

 | Congenital Heart Disease |  |
DOI: 10.32604/CHD.2020.011520
ARTICLE
Use of Implantable Cardioverter-Defibrillators in Congenital Heart Disease and Pediatric Patients: Results from the German National Registry for Congenital Heart Defects
1Department of Cardiology III-Adult Congenital and Valvular Heart Disease, University Hospital of Muenster, Muenster, Germany
2Department of Cardiology II-Electrophysiology, University Hospital of Muenster, Muenster, Germany
3National Register for Congenital Heart Defects, Berlin, Germany
4Deutsches Zentrum für Herz-Kreislauf-Forschung, Berlin, Germany
*Corresponding Author: Alicia Jeanette Fischer. Email: fischera@ukmuenster.de
#Shared Authorship
Received: 14 May 2020; Accepted: 22 June 2020
Abstract: Background: Sudden cardiac death is a leading cause of death in patients with congenital heart disease (CHD). Risk stratification for implantable cardioverter defibrillators (ICD) remains difficult due to limited data about use and outcome of device therapy in CHD patients in larger community-based cohorts. Methods and results: Out of a dataset with more than 50,000 patients registered at the German National Register for Congenital Heart Defects, 109 patients (median age 35.5; IQR 23.75–46.00), 68 (62%) male) with an ICD were identified and were retrospectively analyzed. Although the number of implantations increased steadily throughout the investigated time interval from 2001 to 2015, only 0.2% of the CHD patients in the national register received an ICD. Indication for ICD implantation was secondary prevention in 84 patients (78%) and primary prevention in 24 patients (22%). 23 patients (21%) of the ICD patients received appropriate ICD therapy. 7 patients (6%) received an inappropriate ICD therapy. In 23 patients (21%) device complications were documented with a high number of lead fractures and insulation defects (n = 14, 13%). Conclusion: The current study investigates the clinical uptake and use of ICD therapy based on a large national registry for CHD patients. Despite a steady increase in the number of implanted devices, ICD uptake remains relatively low, particularly for primary prevention. The data suggests a potential reluctance in utilization of device therapy in this patient cohort for primary prevention. Selecting patients in whom benefits outweigh the risks associated with lifelong ICD therapy remains challenging.
Keywords: Adult congenital heart disease; congenital heart disease; mortality; sudden cardiac death; implantable cardioverter defibrillator; device therapy
Globally, there is a steady increase in the prevalence of patients with congenital heart disease (CHD) [1]. More than 90% of children with CHD survive into adulthood in Western countries [2]. The higher life expectancy leads to an increased cumulative risk for complications such as arrhythmia. Malignant ventricular arrhythmias and sudden cardiac death (SCD) represent one of the major causes of death in CHD patients accounting for up to 25% of deaths in this cohort [3,4]. In a representative study on patients with repaired Tetralogy of Fallot (ToF) prevalence of ventricular arrhythmia has been reported to be as high as 14.2% [5]. Risk for SCD increases with complexity of disease [4].
As the cohort of CHD patients is heterogeneous, identifying uniform risk markers for primary prevention of SCD remains a challenge. Current guidelines uniformly recommend ICD implantation in primary prevention based on data extrapolated from patients without congenital heart disease [6–8]. Guidance for risk stratification exists for selected defects and focuses mainly on patients with repaired ToF [9]. Whether and how the identified ToF risk factors/scores can be applied to other congenital heart defects remains a matter of discussion. Despite the perceived benefits of primary prevention, the relevant rate of device-related long-term complications [10] has to be carefully considered when weighing risks and benefits of ICD treatment, particularly as younger age has been identified as an independent risk factor for complications. It is current consensus, that, although the risk score introduced for patients with the most common defect, the ToF, by Khairy et al. [9] facilitates identification of high-risk patients, the actual ICD indication has to be made on the basis of individual clinical judgment [11]. While various small retrospective analyses mainly based on single-center data have been published, the uptake and indications for ICD therapy remain unclear. Currently, there are aims to gather prospective data in a single center setting to implement a uniform risk score for patients with CHD [12].
The aim of our retrospective analysis is to identify indications for ICD implantation, incidence and type of related complications and appropriate therapies as a surrogate for benefit from ICD therapy in a nationwide dataset from the German National Register for Congenital Heart Defects (NRCHD).
The dataset of the NRCHD has been described in detail previously and has been proven to be comparable to the general prevalence of CHD in Germany with a slight overrepresentation of complex defects [13]. The dataset includes demographic and comprehensive medical data of more than 50,000 patients with CHD or acquired pediatric heart disease such as valvular heart disease. All patients in the NRCHD were systematically screened to identify individuals who received an ICD. Identification of ICD patients was based on the International Pediatric and Congenital Cardiac Code published by the International Society for Nomenclature of Pediatric and Congenital Heart Disease (http://www.ipccc.net) codes for ICD (12.42.31, 12.42.33, 12.42.35, 12.42.39, 12.42.61, 12.42.64 or 12.42.65) [14]). When electronically identified as ICD recipient, data were examined manually, and all pertinent demographic and clinical details were collected. Complexity of CHD was classified according to the 32nd Bethesda conference classification [15]. We analyzed the selected patients concerning patient demographics, type of congenital defect as well as repair, associated conditions, indication for ICD and heart failure medication. The patients provided written informed consent for inclusion in the National Register.
The descriptive analysis is presented as medians with interquartile ranges (IQR; 25th and 75th percentile). Standard methods of descriptive statistics were used. Comparisons between groups were performed using Chi-square test. Statistical analysis was performed with MedCalc 10.1.2.0 (MedCalc Software, Mariakerke, Belgium) and GraphPad Prism 7.0 software. For all analyses, a two-tailed probability value P < 0.05 was considered as statistically significant.
Between 2001 and 2015, more than 50,000 patients were registered in the NRCHD. Of those, ICD therapy had been implemented in 109 patients (0.2% of the entire cohort). 13 patients were < 18 years of age. The median age of ICD patients was 35.5 years (IQR 23.74–46.00) with a slight dominance of male patients (n = 68; 62%). As displayed in (Tab. 1), the analyzed cohort was heterogeneous with regard to the baseline characteristics.
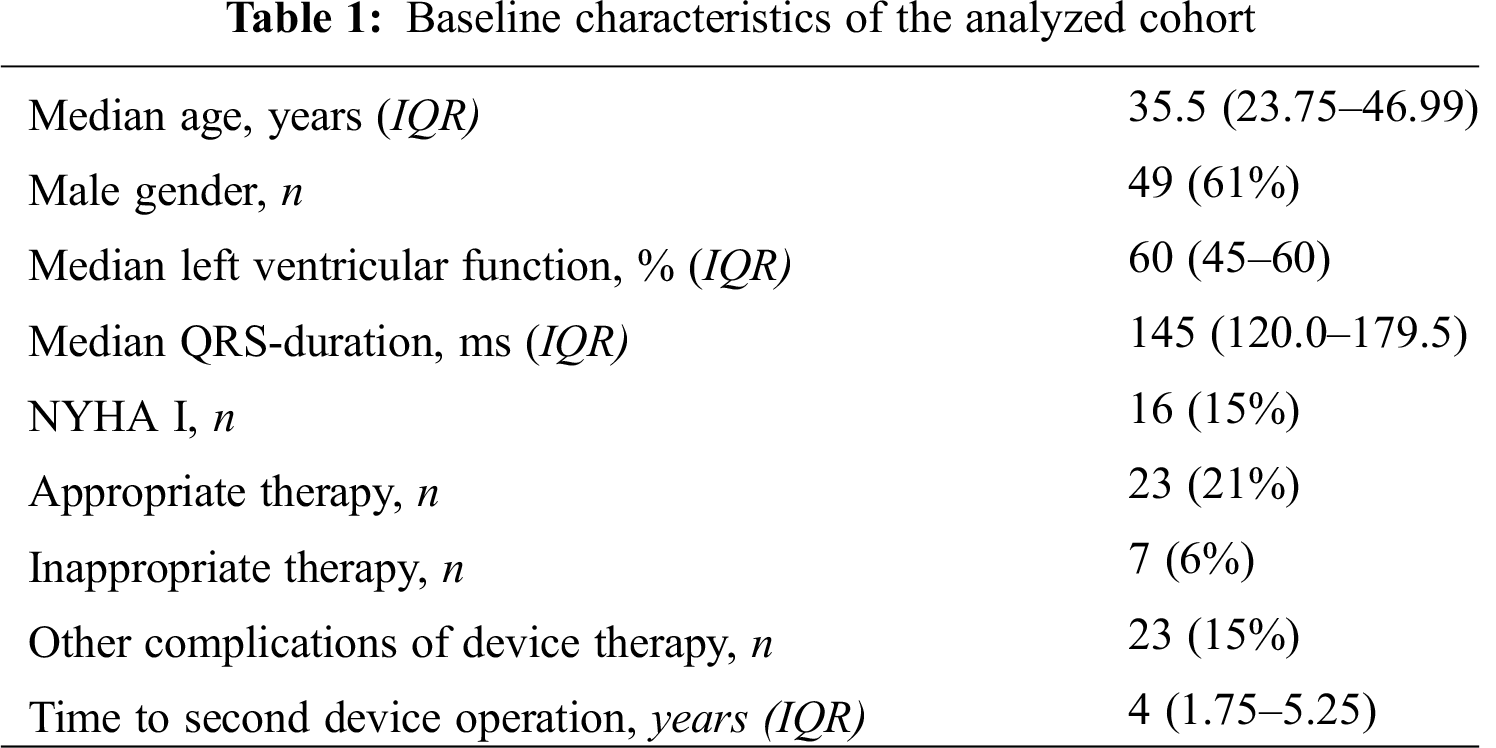
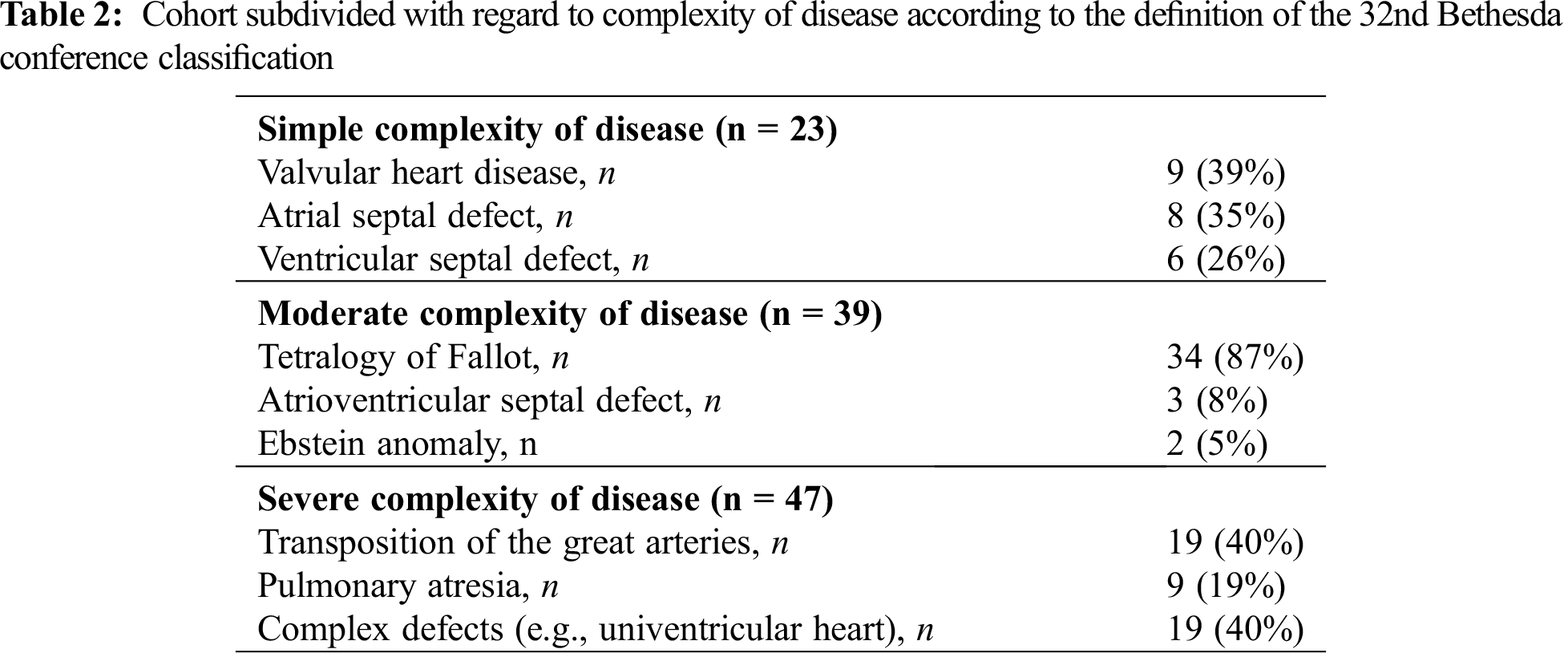
Systemic ventricular systolic function pre-ICD implantation was documented in 61 patients (60%). Ranges and cutoff values of ventricular systolic function were defined according to current recommendations [16]. Overall, systolic ventricular function was most often preserved (n = 31; 51%), mildly reduced in 12 patients (20%), moderately impaired in 10 patients (16%) and severely impaired in 8 patients (13%). The majority pf patients presented with a symptomatic limitation of physical activity NYHA II–III (n = 55, 72%). Across the spectrum of disease, the median QRS duration was 145 ms (IQR 120.0–179.5) in our study. Heart failure medication was not universally prescribed. Overall, 37 patients (34%) did not receive any heart failure drug, while 72 patients (66%) had a prescription for at least one heart failure drug. Only few patients (n = 9, 10%) received antiarrhythmic drug treatment. Amiodarone was prescribed in 7 of these 9 patients while two patients received sotalol and one patient flecainide. (Tab. 2) displays the distribution of congenital heart defect according to their complexity.
The most common indication for ICD implantation was secondary prevention (n = 84, 78%). Ventricular tachycardia was most often the reason for secondary prevention (n = 53 (65%)). In 21 patients (26%), the indication was ventricular fibrillation. Primary prevention was assumed if the clinical criteria met the recommendations of current guidelines and no malignant arrhythmia had been documented [6–8]. In 24 patients (22%), indication for ICD implantation was primary prevention. For one patient the available information was inconclusive. The distribution of primary and secondary prevention subdivided regarding the underlying congenital heart defect is displayed in (Fig. 1).
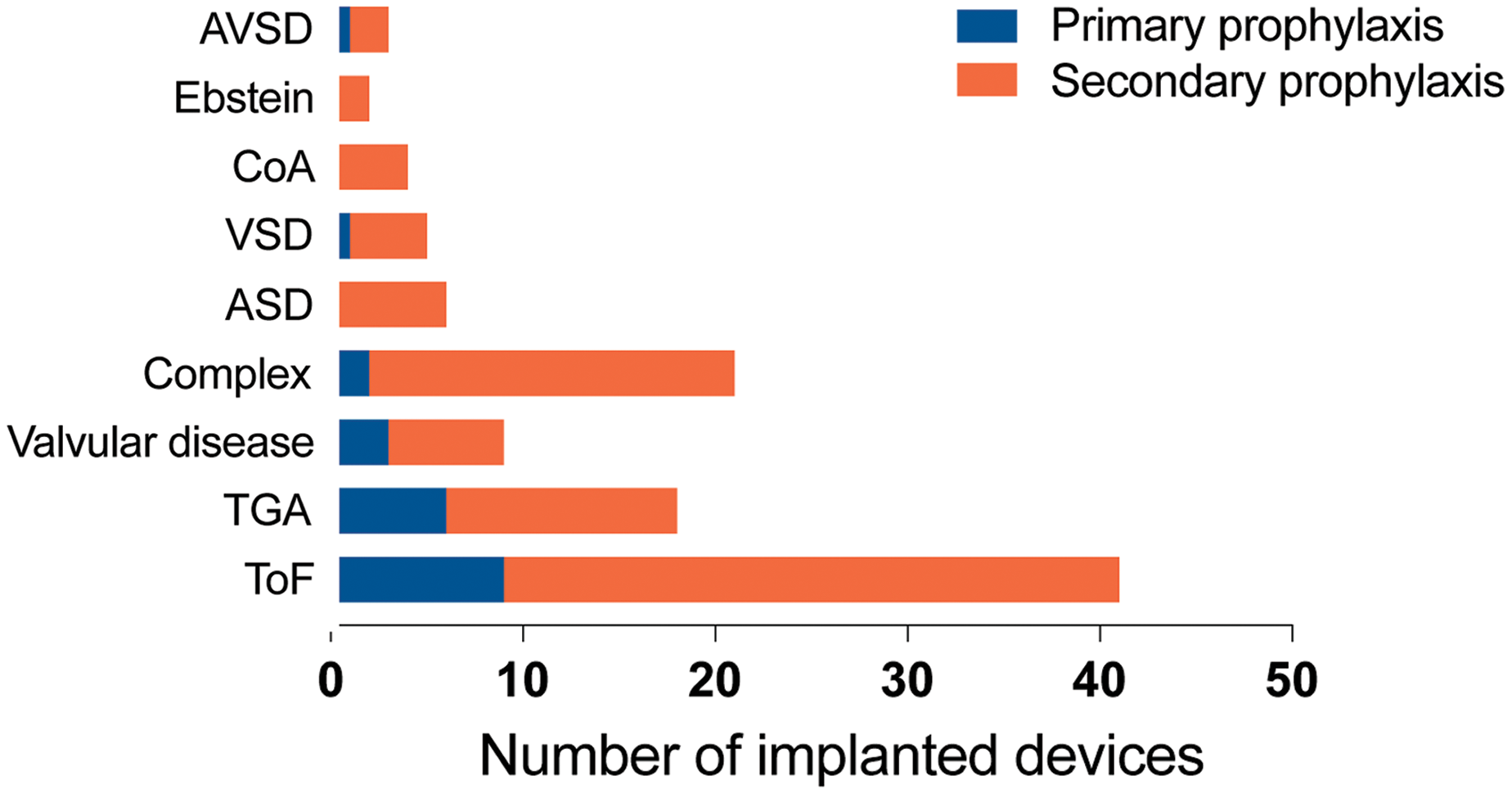
Figure 1: ICD carriers listed depending on underlying congenital heart defect. (ToF = Tetralogy of Fallot, TGA = Transposition of the great arteries, ASD = Atrial septal defect, VSD = Ventricular septal defect, CoA = Coarctation of the aorta, AVSD = Atrioventricular septal defect)
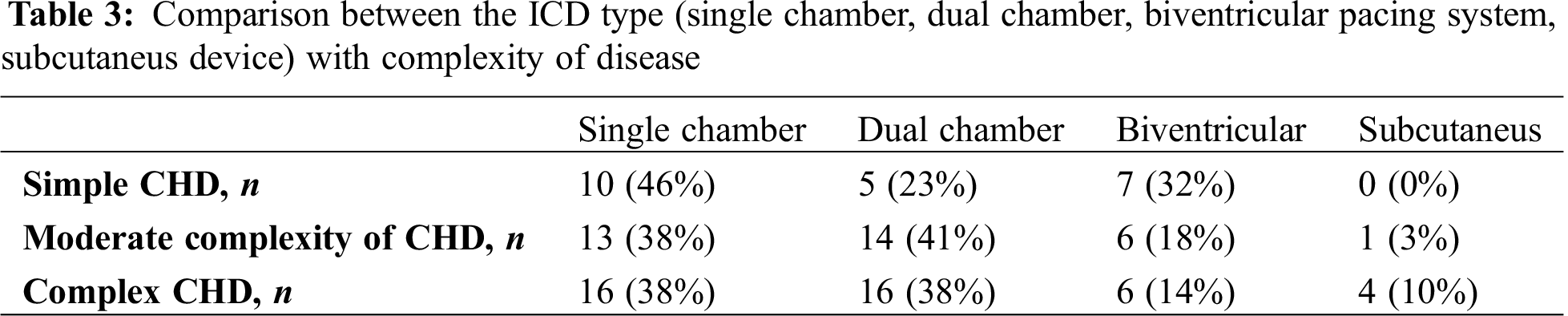
Overall, 23 ICD patients in the analyzed cohort (21%) had a simple heart defect. Of these, 4 patients (17%) had received the ICD for primary prevention. Thirty-nine patients (36%) presented with congenital heart defect of moderate complexity. Eleven patients (28%) in this cohort had received the ICD for primary prevention. Forty-seven patients (43%) with an ICD were identified as patients with a defect of severe complexity and primary prevention was the indication in eight (17%) of these patients. The relative proportion of patients with primary prevention was highest in patients with moderate disease complexity, although the difference did not reach statistical significance (p = 0.40). The majority of patients (n = 39, 36%) received a single chamber ICD, while 35 of patients (32%) underwent implantation of a dual chamber ICD, and 19 patients (17%) were provided with a device with adjunctive cardiac resynchronization therapy (CRT). Five patients (5%) received an entirely subcutaneous ICD. In 11 patients (10%), prior pacemaker therapy was documented before upgrading the device for ICD therapy. The difference in ICD type between the complexity groups was not statistically significant (p = 0.32). It is displayed in Tab. 3.
The annual number of device implantation increased gradually throughout the analyzed time interval as shown in (Fig. 2).
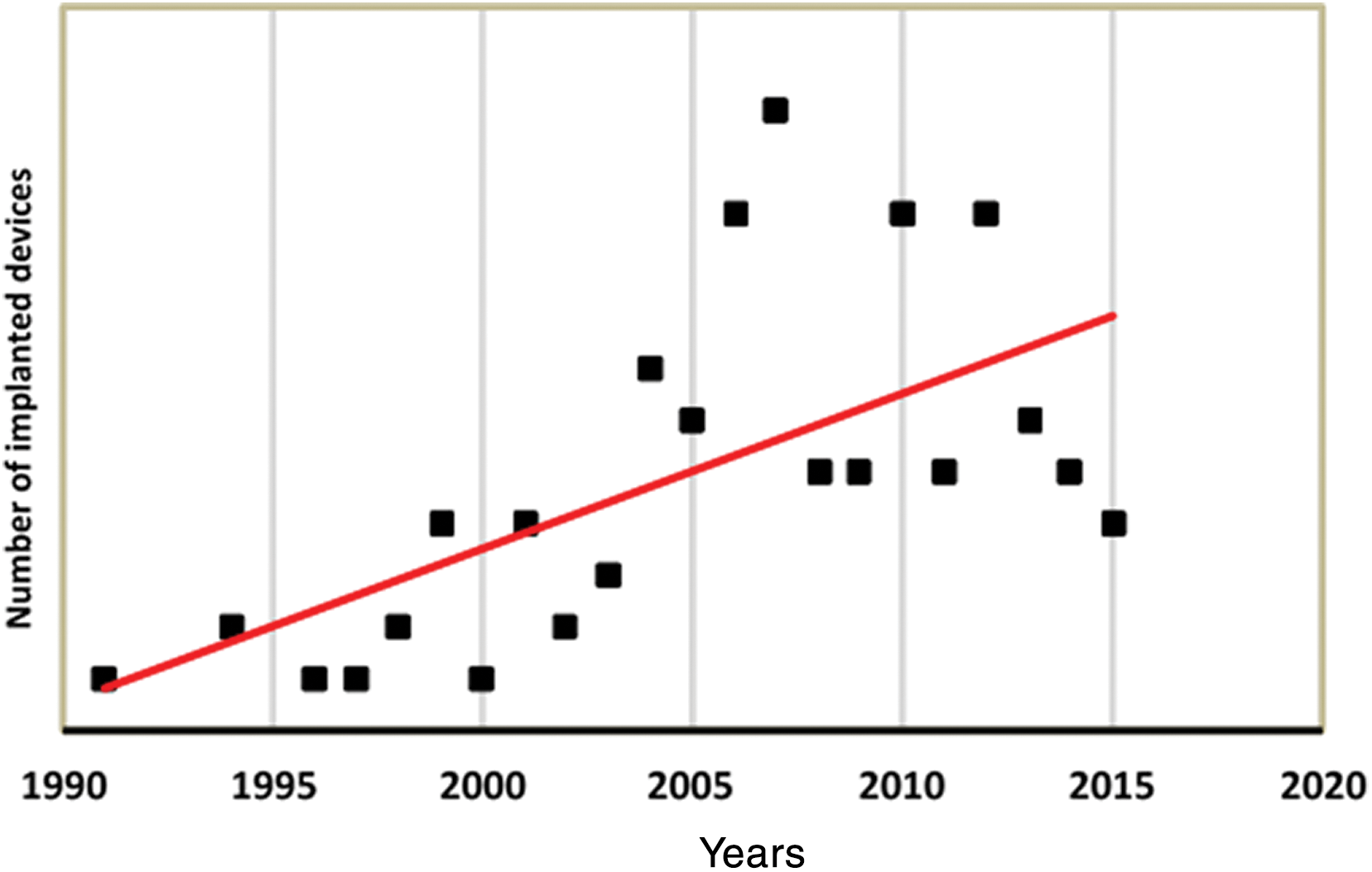
Figure 2: Number of ICD implantations during the investigated time interval
The median length of follow-up of the patients with CHD that received an ICD was 2.5 years (IQR 1–7.25). After a median time of 19.5 months (IQR 8–65), 23 patients (21%) experienced appropriate therapy. Of these, 11 patients (48%) received one ICD therapy, whereas in 12 patients (52%) more than one therapy had been applied with a cumulative number of 45 delivered shocks. Overall, 7 patients (6%), that were provided with an ICD experienced inappropriate therapy. The reason was lead dysfunction in two patients (29%). In 3 patients (43%), an atrial tachycardia was mistakenly diagnosed as a malignant arrhythmia by the device. In two patients (29%) the reason for inappropriate therapy has not been documented. Of the group of patients with inappropriate therapy, one patient had received the ICD for primary prevention (14% of all inappropriate therapies). In the analyzed cohort there was a small number of CHD patients with simple defects who received appropriate therapy (n = 3, 13%). The distribution of appropriate ICD therapies depending on complexity of disease and indication is displayed in (Fig. 3).
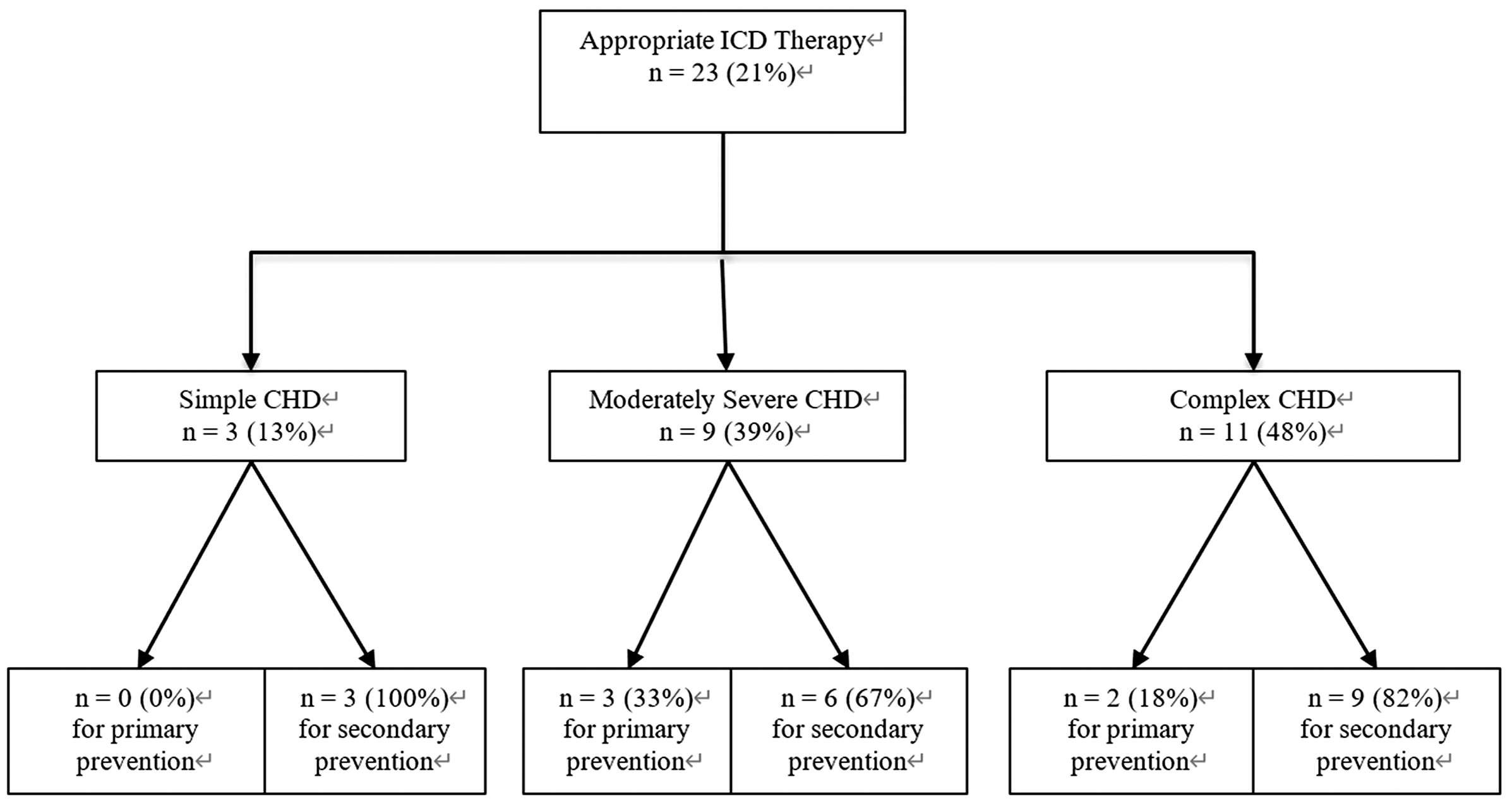
Figure 3: Distribution of appropriate ICD therapies depending on complexity of disease and indication
In 23 patients (15%), a device related complication was documented. The most frequent complication was lead dysfunction (n = 11, 48%). Infection was documented in 2 patients (9%). Overall, 30 patients required revision of the ICD system for battery depletion or system-related complications. In these patients the median time to second operation was 4 years (IQR 1.75–5.25). Mortality in the analyzed patient cohort was low as displayed in (Fig. 4). Twelve ICD patients died during follow-up at a median age of 46 years (IQR 38.75–52.75). In none of these patients, however, there was a definitive documented causal relationship between death and a complication or malfunctioning of the ICD device. When documented, cause of death was septic shock (n = 3), cancer (n = 1) and incessant VT (n = 1).
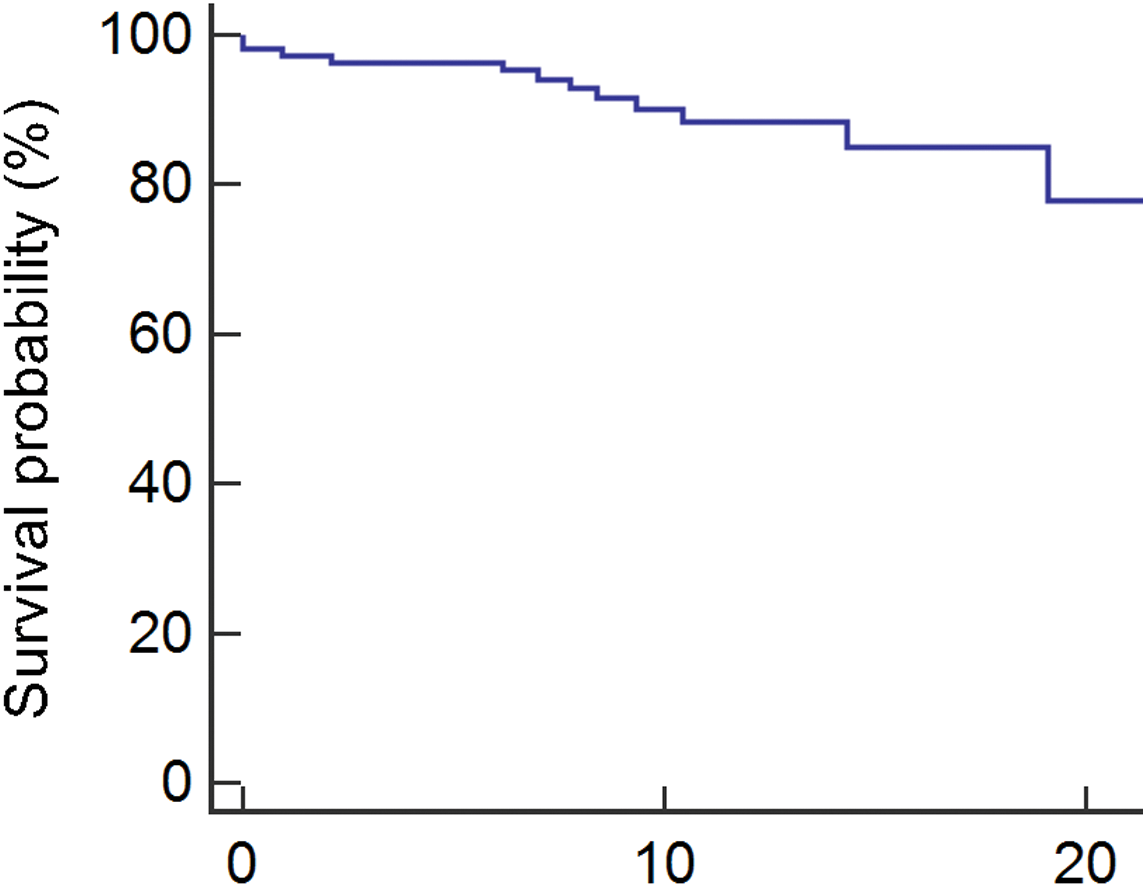
Figure 4: Kaplan-Meyer survival analysis of the analyzed patient cohort (n = 109)
The current study based on a large national register for congenital heart disease shows that ICD therapy is feasible in selected patients presenting with CHD. Implantation rates increased over the investigated time, although uptake of ICD therapy was still surprisingly low with only 0.2% of patients receiving an ICD. In the majority of patients, ICD therapy was implemented for secondary prevention, arguably the setting where indication of ICD implantation is most strongly supported by current guidelines [6–8]. Only 22% of the analyzed patients received an ICD for primary prevention. Although speculative, the reason for the relatively higher use of ICDs for primary prevention in moderate complexity, especially ToF patients is likely to be the availability of a dedicated risk score, published by Khairy et al. [11]. This scoring system facilitates decision making in clinical practice and may lead to a lower threshold for ICD implantation as primary prevention. Overall, the low implantation rate for primary prevention across the spectrum of disease, however, suggests a general reluctance of ICD therapy due to a lack of evidence-based data. Only a minority of patients in our study was treated with antiarrhythmic drugs despite the relatively high reported prevalence of arrhythmias in CHD leading to hospital admission [17]. When deciding for ICD therapy as primary prevention it is current consensus that ICD therapy is recommended in adults with CHD and a systemic LVEF ≤ 35%, biventricular physiology and NYHA Functional Class II or III [6–8,18]. This recommendation is adopted from the conventional criteria for ICD therapy [18]. As seen in the data from this register, only a small number fulfill the conventional criteria with only 13% of the patients suffering from severe impairment of systemic ventricular function. Of those, only one patient had received the ICD for primary prevention. Most patients presented with symptoms of heart failure and 66% received at least one heart failure drug. This illustrates, that life-threatening arrhythmias may appear before the affected patient meets current guidelines and presents with severe impairment of systemic ventricular function. Depending on the complexity of the disease, amount of scarring due to one or more reparative surgeries and altered hemodynamics, the underlying mechanism and risk for occurrence of malignant arrhythmia may vary [19]. Generally, there are data supporting the assumption that the risk of SCD increases with complexity of the disease [20]. In our study, patients with complex defect received ICD therapy more often irrespective of indication. Complex disease includes patients with TGA with a systemic right ventricle. There is data supporting that patients with systemic right ventricle are particularly at risk for malignant arrhythmia [21]. The type of ICD that had been implanted was highly variable throughout the groups of complexity of CHD. The relatively high number of dual-chamber ICDs or biventricular pacing systems suggests that, in addition to prevention of SCD, often there may have been a necessity for concomitant pacing therapy. In the analyzed cohort, 11 patients (10%) were provided with a pacemaker before receiving an upgrade for ICD therapy presumably owed to the high rate of conduction disturbances compared to patients without CHD [6]. Other data based on the NRCHD suggest that the general uptake of resynchronization therapy, however, is low in patients with CHD [22]. In our study, there is a low number of patients with subcutaneous devices. In general, an entirely subcutaneous device appears to be an attractive alternative, particularly in patients without the necessity for pacemaker therapy. In small single- center studies, it has been shown to be a safe and feasible treatment option in patients with CHD [23,24]. The perceived advantages of an entirely subcutaneous device are the reduced risk for endocarditis due to no intracardial lead placement as well as uncomplicated access in case of reoperation. Despite this, in our study, there were only 5% patients provided with a subcutaneous device. Possible reasons may be that ICD therapy had been implemented before subcutaneous devices became widely available on the German market (the first subcutaneous ICD was implanted in Germany in 2010 [23]) and that patients may need adjunctive pacing therapy. In addition, conventional therapy with transvenous or epimyocardial leads offers the possibility of overdrive stimulation of monomorphic ventricular tachycardias that are particularly common in patients with repaired ToF [5,25]. In total, 15% of the analyzed cohort experienced a device-related complication during follow-up. 7 patients experienced inappropriate therapy, whereas the majority was caused by fault detection of atrial tachycardia. It has been shown in other cohorts, that inappropriate therapy is associated with a significantly high mortality stressing the importance to avoid occurrence of these incidents [26]. Other studies show a generally higher complication rate of device therapy in CHD patients compared to other patient cohorts [6]. One possible explanation may be, that there is an additional strain on the leads compared to older, more immobile conventional heart failure patients. The time to reoperation was approximately 4 years. In the analyzed patient group device therapy had been initiated at a mean age of 38 years. Thus, these patients will presumably need many reoperations with the associated risks. This emphasizes the importance of careful risk stratification and choice of the device type.
This is a study based on the German National Register for Congenital Heart Defects. Despite the size of the Register, only 109 patients with an ICD device could be identified, thus limiting the statistical power of analyses. This was a descriptive retrospective study aimed at providing mainly descriptive data rather than establishing risk tools and indications for ICD implantation in CHD patients. In addition, the heterogeneity of the patients included in terms of age and anatomy limits the generalisability of our results.
Although device therapy is implemented increasingly in patients with congenital heart disease, there still appears to be reluctance to initiate ICD therapy for primary prevention most likely due to uncertainty about reliable and uniform risk factors. Patients presenting with congenital heart disease pose a specific therapeutic dilemma as they are particularly prone to life-threatening arrhythmia but implantable cardioverter defibrillator therapy in these young patients is associated with a high complication rate need for reoperations and considerable impact on physical and psychological well-being. The results of this study, thus, highlight the need for more prospective data, better risk stratification tools and improvement in device technology to optimize ICD therapy for patients with congenital heart disease.
Funding Statement: This study was supported by a research grant from the EMAH Stiftung Karla Voellm, Krefeld, Germany and it was supported by the Competence Network for Congenital Heart Defects, which has received funding from the Federal Ministry of Education and Research, grant number 01GI0601 (until 2014), and the DZHK (German Center for Cardiovascular Research; as of 2015).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Liu, Y., Chen, S., Zuhlke, L., Black, G. C., Choy, M. K. et al. (2019). Global birth prevalence of congenital heart defects 1970-2017: Updated systematic review and meta-analysis of 260 studies. International Journal of Epidemiology, 48(2), 455–463. [Google Scholar]
2. Moons, P., Bovijn, L., Budts, W., Belmans, A., Gewillig, M. (2010). Temporal trends in survival to adulthood among patients born with congenital heart disease from 1970 to 1992 in Belgium. Circulation, 122(22), 2264–2272. [Google Scholar]
3. Nieminen, H. P., Jokinen, E. V., Sairanen, H. I. (2007). Causes of late deaths after pediatric cardiac surgery: A population-based study. Journal of the American College of Cardiology, 50(13), 1263–1271. [Google Scholar]
4. Oechslin, E. N., Harrison, D. A., Connelly, M. S., Webb, G. D., Siu, S. C. (2000). Mode of death in adults with congenital heart disease. American Journal of Cardiology, 86(10), 1111–1116. [Google Scholar]
5. Khairy, P., Aboulhosn, J., Gurvitz, M. Z., Opotowsky, A. R., Mongeon, F. P. et al. (2010). Arrhythmia burden in adults with surgically repaired tetralogy of Fallot: A multi-institutional study. Circulation, 122(9), 868–875. [Google Scholar]
6. Hernández-Madrid, A., Paul, T., Abrams, D., Aziz, P. F., Blom, N. A. et al. (2018). Arrhythmias in congenital heart disease: A position paper of the European Heart Rhythm Association (EHRAAssociation for European Paediatric and Congenital Cardiology (AEPCand the European Society of Cardiology (ESC) Working Group on Grown-up Congenital heart disease, endorsed by HRS, PACES, APHRS, and SOLAECE. EP Europace, 20(11), 1719–1753. [Google Scholar]
7. Priori, S. G., Blomström-Lundqvist, C., Mazzanti, A., Blom, N., Borggrefe, M. et al. (2015). 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European society of cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). European Heart Journal, 36(41), 2793–2867. [Google Scholar]
8. Khairy, P., Van Hare, G. F., Balaji, S., Berul, C. I., Cecchin, F. et al. (2014). PACES/HRS expert consensus statement on the recognition and management of arrhythmias in adult congenital heart disease: developed in partnership between the pediatric and congenital electrophysiology society (PACES) and the heart rhythm society (HRS). Endorsed by the governing bodies of PACES, HRS, the American College of Cardiology (ACCthe American Heart Association (AHAthe European Heart Rhythm Association (EHRAthe Canadian Heart Rhythm Society (CHRSand the International Society for Adult Congenital Heart Disease (ISACHD). Heart Rhythm, 11(10), 102–165. [Google Scholar]
9. Khairy, P., Harris, L., Landzberg, M. J., Viswanathan, S., Barlow, A. et al. (2008). Implantable cardioverter-defibrillators in tetralogy of Fallot. Circulation, 117(3), 363–370. [Google Scholar]
10. Ranasinghe, I., Parzynski, C. S., Freeman, J. V., Dreyer, R. P., Ross, J. S. et al. (2016). Long-term risk for device-related complications and reoperations after implantable cardioverter-defibrillator implantation: An observational cohort study. Annals of Internal Medicine, 165(1), 20–29. [Google Scholar]
11. Khairy, P., Dore, A., Poirier, N., Marcotte, F., Ibrahim, R. et al. (2009). Risk stratification in surgically repaired tetralogy of Fallot. Expert Review of Cardiovascular Therapy, 7(7), 755–762. [Google Scholar]
12. Vehmeijer, J. T., Koyak, Z., Zwinderman, A. H., Harris, L., Peinado, R. et al. (2019). Prevention-achd: prospective study on implantable cardioverter-defibrillator therapy and sudden cardiac death in adults with congenital heart disease; rationale and design. Netherlands Heart Journal, 27(10), 474–479. [Google Scholar]
13. Helm, P. C., Koerten, M. A., Abdul-Khaliq, H., Baumgartner, H., Kececioglu, D. et al. (2016). Representativeness of the German national register for congenital heart defects: A clinically oriented analysis. Cardiology in The Young, 26(5), 921–926. [Google Scholar]
14. Beland, M. J., Jacobs, J. P., Tchervenkov, C. I., Franklin, R. C., International Working Group for M et al. (2002). Report from the executive of the international working group for mapping and coding of nomenclatures for paediatric and congenital heart disease. Cardiology in The Young, 12(5), 425–430. [Google Scholar]
15. Webb, G. D., Williams, R. G. (2001). 32nd Bethesda conference: Care of the adult with congenital heart disease. Journal of the American College of Cardiology, 37(5), 1162. [Google Scholar]
16. Lang, R. M., Badano, L. P., Mor-Avi, V., Afilalo, J., Armstrong, A. et al. (2015). Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American society of echocardiography and the European association of cardiovascular imaging. Journal of the American Society of Echocardiography: Official Publication of the American Society of Echocardiography, 28(1), 1–39. [Google Scholar]
17. Kaemmerer, H., Bauer, U., Pensl, U., Oechslin, E., Gravenhorst, V. et al. (2008). Management of emergencies in adults with congenital cardiac disease. The American Journal of Cardiology, 101(4), 521–525. [Google Scholar]
18. Priori, S. G., Aliot, E., Blømstrom-Lundqvist, C., Bossaert, L., Breithardt, G. et al. (2002). Task force on sudden cardiac death. European Society of Cardiology. Europace, 4(1), 3–18. [Google Scholar]
19. Khairy, P., Balaji, S. (2009). Cardiac arrhythmias in congenital heart diseases. Indian Pacing Electrophysiol Journal, 9(6), 299–317. [Google Scholar]
20. Koyak, Z., Harris, L., De Groot, J. R., Silversides, C. K., Oechslin, E. N. et al. (2012). Sudden cardiac death in adult congenital heart disease. Circulation, 126(16), 1944–1954. [Google Scholar]
21. Silka, M. J., Hardy, B. G., Menashe, V. D., Morris, C. D. (1998). A population-based prospective evaluation of risk of sudden cardiac death after operation for common congenital heart defects. Journal of the American College of Cardiology, 32(1), 245–251. [Google Scholar]
22. Flügge, A. K., Wasmer, K., Orwat, S., Abdul-Khaliq, H., Helm, P. C. et al. (2018). Cardiac resynchronization therapy in congenital heart disease: Results from the German national register for congenital heart defects. International Journal of Cardiology, 273, 108–111. [Google Scholar]
23. Willy, K., Reinke, F., Bögeholz, N., Köbe, J., Eckardt, L. et al. (2019). The entirely subcutaneous ICDTM system in patients with congenital heart disease: experience from a large single-centre analysis. Europace, 21(10), 1537–1542. [Google Scholar]
24. Moore, J. P., Mondesert, B., Lloyd, M. S., Cook, S. C., Zaidi, A. N. et al. (2016). Clinical experience with the subcutaneous implantable cardioverter-defibrillator in adults with congenital heart disease. Circulation Arrhythmia and Electrophysiology, 9(9), 1–7. [Google Scholar]
25. Yang, J., Brunnquell, M., Liang, J. J., Callans, D. J., Garcia, F. C. et al. (2019). Long term follow-up after ventricular tachycardia ablation in patients with congenital heart disease. Journal of Cardiovascular Electrophysiology, 30(9), 1560–1568. [Google Scholar]
26. Van Rees, J. B., Borleffs, C. J., De Bie, M. K., Stijnen, T., Van Erven, L. et al. (2011). Inappropriate implantable cardioverter-defibrillator shocks: incidence, predictors, and impact on mortality. Journal of the American College of Cardiology, 57(5), 556–562. [Google Scholar]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |