

 | Congenital Heart Disease |  |
DOI: 10.32604/CHD.2020.011983
ARTICLE
Causes of Death after Congenital Heart Surgery in Children
1Department of Thoracic and Cardiovascular Surgery, Shanghai Children’s Medical Center, Shanghai Jiao Tong University School of Medicine, Shanghai, China
2Department of Pediatric Surgery, Shanghai Children’s Medical Center, Shanghai Jiao Tong University School of Medicine, Shanghai, China
*Corresponding Author: Nan Bao. Email: bnscmc@shsmu.edu.cn
Received: 16 June 2020; Accepted: 14 August 2020
Abstract: Background: This retrospective cohort study aimed to explore the causes of death in children with congenital heart disease (CHD) after cardiac surgery in one of the biggest cardiac centers for children with CHD in China. Methods: A total of 26,856 children undergoing cardiac surgery from January 1, 2012 to December 31, 2019 were included. Based on the clinical data, the causes of death were divided into ten categories and further compared among different periods, types of CHD and surgical procedures. Results: Of all patients, 513 (1.9%) died (median age 162 d, median weight 5.6 kg). The mortality in 2016–2019 was lower than that in 2012–2015 (1.4 ± 0.3% vs. 2.5 ± 0.3%, p = 0.005). A total of 42.5% of children died of heart failure, and 32.9% died of residual anatomic defects. Patients with transposition of the great arteries tended to die from residual anatomic defects (21.9%), while those with double-outlet right ventricle (20%) and single ventricle (20%) tended to die from pulmonary hypertension (PH) (p = 0.006). After biventricular repair, children tended to die from heart failure (90.4%), while after single-ventricle repair, children tended to die from PH (50%) (p < 0.0001). There is a negative correlation between mortality and the ECMO implantation rate (r = −0.898, p = 0.002). Conclusions: Heart failure and residual anatomic defects were the main causes of death after cardiac surgery. The cause of death patterns differed among CHD types and surgical strategies. ECMO may be a life-saving tool when other conventional therapies do not work.
Keywords: Congenital heart disease; causes of death; cardiac surgery
The global prevalence of congenital heart disease (CHD) at birth is estimated to be 1 per 1000 live births [1]. The diagnostic and treatment methodologies for CHD have dramatically improved over the past 80 years, and the survival rate of infants with critical CHD has improved by almost 20% in a survey of North America [2]. Reports from Belgium and Sweden found that 90–95% of children with CHD survived into adulthood [3,4].
However, in China, the overall prevalence of CHD is 8.98 per 1000 live births and that of critical CHD is 1.46 per 1000 live births, as reported by a national survey [5]. The first specialized CHD team was established in the mid-1970s. After nearly 40 years of hard work, an increasing number of children’s heart centers have been founded. At present, the mortality of CHD has dropped to 2–3% in a few professional pediatric heart centers that have reached the advanced international level [6].
The rapid development of cardiac centers has benefited from the following aspects: improved surgical skills, myocardial protection from cardiopulmonary bypass (CPB), and advanced postoperative monitoring [7,8]. Most importantly, children with extremely unstable circulation can survive through extracorporeal membrane oxygenation (ECMO).
However, literature on mortality and causes of death after cardiac surgery in children with CHD is very limited [9–11]. As one of the largest children’s CHD centers in China, our heart center treats more than 3,700 surgical cases every year. The patients are getting much younger and more complicated. The purpose of this study was to analyze the recent mortality data and explore the main causes of death in children with CHD after cardiac surgery.
This was a retrospective study of children undergoing cardiac surgery from January 1, 2012 to December 31, 2019 and was approved by the medical ethics committee of Shanghai Children’s Medical Center, School of Medicine, Shanghai Jiaotong University (SCMCIRB-W2020008) on February 27, 2019. The requirement for patient consent was waived. The age of the patients was under 18 years. In-hospital death was defined as any death occurring after the cardiac surgical procedure but before hospital discharge. ECMO might be initiated immediately after surgery in the operating room, as cardiopulmonary resuscitation in the cardiac intensive care unit (CICU), or selectively due to circulatory instability in the CICU. The ECMO implantation rate referred to the ratio of the number of ECMO cases to the number of cardiac surgeries in the same year.
The patient’s diagnosis was based on echo, CT, MRI, or cardiac catheterization, combined with intraoperative findings or pathology. The surgical procedure was divided into single-ventricle repair and biventricle repair. Single-ventricle surgery included systemic-to-pulmonary shunt, Glenn, and Fontan surgeries. Patients with an unstable hemodynamic status were considered to have critical CHD by doctors in the CICU and a high pediatric risk of mortality (PRISM) score.
The causes of death were discussed and concluded by the surgeons and the doctors in the CICU. Based on the clinical data, especially development of the disease and treatment in the CICU, the main causes of death were divided into ten categories. The causes of death mainly included heart failure, residual anatomic defect, cardiac arrest, pulmonary hypertension (PH), sepsis, abandoned therapy by the parents or guardians with custody, respiratory failure, neonatal necrotizing enterocolitis (NEC), disseminated intravascular coagulation (DIC) and brain death.
Heart failure often manifested as low cardiac output referred to a dramatic decrease in cardiac output after cardiac surgery, manifested by systemic, pulmonary venous congestion, and inadequate blood flow for organ perfusion. Typical manifestations were tachycardia, hypotension, oliguria (<1 ml/kg/h), hepatomegaly, cold limbs and central hyperthermia. Postoperative anatomical residues included aberrant coronary artery, residue obstruction (generally included stenosis of pulmonary vein, pulmonary artery, left and right ventricular outflow tract, aortic stenosis and the stenosis of the implanted conduit), valvular regurgitation and stenosis, imbalanced blood flow ratio of systemic circulation and pulmonary circulation. Cardiac arrest referred to sudden cardiac arrest with unknown reason. Pulmonary hypertension mainly referred to postoperative hypertensive crisis after bi-ventricle repair or circulation failure caused by increased pulmonary arterial pressure after single ventricle repair.
The data were analyzed using SPSS 22.0 (IBM, Armonk, NY, USA). Data with a normal distribution are presented as the means ± standard deviation (SD). Non-normally distributed values are presented as medians and interquartile ranges (IQRs, 25th and 75th percentiles), and the medians of the two groups were compared using the Mann-Whitney U test. Categorical data are represented as frequencies and percentages, and the chi-square test was used for testing. Correlation analyses of mortality, incidence of heart failure, and ECMO implantation rate were performed by Pearson correlation analysis. A p-value <0.05 was considered statistically significant.
The patient characteristics are summarized in Tab. 1. A total of 26,856 children underwent cardiac surgery from January 1, 2012 to December 31, 2019. The age of the cohort was 286.7 ± 62 d and the weight was 12.9 ± 7.6 kg with the ratio of male to female 8:7. 10,385 (39.1%) had critical CHD. Overall, 513 children died, and the total mortality rate was 1.9%. Among the patients who died, 209 were female (40.7%), and 304 were male (59.3%). The median age was 162 (IQR 60-396) d. Children <6 m accounted for half of the population (50.9%), 6 m–1 y accounted for 22.6%, and >1 y accounted for 26.5%. The median weight was 5.6 (IQR 3.8-8.1) kg. Children <5 kg accounted for nearly half of the population (44.8%), those 5–10 kg accounted for 36.3%, and those >10 kg accounted for 18.9%. Biventricular repair was performed in 82.1% of the patients who died, while single-ventricle repair was performed in 17.9%.
Table 1: Characteristics of death data after cardiac surgery
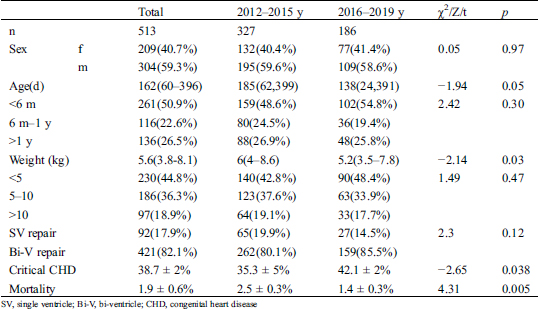
A total of 327 patients died from 2012 to 2016, and 186 died from 2016 to 2019. The mortality rate in 2016–2019 was significantly lower than that in 2012–2015 (1.4 ± 0.3% 2.5 ± 0.3%, p = 0.005), but the critical CHD rate in 2016–2019 was significantly higher than that in 2012–2015 (42.1 ± 2% vs. 35.3 ± 5%, p = 0.038). Although the median age of 138 d in 2016–2019 was younger than the median age of 185 d in 2012–2015, there was no significant difference in age between the two groups (Z = −1.94, p = 0.05). The distribution of age was not significantly different in the <6 m, 6 m–1 y, or > 1 y subgroups between 2012–2015 and 2016–1019 (χ2 = 2.42, p = 0.3). The weight of 5.2 (IQR3.5–7.8) kg in 2016–2019 was significantly lighter than that the 6 (IQR4–8.6) kg in 2012–2016 (Z = -2.14, p = 0.03). However, there were no significant differences in body weight distribution between 2012–2015 and 2016–2019 in the subgroups <5 kg, 5–10 kg, and >10 kg (χ2 = 1.49, p = 0.47).
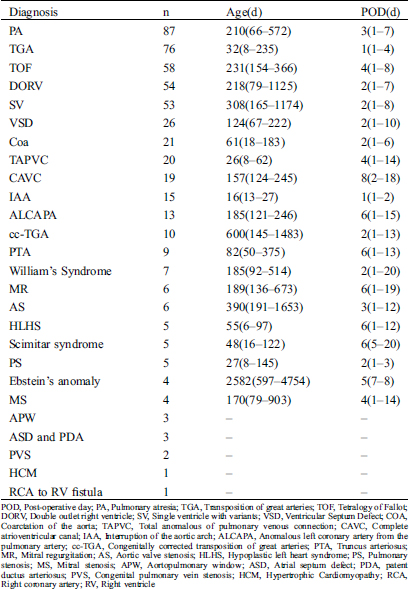
There were 26 types of CHD Tab. 2. IAA died in the younger age 16 d (IQR13-27d) but Ebstein’s anomaly died in the older age 2582 d (IQR597-4754). Patients with TGA and IAA was likely to die in the early period after surgery but patients with CAVC was likely to die in the late period after the surgery.
Table 2: Time of death among CHD disease
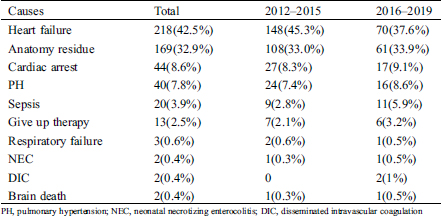
42.5% of children died of heart failure, followed by residual anatomic defects (32.9%), cardiac arrest (8.6%), PH (7.8%), sepsis (3.9%), and abandoned therapy by the parents or guardians with custody (2.5%) (Tab. 3). The mortality of respiratory failure, NEC, DIC and brain death was less than 1%. Comparing the distribution of causes of death across different periods, there was no significant difference in the distribution of causes of death between 2012–2015 and 2016–2019 (χ2 = 5.87, p = 0.319).
Table 3: Comparison of causes of death
Left ventricle failure (LVF) accounted for 75.2% of heart failure cases, and right ventricle failure (RVF) accounted for 24.8%. Among the residual anatomic defects, aberrant coronary artery accounted for 30.8%, residue obstruction accounted for 26.7%, an imbalanced blood flow ratio between systemic circulation and pulmonary circulation accounted for 24.3%, and valvular dysfunction accounted for 18.2% (Fig. 1).

Figure 1:
The main cause of death after PA was heart failure, and most of the patients RVF (26/33). The main cause of death from TGA was residual anatomic defects (30/76). The main cause of death after TOF repair was heart failure, but approximately half of these patients had LVF (16/30). However, the analysis of the distribution of causes of death showed that patients with TGA tended to die from residual anatomic defects (37/169, 21.9%); 28 of the 37 patients had aberrant coronary arteries; in contrast, those with DORV (8/40, 20%) and SV (8/40, 20%) tended to die from PH (χ2 = 38.73, p = 0.006) (Fig. 2).

Figure 2: Causes of death after different types of CHD repair
In terms of different surgical procedures, children who underwent biventricular repair tended to die from heart failure (197/218, 90.4%), while children who underwent single-ventricle repair tended to die from PH (20/40, 50%) (χ2 = 37.1, p < 0.0001) (Fig. 3). Among the 92 deaths after single-ventricle repair, the number of deaths from heart failure, residual anatomic defect, cardiac arrest, PH, sepsis, abandoned therapy by the parents or guardians with custody, respiratory failure, NEC, DIC and brain death were 21, 36, 5, 20, 2, 4, 1, 1, 1, and 1, respectively. Among 421 deaths after biventricular repair, the number of deaths was 197, 133, 39, 20, 18, 9, 2, 1, 1, and 1, respectively.

Figure 3: Causes of death after different surgical repairs
3.4 Correlation between ECMO and Mortality
The mortality rates from 2012 to 2019 were 2.98%, 2.41%, 2.08%, 2.34%, 1.65%, 1.57%, 1.39%, and 0.94%, while the implantation rates of ECMO were 0.03%, 0.06%, 0.12%, 0.15%, 0.40%, 0.90%, 1.07% and 1.14%, respectively. The curve of ECMO implantation showed a rising trend while the mortality showed a decreasing trend. Correlation analysis showed a significant negative correlation between mortality and the ECMO implantation rate from 2012 to 2019 (r = −0.898, p = 0.002) (Fig. 4). Among the different causes of death, only heart failure had a correlation with ECMO implantation, with a negative correlation (r = −0.816, p = 0.013). There was no significant correlation between other causes of death and ECMO: residual anatomic defect (r = 0.66, p = 0.075), cardiac arrest (r = 0.39, p = 0.34), PH (r = −0.445, p = 0.269), sepsis (r = 0.23, p = 0.58), or abandoned therapy (r = −0.18, p = 0.68).

Figure 4: Correlation analysis of ECMO and mortality
In our study, the overall mortality after CHD surgery was 1.9%, and the mortality rate has decreased significantly in recent years (2016–2019 1.4 ± 0.3% vs. 2012–2015 2.5 ± 0.3%, p = 0.005). Compared with the mortality rates of other heart centers in the world, the mortality rate in our center was similar [9]. In a registry of 123 centers in North America (120 in the United States and 3 in Canada), the mortality rate after CHD surgery was 3.7% from 2010 through 2012 [10]. Another study with a mortality rate of 3.6% showed that there was no relation between surgical volume and mortality between 2003 and 2011 [11]. Fuwai Cardiovascular Center, another famous heart center in China with a similar surgical volume to our center, had a lower mortality rate (<1%) [5]. However, the complexity of disease treated by the two centers was not homogeneous.
The study also found that deaths mainly occurred at a young age and in those with low weight, with children <6 months accounting for half of the deaths (50.9%) and children <5 kg accounting for nearly half of the deaths (44.8%). Moreover, the weight in 2016–2019 was significantly lower than the weight in 2012–2016 (Z = −2.14, p = 0.03). Similarly, Alsoufi [12] reported that underweight (≤2.5 kg) was associated with a significant increase in hospital mortality (OR, 2.15; p = 0.002). Therefore, some new clinical monitoring and treatment methods have focused on this population, aiming to improve the survival rate of younger and low-weight patients. Some studies demonstrated that multichannel near infrared reflectance spectroscopy could non-invasively monitor tissue oxygen supply, including that to the brain, kidneys, gastrointestinal tract and limbs, in real time in CHD patients with younger age and lighter weight [13,14].
The main cause of death after cardiac surgery in our study was heart failure (42.5%), which was characterized by low cardiac output. It was reported low cardiac output occurred in 25–65% of pediatric patients with CHD undergoing cardiac surgery with CPB support [15]. In a report from Boston Children’s Hospital, 19% of the deaths were due to ventricular failure, which was much lower than the rate in our study [16]. Studies showed immature myocardium was extremely sensitive to extracellular calcium compared to mature myocardium [17,18]. This might also be one of the reasons why most of the deaths were concentrated in the young age population in our study.
With regard to the distribution of causes of death among different CHD types, our study showed that those with TGA tended to die from residual anatomic defects (37/169, 21.9%), and 28 of the 37 patients had aberrant coronary arteries. Similarly, another study showed coronary anomalies were present in 33.7% of TGA patients, and coronary sinuses with more than 1 ostium were associated with a significantly increased risk of postoperative death (hazard ratio 2.58) [19]. However, an increasing number of recent studies have demonstrated that aberrant coronary arteries might not impact mortality after TGA repair because of modified surgical techniques using double coronary buttons with an unroofed intramural course, a single coronary button, or aortopulmonary fenestration [20,21].
Another special cause of death that differed among CHD types and surgical repairs was PH in not only patients with SV disease (8/40, 20%) but also after single-ventricle repair (20/40, 50%). PH, as an independent cause of death, was reported in 8% of patients after cardiac surgery in Boston Children’s Hospital, and similarly, the rate was 7.8% in our study [16]. PH may be a risk factor for early and late mortality after both Glenn shunt and Fontan operations [22]. Especially after the Fontan operation, a slight increase in pulmonary vascular resistance would lead to a dramatic decrease in cardiac output as the pulmonary circuit has no pump [23]. Thus, maintaining a low pulmonary vascular resistance (PVR) is essential to guaranteeing durability early after single-ventricle repair in patients with a high risk of PH caused by CPB [24]. The updated consensus statement on the diagnosis and treatment of PH of the European Pediatric Pulmonary Vascular Disease Network suggested that the hemodynamic threshold for operability pre-Fontan surgery was probably a mean transpulmonary pressure gradient ≤6 mmHg in children/young adults with single-ventricle physiology [25].
In addition, our study showed a strong negative correlation between mortality and ECMO implantation rate (r = −0.898, p = 0.002), which indicated that ECMO may be a life-saving tool when other conventional therapies do not work. The use of ECMO for cardiac failure has steadily increased in recent years, and it was reported that up to 2–5% of all children undergoing cardiac surgery require mechanical cardiac support with ECMO in the postoperative period [26,27]. Compared with these data, the ECMO rate in our center was much lower, at 0.03% in 2012 and rising to 1.14% in 2019. The overall survival rate of patients with ECMO support was 40–50% in the literature [28,29]. A study in our hospital showed that 36.4% of patients were discharged successfully after ECMO resuscitation, which suggests that one-third of the patients may survive after resuscitation supported by ECMO [30].
The primary limitation of this study is its retrospective nature and reliance on a review of medical records. Providing the actual causes of death after surgery for CHD is difficult because of the multiple processes involved and the different criteria and points of view of people who are involved in the process of assigning the cause of death. In addition, it was carried out at a highly developed and specialized center with substantial expertise and, therefore, might not reflect the entire situation in China. Despite these limitations, our study provides some important insights into the patterns of causes of death and potential improvements for strategies that might reduce mortality after congenital heart surgery. Specifically, close attention should be paid to these causes before surgery, and top priority should be given to the careful evaluation of these factors related to different types of CHD and surgical procedures.
Data Availability Statement: Data are available on reasonable request.
Funding Statement: Supported by National Natural Science Foundation of China (81771934).
Conflicts of interest: The authors declare that there is no conflict of interest.
1. GBD 2017 Congenital Heart Disease Collaborators. (2020). Global, regional, and national burden of congenital heart disease, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet, 4(3), 185–200.
2. Oster, M. E., Lee, K. A., Honein, M. A., Riehle-Colarusso, T., Shin, M. et al. (2013). Temporal trends in survival among infants with critical congenital heart defects. Pediatrics, 131(5), e1502–e1508. DOI 10.1542/peds.2012-3435.
3. Moons, P., Bovijn, L., Budts, W., Belmans, A., Gewillig, M. (2010). Temporal trends in survival to adulthood among patients born with congenital heart disease from 1970 to 1992 in Belgium. Circulation, 122(22), 2264–2272. DOI 10.1161/CIRCULATIONAHA.110.946343.
4. Mandalenakis, Z., Rosengren, A., Skoglund, K., Lappas, G., Eriksson, P. et al. (2017). Survivorship in children and young adults with congenital heart disease in Sweden. JAMA, 177(2), 224–230.
5. Zhao, Q. M., Liu, F., Wu, L., Ma, X. J., Niu, C. et al. (2019). Prevalence of congenital heart disease at live birth in China. Journal of Pediatrics, 204, 53–58. DOI 10.1016/j.jpeds.2018.08.040.
6. Li, S. J. (2012). Strive to improve the clinical efficacy of complex congenital heart disease. Chinese Journal of Clinicians (Electronic Edition), 6(22), 7023–7025.
7. Damberg, A., Carino, D., Charilaou, P., Peterss, S., Tranquilli, M. et al. (2017). Favorable late survival after aortic surgery under straight deep hypothermic circulatory arrest. Journal of Thoracic and Cardiovascular Surgery, 154(6), 1831–1839.e1. DOI 10.1016/j.jtcvs.2017.08.015.
8. Harjola, V. P., Parissis, J., Brunner-La Rocca, H. P., Čelutkienė, J., Chioncel, O. et al. (2018). Comprehensive in-hospital monitoring in acute heart failure: applications for clinical practice and future directions for research. A statement from the acute heart failure committee of the heart failure association (HFA) of the European Society of Cardiology (ESC). European Journal of Heart Failure, 20(7), 1081–1099. DOI 10.1002/ejhf.1204.
9. Martin, G. R., Jonas, R. A. (2018). Surgery for congenital heart disease: improvements in outcomes. American Journal of Perinatology, 35(06), 557–560. DOI 10.1055/s-0038-1639358.
10. Jacobs, J. P., O’Brien, S. M., Pasquali, S. K., Kim, S., Gaynor, J. W. et al. (2014). The importance of patient-specific preoperative factors: an analysis of the society of thoracic surgeons congenital heart surgery database. Annals of Thoracic Surgery, 98(5), 1653–1659. DOI 10.1016/j.athoracsur.2014.07.029.
11. Kansy, A., Ebels, T., Schreiber, C., Tobota, Z., Maruszewski, B. (2014). Association of center volume with outcomes: analysis of verified data of european association for cardio-thoracic surgery congenital database. Annals of Thoracic Surgery, 98(6), 2159–2164. DOI 10.1016/j.athoracsur.2014.07.065.
12. Alsoufi, B., Manlhiot, C., Mahle, W. T., Brian, K., William, L. B. et al. (2014). Low-weight infants are at increased mortality risk after palliative or corrective cardiac surgery. Journal of Thoracic and Cardiovascular Surgery, 148(6), 2508–2514.e1. DOI 10.1016/j.jtcvs.2014.07.047.
13. Hickok, R. L., Spaeder, M. C., Berger, J. T., Schuette, J. J., Klugman, D. (2016). Postoperative abdominal NIRS values predict low cardiac output syndrome in neonates. World Journal for Pediatric and Congenital Heart Surgery, 7(2), 180–184. DOI 10.1177/2150135115618939.
14. Green, M. S., Sehgal, S., Tariq, R. (2016). Near-infrared spectroscopy: the new must have tool in the intensive care unit?. Seminars in Cardiothoracic and Vascular Anesthesia, 20(3), 213–224. DOI 10.1177/1089253216644346.
15. Robert, S. M., Borasino, S., Dabal, R. J., Cleveland, D. C., Hock, K. M. et al. (2015). Postoperative hydrocortisone infusion reduces the prevalence of low cardiac output syndrome after neonatal cardiopulmonary bypass. Pediatric Critical Care Medicine, 16(7), 629–636. DOI 10.1097/PCC.0000000000000426.
16. Ma, M., Gauvreau, K., Allan, C. K., Mayer Jr, J. E., Jenkins, K. J. (2007). Causes of death after congenital heart surgery. Annals of Thoracic Surgery, 83(4), 1438–1445. DOI 10.1016/j.athoracsur.2006.10.073.
17. Frank, A., Bonney, M., Bonney, S., Lindsay, W., Michael, K., et al. (2012). Myocardial ischemia reperfusion injury: from basic science to clinical bedside. Seminars in Cardiothoracic and Vascular Anesthesia, 16(3), 123–132.
18. Bautista-Hernandez, V., Karamanlidis, G., McCully, J. D., Del Nido, P. J. (2015). Cellular and molecular mechanisms of low cardiac output syndrome after pediatric cardiac surgery. Current Vascular Pharmacology, 14(1), 5–13. DOI 10.2174/1570161113666151014122557.
19. Kolovos, N. S., Bratton, S. L., Moler, F. W., Bove, E. L., Ohye, R. G. et al. (2003). Outcome of pediatric patients treated with extracorporeal life support after cardiac surgery. Annals of Thoracic Surgery, 76(5), 1435–1441. DOI 10.1016/S0003-4975(03)00898-1.
20. Szymczyk, K., Moll, M., Sobczak-Budlewska, K., Moll, J. A., Stefańczyk, L. et al. (2018). Usefulness of routine coronary CT angiography in patients with transposition of the great arteries after an arterial switch operation. Pediatric Cardiology, 39(2), 335–346. DOI 10.1007/s00246-017-1761-z.
21. Koshiyama, H., Nagashima, M., Matsumura, G., Hiramatsu, T., Nakanishi, T. et al. (2016). Arterial switch operation with and without coronary relocation for intramural coronary arteries. Annals of Thoracic Surgery, 102(4), 1353–1359. DOI 10.1016/j.athoracsur.2016.03.031.
22. Ohuchi, H. (2017). Where is the “Optimal” Fontan Hemodynamics?. Korean Circulation Journal, 47(6), 842–857. DOI 10.4070/kcj.2017.0105.
23. Ohuchi, H., Miyazaki, A., Negishi, J., Hayama, Y., Nakai, M. et al. (2017). Hemodynamic determinants of mortality after Fontan operation. American Heart Journal, 189, 9–18. DOI 10.1016/j.ahj.2017.03.020.
24. Castaldi, B., Bordin, G., Padalino, M., Cuppini, E., Vida, V. et al. (2018). Hemodynamic impact of pulmonary vasodilators on single ventricle physiology. Cardiovascular Therapeutics, 36(1), e12314. DOI 10.1111/1755-5922.12314.
25. Hansmann, G., Koestenberger, M., Alastalo, T. P., Apitz, C., Austin, E. D. et al. (2019). 2019 updated consensus statement on the diagnosis and treatment of pediatric pulmonary hypertension: the European Pediatric Pulmonary Vascular Disease Network (EPPVDN), endorsed by AEPC, ESPR and ISHLT. Journal of Heart and Lung Transplantation, 38(9), 879–901. DOI 10.1016/j.healun.2019.06.022.
26. Kane, D. A., Thiagarajan, R. R., Wypij, D., Scheurer, M. A., Fynn-Thompson, F. et al. (2010). Rapid-response extracorporeal membrane oxygenation to support cardiopulmonary resuscitation in children with cardiac disease. Circulation, 122((11 Suppl), ), S241–S248. DOI 10.1161/CIRCULATIONAHA.109.928390.
27. Gupta, P., McDonald, R., Chipman, C. W., Stroud, M., Gossett, J. M. et al. (2012). 20-year experience of prolonged extracorporeal membrane oxygenation in critically ill children with cardiac or pulmonary failure. Annals of Thoracic Surgery, 93(5), 1584–1590. DOI 10.1016/j.athoracsur.2012.01.008.
28. Gupta, P., Robertson, M. J., Beam, B. W., Rettiganti, M. (2015). Outcomes associated with preoperative use of extracorporeal membrane oxygenation in children undergoing heart operation for congenital heart disease: a multi-institutional analysis. Clinical Cardiology, 38(2), 99–105. DOI 10.1002/clc.22358.
29. Schmidt, M., Burrell, A., Roberts, L., Bailey, M., Sheldrake, J. et al. (2015). Predicting survival after ECMO for refractory cardiogenic shock: the survival after veno-arterial-ECMO (SAVE)-score. European Heart Journal, 36(33), 2246–2256. DOI 10.1093/eurheartj/ehv194.
30. Guo, Z., Yang, Y., Zhang, W., Shen, J., Jiang, L. et al. (2019). Extracorporeal cardiopulmonary resuscitation in children after open heart surgery. Artificial Organs, 43(7), 633–640. DOI 10.1111/aor.13408.
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |