

 | Congenital Heart Disease |  |
DOI: 10.32604/CHD.2020.011771
ARTICLE
Longitudinal Health-Related Quality of Life Assessment in Children with Congenital Heart Disease
1Hospital Materno Infantil Teresa Herrera, Unit Pediatric Cardiology, A Coruña, Spain
2Department of Pediatric Cardiology and Congenital Heart Disease, German Heart Centre Munich, Technical University Munich, Munich, Germany
3Institute of Preventive Pediatrics, Technical University Munich, Munich, Germany
4Complejo Hospitalario Universitario de A Coruña, Clinical Epidemiology and Statistics Unit, A Coruña, Spain
*Corresponding Author: Jan Müller. Email: j.mueller@tum.de
Received: 29 May 2020; Accepted: 10 August 2020
Abstract: Objective: Health-related quality of life (HRQoL) has become an important outcome measure for patients with congenital heart disease (CHD). The aim of this study was to evaluate the natural course of HRQoL from longitudinal assessment in children with CHD. Patients and Methods: From July 2014 to February 2020 this longitudinal study recruited 317 children with CHD (113 girls, 35.6%) aged 6 to 18 years (11.6 ± 2.9 years). HRQoL was assessed with the generic, self-reported and age-adapted KINDL® questionnaire. During a mean follow-up period of 2.2 ± 1.3 years, 195 patients had one HRQoL re-assessment, 70 two, 40 three and 12 patients four or more re-assessment, respective. Results: Overall HRQoL at baseline was 78.7 ± 9.3. During follow-up there were no changes in HRQoL over time (0.03 [–0.01–0.07]; p = 0.195). In a linear mixed model neither CHD severity, the diagnostic subgroup, age, BMI, surgical history nor gender could be linked to a change in HRQoL during the follow-up time. Only children with higher age baseline (–0.48 [–0.85––0.11]; p = 0.010) had lower HRQoL. Same trend was seen for BMI (–0.19 [–0.41–0.03]; p = 0.099). Conclusion: Older children with CHD have significantly worse HRQoL, but they evolve similarly to younger children over time. Since no demographic or clinical variable could be linked to the course of HRQoL, it seems that individual HRQoL courses are not predictable and routine HRQoL evaluations seem to be necessary for acute decision making in clinical practice.
Keywords: Health-related quality of life; longitudinal; serial; children; congenital heart disease
Due to the enormous improvement in surgical treatment and aftercare, which has led to an increase in life expectancy, health-related quality of life (HRQoL) has become an important measure for functional outcome in patients with congenital heart disease (CHD). Nowadays, self-perceptions of well-being and functioning are recognized as a relevant end-point, help to improve the relationship of patients and physicians, and provide decision-making assistance in clinical practice [1,2].
Many studies have been conducted within the last decade, nevertheless there is still debate in HRQoL research in the field of CHD owed to many studies with various populations, small sample sizes and lack of generic and standardized questionnaires [3,4]. Those reasons had led to many studies claiming a superior [5–9] or equal [1,10,11]. HRQoL in patients with CHD in comparison to healthy peers and at the same time to a similar number of studies contradicting these findings [12,13]. The latter is also the result of the most recent meta-analysis from Ladak and colleagues [14] which outlined worse HRQoL in children and adolescents with CHD by comprising 20 studies.
However, during this debate it is often neglected that the results are derived from cross-sectional investigations and forecasts over time courses are therefore not permissible. However, it is extremely important to be able to consider these changes not only from a cross sectional point of view, but also from a longitudinal perspective in order to be able to take family or clinical measures. Unfortunately, In children and adolescents with CHD there are only a few studies available that dealt with longitudinal assessment [11,15–17]. Especially working groups around Moons have taken care of this topic. They found relatively stable HRQoL in adolescents aged 14 to 18 years. More than that, they found symptoms, cognitive functioning, and communication problems to be predictors of perceived health domains over time [11]. But since the observation period was four years, this study characterizes more of a transition of HRQoL from adolescents to early adulthood. However, it should be mentioned that all of the four studies dealt with the same 429 patients, and conclusions should therefore be carefully chosen. Moreover, mean age was almost 16 years at inclusion and information on children is missing.
Therefore, the aim of the current study was to assess the natural course of HRQoL in children and young adolescents with CHD with respect to clinical and demographical variables and to determine factors associated with change in HrQOL.
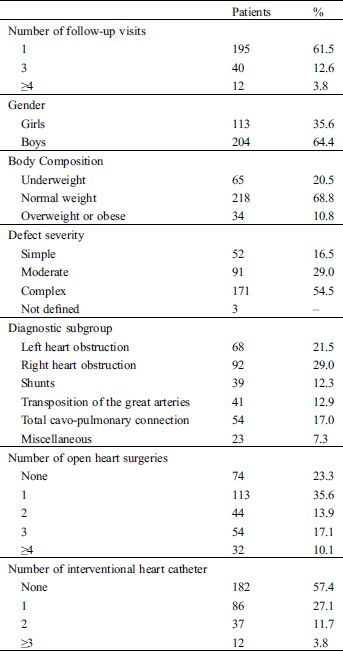
From July 2014 to February 2020, we prospectively examined 317 children and adolescents between 6 and 18 years with various CHD (113 girls, mean age 11.6 ± 2.9 years at baseline) from their regular outpatient visit for their HRQoL by using the self-report KIDNL® questionnaire. All children and adolescents have filled out the questionnaire alone and without the help of their parents (Fig. 1).

Figure 1: Patient inclusion CHD: congenital heart disease, HRQoL: health-related quality of life
During this time period 195 patients had one HRQoL re-assessment, 70 two, 40 three and 12 patients four or more re-assessment, respective.
In total this corresponds to 515 re-assessments of HRQoL and a follow-up length of 2.2 ± 1.3 years. The follow-up re-assessments took place at the routinely after-care appointments at our outpatient department. Patients with syndromes, mental or psychomotor retardation as well as all children unable to fill out the German form of the questionnaire or patients currently hospitalized were excluded from participation. All patients did not have any interventions or surgery during the follow-up period.
68 patients had left heart obstruction (Aortic Stenosis or Coarctation of the aorta), 92 right heart obstruction (Tetralogy of Fallot or pulmonary stenosis), 39 isolated shunts, 41 Transpositions of the great Arteries, 54 had a total cavo-pulmonary connection and 23 various diagnosis.
According to the American College of Cardiology (ACC) criteria [18] 52 patients had simple, 91 moderate and 171 complex severity class of heart defects (3 could not be classified according to the ACC criteria). All demographic and clinical variables of the 317 children and adolescents included in the study are displayed in Tab. 1.
Table 1: Demographic and clinical characteristics of the 317 children and adolescents included in the study
Written informed consent was obtained from all participants and their guardian. The study was approved by the local ethical board of the Technical University of Munich (Project Number: 314/14) and is part of the FOOTLOOSE (Functional outcome in children and adolescents with congenital heart disease) project (Deutsches Register Klinische Studien: DRKS00018853).
Parts of the data were already published in our cross-sectional findings on HRQoL in patients with CHD [7].
2.2 Health-Related Quality of Life (HRQoL)
HRQoL was evaluated with the German self-report of the KINDL® questionnaire. The KINDL® is a generic instrument and it can be used to assess HRQoL in healthy as well as in ill children and adolescents. The two versions one for children aged 7–13 and one for adolescents aged 14–17 years were used. Those six patients younger than seven years of age also received the children form. Both questionnaire consists of 24 items that could be answered on a five point Likert scale (never, seldom, sometimes, often, and always). From those questions a total quality of life score was generated as well as six subscales for physical and emotional well-being, self-esteem, family, friends and everyday functioning. Values range from 0 (worst) to 100 (best).
The KINDL® is a common, international, standardized questionnaire. There are two age-adapted forms available for children aged seven to eighteen that evaluate HRQoL from a subjective perspective [19–21]. The KINDL is internationally recognized and prior studies have indicated that it can provide reliable and valid findings when used in healthy children and children with chronic conditions [19,22].
Descriptive data is shown as means ± standard deviations, or percentage where appropriate and regression coefficients and 95% confidence intervals.
Students t-test or ANOVA tests were performed to test whether HRQoL scores differed among patients’ subgroups. Spearman’s correlation coefficient was computed to determine the association of HRQoL scores with age and BMI.
Linear mixed model analysis was performed to examine the course of HRQoL using the lmer function from the lme4 R package. Association of demographic and clinical variables with HRQoL over time was assessed, with child modelled as a random effect, whilst the explanatory variables were entered as fixed effects.
The initial model was a random intercept and random slope model without adjusting for additional covariates. Since no significant correlation was found between random intercept and slope, a model with uncorrelated random effects was selected. Demographic and clinical variables were then entered one by one into the initial model. Any first-order interaction effect with time was statistically significant, so the final model included only the main fixed effects.
SPSS version 24.0 and RStudio version 1.1.463 were used for all analyses. All reported p-values were two-sided and considered statistically significant at p ≤ 0.05.
Overall HRQoL at baseline was 78.7 ± 9.3 and higher age at baseline was associated with worse HRQoL (r = –0.193; p = 0.001). Baseline HRQoL also tended to be lower when BMI was higher (r = –0.137; p = 0.015). No other association to HRQoL to any demographic or clinical variable could be found (Tab. 2).
Table 2: Association of baseline health-related quality of life with demographic and clinical characteristics


In terms of the linear mixed model there was considerable variation in the intercept (estimated basal HRQoL) across children, with barely variation in slope (change in HRQoL with time). Therefore, less evidence exist of a relationship between intercept and slope which suggests, that change in HRQoL are not related to basal HRQoL.
As illustrated in the linear mixed models in Tab. 3, there were no changes over time and neither CHD severity, the diagnostic subgroup, age, BMI, surgical history nor gender could be linked to a change in HRQoL during the follow-up time.
Table 3: Linear mixed model results for longitudinal regression models of HRQoL predicted by time, demographic and clinical variables


Only age at baseline (–0.60 [–0.94 – –0.26]; p = 0.001) was associated with worse HRQoL, which means that older children with CHD have significantly worse HRQoL, but evolve similarly to younger children over time. The same applies for baseline BMI (–0.37 [–0.61 – –0.14]; p = 0.002).
In the final linear mixed model including all co-variates (Tab. 4) only age (–0.59 [–1.00 – –0.18]; p = 0.006) was associated with lower HRQoL but without changes over time.
Table 4: Linear mixed model results for longitudinal regression model of HRQoL predicted by time, demographic and clinical variables

The present study could not show significant changes in HRQoL over time throughout a follow-up period of over two years. Also neither CHD severity, the diagnostic subgroup, age, BMI, surgical history nor gender could be linked to HRQoL during follow-up. Older children with CHD have significantly worse HRQoL, but they evolve similarly to younger children over time. The same seems to apply for patients with higher baseline BMI. Individual HRQoL courses are therefore not predictable and routine HRQoL evaluations seem to be necessary for acute decision making in clinical practice.
First of all, comparable to our previously published study on HRQoL in children and adolescents with CHD, our longitudinal cohort also showed good HRQoL at baseline level when compared to the reference cohort, that was assessed with the same questionnaire and under similar circumstance without era effects [7]. There is a body of literature available that confirms the beneficial HRQoL results in CHD [5–10] even if the most recent meta-analysis [14] contradicts this. However, it is difficult to compare studies on HRQoL because of fundamental differences in sampling and methods [4] which makes a comparison difficult. Very often the studies miss a control group as well or compare values derived from different questionnaires. Since there is no gold standard measurement like cardiopulmonary exercise testing for assessing exercise capacity, it will still be difficult in the future to compare findings from different studies.
Apart from the contradictions in cross sectional measurement, our results are well in line with the only longitudinal study on HRQoL in children and adolescents with CHD [11]. Although the PedsQL questionnaire was used in this study [11], which has only one less question than the KINDL®. Moreover, the HRQoL is also similar presented on a scale from 0 to 100, as well as the linear analog scale (LAS) questionnaire for self-rated health. The results at the baseline are quite similar to ours. Compared to our report where we found no change in HRQoL, Bratt and colleagues [11] reported on a marginal decline of HRQoL during their four follow-up visits over 27 month. However, they had to conclude that these changes are not clinically relevant at group level. Moreover, it could not be ruled out that the only marginal longitudinal decline of HRQoL in CHD is just the same as the natural decline of HRQoL in healthy children as cross-sectional data suggests [23,24].
Irrespective of the good HRQoL attention should be paid to vulnerable subgroups, that are prone to low HRQoL or prone to an exacerbated decline. Locating those is a major challenge, unfortunately this study could not find any predictor or parameter, using a linear mixed model that were associated with changing HRQoL during the mean follow-up of two years. In comparison to Bratt and colleagues [11] that outlined that symptoms, cognitive functioning, and communication problems turned out to deserve specific attention. However, associations were weak and parameters could not serve as established predictors so far. On the other hand, we focused more on clinical and demographical variables, but we could not verify with our analysis, for example that girls are subject to a strong role pressure (especially during puberty) and decline disproportionately in their HRQoL. Maybe just due to the effect that coping strategies and stronger social bonding exists in this CHD cohort making those patients more resilient [25–27]. This may also be an explanation for the fact that overweight children with CHD have no altered course in HRQoL.
The subgroup analysis in this study also did not provide significant results or predictors, and the lone descriptive values should not mislead. Many studies in children and adults with CHD have shown that perceived health status is independent of the diagnosis and severity [7,28–30] or only slightly associated when using generic questionnaires [1,13]. Nevertheless the longitudinal course was also not different, only the baseline values just a bit lower in moderate and complex CHD.
Therefore, it seems that the detection of vulnerable patients with low HRQoL can only be achieved whenever the patient is present at an outpatient visit as so far no clinical or demographical variables can predict changes in longitudinal HRQoL.
This study did not perform longitudinal HRQoL assessment in healthy children and therefore we cannot rule out that the only marginal decline in CHD mimics that of healthy children like cross-sectional data suggests [23,24].
The follow up period with two years is rather small but will be extended for further long-term analysis. Maturation and puberty are an important factors for HRQoL and an examination of the tanner status or endocrinological level is helpful to prove changes in this sensitive phase. Moreover, we were just able to exclude patients that had interventions or surgery during follow-up and no further information could be provided in terms of other clinical parameters that might have deteriorated and therefore effect HRQoL as well. Determinants and predictors of patient-reported health were not assessed in this study. However, with these standard deviations and spread of the data it is questionable whether precise predictors could be figured out in general.
Older children with CHD have significantly worse HRQoL, but they evolve similarly to younger children over time. Since no demographic or clinical variable could be linked to the course of HRQoL, it seems that individual HRQoL courses are not predictable and routine HRQoL evaluations seem to be necessary for acute decision making in clinical practice.
Acknowledgement: none.
Data Sharing: The data can be requested from the corresponding author.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Amedro, P., Dorka, R., Moniotte, S., Guillaumont, S., Fraisse, A. et al. (2015). Quality of life of children with congenital heart diseases: a multicenter controlled cross-sectional study. Pediatric Cardiology, 36(8), 1588–1601. DOI 10.1007/s00246-015-1201-x.
2. Rumsfeld, J. S., Alexander, K. P., Goff, D. C., Graham, M. M., Ho, P. M. et al. (2013). Cardiovascular health: the importance of measuring patient-reported health status: a scientific statement from the American Heart Association. Circulation, 127(22), 2233–2249. DOI 10.1161/CIR.0b013e3182949a2e.
3. Fteropoulli, T., Stygall, J., Cullen, S., Deanfield, J., Newman, S. P. (2013). Quality of life of adult congenital heart disease patients: a systematic review of the literature. Cardiology in the Young, 23(4), 473–485. DOI 10.1017/S1047951112002351.
4. Bratt, E. L., Moons, P. (2015). Forty years of quality-of-life research in congenital heart disease: temporal trends in conceptual and methodological rigor. International Journal of Cardiology, 195, 1–6. DOI 10.1016/j.ijcard.2015.05.070.
5. Teixeira, F. M., Coelho, R. M., Proença, C., Silva, A. M., Vieira, D. et al. (2011). Quality of life experienced by adolescents and young adults with congenital heart disease. Pediatric Cardiology, 32(8), 1132–1138. DOI 10.1007/s00246-011-0039-0.
6. Areias, M. E., Pinto, C. I., Vieira, P. F., Castro, M., Freitas, I. et al. (2014). Living with CHD: quality of life (QOL) in early adult life. Cardiology in the Young, 24(S2), 60–65. DOI 10.1017/S1047951114001218.
7. Reiner, B., Oberhoffer, R., Ewert, P., Müller, J. (2019). Quality of life in young people with congenital heart disease is better than expected. Archives of Disease in Childhood, 104(2), 124–128. DOI 10.1136/archdischild-2017-314211.
8. Culbert, E. L., Ashburn, D. A., Cullen-Dean, G., Joseph, J. A., Williams, W. G. et al. (2003). Quality of life of children after repair of transposition of the great arteries. Circulation, 108(7), 857–862. DOI 10.1161/01.CIR.0000084547.93252.9A.
9. Hock, J., Reiner, B., Neidenbach, R. C., Oberhoffer, R., Hager, A. et al. (2018). Functional outcome in contemporary children with total cavopulmonary connection—health-related physical fitness, exercise capacity and health-related quality of life. International Journal of Cardiology, 255, 50–54. DOI 10.1016/j.ijcard.2017.11.092.
10. Schrøder, M., Boisen, K. A., Reimers, J., Teilmann, G., Brok, J. (2016). Quality of life in adolescents and young adults with CHD is not reduced: a systematic review and meta-analysis. Cardiology in the Young, 26(3), 415–425. DOI 10.1017/S104795111500181X.
11. Bratt, E. L., Luyckx, K., Goossens, E., Budts, W., Moons, P. (2015). Patient-reported health in young people with congenital heart disease transitioning to adulthood. Journal of Adolescent Health, 57(6), 658–665. DOI 10.1016/j.jadohealth.2015.07.021.
12. Wilmot, I., Cephus, C. E., Cassedy, A., Kudel, I., Marino, B. S. et al. (2016). Health-related quality of life in children with heart failure as perceived by children and parents. Cardiology in the Young, 26(5), 885–893. DOI 10.1017/S1047951115001468.
13. Mellion, K., Uzark, K., Cassedy, A., Drotar, D., Wernovsky, G. et al. (2014). Health-related quality of life outcomes in children and adolescents with congenital heart disease. Journal of Pediatrics, 164(4), 781–788.e1. DOI 10.1016/j.jpeds.2013.11.066.
14. Ladak, L. A., Hasan, B. S., Gullick, J., Gallagher, R. (2019). Health-related quality of life in congenital heart disease surgery in children and young adults: a systematic review and meta-analysis. Archives of Disease in Childhood, 104(4), 340–347. DOI 10.1136/archdischild-2017-313653.
15. Apers, S., Luyckx, K., Rassart, J., Goossens, E., Budts, W. et al. (2013). Sense of coherence is a predictor of perceived health in adolescents with congenital heart disease: a cross-lagged prospective study. International Journal of Nursing Studies, 50(6), 776–785. DOI 10.1016/j.ijnurstu.2012.07.002.
16. Luyckx, K., Missotten, L., Goossens, E., Moons, P. (2012). Individual and contextual determinants of quality of life in adolescents with congenital heart disease. Journal of Adolescent Health, 51(2), 122–128. DOI 10.1016/j.jadohealth.2011.11.007.
17. Luyckx, K., Goossens, E., Rassart, J., Apers, S., Vanhalst, J. et al. (2014). Parental support, internalizing symptoms, perceived health status, and quality of life in adolescents with congenital heart disease: influences and reciprocal effects. Journal of Behavioral Medicine, 37(1), 145–155. DOI 10.1007/s10865-012-9474-5.
18. Warnes, C. A., Liberthson, R., Danielson, G. K., Dore, A., Harris, L. et al. (2001). Task force 1: the changing profile of congenital heart disease in adult life. Journal of the American College of Cardiology, 37(5), 1170–1175. DOI 10.1016/S0735-1097(01)01272-4.
19. Ravens-Sieberer, U., Ellert, U., Erhart, M. (2007). Gesundheitsbezogene lebensqualität von kindern und jugendlichen in Deutschland. Bundesgesundheitsblatt-Gesundheitsforschung-Gesundheitsschutz, 50(5–6), 810–818. DOI 10.1007/s00103-007-0244-4.
20. Ellert, U., Ravens-Sieberer, U., Erhart, M., Kurth, B. M. (2011). Determinants of agreement between self-reported and parent-assessed quality of life for children in Germany-results of the German Health Interview and Examination Survey for Children and Adolescents (KiGGS). Health and Quality of Life Outcomes, 9(1), 102. DOI 10.1186/1477-7525-9-102.
21. Erhart, M., Ellert, U., Kurth, B. M., Ravens-Sieberer, U. (2009). Measuring adolescents’ HRQoL via self reports and parent proxy reports: an evaluation of the psychometric properties of both versions of the KINDL-R instrument. Health and Quality of Life Outcomes, 7(1), 77. DOI 10.1186/1477-7525-7-77.
22. Ravens-Sieberer, U., Bullinger, M. (1998). Assessing health-related quality of life in chronically ill children with the German KINDL: first psychometric and content analytical results. Quality of Life Research, 7(5), 399–407. DOI 10.1023/A:1008853819715.
23. Meyer, M., Oberhoffer, R., Hock, J., Giegerich, T., Müller, J. (2016). Health-related quality of life in children and adolescents: current normative data, determinants and reliability on proxy-report. Journal of Paediatrics and Child Health, 52(6), 628–631. DOI 10.1111/jpc.13166.
24. Ravens-Sieberer, U., Erhart, M., Wille, N., Bullinger, M. (2008). Health-related quality of life in children and adolescents in Germany: results of the BELLA study. European Child & Adolescent Psychiatry, 17(S1), 148–156. DOI 10.1007/s00787-008-1016-x.
25. Tak, Y. R., McCubbin, M. (2002). Family stress, perceived social support and coping following the diagnosis of a child’s congenital heart disease. Journal of Advanced Nursing, 39(2), 190–198. DOI 10.1046/j.1365-2648.2002.02259.x.
26. Jackson, A. C., Frydenberg, E., Liang, R. P., Higgins, R. O., Murphy, B. M. (2015). Familial impact and coping with child heart disease: a systematic review. Pediatric Cardiology, 36(4), 695–712. DOI 10.1007/s00246-015-1121-9.
27. Neuner, B., Busch, M. A., Singer, S., Moons, P., Wellmann, J. et al. (2011). Sense of coherence as a predictor of quality of life in adolescents with congenital heart defects: a register-based 1-year follow-up study. Journal of Developmental and Behavioral Pediatrics, 32(4), 316–327. DOI 10.1097/DBP.0b013e31821102ee.
28. Müller, J., Hess, J., Hager, A. (2012). Minor symptoms of depression in patients with congenital heart disease have a larger impact on quality of life than limited exercise capacity. International Journal of Cardiology, 154(3), 265–269. DOI 10.1016/j.ijcard.2010.09.029.
29. Müller, J., Hess, J., Hager, A. (2013). General anxiety of adolescents and adults with congenital heart disease is comparable with that in healthy controls. International Journal of Cardiology, 165(1), 142–145. DOI 10.1016/j.ijcard.2011.08.005.
30. Utens, E. M., Bieman, H. J., Verhulst, F. C., Meijboom, F. J., Erdman, R. A. et al. (1998). Psychopathology in young adults with congenital heart disease. Follow-up results. European Heart Journal, 19(4), 647–651. DOI 10.1053/euhj.1997.0824.
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |