

 | Congenital Heart Disease |  |
DOI: 10.32604/CHD.2020.012927
ARTICLE
The Effect of Carnitine Supplementation on Left Ventricular Function: Lessons from Current Evidence and Insights for Future Studies
1Division of Cardiology, Advocate Children’s Hospital, Chicago, IL, USA
2Department of Pediatrics, Chicago Medical School, Rosalind Franklin University of Medicine and Science, Chicago, IL, USA
3Tecnologico de Monterrey, Escuela de y Ciencias de la Salud, Monterrey, Nuevo Leon, Mexico
4Department of Pediatrics, University of Chicago, Chicago, IL, USA
5Department of Pediatrics, Baylor College of Medicine, Houston, TX, USA
6Division of Critical Care, Texas Children’s Hospital, Houston, TX, USA
*Corresponding Author: Enrique G. Villarreal. Email: a01191417@itesm.mx
Received: 20 July 2020; Accepted: 09 September 2020
Abstract: Introduction: In children, data on the effects on carnitine supplementation and myocardial function are limited. A few studies have investigated the relationship between serum carnitine levels in the setting of depressed cardiac function and have demonstrated possible benefits. As such, this systematic review and meta-analyses aimed to assess the effects carnitine supplementation on left ventricular function. Materials and Methods: A systematic review of the literature was performed to identify full text manuscripts in English. PubMed, EMBASE, and the Cochrane databases were queried. Studies were included with data from pediatric patients, that used carnitine supplementation and included pre- and post-carnitine data for at least one of the outcomes of interest. Results: A total of six studies including 144 patients were included. Carnitine dosage ranged from 50 to 100 mg/kg/day. The average duration of carnitine therapy was 9.8 months. Left ventricular ejection fraction was higher after carnitine supplementation with a mean difference between groups of 3.56 [95% confidence interval 0.06–7.06, p-value 0.04]. Left ventricular shortening fraction was higher after carnitine supplementation with a mean difference between groups of 3.68 [95% confidence interval 1.22–6.15, p-value 0.01]. Left ventricular end diastolic diameter was higher after carnitine supplementation, but the difference did not reach significance. Conclusion: Carnitine supplementation may augment left ventricular ejection fraction and shortening fraction. Those with lower baseline ejection fraction and shortening fraction appear to benefit the most from carnitine supplementation. Additional studies of the effects of carnitine on cardiac function are warranted.
Keywords: Carnitine; left ventricular function; ventricular ejection fraction; pediatrics; dietary supplement
Carnitine is a conditionally essential amino-acid endogenously synthesized in the liver, kidney and brain from lysine or methionine [1]. It functions as a transporter of long-chain fatty acids into mitochondria and participates in cellular energy metabolism [2]. Carnitine deficiency has been associated with cellular inability to use fat as fuel and a decrease in energy availability in vital organs [3]. In adults, it was shown that carnitine myocardial levels are quickly diminished during an ischemic event [4,5], whereas exogenous supplementation of carnitine replenish depleted levels and improve myocardial metabolic and left ventricular function [6]. In children, data on the effects on carnitine supplementation and myocardial function are limited. A few studies have investigated the relationship between serum carnitine levels in the setting of depressed cardiac function and have demonstrated possible benefits [7–12]. As such, this systematic review and meta-analyses aimed to assess the effects carnitine supplementation on left ventricular function.
A systematic review was conducted to identify studies pertaining to cardiac function in response to carnitine supplementation in children. A new review protocol was used for this study as there was no existing protocol. PubMed, EMBASE, and the Cochrane databases were queried to identify such studies. The following keywords were used individually and in various combinations with one another: “carnitine”, “levocarnitine”, “L-carnitine”, “children”, “cardiac function”, “ejection fraction”, and “fractional shortening”. Two authors (RL, SF) reviewed the abstracts and titles of resulting studies. Full-text manuscripts of studies found to be pertinent to study questions were then obtained. These were reviewed for quality using the Newcastle-Ottawa Scale and assessed for risk of bias using the Cochrane Risk of Bias tool. Any discrepancies in these evaluations between the two authors were then reviewed by both authors and a consensus achieved. Studies of adequate quality and low risk of bias were then considered for inclusion.
Studies were included in the final pooled-analyses if they met the following requirements: (1) utilized carnitine; (2) included pediatric patients (under 18 years of age); (3) contained echocardiographic data regarding cardiac function; (4) contained data for the same patients before and after carnitine supplementation; (5) had published data; (6) were in English language. Studies that met these criteria were then deemed appropriate for inclusion. Two studies comparing separate cohorts (those having received carnitine versus those who did not) were excluded as it was decided that simply including data from before and after carnitine supplementation would be most ideal. Any discrepancies in appropriateness of inclusion between the two authors were reviewed by both authors and a consensus achieved.
Reported endpoints from the studies deemed appropriate for inclusion were outlined. The number of studies reporting each endpoint were then reviewed and a decision was made to include endpoints that had data from three or more studies. The following endpoints were identified for pooled-analyses: left ventricular ejection fraction (%), left ventricular shortening fraction (%), and left ventricular end diastolic dimension (cm).
Data was extracted using an electronic data extraction tool. Data was extracted by two authors (RL and EV) independently. Since all variables were continuous, means and standard deviations for the pre- and post-milrinone data were recorded. The individually extracted data was then compared to identify any discrepancies. Discrepancies in the extracted data were then reviewed by the authors together and discrepancies resolved by consensus.
Once data was extracted each endpoint underwent evaluation of heterogeneity. A Q-statistic and its resulting p-value were calculated as was an I2 statistic. If the p-value for the Q-statistic was less than 0.05 or if the I2 value was greater than 50%, heterogeneity was deemed significant. In the absence of significant heterogeneity, a fixed-effects model was used for the pooled analyses while a random-effects model was used if there was significant heterogeneity present. All endpoints were continuous in nature and thus pooled analyses were conducted to determine the mean difference and 95% confidence interval. Publication bias was not assessed due to the low number of pooled studies. Thus, the units for the mean difference is the same as the endpoint itself.
Meta-regression was not conducted due to the low number of pooled studies. Sensitivity analyses were conducted to determine the effect of carnitine dose, duration of carnitine therapy, age, patient population, and baseline values of each specific endpoint on the effect of carnitine on the endpoint.
All statistical analyses were done using RevMan 5.0. A p-value of less than 0.05 was considered statistically significant.
A total of six studies including 144 patients were included in the final analyses (Fig. 1). Studies were published between 1995 and 2018. Carnitine supplementation was usually either 50 mg/kg/day or 100 mg/kg/day. Four of the included studies used the same dose for all patients while two studies utilized different doses for study patients. The average duration of carnitine supplementation before obtaining repeat measures was 9.8 months. The mean age at time of carnitine supplementation was 7.1 years and 60% of patients were male. All studies, as based on an a priori decision were single cohort studies and compared echocardiographic values before and after carnitine supplementation.
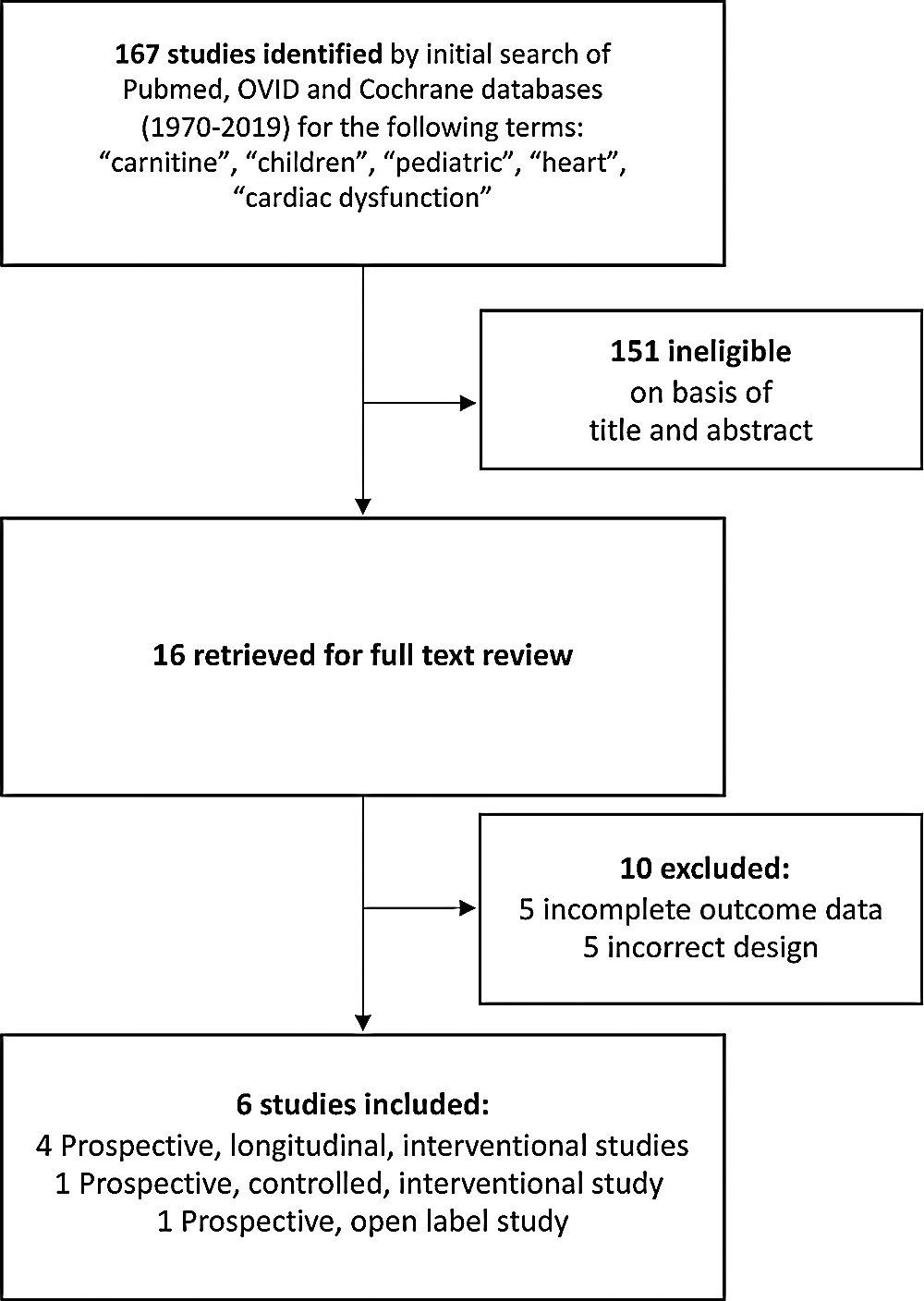
Figure 1: Flowchart demonstrating the search strategy and search results for published manuscripts
The included studied had slightly different study population. One study was investigating patients with sickle cell anemia and pulmonary arterial hypertension, another study was investigating patients with chronic renal failure undergoing hemodialysis, another study was investigating oncology patients undergoing anthracycline patients, and three studies were investigating patients with cardiomyopathy (Tab. 1). As such, ventricular dysfunction did not have to be present at baseline in some of these patients and in the patients with normal ventricular function the carnitine supplementation was being studied to see its effect on preserving ventricular function in settings in which left ventricular function may decrease.
Table 1: Study characteristics describing sample size, dosage, duration, type of study and specific patient population
3.2 Left Ventricular Ejection Fraction
A total of five studies with data from 109 patients were included in the final analysis for left ventricular ejection fraction [7–11]. Heterogeneity analysis resulted in a Q-statistic with a p-value of less than 0.01 and an I2 value of 79%, indicating the presence of significant heterogeneity. Thus, a random-effects model was used. Left ventricular ejection fraction was found to be significantly higher after carnitine supplementation. The mean difference in left ventricular ejection fraction was 3.56 with a 95% confidence interval 0.06 to 7.06 (p-value 0.04) (Fig. 2). Thus, left ventricular ejection fraction (measured in percent) increased by an absolute value of 3.56. Sensitivity analyses demonstrated that the overall effect of carnitine does not appear to be impacted by time, dose, or age. Those with a lower baseline left ventricular ejection fraction and longer duration of carnitine supplementation do seem to experience greater increase left ventricular ejection fraction. Those with cardiomyopathy also experienced greater increase in left ventricular ejection fraction.
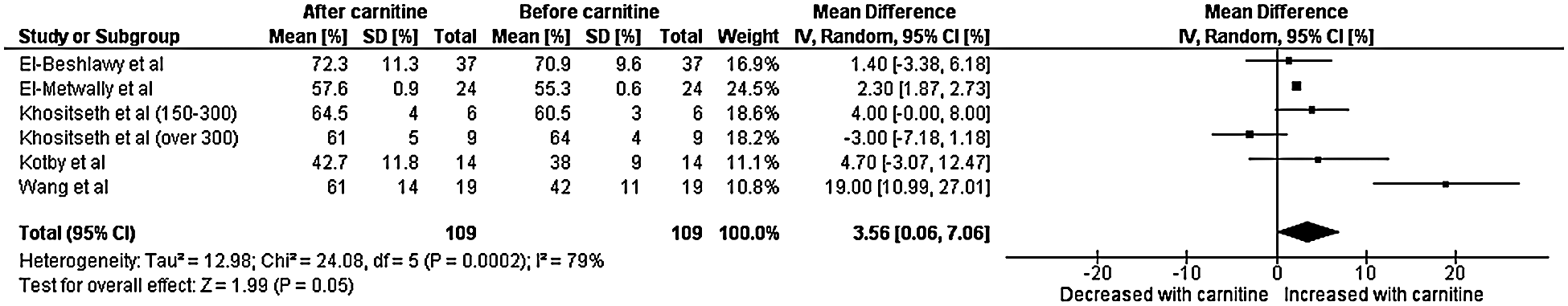
Figure 2: Forest plot displaying the change on left ventricular fraction after the administration of carnitine
3.3 Left Ventricular Shortening Fraction
A total of six studies with data from 144 patients were included in the final analysis for left ventricular shortening fraction [7–12]. Heterogeneity analysis resulted in a Q-statistic with a p-value of less than 0.01 and an I2 value of 94%, indicating the presence of significant heterogeneity. Thus, a random-effects model was used. Left ventricular shortening fraction was found to be significantly higher after carnitine supplementation. The mean difference in left ventricular shortening fraction was 3.68 with a 95% confidence interval 1.22 to 6.15 (p-value less than 0.01) (Fig. 3). Thus, left ventricular shortening fraction (measured in percent) increased by an absolute value of 3.68. Sensitivity analyses demonstrated that the overall effect of carnitine does not appear to be impacted by time, dose, or age. Those with a lower baseline left ventricular shortening fraction and longer duration of carnitine supplementation do seem to experience greater increase left ventricular shortening fraction. Those with cardiomyopathy also experienced greater increase in left ventricular shortening fraction. As per this sensitivity analyses, when only the three studies with cardiomyopathy patients are included the mean difference in shortening fraction was 6.5.
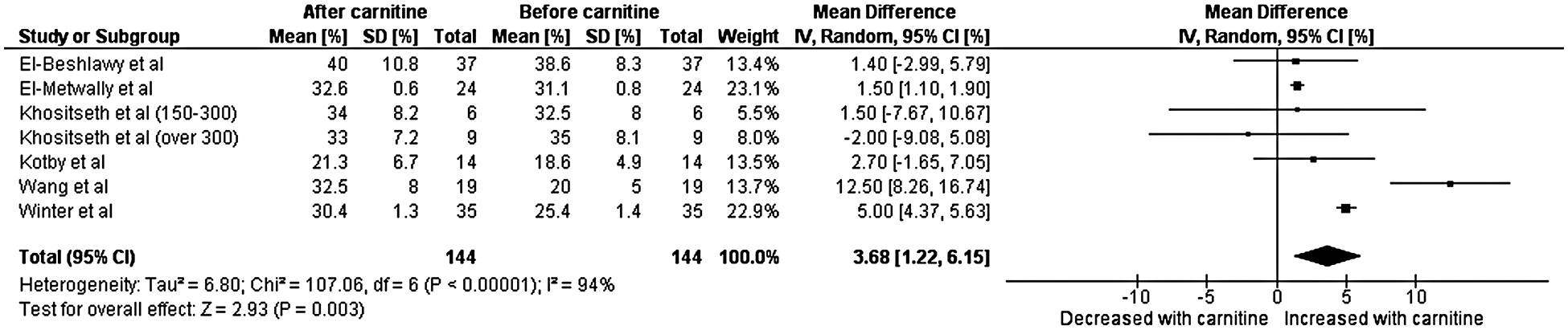
Figure 3: Forest plot displaying the change on left ventricular shortening after the administration of carnitine
3.4 Left Ventricular End Diastolic Diameter
A total of three studies with data from 80 patients were included in the final analysis for left ventricular end diastolic diameter [7,8,11]. Heterogeneity analysis resulted in a Q-statistic with a p-value of less than 0.01 and an I2 value of 92%, indicating the presence of significant heterogeneity. Thus, a random-effects model was used. Left ventricular end diastolic diameter was found to have a non-significant effect after the administration of carnitine supplementation. The mean difference in left ventricular end diastolic dimension was –0.46 cm with a 95% confidence interval –1.16 to 0.24 (p-value 0.20) (Fig. 4). Sensitivity analyses demonstrated that the overall effect of carnitine does not appear to be impacted by time, dose, or age. Those with a higher baseline left ventricular end diastolic dimension and longer duration of carnitine supplementation do seem to experience greater decrease in left ventricular end diastolic dimension. Those with cardiomyopathy also experienced greater decrease in left ventricular end diastolic diameter.
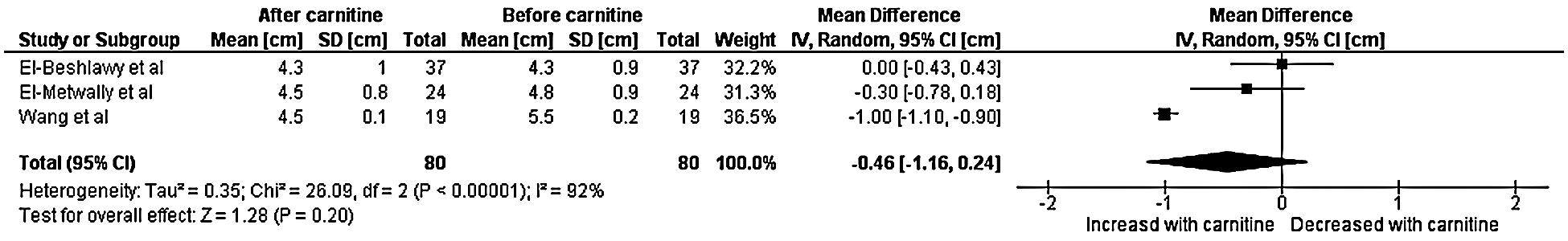
Figure 4: Forest plot displaying the change on left ventricular end diastolic diameter after the administration of carnitine
These meta-analyses demonstrate that carnitine supplementation in children may improve left ventricular function as evidenced by increases in left ventricular ejection fraction and shortening fraction. Pooled results demonstrated that carnitine dosed at 50 to 100 mg/kg/day in children with a mean age of seven years resulted in a significant increase of 3.56 in the absolute value of the ejection fraction and a significant increase of 3.68 in the absolute value of the shortening fraction after 10 months of carnitine supplementation.
A previous study by Wang et al. [11] demonstrated significant improvement in ejection fraction and shortening fraction in children with dilated cardiomyopathy who received carnitine in addition to conventional therapy. This study also demonstrated decreased biventricular diameter in those supplemented with carnitine, suggesting a benefit of carnitine supplementation to conventional therapy [11]. Kotby et al. [10] also reported similar results of increased ejection fraction, shortening fraction, and end diastolic diameter. Both studies also noted improvement of clinical symptoms, particularly improvements in high effort tolerance.
Carnitine has also been shown to improve pulmonary artery systolic pressure and diastolic function as evidenced by improvement in the E/A (Early filling/Atrial filling) ratio. El-Beshlawy et al. [7] demonstrated decrease in E/A ratio in sickle cell patients with cardiac abnormalities treated with carnitine. These results suggest improved diastolic function. This study also demonstrated a significant reduction in pulmonary artery systolic pressure in patients who had pulmonary hypertension before carnitine supplementation [7]. El-Metwally et al. [8] reported a similar reduction in the E/A ratio.
Carnitine plays a significant role in facilitating fatty acid transport across the mitochondrial membrane [1,2]. Fatty acids serve as the primary source of energy for cardiac muscles; thus, fatty acid transport and subsequent beta-oxidation is necessary to maintain proper cardiac function. Carnitine acts as a cofactor and is conjugated with Coenzyme A (CoA) using carnitine acyltransferase I. The acylcarnitine molecule is transferred across the mitochondrial membrane using carnitine acyl translocase. Once the molecule reaches the mitochondrial matrix, Coenzyme A is reformed with the help of carnitine acyltransferase II, undergoes beta oxidation, and then couples with the electron transport pathway to form adenosine triphosphate. Thus, carnitine aids in the formation of adenosine triphosphate [12].
Beta-oxidation involves multiple steps and enzymes. Five enzyme deficiency states have been identified to lead to beat-oxidation defects with four having the potential to cause secondary carnitine deficiency [13]. Three of the four enzyme deficiency states linked to beta-oxidation defects and concurrent carnitine deficiency have been linked to cardiomyopathy: medium chain acyl-CoA dehydrogenase deficiency, long-chain acyl-CoA dehydrogenase deficiency, and long chain l-3-hydroxyacyl-CoA dehydrogenase deficiency. Such deficiencies can lead to low plasma and tissue carnitine levels [13,14]. Supplementation can offset the loss of carnitine in plasma which normalizes mitochondrial energy production and in turn, improves myocardial function [15]. In fact, in the International Society for Heart and Lung Transplantation Guidelines for the Management of Pediatric Heart Failure, metabolic testing including evaluation of carnitine is suggested following the initial tier of testing [16].
While primary carnitine deficiencies are relatively rare, secondary or acquired forms are more common and may be associated with a number of conditions, including forms discussed above but also include several other disease states. These conditions include but are not limited to dialysis, liver or kidney disorders, cardiopulmonary bypass, sepsis, heart failure, malnutrition, as well as deficiencies of vitamin C, iron, niacin or pyroxidine (as necessary cofactors for biosynthesis) [17–19]. While tissues rich in carnitine are the heart, liver and skeletal muscle, the myocardium and skeletal muscle are not capable of carnitine synthesis and rely on liver and kidney production and dietary (animal) sources to supply carnitine as a transport vehicle for fatty acids into the myocardial mitochondria to maintain aerobic metabolism.
These pooled analyses aimed to quantitatively summarize the current data on carnitine supplementation on left ventricular function. While these analyses represent the most comprehensive set of data on carnitine supplementation for augmentation of left ventricular function, it does not offer definitive data. There are limitations of these analyses. Firstly, the patient population has clinical heterogeneity in the pooled studies. Of the six included studies, three studies focused on cardiomyopathy patients with left ventricular dysfunction [10–12] while the other three focused on patients with potentially normal ventricular function but in a therapeutic or clinical situation where they were at risk for decrease in left ventricular function [7–9]. Sensitivity analyses were conducted to offset this and assess if patient population impacted the overall results. Along with clinical heterogeneity there is also statistical heterogeneity present in the pooled analyses. This was offset by utilizing a random-effects model for all the clinical endpoints as there was significant statistical heterogeneity in all. It should be acknowledged that the heterogeneity quantified in the results is statistical and not clinical heterogeneity. Some of this statistical heterogeneity may be rooted in clinical heterogeneity but due to the lower number of studies meta-regression could not be conducted to further evaluate the mechanism of the statistical heterogeneity. Additionally, the included studies varied in the dose and duration of carnitine supplementation, but all doses are within the typical dose range for carnitine supplementation [12,20]. Sensitivity analyses were conducted to evaluate the impact of these on the mean effects. We acknowledge that some of the patients included in this study might be receiving additional vasoactive drugs that could have an impact on left ventricular ejection fraction and shortening fraction, however it’s difficult to control for other interventions with the current study design. Age also differed between the included studies and sensitivity analyses were conducted for each endpoint with respect to age as well. The relatively low number of included studies and, thus, patients also limit the pooled analyses. Finally, it should also be mentioned that the source studies didn’t all assess pre- and post-supplementation carnitine levels so this could not be commented on.
Despite the limitations mentioned above it is felt that these pooled analyses provide a helpful insight into the potential of a relatively inexpensive and safe therapy in prevention or reversal of left ventricular dysfunction. The true value of such pooled analyses lies not only in summarizing the available data but also in being able to provide guidance for future studies. Sensitivity analyses demonstrated that those with lower baseline left ventricular ejection fraction, shortening fraction, and end diastolic dimension experienced greater benefit from carnitine supplementation. Thus, the cardiomyopathy population would be an ideal population in which to attempt to delineate the utility of carnitine supplementation first. Such a study would randomize children with cardiomyopathy to either medical therapy with carnitine supplementation or medical therapy without carnitine supplementation. As dose effect would be of interest a total of three arms could be used for the design of such a study: (1) 50 mg/kg/day of carnitine supplementation in addition to other medical therapy; (2) 100 mg/kg/day of carnitine supplementation in addition to other medical therapy; (3) medical therapy without carnitine supplementation. Endpoints of interest could include vital signs such as heart rate, systolic blood pressure, mixed venous saturations and near infrared spectroscopy; echocardiographic parameters such as estimated right ventricular pressure, right ventricular strain, left ventricular ejection fraction, left ventricular strain, and diastolic function assessment using tricuspid and mitral valve inflow; laboratory evaluations such as N-terminal beta natriuretic peptide and arterial lactate; and functional assessment using the New York Heart Association scale, need for transplant evaluation or transplant, and mortality. Total and free carnitine levels could be obtained prior to the initiation of carnitine supplementation and then subsequently at 6 months and 12 months to determine the impact of duration of carnitine supplementation on effect. Using results of the pooled analysis for left ventricular ejection fraction (mean difference 3.56, 95% confidence interval 0.06 to 7.06, each group would need approximately 100 patients to detect a statistical significance in ejection fraction between timepoints for the same patient with 80% power at a p-value of 0.05. It is not possible to determine how many patients would be needed to power such a study for comparisons between the three separate groups. It seems reasonable that a study with 300 cardiomyopathy patients that are then randomized 1:1:1 to the three arms would be sufficient in answering several clinically relevant questions in a relatively efficient manner.
These analyses of current pooled data regarding the effect of carnitine supplementation in the pediatric population on left ventricular function demonstrates that carnitine supplementation may be a helpful adjunct therapy for augmenting left ventricular ejection fraction and shortening fraction although it may not improve left ventricular end diastolic volume. Those with lower baseline ejection fraction and shortening fraction appear to gain more benefit from carnitine supplementation. Additional studies of the effects of carnitine on pediatric cardiac function are warranted.
Data sharing: No additional data will be available.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Cave, M. C., Hurt, R. T., Frazier, T. H., Matheson, P. J., Garrison, R. N. et al. (2008). Obesity, inflammation, and the potential application of pharmaconutrition. Nutrition in Clinical Practice, 23(1), 16–34. DOI 10.1177/011542650802300116.
2. Inazu, M., Matsumiya, T. (2008). Physiological functions of carnitine and carnitine transporters in the central nervous system. Nihon Shinkei Seishin Yakurigaku Zasshi, 28(3), 113–120.
3. Foster, D. W. (2004). The role of the carnitine system in human metabolism. Annals of the New York Academy Sciences, 1033(1), 1–16. DOI 10.1196/annals.1320.001.
4. Rizzon, P., Biasco, G., Di Biase, M., Boscia, F., Rizzo, U. et al. (1989). High doses of L-carnitine in acute myocardial infarction: Metabolic and antiarrhythmic effects. European Heart Journal, 10(6), 502–508. DOI 10.1093/oxfordjournals.eurheartj.a059519.
5. Liedtke, A. J., DeMaison, L., Nellis, S. H. (1988). Effects of L-propionylcarnitine on mechanical recovery during reflow in intact hearts. American Journal of Physiology, 255(1), 169–176. DOI 10.1152/ajpcell.1988.255.6.C760.
6. Micheletti, R., Giacalone, G., Canepari, M., Salardi, S., Bianchi, G. et al. (1994). Propionyl-L-carnitine prevents myocardial mechanical alterations due to pressure overload in rats. American Journal of Physiology, 266(6), 2190–2197. DOI 10.1152/ajpheart.1994.266.6.H2190.
7. El-Beshlawy, A., Abd El Raouf, E., Mostafa, F., Talaat, M., Isma’eel, H. et al. (2006). Diastolic dysfunction and pulmonary hypertension in sickle cell anemia: Is there a role for L-carnitine treatment? Acta Haematologica, 115(1–2), 91–96. DOI 10.1159/000089472.
8. El-Metwally, T. H., Hamed, E. A., Ahmad, A. R., Mohamed, N. A. (2003). Dyslipidemia, oxidative stress and cardiac dysfunction in children with chronic renal failure: Effects of L-carnitine supplementation. Annals of Saudi Medicine, 23(5), 270–277. DOI 10.5144/0256-4947.2003.270.
9. Khositseth, A., Jirasakpisarn, S., Pakakasama, S., Choubtuym, L., Wattanasirichaigoon, D. (2011). Carnitine levels and cardiac functions in children with solid malignancies receiving doxorubicin therapy. Indian Journal of Medical and Paediatric Oncolology, 32(1), 38–42. DOI 10.4103/0971-5851.81889.
10. Kotby, A., Yamamah, G., Baky, A., El-Kassas, G., Zaghloul, A. (2006). Therapeutic evaluation of L-carnitine in Egyptian children with dilated cardiomyopathy. Journal of Medical Sciences, 6(5), 800–805. DOI 10.3923/jms.2006.800.805.
11. Wang, Y., Xu, Y., Zou, R., Wu, L., Liu, P. et al. (2018). Effect of levocarnitine on the therapeutic efficacy of conventional therapy in children with dilated cardiomyopathy: Results of a randomized trial in 29 children. Paediatric Drugs, 20(3), 285–290. DOI 10.1007/s40272-018-0284-2.
12. Winter, S., Jue, K., Prochazka, J., Francis, P., Hamilton, W. et al. (1995). The role of L-carnitine in pediatric cardiomyopathy. Journal of Child Neurology, 10(Suppl 2), S45–S51. DOI 10.1177/0883073895010002S07.
13. Frigeni, M., Balakrishnan, B., Yin, X., Calderon, F. R. O., Mao, R. et al. (2017). Functional and molecular studies in primary carnitine deficiency. Human Mutation, 38(12), 1684–1699. DOI 10.1002/humu.23315.
14. Mahapatra, S., Ananth, A., Baugh, N., Damian, M., Enns, G. M. (2018). Triheptanoin: A rescue therapy for cardiogenic shock in carnitine-acylcarnitine translocase deficiency. JIMD Reports, 39(4), 19–23. DOI 10.1007/8904_2017_36.
15. Helton, E., Darragh, R., Francis, P., Fricker, F. J., Jue, K. et al. (2000). Metabolic aspects of myocardial disease and a role for L-carnitine in the treatment of childhood cardiomyopathy. Pediatrics, 105(6), 1260–1270.
16. Kirk, R., Dipchand, A. I., Rosenthal, D. N., Addonizio, L., Burch, M. et al. (2014). The International society for heart and lung transplantation guidelines for the management of pediatric heart failure: Executive summary. Journal of Heart and Lung Transplantation, 33(9), 888–909. DOI 10.1016/j.healun.2014.06.002.
17. Carter, A. L., Abney, T. O., Lapp, D. F. (1995). Biosynthesis and metabolism of carnitine. Journal of Child Neurology, 10(Suppl 2), S3–S7. DOI 10.1177/088307389501000114.
18. Flanagan, J. L., Simmons, P. A., Vehige, J., Willcox, M. D., Garrett, Q. (2010). Role of carnitine in disease. Nutrition & Metabolism, 7(1), 30. DOI 10.1186/1743-7075-7-30.
19. Pons, R., De Vivo, D. C. (1995). Primary and secondary carnitine deficiency syndromes. Journal of Child Neurolology, 10(Suppl 2), S8–S24. DOI 10.1177/08830738950100S104.
20. Chaiyakulsil, C., Chantra, M., Katanyuwong, P., Khositseth, A., Anantasit, N. (2018). Comparison of three non-invasive hemodynamic monitoring methods in critically ill children. PLoS One, 13(6), e0199203. DOI 10.1371/journal.pone.0199203.
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |