

 | Congenital Heart Disease |  |
DOI: 10.32604/CHD.2020.012092
ARTICLE
Acquired Coronary Artery Disease in Patients with Congenital Heart Disease: Issues in Diagnosis and Management
1Department of Pediatric Cardiology & Adult Congenital Heart Disease, Onassis Cardiac Surgery Center, Athens, Greece
2First Department of Cardiology, University of Athens, Athens Medical School, Athens, Greece
3Department of Cardiology, Onassis Cardiac Surgery Center, Athens, Greece
*Corresponding Author: Sotiria C. Apostolopoulou. Email: sotiria.apostolopoulou@gmail.com
Received: 16 June 2020; Accepted: 31 August 2020
Abstract: Objective: Acquired coronary artery disease, initially thought to rarely affect survivors of congenital heart disease, is increasingly recognized in this population, as these patients grow in age and numbers in the recent era. This study reports our experience with coronary artery disease in adults with congenital heart disease and discusses treatment issues and the existing literature. Methods: Retrospective review of all charts of adults with congenital heart disease and acquired coronary artery disease was performed. Patients’ clinical characteristics, diagnosis, risk factors, noninvasive and invasive imaging and management data were recorded. Results: Coronary artery disease was diagnosed at 35–70 of age in 17 patients out of a total of 1345 adult congenital heart disease patients followed. Congenital heart disease was moderate or complex in 5 patients (4 repaired Tetralogy of Fallot, 1 repaired ventricular septal defect) and simple unrepaired atrial septal defect diagnosed later in life before or after coronary artery disease identification in 12 patients. Coronary artery disease symptoms were present in 12 patients (8 myocardial infarctions, 4 exercise intolerance), while all patients had 2–3 risk factors for coronary artery disease. Coronary angiography showed 1–3 vessel disease treated with percutaneous coronary intervention in 7 patients, coronary artery bypass graft in 2 patients and both in 2 patients. Patients received appropriate medical therapy and remained stable for 1–17 years, while 2 patients are awaiting surgical pulmonary valve replacement in the near future. Conclusion: Coronary artery disease can develop in adults with congenital heart disease later in life in the presence of traditional risk factors, and prevention, screening and treatment strategies should be applied in this population. Further studies are needed to fully elucidate the extent of this issue in this increasing and ageing population and to determine its optimal medical, interventional and surgical management.
Keywords: Adult congenital heart disease; atherosclerosis; congenital heart defects; coronary intervention; risk factors
Advances in the management of congenital heart disease (CHD) over the last decades have significantly improved the prognosis of these patients resulting in a continually increasing and ageing population of adults with CHD [1]. Acquired coronary artery disease (CAD) may not have been perceived as a major issue in this population in the past, as CHD patients had decreased survival into adulthood but, as their life expectancy increased, acquired medical conditions started to play an important role in their morbidity and mortality [2]. Adults with CHD may have several risk factors for development of CAD such as hypertension, endothelial dysfunction, prior coronary artery interventions, left ventricular hypertrophy and aortopathy [3], and the burden of CAD has been well recognized in adults with CHD nowadays [4]. Atherosclerotic CAD was present in approximately 1% of adult CHD patients, with most common diagnoses atrial septal defect (35%), bicuspid aortic valve (18%), Tetralogy of Fallot (9%) and coarctation of the aorta (7%) but was also identified in a variety of other CHD defects [5]. Although patients with cyanotic CHD and Eisenmenger syndrome are traditionally perceived to be less affected by acquired CAD [4], subclinical atherosclerosis exists in these patients as well, without differences from the general population [6]. In addition, symptoms of CAD may easily be attributed to hemodynamic issues or heart failure related to CHD with or without repair [7], with the risk of overlooking myocardial ischemia in these patients. Indications for invasive or computed tomography coronary angiography as well as medical, interventional and surgical management of documented CAD in adults with CHD may not follow the guidelines of the general population and may depend on symptoms, diagnosis, severity and complexity of CHD, as well as previous or anticipated operations [8].
This study describes our experience with documented CAD in adults with CHD and discusses issues in detection, diagnosis and medical, interventional or diagnostic management, which may differentiate them from the general population, while stressing the need for adequate screening and prevention strategies in adult CHD.
All patients with CHD and acquired CAD were included in the study and retrospective review of their hospital charts was performed. Patients were identified based on the recollection of their physicians and their being followed by both the adult CHD and the adult cardiology departments. Data gathered included demographics, CHD diagnosis, previous cardiovascular operations, CAD presentation, cardiovascular risk factors, presence of arrhythmia, heart failure and carotid or peripheral vascular disease, medication use, results of noninvasive and invasive cardiac studies. Cardiovascular risk factors included hyperlipidemia, hypertension, diabetes mellitus, smoking history and family history of CAD. Medical, interventional or surgical management of CAD was recorded whether it was isolated or combined with repair of the congenital heart defect.
CAD presented at 35–70 years of age in 17 patients with CHD (11 males, 6 females) out of a total of 1345 adult CHD patients followed Tab. 1. Diagnoses were Tetralogy of Fallot (TOF) repaired at 4–27 years of age in 4 patients, repaired ventricular septal defect (VSD) in 1 patient, and unrepaired atrial septal defect (ASD) in the remaining 12 patients, one of which also had pulmonary stenosis. As seen in the Tab. 1, 5 patients had moderate or complex repaired CHD with CAD diagnosed during follow-up, while 12 patients had atrial septal defect diagnosed later in life before or after CAD identification. Within this population, CAD symptoms were present in 12 patients, 8 with classic myocardial infarction (MI) presentation and 4 with decreased exercise tolerance, initially attributed to right ventricular dysfunction due to their CHD. The remaining 5 patients were asymptomatic and were discovered to have CAD during diagnostic cardiac catheterization in preparation for transcatheter or surgical treatment of their CHD. All cases of MI were type 1 with 3 STEMI and 5 NSTEMI presentations. Regarding the patients presenting with reduced exercise tolerance, symptomatology was attributed to their CAD instead of their CHD in retrospect after identification and PCI treatment of CAD resulted in improvement of symptoms.
Table 1: Patients’ characteristics
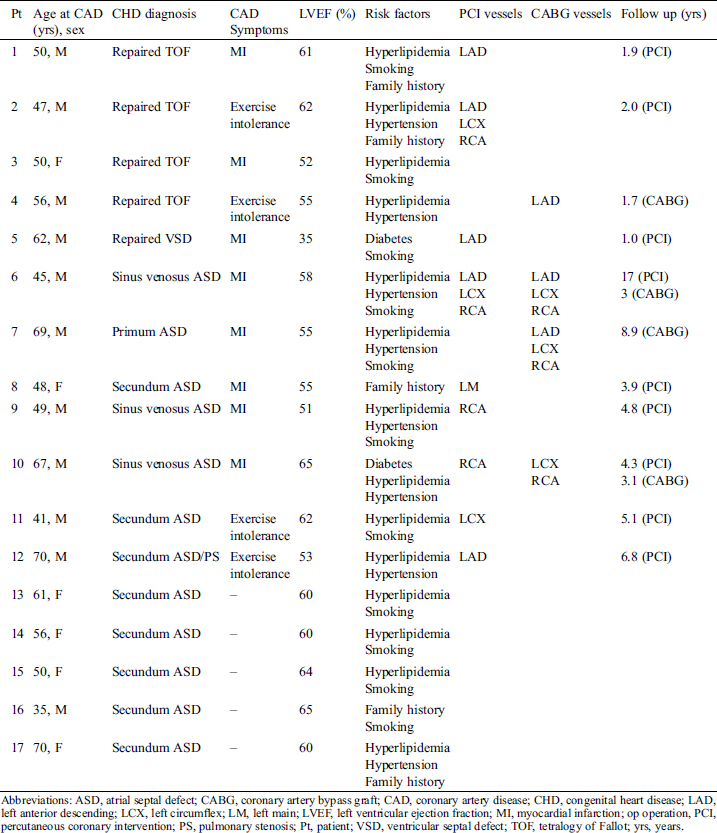
At the time of CAD diagnosis, all patients had 2–3 traditional risk factors for CAD as seen in the Tab. 1. The most common risk factors of hyperlipidemia and hypertension were largely unrecognized in our population and were not treated appropriately prior to CAD identification. Right ventricles were dilated in the patients with TOF and ASD and all but 1 patient had relatively preserved left ventricular function, while only the patient with repaired VSD had new onset decreased left ventricular ejection fraction attributed to his CAD.
3.2 Extent of CAD and Coronary Interventions
Coronary angiography showed 1–3 affected vessels. Percutaneous coronary intervention (PCI) alone in 1–3 affected vessels was performed in 7 patients, coronary artery bypass graft (CABG) during CHD repair in 1 patient and CABG alone in 1 patient as his CHD was not recognized at the time of surgery. Two patients underwent initially PCI but developed CAD progression and were treated with CABG concomitantly with their surgical ASD repair 1 and 15 years after PCI respectively. One repaired TOF patient presenting with MI received only medical treatment as her RCA lesion was in a distant small vessel that did not warrant intervention. The remaining 5 ASD patients without CAD related symptoms had lesions discovered in preoperative coronary angiography that did not need transcatheter or surgical intervention, therefore they underwent isolated surgical ASD repair. In total, CAD was not suspected and was an incidental finding at elective preoperative coronary angiography in 7 ASD patients, 2 of whom had significant lesions and underwent PCI. Coronary intervention alone was performed in 2 patients after MI as their CHD was not recognized at the time. One of these patients underwent CABG along with ASD repair 15 years later due to CAD progression, while the other has not had his ASD repaired to date. Patients treated with PCI alone underwent invasive coronary angiography 1 year after the procedure, showing patency of all treated vessels.
During 3 years of follow up after CAD diagnosis, 2 patients with repaired TOF have developed worsening pulmonary regurgitation leading to severe right ventricle dilation and dysfunction and are scheduled for surgical pulmonary valve replacement as their dilated right ventricular outflow tracts preclude transcatheter treatment.
CAD is increasingly recognized in adults with CHD [4], most commonly associated with atrial septal defects and bicuspid aortic valve disease, probably due to these diagnoses’ high prevalence and excellent survival [5]. This small study of adults with CHD and documented CAD treated with medical and interventional therapy with good outcome delineates the importance of recognition, workup and possibly different management of CAD in this population.
Traditional cardiovascular risk factors, such as diabetes mellitus, systemic hypertension, hyperlipidemia, obesity and smoking history are present in adults with CHD where sedentary lifestyle, obesity and diabetes mellitus occur more frequently and prematurely than in the general population [9,10]. Additional conditions promoting CAD in adult CHD may be pre-existing coronary artery abnormalities and surgical manipulations, left-sided obstructive lesions with systemic hypertension, left ventricular hypertrophy and diastolic dysfunction and aortopathy with its negative influence on coronary flow [3]. Subclinical atherosclerosis is identifiable on CT in patients with coarctation of the aorta more often than in controls [11], suggesting the possible need for assessment of the coronaries and calcium burden by computed tomography angiography during the regular follow up of CHD patients.
Risk factors frequently remain undertreated in adult CHD due to decreased awareness and screening on the part of physicians as well as the assumption that prevention may be less important in a population perceived as having decreased life expectancy [12]. Indeed, our patients were not receiving adequate modification therapy for all their risk factors prior to CAD detection and only received adequate medical therapy after recognition of CAD. These observations stress the importance of establishing organized adult CHD programs with increased awareness and adherence to the established prevention, screening and detection guidelines [13] in the adult CHD population.
CAD was traditionally considered to occur less frequently in cyanotic CHD, as these patients have dilated coronary arteries with increased basal flow and the potential for hyperemia, as well as decreased coronary atherogenesis due to the beneficial effects of increased nitric oxide levels, decreased platelets, hypocholesterolemia and hyperbilirubinemia [14]. But as the cyanotic CHD population ages and is further exposed to risk factors, newer large studies demonstrate that these patients have the same, if not increased, risk for CAD and should therefore be treated accordingly in terms of awareness, screening and prevention strategies [15].
Symptomatology may not be very helpful in suspecting CAD in patients with CHD as it can be insidious, nonspecific and mistakenly attributed to residual abnormalities, such as ventricular volume and pressure overload, great vessel dilation, external compression, or coronary artery anomalies [4]. Moreover, classic angina may not manifest even in the presence of significant CAD due to impaired autonomic function after CHD surgery [16]. Increased vigilance and education of physicians and patients are needed in order to suspect and correctly interpret symptoms and proceed to adequate diagnostic workup in this patient population. In view of the paucity and ambiguity of symptoms, coronary angiography should be performed before elective CHD surgery in older subjects with cardiovascular risk factors [4], in analogy with patients undergoing valvular heart surgery [17]. On the other hand, CHD was not recognized in 2 of our CAD patients prior to coronary intervention. Therefore, complete workup for coexistence of congenital defects is advised in CAD patients, as presence of CHD may influence timing and mode of CAD intervention.
Revascularization in CAD is advocated in the setting of ischemic symptoms, left ventricular dysfunction, or left main coronary artery involvement, according to specific guidelines in the general population [18]. Such guidelines may not entirely apply to adult CHD with possible pre-existing myocardial injury and dysfunction due to suboptimal myocardial protection during cardiopulmonary bypass, prolonged cyanosis, chronic ventricular volume overload or interventricular interaction [19]. PCI has been used successfully to treat CAD in the setting of repaired CHD [20], while CABG may be performed concomitantly with elective CHD repair with good survival and late outcome [21]. Treating CAD by PCI prior to CHD surgery may significantly shorten the duration of the operation, on the other hand, CABG including grafting of the left internal mammary artery to the left anterior descending artery has a survival benefit compared to PCI in complex patients [22] and may be performed at the time of CHD repair. Number and nature of previous operations, increased risk and difficulty of CABG in the reoperation setting, and anticipated future reoperations for CHD need to be taken into account during decisions about surgical or interventional revascularization in these complex patients. These are probably some of the reasons why PCI was used more frequently than CABG in our cohort.
Dual antiplatelet therapy may carry increased risk in adults with CHD due to the common use of anticoagulants to treat chronic atrial fibrillation in this population as well as the hemorrhagic diathesis of complex and cyanotic patients [23]. If PCI is indicated, newer generation stents (thin strut) should probably be used as shorter duration dual antiplatelet therapy is required.
MI is increasingly occurring in our ageing adult CHD patients, possibly with higher incidence and mortality compared to the general population [24]. Significant CAD was an independent risk factor for mortality in adults with repaired TOF, a large percentage of whom had left ventricular dysfunction at CAD diagnosis [20]. Although the cause of left ventricular dysfunction may be multifactorial and related to CHD, early detection and revascularization may positively influence the outcome of these patients.
This study has several limitations. It is a small retrospective study with non-homogeneous population in terms of original diagnosis, clinical picture and management. Patients were identified based on the recollection of their physicians and their being followed by both the adult CHD and the adult cardiology departments, therefore the actual prevalence of CAD in adults with CHD may have been underestimated in this study. The decision to perform coronary angiography was not part of a protocol but rather the decision of the primary cardiologist in response to symptoms or during a preoperative or necessary hemodynamic evaluation. As coronary angiography is not routinely performed in our patients, asymptomatic or minimally symptomatic CAD may have been overlooked.
As advances in medical and surgical management of CHD improve the long-term survival of these patients, acquired CAD is increasingly recognized as this population ages and acquires cardiovascular risk factors. Increased awareness of physicians and patients, screening and detection strategies and adequate preventive and therapeutic management are required in order to improve cardiovascular morbidity and mortality in adult CHD patients. Careful assessment and selection of a combination of medical, transcatheter and surgical treatment are warranted for optimization of outcome for this complex patient population.
Availability of Data and Materials: All data used in the study can be accessed after request to the authors.
Author Contributions: SCA and SB were involved with conceptualizing the idea. SCA and ES performed data acquisition and data analysis and drafted the manuscript. SB, DT and KT helped in drafting and revising the manuscript critically. All authors approve the submitted and final version of the manuscript.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest with the contents of this article.
1. Van Der Bom, T., Bouma, B. J., Meijboom, F. J., Zwinderman, A. H., Mulder, B. J. (2012). The prevalence of adult congenital heart disease, results from a systematic review and evidence-based calculation. American Heart Journal, 164(4), 568–575. DOI 10.1016/j.ahj.2012.07.023.
2. Afilalo, J., Therrien, J., Pilote, L., Ionescu-Ittu, R., Martucci, G. et al. (2011). Geriatric congenital heart disease. burden of disease and predictors of mortality. Journal of the American College of Cardiology, 58(14), 1509–1515. DOI 10.1016/j.jacc.2011.06.041.
3. Niwa, K. (2019). Metabolic syndrome in adult congenital heart disease. Korean Circulation Journal, 49(8), 691–708. DOI 10.4070/kcj.2019.0187.
4. Giannakoulas, G., Dimopoulos, K., Engel, R., Goktekin, O., Kucukdurmaz, Z. et al. (2009). Burden of coronary artery disease in adults with congenital heart disease and its relation to congenital and traditional heart risk factors. American Journal of Cardiology, 103(10), 1445–1450. DOI 10.1016/j.amjcard.2009.01.353.
5. Yalonetsky, S., Horlick, E. M., Osten, M. D., Benson, L. N., Oechslin, E. N. et al. (2013). Clinical characteristics of coronary artery disease in adults with congenital heart defects. International Journal of Cardiology, 164(2), 217–220. DOI 10.1016/j.ijcard.2011.07.021.
6. Tarp, J. B., Sγέrgaard, M. H., Christoffersen, C., Jensen, A. S., Sillesen, H. et al. (2019). Subclinical atherosclerosis in patients with cyanotic congenital heart disease. International Journal of Cardiology, 277, 97–103. DOI 10.1016/j.ijcard.2018.08.104.
7. Budts, W. (2014). Chest pain in adult congenital heart disease: where does it come from?. Acta Clinica Belgica, 59(2), 68–78. DOI 10.1179/acb.2004.010.
8. Awerbach, J. D., Krasuski, R. A., Camitta, M. G. W. (2018). Coronary disease and modifying cardiovascular risk in adult congenital heart disease patients: should general guidelines apply?. Progress in Cardiovascular Diseases, 61((3–4), ), 300–307. DOI 10.1016/j.pcad.2018.07.018.
9. Pemberton, V. L., Mccrindle, B. W., Barkin, S., Daniels, S. R., Barlow, S. E. et al. (2010). Report of the National Heart, Lung, and Blood Institute’s Working Group on obesity and other cardiovascular risk factors in congenital heart disease. Circulation, 121(9), 1153–1159. DOI 10.1161/CIRCULATIONAHA.109.921544.
10. Madsen, N. L., Marino, B. S., Woo, J. G., Thomsen, R. W., Videbœk, J. et al. (2016). Congenital heart disease with and without cyanotic potential and the long-term risk of diabetes mellitus: a population-based follow-up study. Journal of the American Heart Association, 5(7), 1. DOI 10.1161/JAHA.115.003076.
11. Krishnamurthy, Y., Stefanescu Schmidt, A. C., Bittner, D. O., Scholtz, J. E., Bui, A. et al. (2019). Subclinical burden of coronary artery calcium in patients with coarctation of the aorta. American Journal of Cardiology, 123(2), 323–328. DOI 10.1016/j.amjcard.2018.10.017.
12. Bauer, U., Körten, M. A., Diller, G. P., Helm, P., Baumgartner, H. et al. (2019). Cardiovascular risk factors in adults with congenital heart defects—recognised but not treated? An analysis of the German national register for congenital heart defects. International Journal of Cardiology, 277, 79–84. DOI 10.1016/j.ijcard.2018.08.009.
13. Piepoli, M. F., Hoes, A. W., Agewall, S., Albus, C., Brotons, C. et al. (2016). 2016 European Guidelines on cardiovascular disease prevention in clinical practice. European Heart Journal, 37(29), 2315–2381. DOI 10.1093/eurheartj/ehw106.
14. Perloff, J. K. (2012). Cyanotic congenital heart disease the coronary arterial circulation. Current Cardiology Reviews, 8(1), 1–5. DOI 10.2174/157340312801215836.
15. Segura, T., Gatzoulis, M. A. (2019). Where are we with coronary artery disease for the cyanotic patient with congenital heart disease?. International Journal of Cardiology, 277, 108–109. DOI 10.1016/j.ijcard.2018.10.033.
16. Davos, C. H., Davlouros, P. A., Wensel, R., Francis, D., Davies, L. C. et al. (2002). Global impairment of cardiac autonomic nervous activity late after repair of tetralogy of Fallot. Circulation, 106(1), I69–75. DOI 10.1161/01.CIR.0000020013.73106.D8.
17. Baumgartner, H., Falk, V., Bax, J. J., De Bonis, M., Hamm, C. et al. (2017). 2017 ESC/EACTS Guidelines for the management of valvular heart disease. European Heart Journal, 38(36), 2739–2791. DOI 10.1093/eurheartj/ehx391.
18. Joseph, J., Velasco, A., Hage, F. G., Reyes, E. (2018). Guidelines in review: Comparison of ESC and ACC/AHA guidelines for the diagnosis and management of patients with stable coronary artery disease. Journal of Nuclear Cardiology, 25(2), 509–515. DOI 10.1007/s12350-017-1055-0.
19. Dłużniewska, N., Podolec, P., Miszalski-Jamka, T., Krupiński, M., Banyś, P. et al. (2018). Effect of ventricular function and volumes on exercise capacity in adults with repaired Tetralogy of Fallot. Indian Heart Journal, 70(1), 87–92. DOI 10.1016/j.ihj.2017.07.021.
20. Egbe, A. C., Ananthaneni, S., Jadav, R., Kothapalli, S., Rihal, C. S. et al. (2019). Coronary artery disease in adults with tetralogy of Fallot. Congenital Heart Disease, 14(3), 491–497. DOI 10.1111/chd.12782.
21. Giamberti, A., Lo Rito, M., Conforti, E., Varrica, A., Carminati, M. et al. (2017). Acquired coronary artery disease in adult patients with congenital heart disease. a true or a false problem?. Journal of Cardiovascular Medicine (Hagerstown), 18, 605–609.
22. Thuijs, D., Kappetein, A. P., Serruys, P. W., Mohr, F. W., Morice, M. C. et al. (2019). Percutaneous coronary intervention versus coronary artery bypass grafting in patients with three-vessel or left main coronary artery disease. 10-year follow-up of the multicentre randomised controlled SYNTAX trial. Lancet, 394(10206), 1325–1334. DOI 10.1016/S0140-6736(19)31997-X.
23. Chaix, M. A., Gatzoulis, M. A., Diller, G. P., Khairy, P., Oechslin, E. N. (2019). Eisenmenger syndrome: a multisystem disorder—Do not destabilize the balanced but fragile physiology. Canadian Journal of Cardiology, 35(12), 1664–1674. DOI 10.1016/j.cjca.2019.10.002.
24. Olsen, M., Marino, B., Kaltman, J., Laursen, H., Jakobsen, L. et al. (2017). Myocardial infarction in adults with congenital heart disease. American Journal of Cardiology, 120(12), 2272–2277. DOI 10.1016/j.amjcard.2017.08.050.
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |