

 | Congenital Heart Disease |  |
DOI: 10.32604/CHD.2020.013020
ARTICLE
Risk Factor Analysis for Shunt Failure after Systemic Pulmonary Shunt
Beijing Anzhen Hospital, Capital Medical University, Beijing, 100029, China
*Corresponding Author: Junwu Su. Email: sujunwu2019@sina.com
Received: 22 July 2020; Accepted: 18 August 2020
Background: To identify factors associated with shunt failure in patients with a systemic pulmonary shunt (SPS). Methods: We retrospectively assessed 451 patients who received SPS. Perioperative parameters such as hemoglobin, Nakata Index, and shunt size were assessed, and factors influencing shunt failure after an initial palliative shunt operation were analyzed. Results: We analyzed 451 patients who underwent isolated SPS surgery at Anzhen Hospital. Of these, shunt failure occurred in 48 (10.6%) cases after a median of 6.5 days. The 30-day mortality rate was 2.1%. Univariate and logistic regression analysis revealed that a Nakata Index ≤ 105 and vasoactive-inotropic score (VIS) ≥ 8.5 were risk factors for shunt failure. Conclusion: Nakata Index ≤ 105 was identified as a risk factor for shunt failure. A maximum VIS of ≥8.5 over the first 24 h was a good predictor of poor clinical outcomes. After SPS, close monitoring of the balance of systemic and pulmonary circulation, usage of appropriate vasoactive-inotropic agents, and early intervention could reduce the occurrences of shunt failure.
Keywords: Congenital heart disease; systemic-to-pulmonary shunts; shunt failure; modified Blalock-Taussig shunt; central shunt
Despite the development of techniques and progress in patients who undergo total corrective surgery for complex congenital heart disease, many children remain unable to complete primary repair. Systemic pulmonary shunt (SPS), a surgical approach that has been in use for over 70 years, has been shown to increase pulmonary blood flow and improve development of the pulmonary artery [1]. However, SPS is a high-risk surgery; as per a recent report by Nhue et al., [2] the mortality rate of SPS is 4−14% and the incidence of complications, including stenosis and thromboembolism, is 8−13% [3]. The present study aimed to evaluate early outcomes, and thus, reduce the risk of shunt failure.
A total of 451 patients who underwent isolated SPS at Anzhen Hospital, Beijing between March 2010 and July 2018 were selected for this study. The SPS included both modified Blalock-Taussig shunt (MBTS) and central shunt (CS). Patient information was obtained retrospectively from their medical records. Patients who received other major concomitant surgeries, such as pulmonary artery banding and bidirectional cavopulmonary anastomosis, were excluded from analysis. The median age at SPS insertion was 12 months (interquartile range: 17 months). In some areas of China, due to a lack of adequate medical resources, pulmonary hypoplasia patients cannot afford surgery when required. To relieve the symptoms, some patients receive SPS later, when they are older. Patient characteristics have been summarized in Tab. 1.
Table 1: Characteristics of 451 patients

Of the 451 patients, 195 received CS and 256 received MBTS with a polytetrafluoroethylene graft. Of the latter group, 42 patients underwent a cardiopulmonary bypass (CPB). The size of the shunt was determined based on the age of the patient and development of pulmonary vessels; 72 patients received a 4 mm shunt, 310 patients received a 5 mm shunt, 61 patients received a 6 mm shunt and 8 patients received an 8 mm shunt. After the operation, all patients were kept under assisted respiration, monitored for vital signs, and administered intravenous heparin at 5000 IU/m2/day, beginning at 4 h after surgery and lasting until extubation; thereafter, they were administered oral aspirin at 3−5 mg/kg.
After surgery, the patients underwent echocardiographic examination and chest radiography, both during their stay in the intensive care unit and once before discharge. Hourly doses of all vasoactive medications were recorded for the first 24 h after admission to the cardiothoracic intensive care unit and the vasoactive-inotropic score (VIS) was calculated. We defined two study endpoints: the first was shunt failure, defined as shunt thrombosis, stenosis or oversize requiring shunt revision and/or replacement; the second was shunt-related mortality, defined as a patient dying within 30 days due to shunt failure. Patients who reached the above endpoints were assigned to the shunt failure group, and all other patients were assigned to the no shunt failure group.
Statistical analysis was performed using the Statistical Package for Social Sciences (SPSS) 19.0 software (SPSS Inc., Chicago, IL, USA). All data were represented as median (interquartile range) for each group. Based on the distribution of continuous data, Student’s t-test or Chi-square test was used to compare groups. Multivariate analysis was performed using stepwise logistic regression, only including factors identified as significant during univariate analysis to assess independent risk factors for shunt failure. A probability value of less than 0.05 was considered significant.
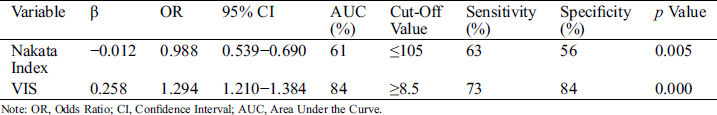
Based on the surgical outcome, 403 patients were assigned to the no shunt failure group and 48 patients (10.6%) were shifted to the failure group after a median of 6.5 days. Shunt-related mortality was 2.1% (n = 12), and two patients died due to hemorrhea. Patient characteristics and variables compared between the groups are depicted in Tab. 2. The progression of patients who experienced shunt failure is depicted in Fig. 1. The median age was significantly lower in patients of the shunt failure group (7.5 months) when compared to the no shunt failure group (12 months, p < 0.05). The median weight was significantly lower in the shunt failure group 7 kg (4.4 kg) as compared to the no shunt failure group 9 kg (4.5 kg, p < 0.05). The McGoon Ratio and Nakata Index at the time of operation were both lower in the shunt failure group and VIS was higher in the shunt failure group. Shunt failure was more prevalent in patients who received platelet transfusions. Multivariate analysis was used to examine the risk factors for shunt failure. Nakata Index ≤ 105 and VIS ≥ 8.5 were identified as risk factors for shunt failure, as shown in Tab. 3.

Figure 1: Shunt failure and treatment
Table 2: Patient characteristics and variables compared between no shunt failure and shunt failure patient groups

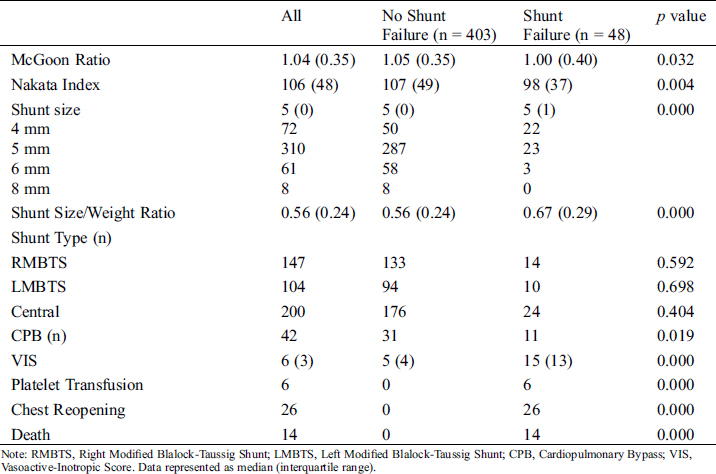
Table 3: Risk factors for shunt failure
Since its invention in the 1940s [4], SPS has benefitted numerous patients. Although surgical techniques, anesthesia, and intensive care have progressed over time, the overall outcome of the SPS remains unsatisfactory. A recent study has reported the mortality rate to be 3.7−14% [5]. In our study, the 30-day mortality rate of SPS was 2.1%. Therefore, in a clinical setting, there is still a need for indicators of poor outcomes to aid in early detection and prevention of unfavorable outcomes. Early intervention is important for improving the prognosis of patients undergoing SPS.
We found that early postoperative mortality was related to shunt pathology; 2.1% (n = 12) of patients died due to shunt-related reasons, of which seven died due to over-shunting. These patients also had a much higher rate of poor outcomes than those who experienced a blocked shunt. Consequently, over-shunting may have caused more severe damage in the brain and other major organs. Kok et al. [6] reported that most patients suffer poor outcomes due to over-shunting following SPS usually within the first 24 h of surgery.
VIS [7] has been proposed as a marker of illness severity after pediatric cardiac surgery. We found that the maximum VIS over the first 24 h was a good predictor of poor clinical outcome. VIS ≥ 8.5 (OR 1.294, 95% CI 1.210−1.384) was identified as risk factor for shunt failure. Specifically, patients who experienced over-shunting had a higher VIS than those who experienced shunt block [20 (9) vs. 10 (13), p < 0.05]. The use of high-dose vasoactive inotropics indicates that the balance between systemic and pulmonary circulation is difficult to maintain after SPS. While we cannot directly implicate vasoactive-inotropic support as a cause of poor outcomes, we believe that high VIS is a potent marker of severity after SPS and it could help to improve clinical care and guide therapeutic decisions.
On the one hand, SPS increases cardiac volume load due to higher pulmonary blood flow. On the other hand, SPS decreases cardiac diastolic blood pressure due to coronary artery hypoperfusion [8]. Appropriate vasoactive-inotropic support can help avoid cardiac insufficiency, especially in those with hypoplastic left heart disease (such as tetralogy of Fallot and pulmonary atresia) [9]. Dirks et al. has found that bigger shunt size may be related to over-shunting, leading to lower diastolic pressure and in turn causing decreased coronary and systemic perfusion [10]. We recommend using appropriate vasoactive-inotropic agents to either maintain systemic perfusion pressure or keep the graft unobstructed. Because of our rich experience in treatment, two patients who experienced over-shunting received shunt clipping and successfully avoided poor clinical outcomes.
Shunt blocks, including shunt thrombosis or stenosis, are a major complication of SPS [11]. Winfield et al. [12] found that most MBTS patients develop stenosis by the time of takedown, and 21% of patients show >50% obstruction. Voravit et al. [13] analyzed patients weighing <3 kg with functional univentricular hearts who received MBTS and found in-hospital shunt thrombosis to be 14% and hospital mortality to be 18%. Shunt block has numerous causes, including preoperative high hemoglobin, weight <3 kg, duct patency after SPS and smaller shunt size, especially ≤3 mm [14,15]. It has also been shown that low postoperative oxygen saturation and high platelet counts are risk factors for shunt thrombosis [16].
In our study, shunt thrombosis was diagnosed in 14 patients and shunt stenosis was diagnosed in 25 patients. Twenty-two patients required reoperation for shunt exchange. In the shunt failure group, a higher proportion of patients received CS, which is especially useful for bilateral small branch pulmonary arteries. CS allows distribution of blood flow to both lungs and better pulmonary artery growth, but it could also increase the risk of over-shunting. The use of smaller shunts may result in less pulmonary blood flow. Bahaaldin et al. [17] suggested that a shunt size/weight ratio of 1.16 mm/kg is more suitable for neonates. However, in our study, the median age was 12 months and the shunt size/weight ratio was 0.56 mm/kg. We, therefore, did not adhere to the recommended standards of other countries. It is believed that a shunt size of at least 4 mm is required for sufficient flow and respective flushing effect to prevent shunt failure. In our center, based on the pulmonary artery diameter and body weight, we recommend use of a 3–4 mm graft for infants and 5–6 mm graft for children. Given that several patients could not receive the surgery during the optimal time window, 69 patients received 6 mm or 8 mm grafts, of which most were assigned to the no shunt failure group. In our previous study [18], older children with cyanotic congenital heart disease benefited from SPS, but we still recommend that the surgery be performed during the optimal time period.
We also found that the McGoon Ratio and Nakata Index at the time of operation were lower in the shunt failure group. Patients with Nakata Index ≤ 120 were not considered for radical surgery. Nakata Index ≤ 105 was identified as an independent risk factor for patients with SPS. Pulmonary vascular development is one of the most important indicators that determine the surgical approach to alleviate complex congenital heart disease. Pulmonary valve stenosis and/or pulmonary artery bifurcation stenosis can often be cured at certain stages, but branch pulmonary artery stenosis needs more careful analysis. Clinically, the Nakata Index is used as an indicator to assess the extent of distal pulmonary development. We observed that, in patients with a small Nakata Index, the shunt size was more likely to not match the pulmonary artery and hence, cause shunt failure. Additionally, patients with poor pulmonary artery development often received CS, making over-shunting hard to avoid and increasing the possibility of pulmonary overperfusion and congestive heart failure [19].
In summary, Nakata Index ≤ 105 was identified as risk factor for shunt failure. A maximum VIS ≥ 8.5 over the first 24 h was a good predictor of poor clinical outcomes. After SPS, close monitoring of the balance of systemic and pulmonary circulation, usage of appropriate vasoactive-inotropic agents and early intervention can reduce the occurrence of shunt failure. Like any retrospective study, our study has certain limitations, including the lack of a control group and lack of cardiac catheterization in all patients, precluding extraction of more specific data.
Ethical Approval:This article does not contain any studies with human participants performed by any of the authors.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. McKenzie, E. D., Khan, M. S., Samayoa, A. X., Vener, D. S., Ishak, Y. M. (2013). The Blalock-Taussig shunt revisited: a contemporary experience. Journal of the American College of Surgeons, 216(4), 699–706. DOI 10.1016/j.jamcollsurg.2012.12.027.
2. Do, N., Hill, K. D., Wallace, A. S., Vricella, L., Cameron, D. et al. (2018). Shunt failure—risk factors and outcomes: an analysis of the society of thoracic surgeons congenital heart surgery database. Annals of Thoracic Surgery, 105(3), 857–864. DOI 10.1016/j.athoracsur.2017.06.028.
3. Bove, T., Vandekerckhove, K., Panzer, J., Groote, K., Wolf, D. et al. (2015). Disease-specific outcome analysis of palliation with the modified Blalock-Taussig shunt. World Journal for Pediatric and Congenital Heart Surgery, 6(1), 67–74. DOI 10.1177/2150135114558690.
4. Blalock, A., Taussig, H. (1945). The surgical treatment of malformations of the heart in which there is pulmonary atresia. Journal of the American Medical Association, 128(3), 189–202. DOI 10.1001/jama.1945.02860200029009.
5. Alkhulaifi, A., Lacour-Gayet, F., Serraf, A., Belli, E., Planché, C. (2000). Systemic pulmonary shunts in neonates: early clinical outcome and choice of surgical approach. Annals of Thoracic Surgery, 69(5), 1499–1504. DOI 10.1016/s0003-4975(00)01078-x.
6. Soo, K. W., Brink, J., d’Udekem, Y., Butt, W., Namachivayam, S. P. (2018). Major adverse events following over-shunting are associated with worse outcomes than major adverse events after a blocked systemic-to-pulmonary artery shunt procedure. Pediatric Critical Care Medicine, 19(9), 854–860. DOI 10.1097/PCC.0000000000001659.
7. Michael, G., James, G., Alberta, H., Michelle, L., Robert, J. (2010). Vasoactive–inotropic score as a predictor of morbidity and mortality in infants after cardiopulmonary bypass. Pediatric Critical Care Medicine, 11(2), 234–238. DOI 10.1097/PCC.0b013e3181b806fc.
8. Fenton, K., Siewers, R., Rebovich, B., Pigula, F. (2003). Interim mortality in infants with systemic to pulmonary artery shunt. Society of Thoracic Surgeons, 76(1), 152–157. DOI 10.1016/s0003-4975(03)00168-1.
9. Xu, Z. M., Yang, Q., Guo, L. L., Su, Z. K. (2011). Risk factors for early failure after systemic-to-pulmonary artery shunt in congenital heart disease. Journal of Shanghai Jiaotong University (Medical Science), 31(9), 1266–1268.
10. Dirks, V., Prêtre, R., Knirsch, W., Buechel, E., Seifert, B. et al. (2013). Modified Blalock Taussig shunt: a not-so-simple palliative procedure. European Journal of Cardio-Thoracic Surgery, 44(6), 1096–1102. DOI 10.1093/ejcts/ezt172.
11. Jason, A., Anshuman, K., Bradford, J., Lois, U., Nishant, D. et al. (2007). Two thousand Blalock-Taussig shunts: a six-decade experience. Annals of Thoracic Surgery, 84(6), 2070–2075. DOI 10.1016/j.athoracsur.2007.06.067.
12. Winfield, J., James, Y., Anjan, S., Hector, M., Colleen, S. et al. (2005). Obstruction in modified Blalock shunts: a quantitative analysis with clinical correlation. Society of Thoracic Surgeons, 79(6), 2072–2076. DOI 10.1016/j.athoracsur.2004.12.050.
13. Chittithavorn, V., Duangpakdee, P., Rergkliang, C., Pruekprasert, N. (2017). Risk factors for in-hospital shunt thrombosis and mortality in patients weighing less than 3 kg with functionally univentricular heart undergoing a modified Blalock-Taussig shunt. Interactive CardioVascular and Thoracic Surgery, 25(3), 407–413. DOI 10.1093/icvts/ivx147.
14. Shibata, M., Itatani, K., Oka, N., Yoshii, T., Nakamura, Y. et al. (2015). Optimal graft size of modified Blalock-Taussig shunt for biventricular circulation in neonates and small infants. International Heart Journal, 56(5), 533–536. DOI 10.1536/ihj.15-042.
15. Gedicke, M., Morgan, G., Parry, A., Martin, R., Tulloh, R. (2010). Risk factors for acute shunt blockage in children after modified Blalock-Taussig shunt operations. Heart and Vessels, 25(5), 405–409. DOI 10.1007/s00380-009-1219-1.
16. Hobbes, B., d’Udekem, Y., Zannino, D., Konstantinov, I., Brizard, C. et al. (2017). Determinants of adverse outcomes after systemic-to-pulmonary shunts in biventricular circulation. Annals of Thoracic Surgery, 104(4), 1365–1370. DOI 10.1016/j.athoracsur.2017.06.043.
17. Bahaaldin, A., Scott, G., Makoto, M., Martha, C., Kirk, K. et al. (2016). Factors affecting death and progression towards next stage following modified Blalock-Taussig shunt in neonates. European Journal of Cardio-Thoracic Surgery, 50(1), 169–177. DOI 10.1093/ejcts/ezw017.
18. Zhang, H., Fan, X., Su, J., Liu, Y., Zhao, L. et al. (2019). The efficiency of systemic-to-pulmonary shunts in older children with hypoplastic pulmonary arteries. Journal of Cardiac Surgery, 34(6), 463–467. DOI 10.1111/jocs.14063.
19. Mohammadi, S., Benhameid, O., Campbell, A., Potts, J., Joza, J. et al. (2008). Could we still improve early and interim outcome after prosthetic systemic-pulmonary shunt? A risk factors analysis. European Journal of Cardio-Thoracic Surgery, 34(3), 545–549. DOI 10.1016/j.ejcts.2008.06.001.
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |