of patients with a rudimentary ventricle considered to be contributing to end-diastolic volume, such as Tricuspid atresia (TA) with a large ventricular septal defect (VSD) or a double inlet left ventricle (DILV) with a smaller outlet chamber, the rudimentary ventricle’s volume was included in the univentricular volume measurement.
For the Simpson’s biplane method end-diastole was defined as the largest volume and end-systole was defined as the smallest volume. The endocardial border was traced at the blood-tissue interface between the compacted myocardium and ventricular cavity, with the contour closed by a straight line between the two opposite sections of the atrioventricular valve ring, measured in the apical four and two chamber views. 2D imaging was performed to maximise the ventricular areas, with views acquired at a depth to optimise focus on the cavity.
For 3D acquisitions, an x-plane of the ventricle was performed to ensure inclusion of the planes within the volume to be acquired. Spatial and temporal resolutions were optimised by narrowing sector width, optimising depth and focus. A 3D full volume setting was applied and images were taken over 4 heart beats in all cases during end-expiratory breath-holding. Volume analysis was performed off-line using the Q-Lab (Philips, Netherlands) 3D left ventricular volume software package for both systemic left and right ventricles; using the same definition for end-diastole and end-systole as for 2D acquisitions (Fig. 1). The mean frame rate used was 27 Hz with a range from 21 to 59 Hz and time taken to acquire the 3D images and perform the post-processing analysis of volumes took between 3 and 5 minutes.
2.2 Cardiac Magnetic Resonance Imaging
CMR was performed according to standard protocols with a 1.5 T scanner (Siemens, Avanto) during end-expiratory breath-holding with retrospective ECG gating using steady state free precession (SSFP) cine imaging (Fig. 1). In brief, following acquisition of scout images in the vertical, horizontal and 3-chamber axes, SSFP images were acquired with 7 mm thickness; 3 mm gap from the atrioventricular valve plane through the apex. Sequence parameters were as follows: echo time 1, 2 ms; repetition time 60 msec, flip angle 79 degrees; 25 phases per cardiac cycle; number of excitation 1; FOV 300 mm, in plane spatial resolution 1.6 mm × 1.2 mm. Analysis of all CMR images was performed by a single observer (level 3, European Association of Cardiovascular Imaging) with 3 years’ experience in CMR in imaging adults with congenital heart disease, using semi-automated quantification (Circle CVi42, Calgary, Canada). The observer was blinded to the results derived from echocardiography. Quantitative parameters (ventricular EDV, ESV, mass, stroke volume) were derived by semi-automated blood-pool thresholding using non-rounded endocardial contours, and including papillary muscles and ventricular trabeculations in the blood pool both in systole and diastole. The standard CMR technique requires that both the systemic and the atretic ventricle are included in volume analysis [14].
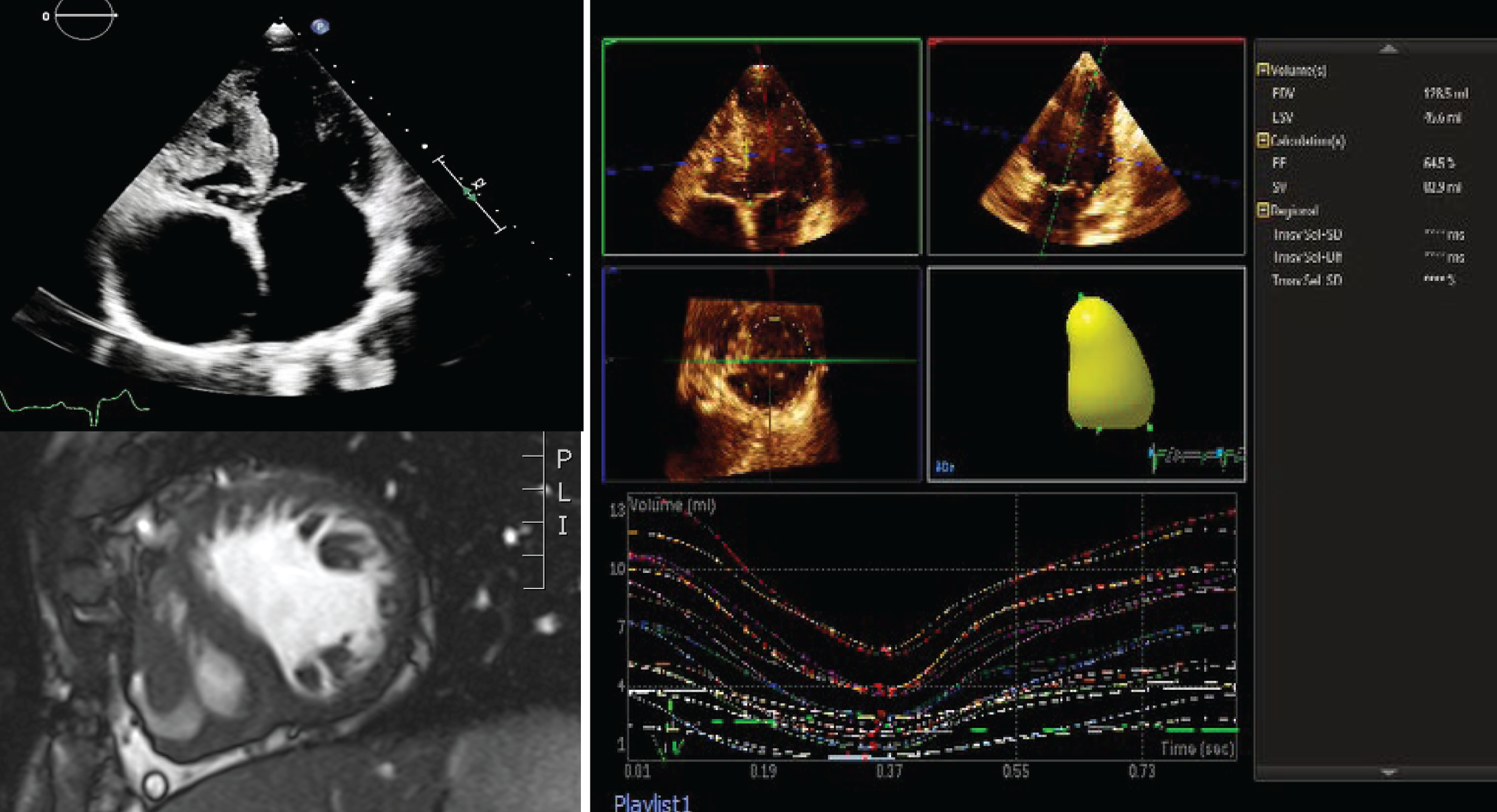
Figure 1: Fontan ventricle in a patient with tricuspid atresia. TTE of the Apical 4 chamber view (top left), CMR short-axis (bottom left) and 3D echocardiography volume of the systematic ventricle (right). (Abbreviations: 2D = two dimensional; 3D = three dimensional; CMR = cardiac magnetic resonance; TTE = transthoracic echocardiography)
2.3 Inter-Observer Reproducibility
To assess inter-observer variability every third echocardiogram was re-analysed by a second observer, equally experienced in adult congenital and 3D echocardiography, who was blinded to the previous measurements. To assess inter-observer variability, every third CMR was re-analysed by a second observer, equally experienced in adult and congenital CMR.
All analysis was carried out in STATA version 14.2. Data was analysed by determining agreement and correlation between matched data sets of 3D and 2D Simpson’s biplane derived ventricular volumes and ejection fractions, with cardiac CMR derived ejection fractions and volumes. A Pearson’s correlation was used to determine the correlation between CMR and both echocardiographic measurements, as well as the correlation between the two echocardiographic methods. An r-value > 0.6 was considered as strong correlation. To assess agreement a Bland-Altman plot was formed. The bias and standard deviations of the difference in value between the echocardiographic method and CMR derived value was calculated and the 95% limits of agreement determined. For inter-observer variability Bland-Altman analysis and coefficient of variation was used. For all analyses a p-value less than 0.05 was used as a cut off for statistical significance.
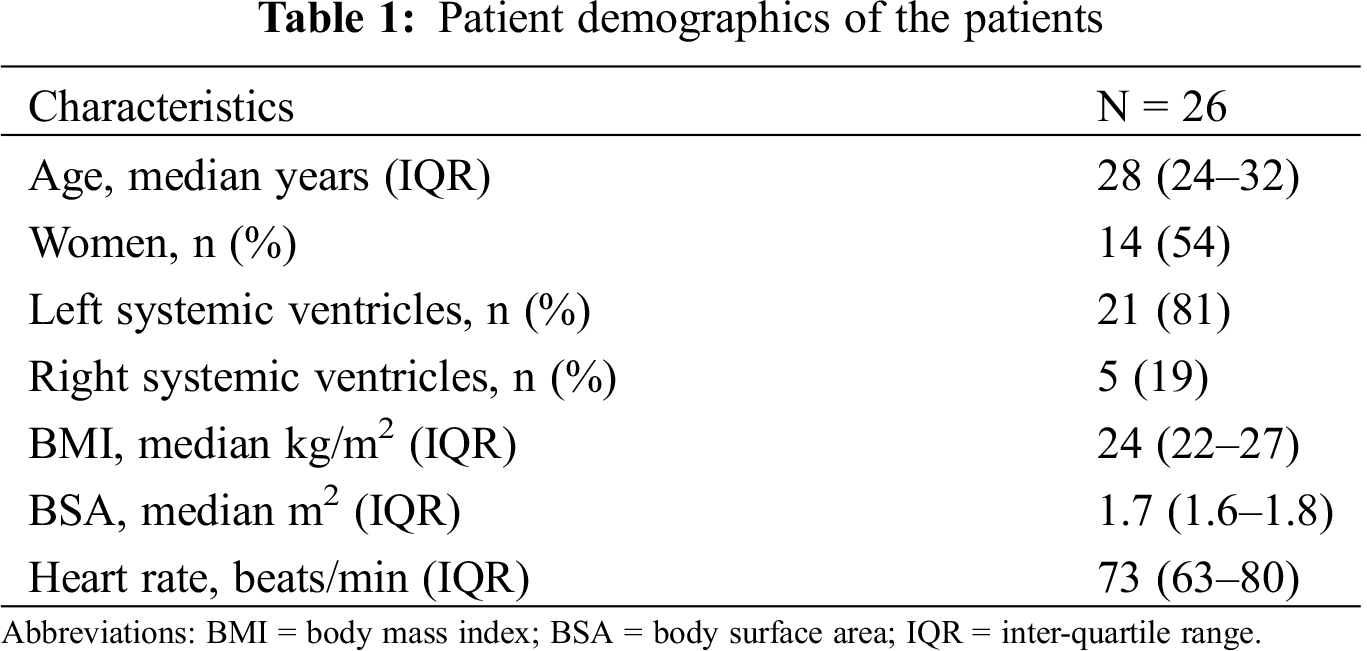
151 patients were screened of which 66 were excluded for the following reasons: CMR contra-indicated (n = 32), arrhythmia (n = 33), unable to provide informed consent (n = 8) and poor echocardiographic window for 3D analysis (n = 48). From the remaining 66 patients, 26 patients were recruited consecutively to the study as they attended their annual echocardiogram. Patient demographics are displayed in (Tab. 1); the median (IQR) for age was 28 years (24–32), BMI 24 kg/m2 (22–27) and 53% of the participants were female (14 females, 12 males). At the time of scanning the median heart rate (IQR) of the patients was 72 beats/minute (63–80), most patients had a left systemic ventricle (21 vs. 5 right systemic ventricles). The remaining patients who met the inclusion criteria (n = 58) were either unwilling to take part or were unable to attend their echocardiogram and CMR within 3 months of each other.
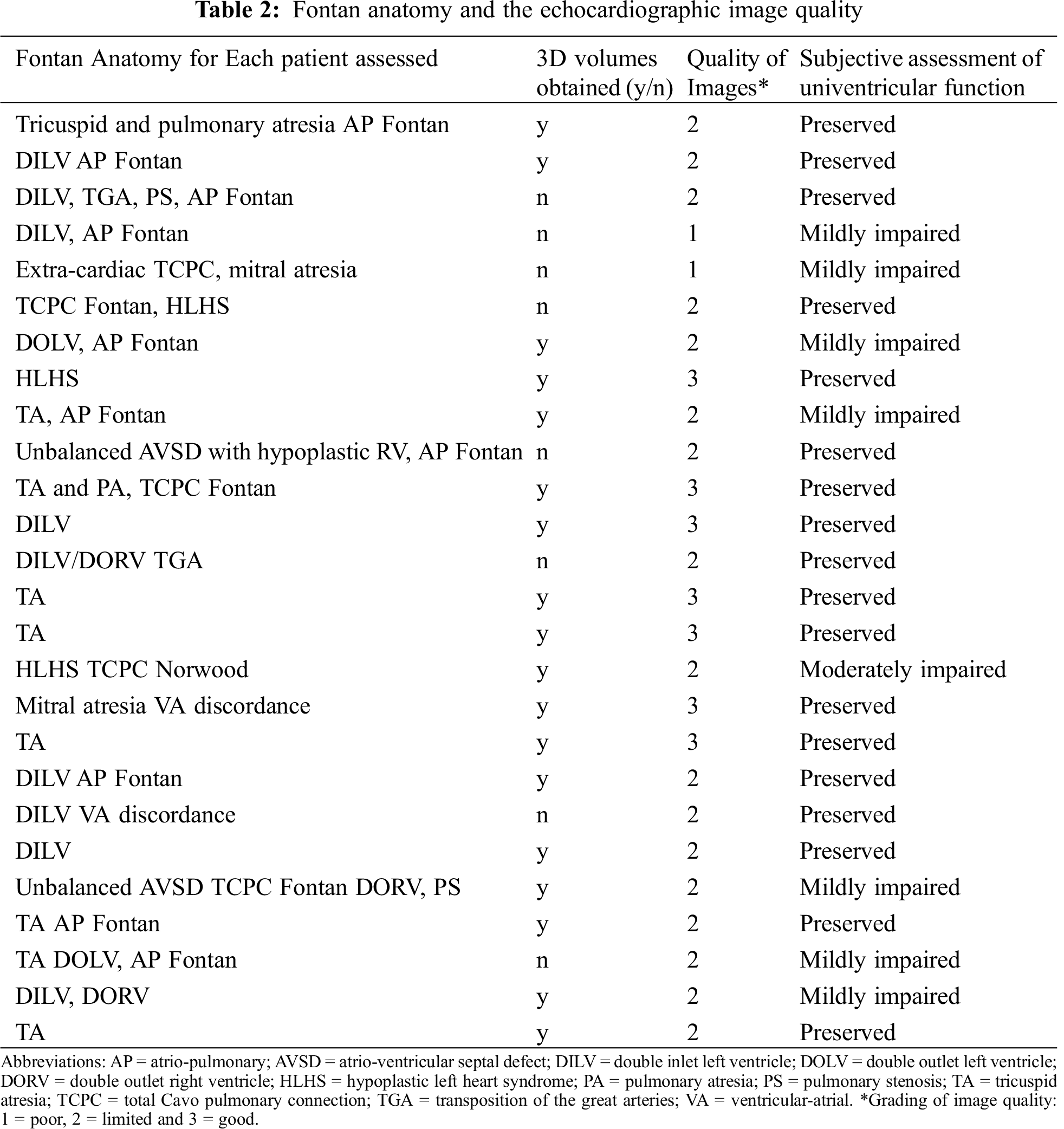
The 26 patients included had a wide range of Fontan anatomies of which 3D volumes were obtainable in 18 of these patients (Tab. 2). Of these 18 patients, there were three with morphological right ventricles and the remainder had morphological left ventricles. There was no substantial modification made to the patients’ pharmacological therapy between the study with 3D echo and CMR. The image quality for the cohort of patients was overall graded as “limited”. In 8 patients image quality was graded as poor and 3D volumes could not be obtained in these patients, as a result of factors such as body habitus (3 patients), abnormal rib cage formation (2 patients) and breathing artefact (3 patients).
3.2 Comparison of 2D and 3D Echocardiography Derived Univentricular Volumes with CMR
Volumes and ejection fractions derived from echocardiography were compared with CMR as the gold-standard technique. The mean difference between time of CMR and TTE was 41 days. Bland-Altman analysis demonstrated a negative bias and wide limits of agreement for comparisons of both 3D and 2D volumes with CMR: for 3D EDV vs. CMR bias (95% limits of agreement) was –47 ml (–108 to 15) and 2D EDV was –53 ml (–114 to 9), for 3D ESV vs. CMR –20 ml (–60 to 20) and 2D ESV was –24 ml (–73 to 26) and for 3D SV vs. CMR –27 ml (–69 to 15) and for 2D SV –29 ml (–66 to 9). Therefore, 3D and 2D echocardiography underestimates volumes when compared to CMR (Tab. 3 and Fig. 2).
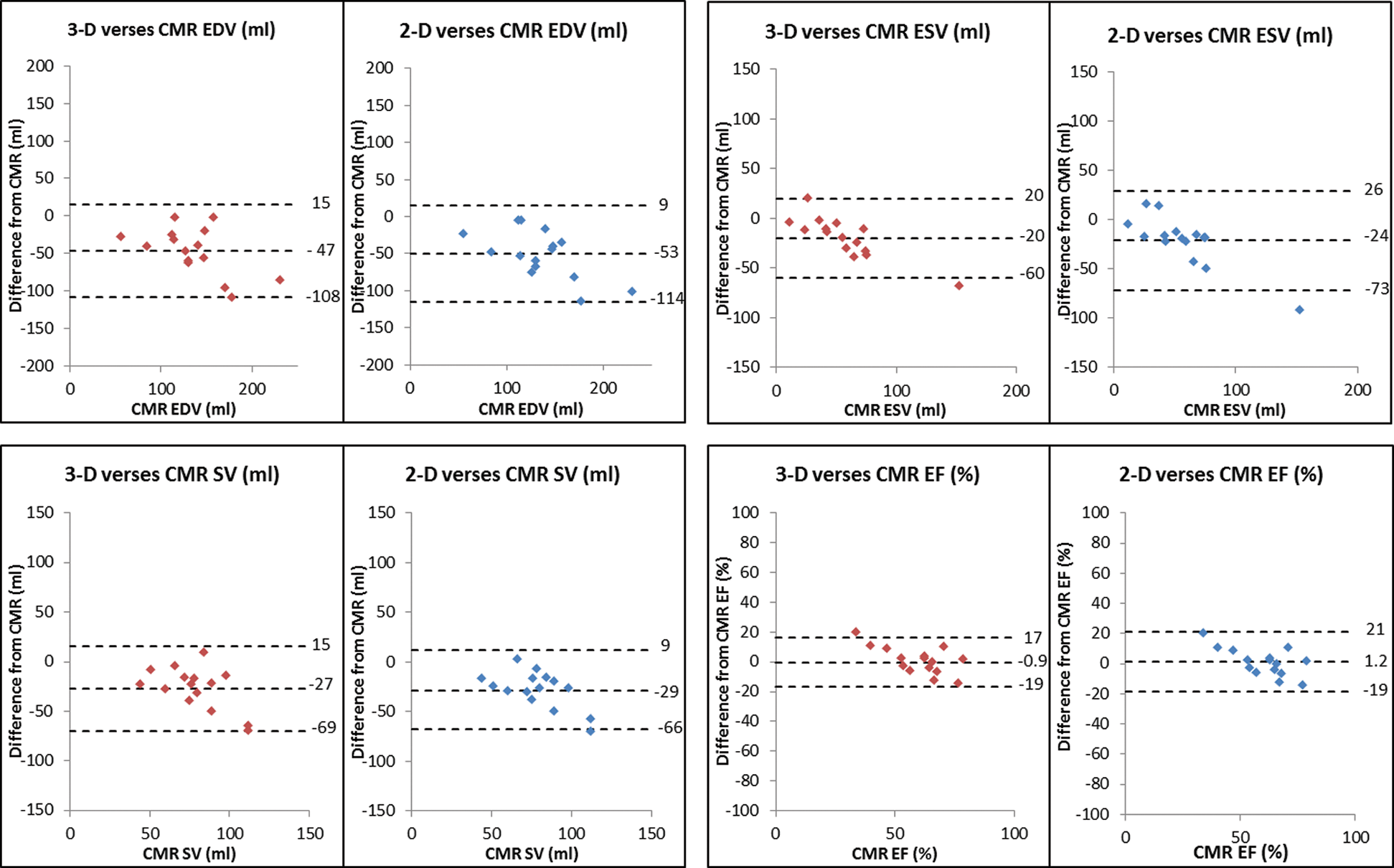
Figure 2: Modified Bland and Atman plots to show the agreement of 3D and 2D EDV, ESV, SV and EF with CMR. (Abbreviations: 2D = two dimensional; 3D = three dimensional; CMR = cardiac magnetic resonance; EDV = end diastolic volume; EF = ejection fraction; ESV = end systolic volume; SV = stroke volume)
It was noted that there was greater underestimation of the volume with larger ventricles (Fig. 2). There was a greater underestimation when 2D Simpson’s biplane was used, in comparison to 3D volumes. Despite the systematic under-estimation of volumes with echocardiography, the correlation between the echocardiographic measurements and cardiac CMR was shown to be strong across all parameters with the exception of stroke volume which showed a weak correlation for both echocardiographic modalities (Tab. 3 and Fig. 3).
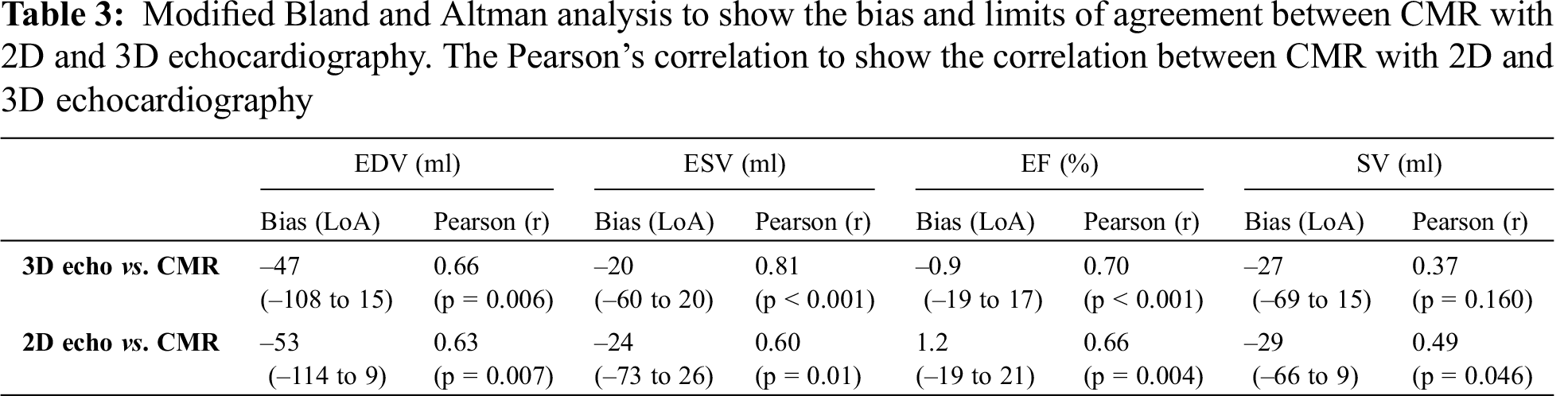
Ejection fraction, the primary measure of ventricular function showed a strong correlation with CMR for both echocardiographic modalities: (Tab. 3). There was no significant bias between CMR and both echocardiographic modalities, however wide limits of agreement were calculated suggesting significant variability within the population studied: for 3D EF vs. CMR bias (95% limits of agreement) –0.9% (–19.0 to 17.0) vs. 2D EF vs. CMR 1.2% (–19.0 to 21.0).
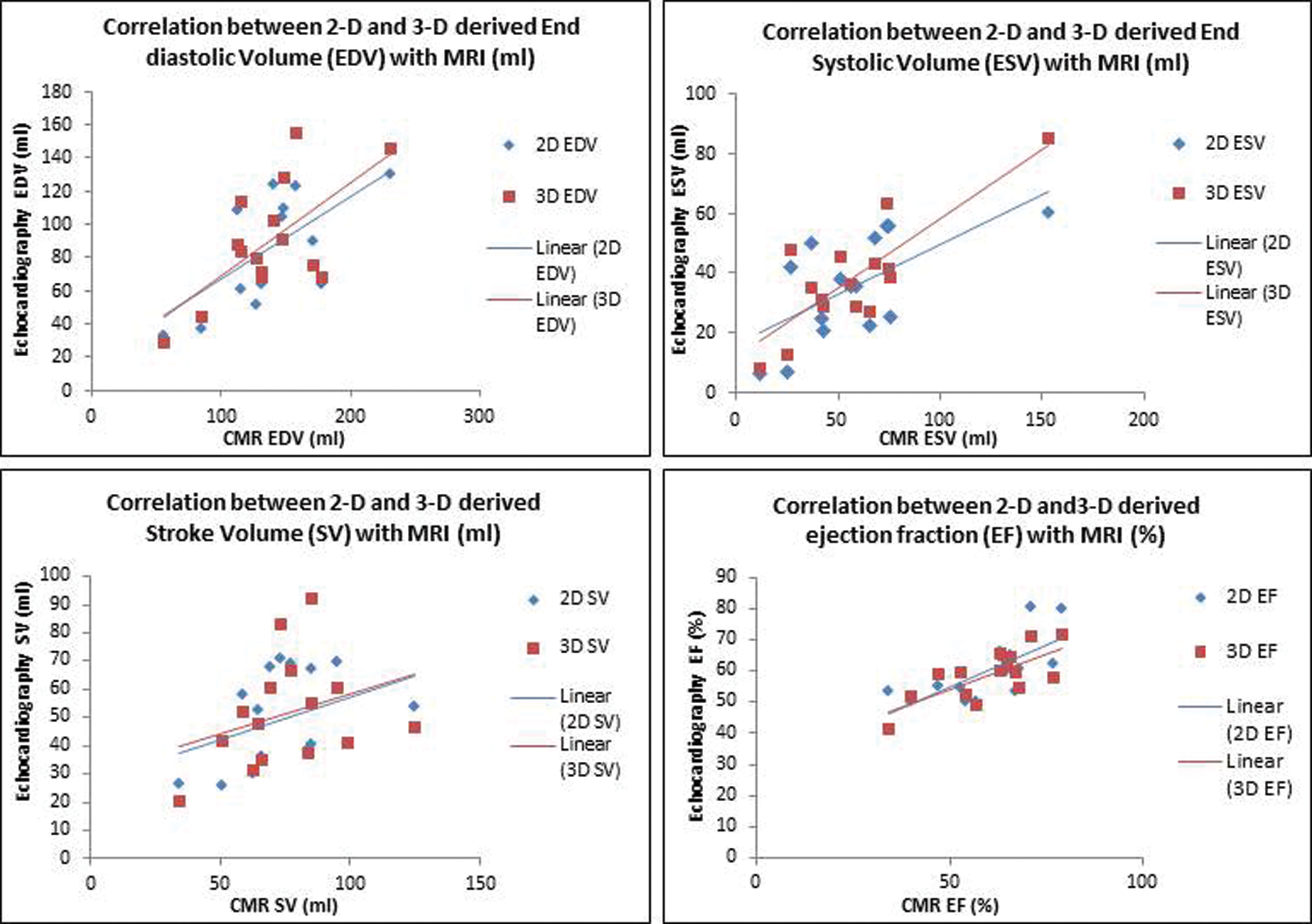
Figure 3: Scatter plots showing the correlation between 3DE and 2DE EDV, ESV, SV and EF with CMR. (Abbreviations: 2D = two dimensional; 3D = three dimensional; CMR = cardiac magnetic resonance; EDV = end diastolic volume; EF = ejection fraction; ESV = end systolic volume; SV = stroke volume)
When 2D and 3D echocardiography derived measurements were compared, they correlated strongly across all parameters when measured by the same operator in the same session, with all r-values above 0.7, p < 0.001 (Fig. 4).
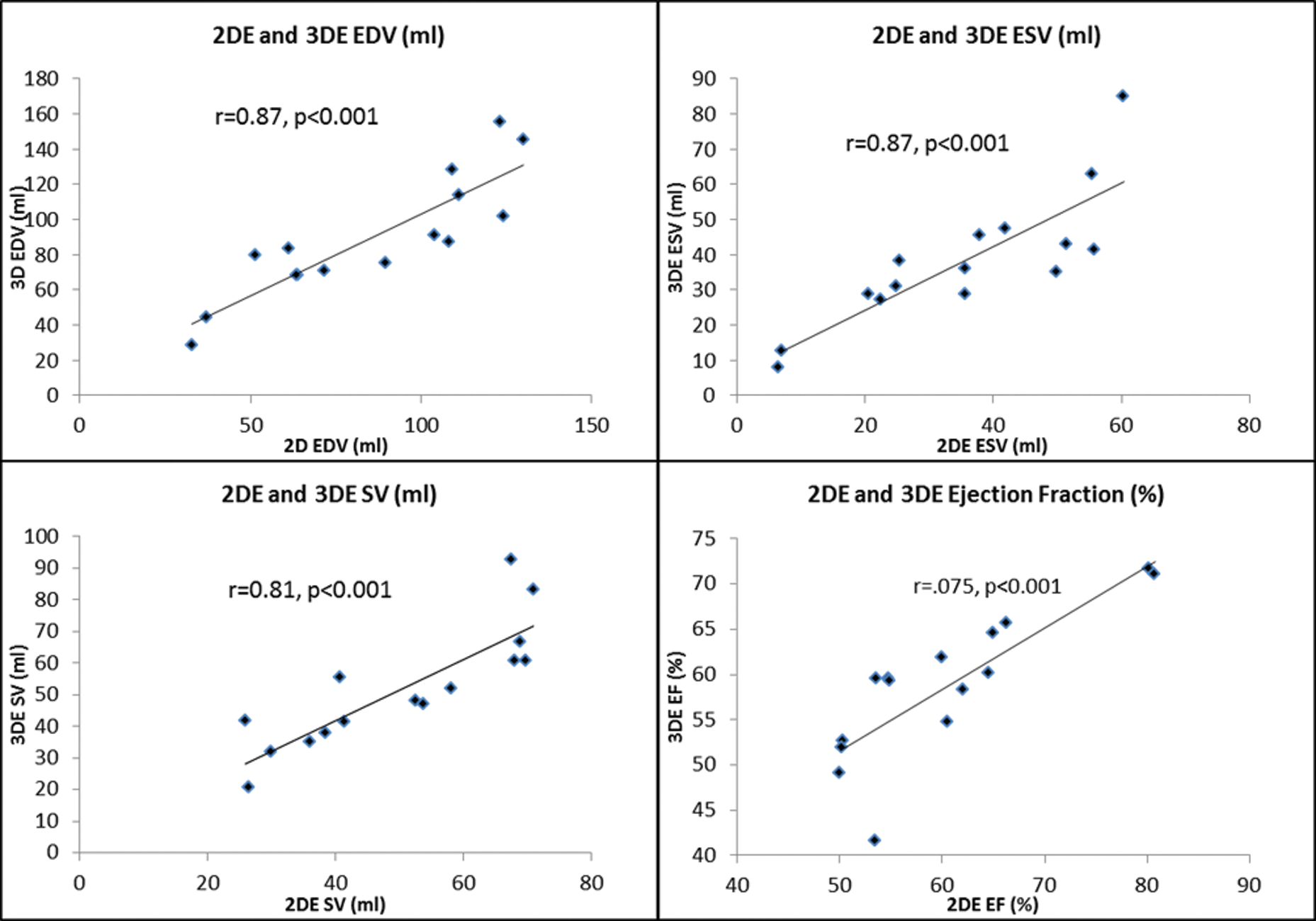
Figure 4: Scatterplots showing the correlation between parameters of 2DE EDV, ESV, EF and SV with 3DE. (Abbreviations: 2D = two dimensional; 3D = three dimensional; EDV = end diastolic volume; EF = ejection fraction; ESV = end systolic volume; SV = stroke volume)
3.3 Inter-Observer Reproducibility
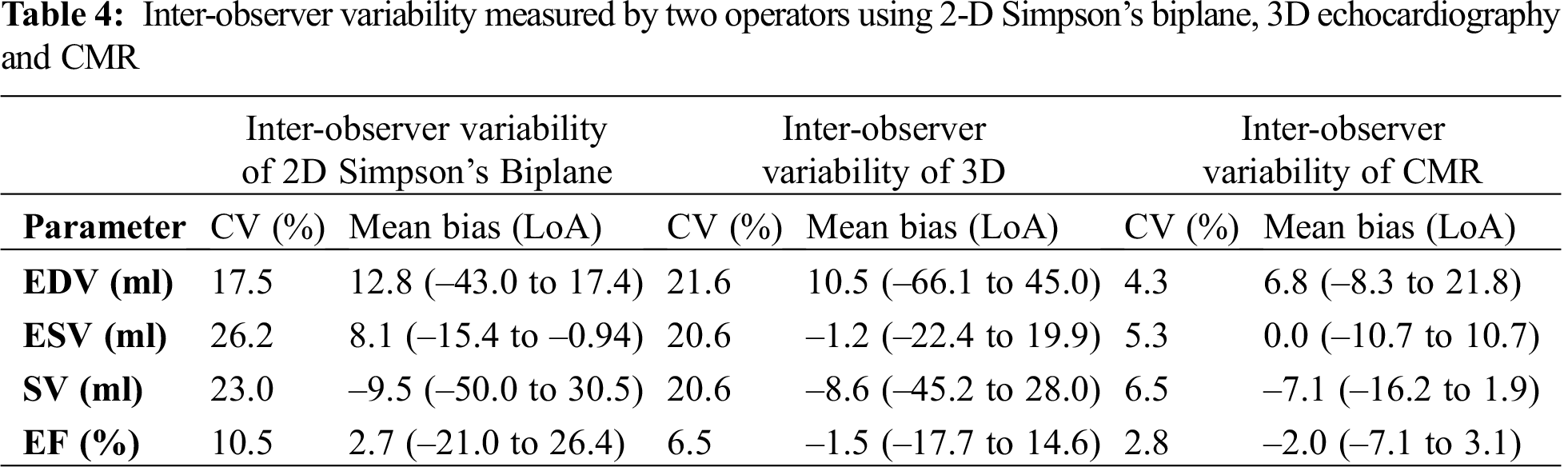
Inter observer variability for echocardiographic measurements were assessed in five patients (Tab. 4). The bias was smaller for 3D between observers across all parameters when compared to 2D Simpson’s biplane method. However, both modalities had wide limits of agreement. Inter-observer reproducibility of CMR was carried out in 7 of the patients with a different observer of equal experience in CMR, re-measured the volumes to obtain EDV, ESV, EF and SV. There was excellent correlation between observers for all parameters (Tab. 4). The limits of agreement between the operators were generally narrow, however, limits of agreement for EDV were shown to be wider (–21.5 to 8.0) (Tab. 4).
This is the first study to our knowledge, to determine the feasibility and accuracy of 3D volumes specifically in adult Fontan patients. 3D volumes could be obtained in the majority of adult Fontan patients with either systemic right or left ventricles; 3D correlated well with CMR but there was a systematic underestimation of volumes. In 69% (n = 18) of the recruited patients it was possible to obtain accurate 3D volumes, which suggests that in the majority of adult Fontan patients 3D echocardiography can be accurately performed in clinical practice.
There is a trend for both 2D and 3D echocardiographic methods to underestimate univentricular volumes when compared to volumes derived by CMR, which is accentuated as the ventricle dilates. This has been previously demonstrated in studies comparing the anatomical left ventricle with CMR, showing a systematic under estimation of 3D obtained volumes when compared to CMR [15]. One of the major contributions to this trend is the superior spatial resolution of CMR, allowing better delineation between the LV cavity and endocardial borders. Therefore, with echocardiography, features such as trabeculae and papillary muscles are often mistaken for the endocardial border, resulting in an under estimation in volume [11]. The Fontan ventricle is also often distorted in shape; the 2D Simpson’s biplane method assumes that the ventricle is cylindrical and so will either under or over-estimate the size. When measuring the 3D volume in larger ventricles, a bigger sector width is required to accommodate the entire ventricle; as a result frame rate reduces and so the resolution of the image will decline, reducing endocardial definition and this may contribute to the greater under-estimation of volumes with larger ventricles [6,16].
3D echocardiography is not routinely used on adult Fontan patients [17]. Volumes and ejection fraction derived by 3D volumes closely correlated with CMR suggesting that there is reliability between measurements, despite a difference in volumes obtained. Therefore, 3D echocardiography could provide an alternative method to CMR for the serial assessment of the size and function of the single ventricle. In a study of paediatric Fontan patients, it has been shown similar to this study that 2D and 3D volumes correlate strongly with each other, in addition to 3D-derived speckle tracking analysis suggesting 3D derived ejection fraction reflects cardiac mechanics as well [18]. CMR is only available in specialised centres and some patients are unable to undergo CMR due to claustrophobia or are contraindicated. In this study alone 32 out of the 151 patients screened had a pacemaker. Although the implantation of MR-compatible and incompatible intra-cardiac devices may increasingly be seen as no longer an absolute contra-indication, the presence of such devices not only complicates the procedure but dramatically reduces the quality of the images that may be acquired. One limitation of this study was that contrast was not used in those with poor quality imaging and this offers a further opportunity to improve applicability [19].
There was a stronger agreement for 3D volumes measured by different observers, than for volumes measured by 2D Simpson’s biplane. The 3D volume method is semi-automated and so there is less user dependence, when compared to the Simpson’s biplane method. The Simpson’s biplane method will have a varied volume depending on how the operator delineates the endocardial border [20]. The use of LVO contrast has been shown to increase the reproducibility between observers, as the endocardial border is more clearly seen [21]. In contrast to the significant inter-observer variability of echocardiography, CMR was shown to have superior reproducibility. This again was most likely due to superior image quality, therefore differences between operators would have resulted from different interpretation of where to trace the endocardial boarder [22].
Previous studies in non-congenitally abnormal left ventricles have shown that the use of LVO contrast in patients with suboptimal acoustic windows, can improve endocardial definition and hence increase the number of patients in which 3D echocardiography can be performed to assess LV volumes and ejection fraction [23–25]. If the use of LVO contrast had been applied in this study, it is likely that both the feasibility and inter-observer reproducibility would improve.
The main limitation is the small sample size, which is expected in such a rare patient population, however this is the first study of 3DE conducted in adult Fontan patients. There was also a proportion of patients who were excluded on the basis of having poor quality echocardiographic images and of the patients in which 3D assessment could be performed, not all had image quality categorised as “good” which may have affected the accuracy of 3D acquisition. A future study involving the use of contrast echocardiography will be required to further examine the utility of 3D echocardiography in the adult Fontan patient, to ensure clearly delineated endocardial boarders for a more accurate 3D assessment. In this study we excluded patients with an arrhythmia to exclude variability caused by a change in haemodynamics. In future studies patients with atrial fibrillation could be included and a single index beat method could be used to prevent stitching artefact [26].
We have shown that 3D echocardiography is a feasible and accurate method of serially assessing univentricular function in adult Fontan patients. It cannot be used interchangeably with CMR due to its systematic underestimation of volumes. However, it correlates more strongly with CMR compared to 2D Simpson’s biplane and so could provide a more accurate assessment of cardiac function, in Fontan patients unsuitable or intolerant to CMR. Whilst this study was performed in adult Fontan patients, this could be a very useful way of monitoring performance in younger children, where endocardial border definition is better than in the adult patient and for whom CMR may require general anaesthesia.
Acknowledgement: We are grateful for the patients who took the time to take part in the study and for the echocardiography and CMR department at the Queen Elizabeth Hospital who supported the facilitation of this study. We are also grateful for Dr Peter Nightingale’s advice on the statistics used in this study.
Author Contributions: KVB was responsible for the co-ordination of the study, recruitment and consenting of patients, collection and analysis of data and leading the production of the manuscript. JG performed all echocardiograms and performed the 3D and 2D Simpson’s biplane measurements and advised on the manuscript. FF analysed the CMR data and advised on the manuscript. RPS and LH advised on the study and provided expert advice on the manuscript. PC was the chief investigator for this study, achieved ethical approval, provided supervision of this study and was the senior author for this manuscript.
Ethical Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards (REC: 15/WS/0046).
Funding Statement: No specific funding used. KB was funded through the National Institute of Health Research (NIHR) fellowship as a Research Assistant and PhD student. The opinions expressed in this paper are those of the authors and do not represent the NIHR or the UK Department of Health.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Clift, P., Celermajer, D. (2016). Managing adult Fontan patients: Where do we stand? An Official Journal of the European Respiratory Society, 25(142), 438–450. [Google Scholar]
2. Gewillig, M. (2005). The Fontan circulation. Heart, 91(6), 839–846. [Google Scholar]
3. Gersony, W. M. (2008). Fontan operation after 3 decades: What we have learned. Circulation, 117(1), 13–15. [Google Scholar]
4. Piran, S., Veldtman, G., Siu, S., Webb, G. D., Liu, P. P. (2002). Heart failure and ventricular dysfunction in patients with single or systemic right ventricles. Circulation, 105(10), 1189–1194. [Google Scholar]
5. Mehrotra, R., Alagesan, R., Srivastava, S. (2013). Quantitative assessment of left ventricular systolic function using 3-dimensional echocardiography. Indian Heart Journal, 65(5), 620–628. [Google Scholar]
6. Margossian, R., Schwartz, M. L., Prakash, A., Wruck, L., Colan, S. D. et al. (2009). Comparison of echocardiographic and cardiac magnetic resonance imaging measurements of functional single ventricular volumes, mass, and ejection fraction (from the Pediatric Heart Network Fontan Cross-Sectional Study). The American Journal of Cardiology, 104(3), 419–428. [Google Scholar]
7. Gatzoulis, M. A., Swan, L., Therrien, J., Pantely, G. A., Braunwald, E. (2005). Adult congenital heart disease—a practical guide. Malden, MA, USA: Blackwell Publishing Ltd. [Google Scholar]
8. Lang, R. M., Badano, L. P., Tsang, W., Adams, D. H., Agricola, E. et al. (2012). EAE/ASE recommendations for image acquisition and display using three-dimensional echocardiography. European Heart Journal Cardiovascular Imaging, 13(1), 1–46. [Google Scholar]
9. McRae, M. E. (2013). Long-term issues after the Fontan procedure. AACN Advanced Critical Care, 24(3), 264–282. [Google Scholar]
10. Badano, L. P., Boccalini, F., Muraru, D., Bianco, L. D., Peluso, D. et al. (2012). Current clinical applications of transthoracic three-dimensional echocardiography. Journal of Cardiovascular Ultrasound, 20(1), 1–22. [Google Scholar]
11. Mor-Avi, V., Jenkins, C., Kuhl, H. P., Nesser, H. J., Marwick, T. et al. (2008). Real-time 3-dimensional echocardiographic quantification of left ventricular volumes: Multicenter study for validation with magnetic resonance imaging and investigation of sources of error. JACC Cardiovascular Imaging, 1(4), 413–423. [Google Scholar]
12. Dorosz, J. L., Lezotte, D. C., Weitzenkamp, D. A., Allen, L. A., Salcedo, E. E. (2012). Performance of 3-dimensional echocardiography in measuring left ventricular volumes and ejection fraction: A systematic review and meta-analysis. Journal of the American College of Cardiology, 59(20), 1799–1808. [Google Scholar]
13. Walker, F., Siu, S. C., Woods, S., Cameron, D. A., Webb, G. D. et al. (2004). Long-term outcomes of cardiac pacing in adults with congenital heart disease. Journal of the American College of Cardiology, 43(10), 1894–1901. [Google Scholar]
14. Rathod, R. H., Prakash, A., Kim, Y. Y., Germanakis, I. E., Powell, A. J. et al. (2014). Cardiac magnetic resonance parameters predict transplantation-free survival in patients with fontan circulation. Circulation Cardiovascular Imaging, 7(3), 502–509. [Google Scholar]
15. Gardner, B. I., Bingham, S. E., Allen, M. R., Blatter, D. D., Anderson, J. L. (2009). Cardiac magnetic resonance vs. transthoracic echocardiography for the assessment of cardiac volumes and regional function after myocardial infarction: An intrasubject comparison using simultaneous intrasubject recordings. Cardiovascular Ultrasound, 7, 38. [Google Scholar]
16. Hung, J., Lang, R., Flachskampf, F., Shernan, S. K., McCulloch, M. L. et al. (2007). 3D echocardiography: A review of the current status and future directions. Journal of the American Society of Echocardiography, 20(3), 213–233. [Google Scholar]
17. Huntgeburth, M., Germund, I., Geerdink, L. M., Sreeram, N., Udink Ten Cate, F. E. A. (2019). Emerging clinical applications of strain imaging and three-dimensional echocardiography for the assessment of ventricular function in adult congenital heart disease. Cardiovascular Diagnosis and Therapy, 9(Suppl2), 326–345. [Google Scholar]
18. Sato, T., Calderon, R. J., Klas, B., Pedrizzetti, G., Banerjee, A. (2020). Simultaneous volumetric and functional assessment of the right ventricle in hypoplastic left heart syndrome after Fontan palliation, utilizing 3-dimensional speckle-tracking echocardiography. Circulation Journal: Official Journal of the Japanese Circulation Society, 84(2), 235–244. [Google Scholar]
19. Mitchell, F. M., Prasad, S. K., Greil, G. F., Drivas, P., Vassiliou, V. S. et al. (2016). Cardiovascular magnetic resonance: Diagnostic utility and specific considerations in the pediatric population. World Journal of Clinical Pediatrics, 5(1), 1–15. [Google Scholar]
20. Cowie, B., Kluger, R., Kalpokas, M. (2013). Left ventricular volume and ejection fraction assessment with transoesophageal echocardiography: 2D vs. 3D imaging. British Journal of Anaesthesia, 110(2), 201–206. [Google Scholar]
21. Malm, S., Frigstad, S., Sagberg, E., Larsson, H., Skjaerpe, T. (2004). Accurate and reproducible measurement of left ventricular volume and ejection fraction by contrast echocardiography: A comparison with magnetic resonance imaging. Journal of the American College of Cardiology, 44(5), 1030–1035. [Google Scholar]
22. Luijnenburg, S. E., Robbers-Visser, D., Moelker, A., Vliegen, H. W., Mulder, B. J. M. et al. (2010). Intra-observer and interobserver variability of biventricular function, volumes and mass in patients with congenital heart disease measured by CMR imaging. The International Journal of Cardiovascular Imaging, 26(1), 57–64. [Google Scholar]
23. Hoffmann, R., Barletta, G., von Bardeleben, S.,Vanoverschelde, J. L., Kasprzak, J. et al. (2014). Analysis of left ventricular volumes and function: A multicenter comparison of cardiac magnetic resonance imaging, cine ventriculography, and unenhanced and contrast-enhanced two-dimensional and three-dimensional echocardiography. Journal of the American Society of Echocardiography, 27(3), 292–301. [Google Scholar]
24. Monaghan, M. J. (2006). Role of real time 3D echocardiography in evaluating the left ventricle. Heart, 92(1), 131–136. [Google Scholar]
25. Nosir, Y. F., Fioretti, P. M., Vletter, W. B., Boersma, E., Salustri, A. et al. (1996). Accurate measurement of left ventricular ejection fraction by three-dimensional echocardiography. A comparison with radionuclide angiography. Circulation, 94(3), 460–466. [Google Scholar]
26. Thavendiranathan, P., Liu, S., Verhaert, D., Calleja, A., Nitinunu, A. et al. (2012). Feasibility, accuracy, and reproducibility of real-time full-volume 3D transthoracic echocardiography to measure LV volumes and systolic function: A fully automated endocardial contouring algorithm in sinus rhythm and atrial fibrillation. JACC Cardiovascular Imaging, 5(3), 239–251. [Google Scholar]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |