

 | Congenital Heart Disease |  |
DOI: 10.32604/CHD.2020.012863
ARTICLE
FFR-Guided PCI in a 17-Year-Old Patient after Arterial Switch Operation for D-Transposition of the Great Arteries
1Pediatric and Congenital Cardiology Unit, Department of Woman and Child’s Health, University of Padua, Via Nicolò Giustiniani 2, Padua, 35128, Italy
2Department of Cardiac, Thoracic, Vascular Sciences, University of Padua, Via Nicolò Giustiniani 2, Padua, 35128, Italy
*Corresponding Author: Biagio Castaldi. Email: biagio.castaldi@aopd.veneto.it
Received: 15 July 2020; Accepted: 08 September 2020
Abstract: Asymptomatic coronary artery obstruction represents a significant diagnostic challenge in patients with Dextro-Transposition of the Great Arteries and history of Arterial Switch Operation. We report the case of a 17-year-old boy with anomalous origin of left circumflex artery from the right coronary artery, who underwent neonatal arterial switch operation and developed silent myocardial ischemia under stress on myocardial scintigraphy. Despite coronary angiogram and intravascular ultrasound showed only intermediate stenosis of the right coronary artery ostium, the physiological analysis, through the employment of pressure wire, demonstrated a severe reduction of coronary fractional flow reserve after pharmacologically induced hyperemia. Thus, the patient was treated with implantation of drug eluting stent. Invasive fractional flow reserve of coronary stenosis may represent a useful tool to guide revascularization strategy in this population.
Keywords: Congenital heart disease; D-TGA; coronary stenosis; fractional flow reserve; myocardial ischemia; coronary stent
Complete Dextro-Transposition of the Great Arteries (D-TGA) represents 5%–7% of all congenital heart defects. In the present era, neonatal arterial switch operation (ASO) constitutes the most common type of surgical correction for D-TGA, and is associated with good early and late outcome results [1]. The most common postoperative late complications are represented by coronary artery obstruction, progressive neo-aortic dilatation, neo-pulmonary stenosis (supravalvular and branch pulmonary stenosis) and right ventricular outflow tract (RVOT) obstruction. Thereafter, current guidelines warrant a prolonged follow-up protocol after ASO, involving noninvasive and invasive diagnostic assessments [2,3]. Nevertheless, the development of silent coronary artery obstruction has been reported in up to 8% of patients and has been associated to increased number of coronary events and late sudden death risk [4,5]. In fact, coronary artery stenosis secondary to ASO shows a completely different physiopathology compared to coronary atherosclerotic disease. In particular, anatomical coronary ostia deformation occurring during rapid growth and the progressive intimal thickening have been advocated as principal causing factors [4]. Furthermore, evidence is lacking regarding assessment of vessel obstruction severity and indication to revascularization in this subset of population with congenital heart disease. Invasive functional analysis of coronary artery stenosis may contribute to the identification of patients at high risk of coronary events and sudden death.
A 17-year-old male, body weight 73 kg, height 190 cm, was electively admitted in our Hospital to undergo invasive coronary angiogram. He had a past history of D-TGA, ventricular septal defect (VSD) and anomalous origin of the left circumflex coronary artery (LCx) from the right coronary artery (RCA). At the age of 2 weeks, he underwent surgical repair with ASO, LeCompte maneuver, coronary artery translocation and VSD closure. As per local protocol to assess coronary anatomy after ASO, at 10 years of age he was referred for elective coronary angiography, showing a 50% lumen stenosis at the origin of RCA. Subsequently, he underwent myocardial perfusion scintigraphy (SPECT) at rest and under pharmacological stress with adenosine infusion, which demonstrated a small perfusion defect of the mid-segment of the anterior wall under stress. Unfortunately, given the presence of right bundle branch block on the electrocardiogram (EKG), repolarization abnormalities assessment during the stress were not reliable for myocardial ischemia diagnosis. Considering the un-correlation of the two diagnostic exams regarding ischemic territory and the absence of symptoms, the decision made was to not treat the stenosis and to follow-up the patient yearly with electrocardiogram (EKG), transthoracic echocardiogram (TTE), and exercise-stress testing. At 17 years of age he repeated a SPECT at rest and under physical exercise stress test, which showed hypo-perfusion of mid- and basal segments of both anterior and inferior wall under stress. TTE showed preserved left ventricle ejection fraction (LVEF biplane 55%), reduced LV global longitudinal strain (GLS −15%), more evident in the inferior and posterior septum wall, and preserved right ventricular (RV) function. Following multidisciplinary team discussion, the patient was admitted for elective coronary angiogram. On admission patient was preloaded with 600 mg of clopidogrel PO. Through femoral artery access, selective angiogram showed dominant RCA with a 50% ostial stenosis (Fig. 1A). IV unfractioned heparin 5000 UI was administered. Thereafter, intravascular ultrasound (IVUS) was employed to better characterize the stenosis from the anatomical standpoint. IVUS (Eagle Eye Platinum ST®, Philips Volcano,) showed mild reduction of RCA ostium minimal lumen area (MLA 8.8 mm2) with mild and eccentric neo-intimal proliferation, in absence of clear atherosclerotic plaque (Fig. 1B). Subsequently, we performed a functional analysis of the stenotic segment using a coronary pressure wire (Verrata Plus®, Philips Volcano). Firstly, we measured the instantaneous wave-free ratio (iFR) which resulted 0.89, falling in the so-called “grey zone”, and then the fractional flow reserve (FFR) pre- and post-adenosine induced (400 mcg intracoronary) hyperaemia which was 0.89 and 0.63 respectively (Figs. 1C and 1D). Given the FFR positive result, 250 mg of aspirin IV were given, and then a 4 × 15 mm zotarolimus eluting stent (Resolute Onyx®, Medtronic, CA, USA) was implanted at 18 Atm. Post dilation of the stent was performed with a 4 × 8 mm non-compliant balloon (NC Euphora®, Medtronic, CA, USA) with excellent angiographic result (Fig. 2A). Post-stenting IVUS evaluation displayed good apposition of the stent struts to the coronary wall and minimal protrusion (<1 mm) in the aortic root (Fig. 2B). Finally, functional analysis was repeated, showing an FFR value post-adenosine infusion of 0.83 (Figs. 2C and 2D). Patient was transferred back to the ward and initiated on aspirin 100 mg OD and clopidogrel 75 mg OD the following day. Pre-discharge TTE demonstrated preserved LVEF and mild improvement of GLS to −16.5%. He was discharged home on day 1 after PCI in good condition with a 12-month plan of dual antiplatelet therapy (DAPT).
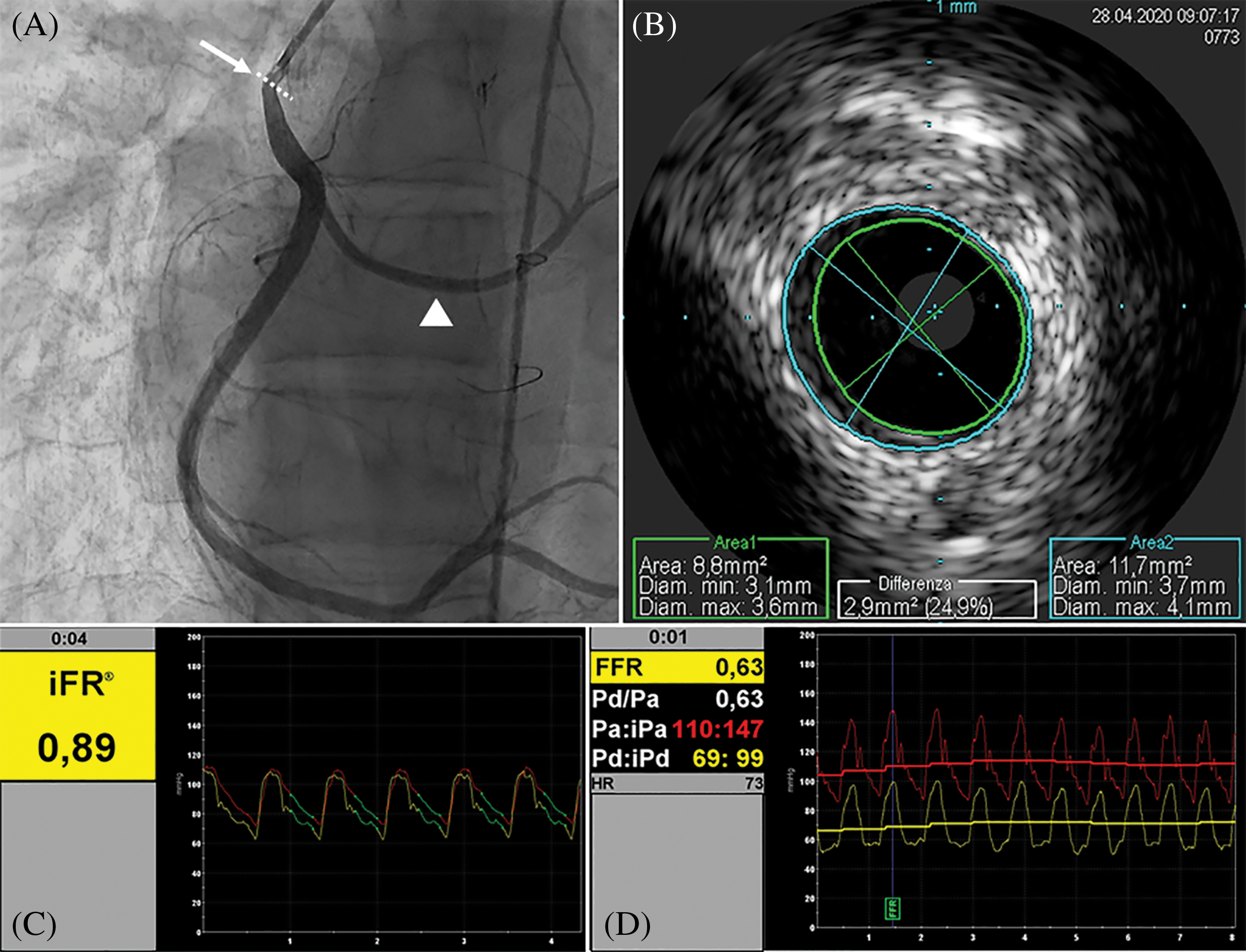
Figure 1: Basal assessment. A, Angiography showing intermediate ostial stenosis of RCA (arrow), anomalous origin of LCx (head arrow) from the RCA. B, IVUS evaluation of the stenotic segment, showing mild eccentric neointimal hyperplasia causing mild stenosis and a MLA of 8.8 mm2. C, iFR resulting 0.89, falling in the so-called “grey zone”, and then in D the FFR after intracoronary infusion of adenosine significantly reduced (0.63). Right coronary artery (RCA). Left circumflex artery (LCx). Intravascular ultrasound (IVUS). Minimal lumen area (MLA). Instantaneous wave-free ratio (iFR). Fractional flow reserve (FFR)
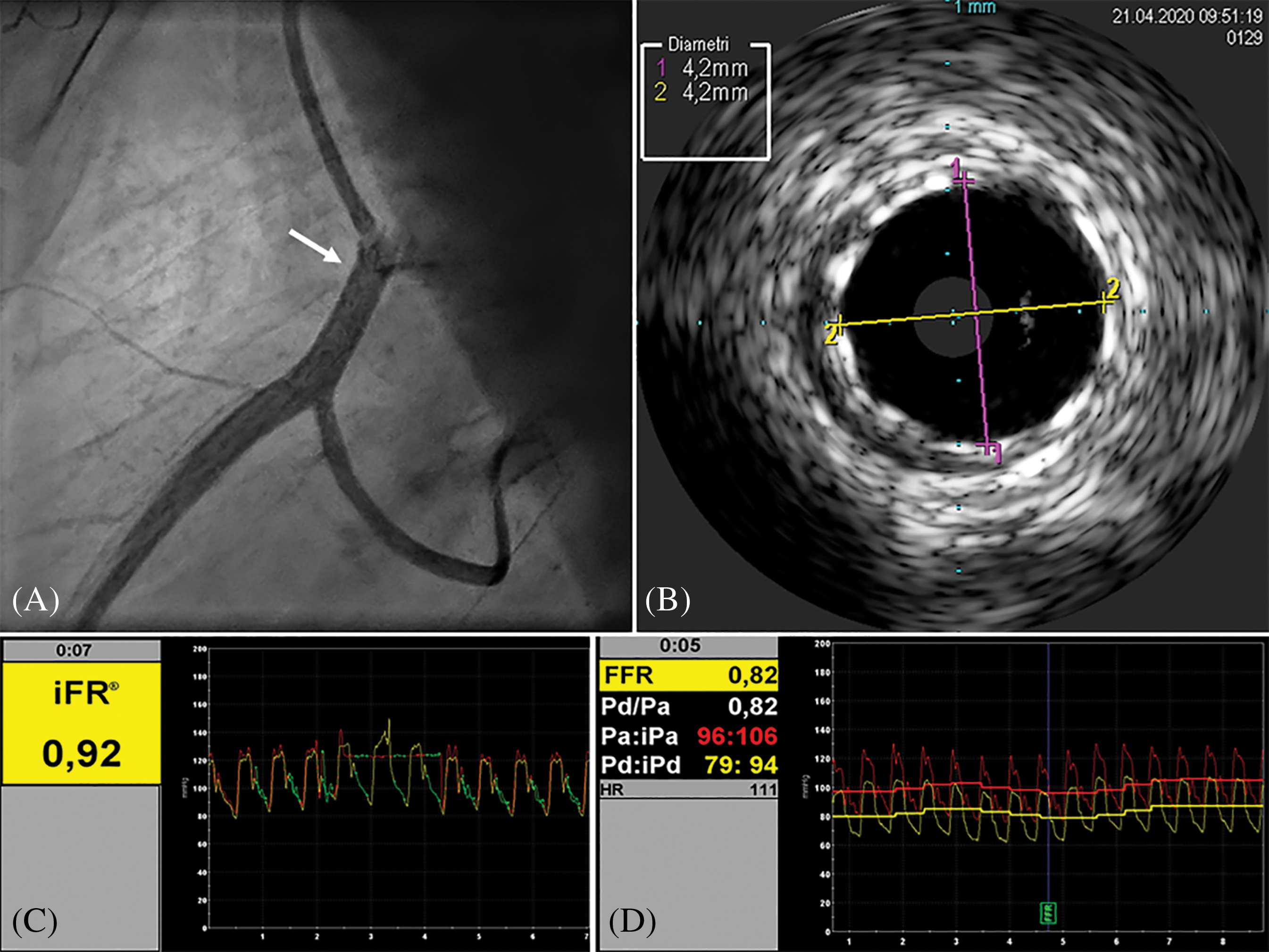
Figure 2: Post-stenting assessment. A, Angiography showing good result of stent implantation (arrow) of the RCA ostium, extended to the proximal segment. B, IVUS evaluation displaying good apposition of the stent’s struts to the arterial wall, and increased MLA (13.8 mm2). C, iFR showing value in the range of normality. D, FFR after intracoronary infusion of adenosine significantly increased after stenting and in the normal range (0.82). Right coronary artery (RCA). Intravascular ultrasound (IVUS). Minimal lumen area (MLA). Instantaneous wave-free ratio (iFR). Fractional flow reserve (FFR)
The present case highlights the value of different non-invasive and invasive imaging modalities in order to evaluate coronary artery stenosis late after ASO. The non-invasive documentation of myocardial ischemia by SPECT prompted the request for an invasive coronary angiography, which demonstrated an intermediate stenosis of the RCA ostium. In agreement with the findings by Pedra et al., IVUS study showed that the obstruction was secondary to eccentric intimal thickening [6]. However, based on IVUS MLA, the stenosis was not considered significant. In fact, for comparable left main coronary artery (LMCA) lesions due to atherosclerotic disease, it appears reasonable to defer revascularization in presence of an MLA larger than 6 mm2 [7]. Nonetheless, the myocardium subtended by the stenosis and the downstream vascular arborisation appeared remarkably extended due to the original coronary anomaly, LCx origin from the RCA, which represents the most common coronary anomaly associated with D-TGA [8]. For these reasons, we performed a functional analysis with the iFR/FFR hybrid approach, which showed a significant reduction of FFR value after induced coronary vascular hyperemia (FFR 0.63), and guided further revascularization with stent implantation. An alternative revascularization strategy is represented by coronary artery bypass grafting (CABG). This treatment should be preferred in the presence of sub-occlusive proximal coronary artery stenosis in order to preserve graft patency from native coronary artery competitive blood flow. Moreover, CABG may be considered as first-line strategy in case of stenosis of the proximal left coronary artery, given the excellent long-term graft patency. However, in our case, a percutaneous approach was preferred due to location and severity of coronary artery obstruction.
In conclusion, given the relatively elevated prevalence of high-risk coronary obstructions in patients with D-TGA and previous ASO, multimodality diagnostic approach for silent myocardial ischemia is requested. Furthermore, this case suggests that, among invasive tools, fluoroscopic and anatomical evaluation with IVUS might be not sufficient to estimate coronary obstruction severity, thus functional assessment through invasive FFR analysis may play a pivotal role in guiding PCI in this population.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Vida, V. L., Zanotto, L., Zanotto, L., Triglia, L. T., Bellanti, E. et al. (2019). Arterial switch operation for transposition of the great arteries: A single-centre 32-year experience. Journal of Cardiac Surgery, 34(11), 1154–1161. DOI 10.1111/jocs.14045.
2. Warnes, C. A., Williams, R. G., Bashore, T. M., Child, J. S., Connolly, H. M. et al. (2008). ACC/AHA, 2008 guidelines for the management of adults with congenital heart disease: Executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines on the Management of Adults with Congenital Heart Disease). Developed in Collaboration with the American Society of Echocardiography, Heart Rhythm Society, International Society for Adult Congenital Heart Disease, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Journal of the American College of Cardiology, 52(23), e143–e263. DOI 10.1016/j.jacc.2008.10.001.
3. Baumgartner, H., Bonhoeffer, P., De Groot, N. M. S., de Haan, F., Deanfield, J. E. et al. (2010). ESC guidelines for the management of grown-up congenital heart disease (new version 2010). European Heart Journal, 31(23), 2915–2957. DOI 10.1093/eurheartj/ehq249.
4. Bonnet, D., Bonhoeffer, P., Piéchaud, J. F., Aggoun, Y., Sidi, D. et al. (1996). Long-term fate of the coronary arteries after the arterial switch operation in newborns with transposition of the great arteries. Heart (British Cardiac Society), 76(3), 274–279. DOI 10.1136/hrt.76.3.274.
5. Legendre, A., Losay, J., Touchot-Koné, A., Serraf, A., Belli, E. et al. (2003). Coronary events after arterial switch operation for transposition of the great arteries. Circulation, 108(Suppl 1), II186–II190. DOI 10.1161/01.cir.0000087902.67220.2b.
6. Pedra, S. R. F. F., Pedra, C. A. C., Abizaid, A. A., Braga, S. L. N., Staico, R. et al. (2005). Intracoronary ultrasound assessment late after the arterial switch operation for transposition of the great arteries. Journal of the American College of Cardiology, 45(12), 2061–2068. DOI 10.1016/j.jacc.2005.02.076.
7. Johnson, T. W., Räber, L., Di Mario, C., Bourantas, C. V., Jia, H. et al. (2019). Clinical use of intracoronary imaging. Part 2: Acute coronary syndromes, ambiguous coronary angiography findings, and guiding interventional decision-making: An expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. EuroIntervention, 15(5), 434–451. DOI 10.4244/EIJY19M06_02.
8. Pasquali, S. K., Hasselblad, V., Li, J. S., Kong, D. F., Sanders, S. P. (2002). Coronary artery pattern and outcome of arterial switch operation for transposition of the great arteries: A meta-analysis. Circulation, 106(20), 2575–2580. DOI 10.1161/01.CIR.0000036745.19310.BB.
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |