

 | Congenital Heart Disease |  |
DOI: 10.32604/CHD.2020.012249
ARTICLE
Pseudoaneurysm after Tetralogy of Fallot Repair Using Right Ventricular Outflow Tract Patch
Pediatric Cardiac Center, Beijing Anzhen Hospital, Capital Medical University, Beijing Institute of Heart Lung and Blood Vessel Diseases, Beijing, 100029, China
*Corresponding Author: Junwu Su. Email: sujunwuaz@163.com
Received: 24 June 2020; Accepted: 21 August 2020
Abstract: Background: Pseudoaneurysm complicating right ventricular outflow tract (RVOT) with conduit placement was an infrequent complication but with potential for significant morbidity and mortality, and a more unusual pseudoaneurysm after RVOT patching was investigated here. Methods: Patients diagnosed as pseudoaneurysms at our institution from 2010 to 2019 were reviewed and their clinical characteristics were analyzed. Results: A total of seven patients developed pseudoaneurysm in RVOT were identified. One pseudoaneurysm arose after placement of a conduit between the right ventricle and the pulmonary artery, and the other six formed after RVOT patching. One patient presented with arrhythmia, one patient had the pseudoaneurysm discovered after the diagnosis of subacute infective endocarditis and mediastinal infection, another patient was diagnosed during the operation for relief of anastomotic stenosis, and the other four patients were diagnosed during follow-up. Three patients had a RVOT pressure gradient immediately postoperatively and a progressive elevation, and another two patients presented with new emergence of RVOT pressure gradient at the discovery of pseudoaneurysm. A new mass in the left superior mediastinum on chest X ray film was showed in 5 patients. The pseudoaneurysm was surgical resected successfully with an uneventful recovery course and without relapse in six patients, and the other one caused by infection was still in a strict follow-up. Conclusion: The increasing elevation or new emergence of RVOT pressure gradient and radiological abnormality with a new mass in superior mediastinum are probable evidences for pseudoaneurysm formation after RVOT patch repair, and the clinicians should pay special attention to patients when indicated.
Keywords: Congenital heart disease; surgery; tetralogy of Fallot; pseudoaneurysm
Pseudoaneurysm formation after right ventricular outflow tract (RVOT) surgical procedures is an unnoticed complication of cardiac surgical repair though one study shows the incidence of 2.1% [1]. The reports for pseudoaneurysm complicating right ventricle-to-pulmonary artery conduit operations are rare, and only two studies from Boston Children’s Hospital [1,2] and several case reports [3–6] have been found. Moreover, the studies of this complication after RVOT patching is rarer, and it might be an overlooked condition in these patients. Pseudoaneurysm may present asymptomatic or as secondary symptoms (primarily respiratory symptoms or shock), and in most cases, the identified pseudoaneurysms were only incidentally diagnosed. However, pseudoaneurysm has not only preoperative risk of infection, thromboembolism, compression of adjoining structures and rupture but also the risk of catastrophic bleeding during repeat median sternotomy. Therefore, the knowledge for preventing, identifying and treating pseudoaneurysm in an appropriate manner need furtherly accumulate. Here we reported our experiences for this complication in recent years, and most of the patients diagnosed as TOF/PS (Tetralogy of Fallot with Pulmonary Stenosis) and underwent complete repair using a transannular or infundibular patch, which differ from the experiences from patients underwent conduit placement in previous reports [1–6].
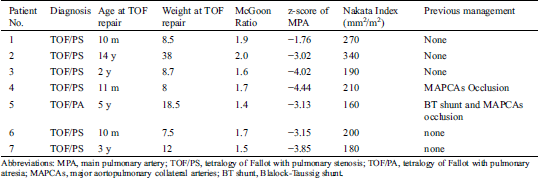
A retrospective study was conducted in this institution with approval of the ethics committee for the analysis and publication of patient data in anonymized fashion, and the requirement for informed consent was waived. From March 2010 through May 2019, seven consecutive patients diagnosed as right ventricular pseudoaneurysms admitted to our hospital were included. The incidence of pseudoaneurysm in RVOT after TOF repair during this period was investigated as well. The patients primarily diagnosed as functional single ventricle, left-looped ventricles, or with catheter-related and palliative procedure-related pseudoaneurysm were excluded in current study. All patients, except the one diagnosed as TOF/PA (Tetralogy of Fallot with Pulmonary Atria) had a bovine pericardial conduit placed between the right ventricle and the pulmonary artery, underwent a radical procedure for TOF/PS repair using a trans-annular or infundibular patch. For all the included patients, medical records were reviewed in details. The variables of the initial TOF repair including any palliative procedures before TOF repair, age, weight at TOF repair, size of the main pulmonary artery, McGoon ratio, Nakata index, the z-score of both preoperative and postoperative size of main pulmonary artery, the postoperative RVOT pressure gradient, intensive care unit and hospital lengths of stay were collected and analyzed. In addition, we reviewed the time interval between the date of the most recent surgical repair and diagnosis of pseudoaneurysm, whether the patients with or without symptoms at the time of diagnosis, the method for discovering the pseudoaneurysm, anatomy of the PSA, treatment and prognosis.
3.1 Clinical Characteristics of Patients
During this period, 1799 TOF repair operations were performed at our institution, and 61 cases used conduit placement and 1738 cases used patch repair. One of the patients with pseudoaneurysm had undergone TOF repair surgery at another hospital. This patient was therefore excluded from the calculation of the incidence of pseudoaneurysm formation. The observed total incidence of pseudoaneurysm formation was thus 0.33% (6/1799), and 0.28% in patients with patch repair, 1.6% in patients with conduit placement. The difference in incidence was insignificant by chi-square test (p = 0.217). Median age of these patients at TOF repair surgery was 1 (interquartile range, 0.75–2) years. The preoperative characteristics of the seven included patients were summarized in (Tab. 1). The age at the correction of TOF ranged from 10 months to 14 years (median: 2 years), and the weight ranged from 7.5 kg to 38 kg (median: 8.7). The branch pulmonary arteries in the seven included patients developed acceptable before the complete repair as indicated by McGoon ratio and Nakata index, but the main pulmonary artery was hypoplastic with the z-score of −1.76 to −4.76 in the patients diagnosed as TOF/PS. The patient with TOF/PA was absence of the main pulmonary artery attaching to right ventricular infundibulum. One patient (Patient No.5) underwent a modified Blalock-Taussig shunt with 5 mm thin-walled Gore-Tex graft four years before the complete repair, and two patients (Patient No. 4 and No. 5) received transcatheter occlusion for major aortopulmonary collateral arteries shortly before the correction. Patient No. 2 get pregnant for three times and delivered one baby vaginally during the interval between the repair of TOF and the discovery of pseudoaneurysm.
Table 1: Clinical characteristics to patients with pseudoaneurysms diagnosed
The procedures for TOF repair included one Rastelli procedure using bovine pericardial conduit with tricuspid valve, five trans-annular patch repair and one infundibular patch repair (Tab. 2). The z-score in diameters of the main pulmonary artery after correction measured by inserting calibrated dilators ranged from −0.15 to 0.76, indicating a satisfactory construction of main pulmonary arteries. Three patients had a systolic pressure gradient ranging from 26 to 64 mmHg between the right ventricular chamber and the branch pulmonary arteries immediately after the repair procedures. At the time of diagnosis for pseudoaneurysm, these three patients showed a significant elevation of pressure gradient and two other patients displayed new emergence of RVOT systolic pressure gradient. The pressure gradient generated at the proximal anastomosis in 4 patients and the distal anastomosis in 1 patient. All patients, except one (patient No. 4) suffered from pericardial tamponade, subacute infective endocarditis and mediastinal infection, have an uneventful postoperative course after the complete repair, with 16–31 hours of mechanical ventilation time, 3–6 days of ICU stay and 7–11 days of postoperative hospital stay. In patient No. 2, a residual ventricular septal defect with diameter of 8 mm was observed postoperatively. In patient No. 4, pericardial tamponade due to large amount of thrombosis was detected by echocardiography on the third postoperative day, and a reoperation was performed to clean the thrombus on the following day. She presented surgical wound infection, dehiscence and hematoma on the sixth day after the procedure, and recovered well after wound cleansing and dressing. Seven weeks after the complete repair of TOF, she developed fever with the highest temperature of 39°C, and the temperature was abnormal for nearly a week even timely antibiotic treatment with cefoperazone/sulbactam. Mediastinitis was discovered one week after she developed fever, with positive blood cultures for Staphylococcus epidermidis, and simultaneously, transthoracic echocardiography revealed the presence of a pulmonary valvular vegetation. Correspondingly, the antibiotic therapy was changed to conform to the results of the blood culture. One month later, the pulmonary valve endocarditis extended downward to the RVOT, presenting by a new vegetation on the RVOT patch, and the pseudoaneurysm was diagnosed at the same time. Treatment with intravenous linezolid was commenced and lasted for 8 weeks after the temperature returned to normal and then oral linezolid substituted for injection.
Table 2: Surgical data of the procedure before pseudoaneurysm developed in patients
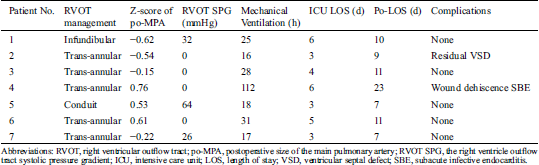
Bovine pericardium (Balance Medical, Beijing, China) was used for infundibular or trans-annular patch in five patients, a bovine epicardial conduit (Balance Medical, Beijing, China) was used in one patient, and the details of patch material was not available in the patient No. 2. Prolene with the metric size 5–0 (Ethicon Inc, Somerville, NJ) was routinely used in all cases.
3.3 Clinical Features of Pseudoaneurysm
The clinical features of the patients with pseudoaneurysm are presented in Tab. 3. The patients had pseudoaneurysms diagnosed from 2 months to 8 years after the complete repair for cardiac abnormality. Only one patient had symptoms at the presentation (patient No. 2 presented with arrhythmia). Patient No. 2 was admitted to hospital 3 times for treatment of arrhythmia in the last two years before the pseudoaneurysm diagnosed, and the interval between the most recent surgical procedure and discovery of the pseudoaneurysm was up to 8 years. The pressure gradient between the myocardium and the trans-annular patch increased from 0 after surgery to 53 mmHg at the discovery of pseudoaneurysm in this patient, associating with the progressive emergence of paroxysmal atrial arrhythmia or ventricular arrhythmia. She had the pseudoaneurysm discovered during the echocardiographic evaluation of cardiac function in the preparation for radiofrequency ablation. The pseudoaneurysm was identified on routine echocardiogram one month after the diagnosis of subacute infective endocarditis in patient No. 4 as previous described. In patient No. 5, the pseudoaneurysm was not discovered until a reoperation for the relief of anastomotic stenosis was performed, even if she had undergone echocardiography, computed tomographic scan and cardiac catheterization. The other 4 patients had no symptoms and were diagnosed as pseudoaneurysm during routine follow-up.
Table 3: The details of the pseudoaneurysm
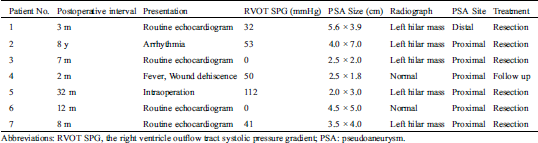
Five patients had a new mass in the left superior mediastinum on chest X ray film (Figs. 1A and 1B), and the other 2 patients had a nearly normal radiograph. The size of aneurysms ranged from 7.6 mm × 7.6 mm to 40 mm × 70 mm. The computed tomography scan showed that the pseudoaneurysm compressed the main pulmonary artery obviously in the patient No. 2 (Fig. 1C). In the patient No. 4 (infection caused pseudoaneurysm), the pseudoaneurysm decreased from 25 mm × 18 mm at the discovery to 7.6 mm × 7.6 mm after treatment with antibiotics for two months. Neither of the pseudoaneurysm sac contained visible thrombus. Although the pseudoaneurysm was detected by echocardiography in most of the patients (6/7), a computed tomography was taken to identify the diagnosis. The echocardiography and computed tomography showed that the pseudoaneurysms were off to the left of the sternum but not in the midline, as indicated in Fig. 1D, therefore, the concern about injury to the pseudoaneurysm and cardiac structures during the sternotomy did not occur.
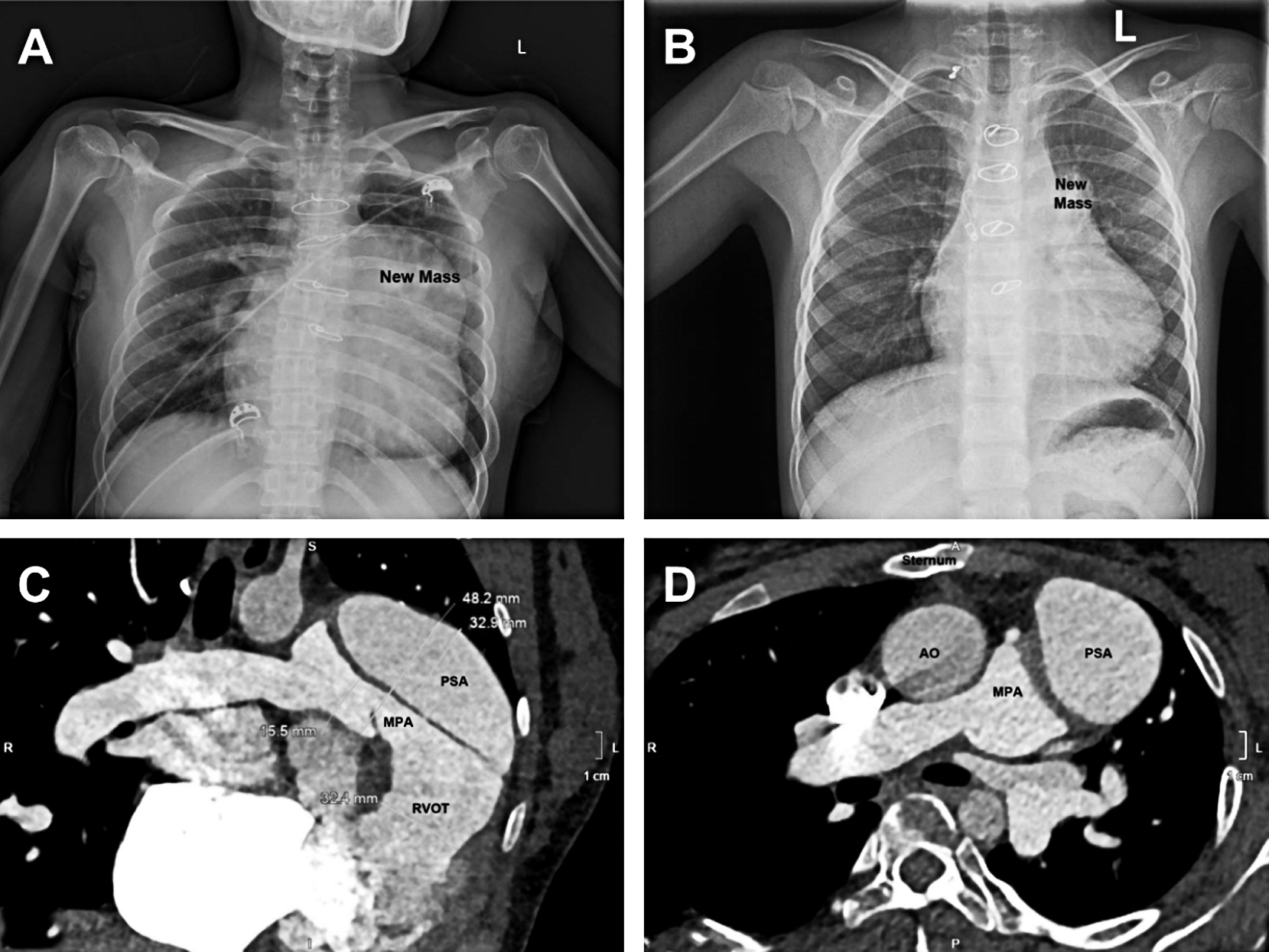
Figure 1: Radiological feature of pseudoaneurysm. (A) a giant new mass formed in the left superior mediastinum. (B) a small new mass was showed. (C) the giant pseudoaneurysm compressed the main pulmonary artery obviously. (D) the pseudoaneurysms are off to the left of the sternum but not in the midline
Six patients underwent surgical resection of the pseudoaneurysm and reconstruction of RVOT and pulmonary artery. Patient No. 4 is still under investigation because of small age and pseudoaneurysm size, with low risk for catastrophic consequences. All 6 patients had uneventful postoperative course, and showed no relapse of pseudoaneurysm during the follow-up for 7–45 months.
Pseudoaneurysm is a rarely reported complication of RVOT reconstruction using conduit placement [2,6], and much less report has been found in patients with TOF/PS using infundibular or trans-annular patch. Here, we report our series of patients developed pseudoaneurysm in patients received patch repair of TOF. This complication has a very low incidences (0.28) in TOF/PS patients using patching and a relative higher incidence in TOF/PA with conduit placement (1.6%) (though the statistic difference in incidence between the two types of patients was insignificant). The low incidence of pseudoaneurysm formation may be the reason for being ignored, but the probability of increase in the risk of catastrophic complications infundibular or trans-annular patch repair, indicates its importance in TOF/PS.
Pseudoaneurysm, also known as a false aneurysm, resulted from a small disruption in the wall of a vessel or cardiac chamber which allows blood to leak into the surrounding space, generating locally contained hematoma with turbulent blood flow and a neck that connects to the real chamber with certain size [7]. The wall of a pseudoaneurysm develops with the products of the clotting cascade and forms with fibrin/platelet crosslinks, but contains no vessel wall, patch or myocardium, which is ultimately weak with high risk of rupture [7]. However, the occurrence of rupture is rare in patients with pseudoaneurysm that developed after RVOT procedure [1,2], and none of ruptured pseudoaneurysm was observed in our patients as well. In the largest series of pseudoaneurysms (24 cases) complicating right ventricle-pulmonary artery conduit procedure reported by Sykes et al. [1], only one patient had a ruptured pseudoaneurysm diagnosed at autopsy. As our experiences and analysis, the low incidence of rupture is probably related to the lower pressure of pulmonary circulation and loosened tissues surrounding the RVOT and pulmonary artery that ease the tension of the pseudoaneurysm through incrementing the size. The experiences of pseudoaneurysm in the high-pressure left-heart circulation including left ventricular pseudoaneurysm complicating myocardial infarction [8], visceral artery pseudoaneurysms [9], aortic and femoral pseudoaneurysms etc. are more sophisticated, which suggests a higher propensity to rupture. Nevertheless, the possibility of undiscovered ruptured pseudoaneurysms complicating RVOT cannot be ruled out, because of the rare report of this complications.
Besides the risk of rupture, the compression on structures surrounding the RVOT and main pulmonary artery is another concern. As previous reported [1,2,6], the large airways and the phrenic nerve were squeezed frequently by the enlarged pseudoaneurysm, which contributed to the emergence of symptoms. In our series of patients, patient No. 2 was referred to hospital eight years after the total correction because of arrhythmia, while clinical symptoms related to compression of airway or phrenic nerve were not shown in this patient, the reason for which is probable that the adult patients have larger chest chamber and the arrhythmic symptoms masked the compression symptoms. The elevated right ventricular pressure is a risk factor for arrhythmia [10], and the long-term compression of RVOT by pseudoaneurysm is an underlying cause of high right ventricular pressure. Although a right ventricular incision is also associated with high risk of arrhythmia, we have not resected an arrhythmic isthmus during surgery on purpose. In addition, the surgical management for pseudoaneurysm need remove little myocardium. Therefore, RVOT obstruction due to pseudoaneurysm is probable the main cause for refractory arrhythmia in this patient, and the disappear of arrhythmia after the surgical resection has partially proved it at least. Therefore, the lesions to myocardium arising from right ventricular outflow obstruction is a new concern in pseudoaneurysm patients without timely discovery.
The analysis of risk factors is necessary to reduce the pseudoaneurysm development, and discover and handle it in a timely manner. The data presented in this report indicated that the diagnosis of Tetralogy of Fallot may predispose to pseudoaneurysm development, which is consistent with the previous report by Sykes et al. [1]. As their view, the high incidence of significant branch or peripheral pulmonary arteries stenosis in Tetralogy of Fallot resulting in postoperative right ventricular hypertension favored the development of a pseudoaneurysm. In previous reports, the postoperative near-systemic right ventricular pressure was believed to be an important factor contributing to the formation of pseudoaneurysms [1,2]. The lack of postoperative right ventricular pressure data is a shortage of this study, but the uneventful of postoperative course suggests that at least the pressure of the right ventricle chamber is not that high. A residue pressure gradient remained at the anastomotic site in 3 patients in the immediate postoperative period, but the systolic pressure gradient only ranged from 26 to 64 mmHg, which was another evidence of lower right ventricular pressure before the pseudoaneurysm formation in this series of patients. Therefore, right ventricular hypertension may not be the primary culprit for pseudoaneurysm in these series of cases, and other factors related to TOF in itself and the procedure may lead to the development of pseudoaneurysm.
Recently, Rao et al. [11] highlighted that the failure of host integration after RVOT construction using an explant was an important mechanism in pseudoaneurysm formation. The intense inflammation reaction to foreign materials is to blame for the failure of the patch material to cellularize and regenerate effectively. Chowdhury et al. [12] reported that hypertrophic, degenerative, and fibrotic changes developed widely in myocardial tissues from RVOT in TOF, and these lesions may reduce the growth potential of cells. In addition, the surgical operation may also damage the myocardium and affect the growth potential. The use of bovine pericardial patch or conduit for RVOT enlargement or reconstruction favor the pseudoaneurysm develop may through these mechanisms though the native cellular remodeling was not checked by histopathologic examination here. In this report, local infection contributed to pseudoaneurysm formation in one patient who suffered from mediastinal infection and subsequent infective endocarditis, which is a rare cause of this complication. In a case-control study [1], patients with younger age when received conduit placement were identified to tend to develop pseudoaneurysm, but in our series of case, patients were older than the currently recommended age for correction. We therefore cannot believe that age is a predisposing factor for pseudoaneurysm formation in these patients. The other possible factors favored incisional pseudoaneurysm as previous reported cannot be identified in this series of patients, but it no doubt that the development of pseudoaneurysm is a multifactorial process.
In most cases, patients developed pseudoaneurysm without symptoms, and was diagnosed during follow-up. Echocardiography and computed tomographic scan were the mainstay of diagnosis, and in this study, the pseudoaneurysm was discovered in six patients through computed tomographic scan following echocardiography. A progressive increase or new emergence of pressure gradient across anastomosis was shown in five patients, which might be a consequence of increasing enlargement of the pseudoaneurysm and a clue of pseudoaneurysm formation. One patient missed the diagnosis of pseudoaneurysm before a reoperation performed for the relief of anastomotic stenosis, even he received both these two examinations and cardiac catheterization. Both the serious anastomotic stenosis and the relatively small size of the pseudoaneurysm may the causes for missed diagnosis in this patient. Five patients showed a new emerging abnormality in the chest radiograph. Therefore, we recommended that the clinicians should pay special attention to the patients with a significant pressure gradient across anastomosis, especially in patient with new emergence or increasing elevation of the pressure gradient and abnormal chest radiograph.
The surgical repair of pseudoaneurysm can be safely performed according to our experiences and previous reports [1,2] except for the attention needs to be given to the sternotomy in the reoperation. The determination of when to repair pseudoaneurysm caused by infections is difficult, because of a high risk of threatened rupture, fatal bleeding during the operation process in the early stage of pseudoaneurysm formation. Luckily, the size of pseudoaneurysm was small in this patient, and she was hemodynamically stable and in good general condition. She has received long-time continued antibiotic with strict follow-up and will be operated during the follow-up if the pseudoaneurysm is progressively enlarging. We believe that it is a suitable strategy for this infant case. Our and other’s experiences [1,2] support timely resection of pseudoaneurysm when discovered in patient with or without symptoms unless special risk factors existed. Although the pseudoaneurysm locate rarely in the midline, the details of the structure behind the sternum should be well delineated by cross section image before the chest is opened and a femoral cannulation can be used to establish bypass when necessary.
Although there are limitations including limited number of cases and incomplete medical data, we believe that some meaningful conclusions can be drawn from this retrospective study. Clinicians should highly pay attention to pseudoaneurysm in patients after the repair of TOF/PS using infundibular patch or trans-annular patch because this neglected complication will result in serious consequence if not timely discovered and treated. As Sykes et al. suggested [1], a rigorous post-surgical follow-up program will be help to timely discover pseudoaneurysm in patients with infundibular or trans-annular patch. New emergence and progressive increase of anastomotic pressure gradient revealed by echocardiography or new mass in the superior mediastinum on chest X ray film might be an evidence of pseudoaneurysm formation, and additional computed tomographic scan should be offered if indicated. The experience for pseudoaneurysm complicating TOF is limited until now, and our experience will help to discover and treat it.
Data Availability Statement:Data are available on reasonable request.
Author Contributions: Gang Li and Junwu Su contributed to the research conception and design, data analysis and interpretation, manuscript drafting, and critical revision of the manuscript. Han Zhang, Yao Yang, Yang Liu and Aijun Liu contributed to data acquisition, data interpretation, manuscript drafting and critical revision of the manuscript. Xiangming Fan, and Pei Cheng contributed to critical revision of the manuscript. All authors had full access to all of the statistical analyses, graphs and tables in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis.
Funding Statement: This work was supported by National Natural Science Foundation of China (81600383) and Beijing Municipal Administration of Hospitals (QML20170601).
Conflict of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Sykes, M. C., Nathan, M., Sanders, S. P., Gauvreau, K., Pigula, F. A. et al. (2017). Pseudoaneurysm complicating right ventricle–to–pulmonary artery conduit surgery: Incidence and risk factors. Journal of Thoracic and Cardiovascular Surgery, 154(6), 2046–2049. DOI 10.1016/j.jtcvs.2017.08.014.
2. Levine, J. C., Jr, M. J. E., Keane, J. F., Spevak, P. J., Sanders, S. P. (1995). Anastomotic pseudoaneurysm of the ventricle after homograft placement in children. Annals of Thoracic Surgery, 59(1), 60–66. DOI 10.1016/0003-4975(94)00569-S.
3. Herbert, C., Ikemba, C., Nugent, A. (2014). Device closure of a pseudoaneurysm of the right ventricular outflow tract in an infant with right ventricle-to-pulmonary artery homograft. Catheterization and Cardiovascular Interventions, 83(4), 587–590. DOI 10.1002/ccd.24990.
4. Maxwell, B. G., Wise-Faberowski, L. (2014). Management of high-risk reentry sternotomy in an infant for repair of a giant pseudoaneurysm of the right ventricular outflow tract. Annals of Cardiac Anaesthesia, 17(1), 59–61. DOI 10.4103/0971-9784.124145.
5. Pillai, S. K., Reddy, H. P., Kulkarni, S., Murthy, K. S., Cherian, K. M. (2004). Pseudoaneurysm of homograft placed in right ventricular outflow tract. Annals of Thoracic Surgery, 78(3), 1068–1070. DOI 10.1016/S0003-4975(03)01583-2.
6. Sadiq, M., Fenton, A. C., Firmin, R. K. (1994). False aneurysm of the right ventricular outflow tract after total correction of tetralogy of Fallot: Diagnosis by echocardiography and successful repair by neck cannulation for cardiopulmonary bypass. Heart, 71(6), 566–568. DOI 10.1136/hrt.71.6.566.
7. Rivera, P. A., Dattilo, J. B. (2020). Pseudoaneurysm. Treasure Island, FL: StatPearls Publishing.
8. Faiza, Z., Lee, L. (2020). Left ventricular false aneurysm. Treasure Island, FL: StatPearls Publishing.
9. Etezadi, V., Gandhi, R. T., Benenati, J. F., Rochon, P., Gordon, M. et al. (2011). Endovascular treatment of visceral and renal artery aneurysms. Journal of Vascular and Interventional Radiology, 22(9), 1246–1253. DOI 10.1016/j.jvir.2011.05.012.
10. Shiraishi, S., Takahashi, M., Sugimoto, A., Tsuchida, M. (2017). Predictors of ventricular tachyarrhythmia occurring late after intracardiac repair of tetralogy of Fallot: Combination of QRS duration change rate and tricuspid regurgitation pressure gradient. Journal of Thoracic Disease, 9(12), 5112–5119. DOI 10.21037/jtd.2017.11.53.
11. Rao, S., Stewart, R. D., Pettersson, G., Tan, C., Golz, S. et al. (2020). Failure of cellularization of ventriculotomy patch leading to right ventricular pseudoaneurysm. World Journal for Pediatric and Congenital Heart Surgery, 11(1), 123–126. DOI 10.1177/2150135119880547.
12. Chowdhury, U. K., Jha, A., Ray, R., Kalaivani, M., Hasija, S. et al. (2019). Histopathology of the right ventricular outflow tract and the relation to hemodynamics in patients with repaired tetralogy of Fallot. Journal of Thoracic and Cardiovascular Surgery, 158(4), 1173–1183.e5. DOI 10.1016/j.jtcvs.2019.03.118.
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |