

 | Congenital Heart Disease |  |
DOI: 10.32604/CHD.2020.012994
ARTICLE
Health-Related Quality of Life, Emotional and Behavioral Problems in Children and Adolescents with Ebstein Anomaly
1Department of Pediatric Cardiology, Amalia Children’s Hospital, University Medical Center Nijmegen, Nijmegen, 6525 GA, The Netherlands
2Department of Pediatric Cardiology, Wilhelmina Children’s Hospital, University Medical Center Utrecht, Utrecht, 3584 EA, The Netherlands
3Academic Center for Child and Adolescent Psychiatry the Bascule/Department of Child and Adolescent Psychiatry, Amsterdam University Medical Center, Amsterdam, 1105 AZ, The Netherlands
4Department of Child and Adolescent Psychiatry/Psychology, Erasmus Medical Center, Rotterdam, 3015 GD, The Netherlands
5Center for Congenital Heart Diseases, University Medical Center Groningen, Groningen, 9713 GZ, The Netherlands
6Department of Pediatric Cardiology, Academic Medical Center Amsterdam, Amsterdam, 1105 AZ, The Netherlands
7Department of Pediatric Cardiology, Maastricht University Medical Center, Maastricht, 6229 HX, The Netherlands
8Department of Pediatric Cardiology, Sophia Children’s Hospital, Erasmus Medical Center, Rotterdam, 3015 GD, The Netherlands
9Department of Pediatrics, Máxima Medical Center Veldhoven, Veldhoven, 5504 DB, The Netherlands
10Department of Radiology, Medical Ultrasound Imaging Center, University Medical Center Nijmegen, Nijmegen, 6525 GA, The Netherlands
11Pediatric Cardiology Unit, Department of Pediatrics, Dana-Dwek Children’s Hospital, Tel Aviv Sourasky Medical Center, Tel Aviv University, Tel Aviv, 6423906, Israel
12Department of Medical Psychology, Amalia Children’s Hospital, University Medical Center Nijmegen, Nijmegen, 6525 GA, The Netherlands
*Corresponding Author: Lianne M. Geerdink. Email: l.m.geerdink@umcutrecht.nl
Received: 21 July 2020; Accepted: 22 September 2020
Abstract: Background: Due to the improved survival rates of children and adolescents with congenital heart disease (CHD), more attention is now being directed towards their health-related quality of life (HRQoL), emotional and behavioral problems. Ebstein anomaly (EA) is a rare CHD with a broad clinical spectrum. The aim of the current study is to evaluate self- and proxy-reported HRQoL and emotional and behavioral problems in children and adolescents with EA. Methods: In this cross-sectional, multicenter study, we included EA patients (aged 8–17 years), who underwent routine clinical assessments in Dutch university hospitals between May 2017 and March 2019. The Generic Pediatric Quality of Life Inventory 4.0 (for ages 8–12/13–17 years) was used to assess HRQoL. The Child Behavior Checklist, Youth Self-Report and Teacher’s Report Form were completed to measure emotional and behavioral problems. Results: Questionnaires for thirty-eight patients (median age: 13 years; 48% male) were completed by patients themselves, parents and teachers. Compared to normative data, self-reported HRQoL was not significantly impaired, except for social functioning in children aged 8–12 years. Gender, severity of tricuspid regurgitation or previous surgery did not predict HRQoL. Parents reported significantly more internalizing (anxiety/depression), attention and thought problems in children with EA. These problems, however, were not reported by adolescents themselves. School teachers reported significantly fewer externalizing problems compared to the norm. Conclusions: Overall, despite satisfactory HRQoL, children and adolescents show emotional and behavioral problems. We recommend routine HRQoL and psychological assessment to screen for less apparent internalizing and psychosocial problems.
Keywords: Ebstein anomaly; pediatric; health-related quality of life; behavioral problems
Due to the improved survival rates of children and adolescents with congenital heart disease (CHD), more attention is now being directed towards their health-related quality of life (HRQoL). HRQoL includes the subjective perception of physical, social, emotional and cognitive functioning [1]. Children and adolescents with CHD are at increased risk of impaired HRQoL [2–10]. Moreover, children with CHD are about two times more likely to develop emotional and behavioral problems than healthy children, independent of the type of CHD [11,12]. These problems can be separated into internalizing and externalizing behavioral difficulties [13]. The first are characterized by internally-focused or emotional problems such as somatic complaints, anxiety, depression and social withdrawal. The latter include externally-focused or behavioral problems such as hyperactivity, delinquency and aggressive behavior. HRQoL and emotional and behavioral problems can be assessed by means of self- or proxy-reports. The latter often include parents’ and schoolteachers’ perceptions.
If left unaddressed, these problems can persist into young adulthood. Increased likelihood of social disadvantages, such as lower educational and occupational status, more unemployment and being in a relationship less often have been reported for adult patients [14,15]. These poorer outcomes have been seen irrespective of cardiac diagnosis.
Ebstein anomaly (EA) is a rare CHD characterized by an apical displacement of the functional tricuspid valve towards the right ventricular outflow tract. When diagnosed in childhood, it can be a serious condition: almost one fifth of all patients die before reaching adulthood [16]. Severity of disease is usually assessed by a short interview and physical examination at the outpatient clinic, an electrocardiogram and an echocardiography. Children with EA might suffer reduced exercise tolerance, which might hinder them in playing with healthy peers or participation in team sports may be limited. They might miss school due to hospital stays or visits at the outpatient clinic. They often require one or more surgical interventions, which might produce high levels of emotional stress. Altogether, we are concerned that children with EA experience reduced HRQoL. Though a substantial amount of research has been conducted on the HRQoL and emotional and behavioral problems in children with CHD, very little is known about those factors in patients with EA specifically. Since EA is uncommon and patients present with a broad clinical spectrum, study cohorts are relatively small and heterogenous with limited research. Nevertheless, this should not hinder men from undertaking the effort to evaluate this population as good as possible. Even more, the uncertainties regarding the natural course of disease and the patient’s future perspectives, might negatively influence the patient’s well-being. While encountering EA patients at the outpatient clinic for regular follow-up, we should not only assess clinical parameters, but also be aware of the impact of this chronic disease on the individual patient. Therefore, we were motivated to bridge this gap in knowledge by means of multi-informant questionnaires completed by patients, parents and teachers.
The goals of the current study were to (1) evaluate self-reported HRQoL in children and adolescents with EA and compare this to the general population; (2) identify biographical or medical predictors of HRQoL; (3) study the relation between self-reported and proxy-reported HRQoL; and (4) evaluate emotional and behavioral problems in children and adolescents with EA and compare them to the general population.
This was a cross-sectional, multicenter study. We included children and adolescents with EA (aged 8–17 years) who underwent routine clinical assessments in one of six participating Dutch university hospitals between May 2017 and March 2019. Children and parents unable to speak Dutch, and thus unable to complete questionnaires, were excluded from this study. Only proxy-reported questionnaires were included for patients functioning on an estimated or documented cognitive level below 8 years of age.
Before the hospital visit, we asked the patients and their parents to complete questionnaires about general HRQoL and emotional and behavioral problems. If parents, teachers and/or children required any assistance in filling out the questionnaires or had any questions, they could contact the principal investigator (LMG) by mail or by telephone. During the hospital visit, we took a clinical history and performed a physical examination, electrocardiogram and echocardiography. As age- and gender-matched normative data are available, we did not recruit a healthy control group.
Demographic variables on patients and parents were collected by telephone, during the hospital visit or from medical files, and included the patients’ gender, age, medical history, known mental disorders and type of education. The severity of the TR was determined by the local experienced pediatric cardiologist and the principal investigator (LMG, who was blinded to other reports), using the approach as recommended by the American Society of Echocardiography [17]. This included color jet area, vena contracta width, density of continuous doppler jet and hepatic vein flow pattern. Evaluation of RV filling dynamics or annular diameter and RA/RV size, were found to be too challenging in our EA patients. Demographic variables from the parents included sex, marital and socioeconomic status. Socioeconomic status was based on the highest of both parents’ occupations and categorized into low, low to middle, middle, or high according to the international classification system [18].
The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki [19] and was approved by the Medical Ethics Committee of the Erasmus Medical Center (protocol number MEC-2016-752) and by the institutional review boards of all participating centers. Written informed consent was obtained from legal guardians of all participants and from participants aged 12 or older.
All questionnaires used are (inter)nationally validated, with adequate psychometric properties. The age ranges of the patients corresponded to those for the questionnaires used.
2.1.1 Health-related Quality of Life
To evaluate HRQoL in patients with EA, patients and parents completed the Generic Pediatric Quality of Life Inventory (PedsQL) 4.0 (aged 8–12 or 13–17 years) [20–22]. This 23-item questionnaire evaluates four domains of quality of life: physical (8 items), emotional (5 items), social (5 items) and school functioning (5 items). Children and their parents score each item on a five-point Likert scale: 0-Never a problem, 1-Almost never a problem, 2-Sometimes a problem, 3-Often a problem, 4-Almost always a problem. Scores are converted to a 0–100 scale where 0 = 100, 1 = 75, 2 = 50, 3 = 25 and 4 = 0. Therefore, higher scores indicate better HRQoL. Final results are reflected in summary scores: physical health (equals physical functioning), psychosocial health (the sum of emotional, social and school functioning) and total health score (sum of all domains). Dutch normative values are available for children aged 8 to 18 years [23].
2.1.2 Emotional and Behavioral Problems
The Child Behavior Checklist (CBCL), Youth Self-Report (YSR) and Teacher’s Report Form (TRF) are standardized measures that assess child and adolescent emotional and behavioral problems. The questionnaires are completed by parents, adolescents and teachers, respectively [24–29]. Problems are rated on a 3-point Likert scale: 0-not true, 1-somewhat or sometimes true, 2-very true or often true. Higher scores indicate more problems. Besides a total problem score, broadband scale scores are calculated for internalizing problems (e.g., depression and anxiety) and externalizing problems (e.g., aggression and delinquency). In addition, eight empirical scales and six scales based on the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) are calculated [30]. Scores fall in the normal, borderline or clinical range when compared to the available normative data from the Dutch general population. Internal consistencies of the problem scales, as measured by Cronbach’s alpha, ranged from 0.67 to 0.97 [31].
The CBCL/6–18 is a 120-item parent proxy-report for children aged 6–18 years. The YSR is a 118-item adolescent (aged 11–17 years) self-report. Items that evaluate socially desirable characteristics such as “I like to be fair to others” or “I try to help others when I can” differ from the CBCL. The TRF/6–18 is a 118-item teacher proxy-report for children aged 6–18 years that includes items scoring behavioral problems difficult for parents to judge, such as “does the child have any difficulty in following instructions?”, “How does the child interact with fellow students?” and “Does he or she cause agitation during class?”.
2.2.1 Health-Related Quality of Life
The mean of the subscale scores for physical, emotional, social and school functioning, is computed as the sum of the items divided by the number of completed items. At least 50% of the items have to be accounted for. The mean Psychosocial Health Summary Score is computed as the sum of the items divided by the number of items answered in the emotional, social and school functioning subscales, whereas the Physical Health Summary Score equals the physical functioning score. Finally, the Total Score of all four domains is calculated as the sum of all items divided by the total number of items (i.e., 23). Due to a non-normal distribution, the results are presented as median with the total range. Cronbach’s alpha is calculated for the internal consistency of each subscale, the Health Summary Scores and the Total Score. A value of 0.6 or higher is considered acceptable.
Our patients’ scores were compared to published normative data of age-matched groups (8–12 years and 13–17 years) using the one-sample Wilcoxon test. Agreement between self- and proxy-reported HRQoL was studied using the Intraclass Correlation Coefficient (ICC). Based on previous CHD and EA specific studies, we established multiple variables as possible predictors of HRQoL. We included gender, TR severity and previous CHD surgery. With only one patient in New York Heart Association (NYHA) functional classification III/IV, we had to exclude this important variable from our analysis. We used a linear regression model for these categorical variables. For this analysis we combined child and adolescent HRQoL outcomes.
2.2.2 Emotional and Behavioral Problems
We used the Achenbach System of Empirically Based Assessment (ASEBA) software to transform the raw scores from all three questionnaires into percentile scores. One-sample binomial tests were conducted for each scale of the CBCL/6–18, YSR, and TRF/6–18 to test whether the proportion of children with EA scoring in the non-clinical (i.e., not in the psychopathological range) or in the borderline/clinical range was higher than the proportion in the norm group. For this, percentile scores of 83 or lower on the internalizing, externalizing and total problems broadband scales are considered non-clinical, whereas scores of 84 or higher are considered borderline or clinical. Percentile scores of 92 or lower on the DSM-oriented and the empirical scales are considered non-clinical, whereas scores of 93 or higher fall into the borderline/clinical group. p-values <0.05 were considered statistically significant.
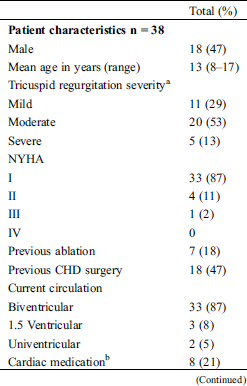
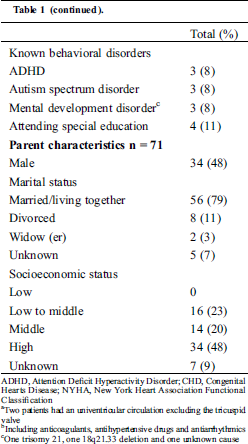
Fifty-three patients aged between 8 and 18 years were eligible to participate and were approached by their treating physician. Nine patients were excluded from the study because the treating physician was unable to contact the parents before the child’s regular visit at the outpatient clinic or because the patient did not show up at his/her visit. Two patients did not want to participate due to several previous (other) study participations and one patient because she was busy studying for her final exams at school. Only one patient was not included because of the child’s condition; she was soon to receive cardiac surgery and the parents had a lot on their mind. Finally, we had to exclude two more patients because they had just turned 18. Finally, thirty-eight patients (median age: 13 years, 48% male) and/or their parents filled out informed consent forms. Almost half the patients had previous CHD surgery (Tab. 1). Previous CHD surgery included TV repair (± annuloplasty, n = 7), TV repair and pulmonary homograft (n = 3), partial cavopulmonary connection (PCPC, n = 3), total cavo-pulmonary connection (TCPC, n = 2), closure of atrial and ventricular septal defect (n = 1), only atrial septal defect closure (China, n = 1) and one patient underwent a lateral coarctectomy for his aortic coarctation. Tricuspid valve regurgitation (TR) was moderate in the majority of the patients.
Table 1: Characteristics of participating pediatric patients with Ebstein anomaly and their parents
3.1 Health-Related Quality of Life
Although self-reported HRQoL was scored lower than the general Dutch population in all but one domain, these differences did not reach statistical significance, except for social functioning in children aged 8–12 years (score 65.0 vs. 86.1 resp., Z = –2.436, p = 0.015, Tab. 2).
Table 2: Results of the pediatric quality of life inventory 4.0 measuring health related quality of life in pediatric patients with Ebstein anomaly
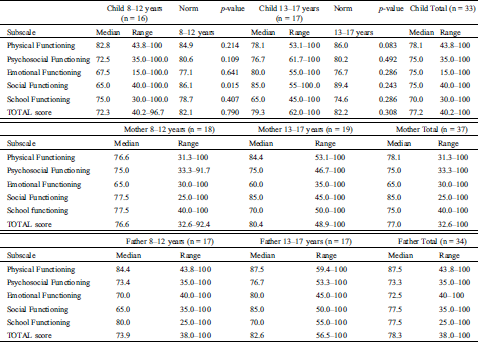
In our sample, patients and their parents rated psychosocial health worse than physical health. There were five patients with extreme low scores on self-reported psychosocial health. In general, a PedsQL score of 70 is seen as a cut-off point for reduced HRQoL. These five patients scored below 60 on at least two out of the three psychosocial subscales. The parent-reports of all these five patients were within normal range.
No significant differences (p = 0.606) in self-reported HRQoL were found between boys and girls. TR severity or history of previous CHD surgery did not predict self-reported HRQoL either (p = 0.846 and p = 0.426 resp.). There was a poor agreement in HRQoL Total Scores between the patients and their mothers (ICC 0.514, F (27) = 3.104, p = 0.002) as well as their fathers (ICC 0.567, F (27) = 3.552, p = 0.01). Agreement between both parents was good (ICC .858, F (27) = 15.948, p = 0.000). Internal consistency was adequate to good for all four subscales of the PedsQL and all three summary scores (Cronbach’s α 0.71–0.94).
3.2 Emotional and Behavioral Problems
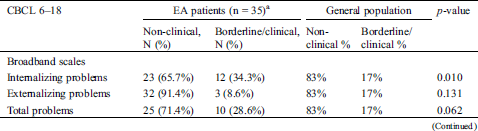
Parents of children with EA reported significantly more internalizing problems than parents in the general population (Tab. 3). These were mainly anxiety/depressive (22.9% versus 8%, p = 0.006), withdrawn/depressive (22.9% vs. 8%, p = 0.006), somatic (28.6% versus 8%, p = 0.000) and thought problems (28.6% vs. 8%, p = 0.000).
Table 3: Distribution of non-clinical versus borderline/clinical emotional and behavioral problems by parents of patients with Ebstein anomaly
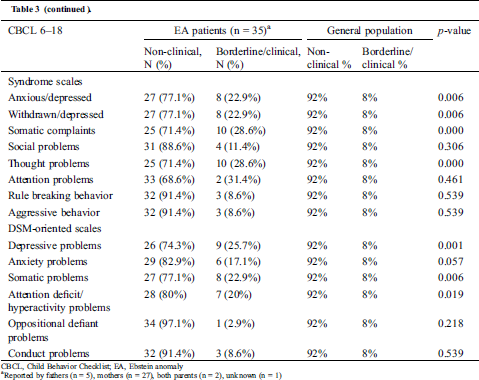
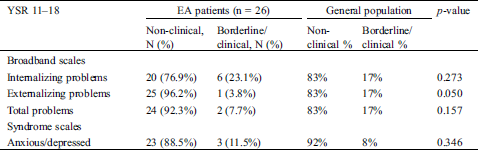
Compared to the general population, a significantly larger proportion of children with EA showed attention deficit/hyperactivity problems (20.0% vs. 8%, p = 0.019). These problems, however, were not self-reported by adolescents. They did not report any internalizing problems and even reported borderline significantly (p = 0.050) fewer externalizing problems than the general population (3.8% vs. 17%). (Tab. 4)
Table 4: Distribution of non-clinical versus borderline/clinical emotional and behavioral problems by adolescents with Ebstein anomaly (age 11–18 years)

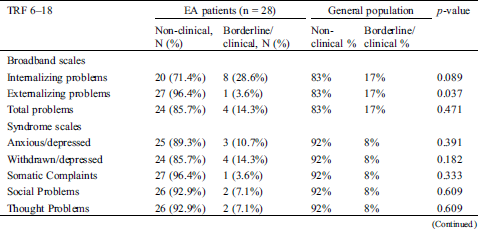
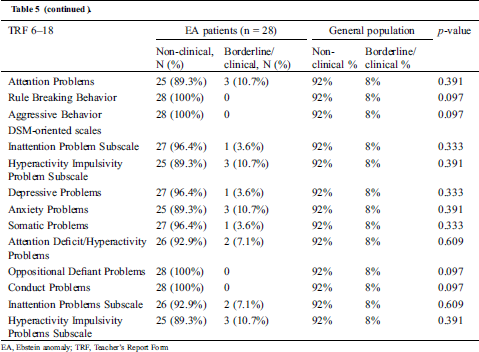
Teachers of children with EA also reported significantly fewer externalizing problems compared to the general population (3.6% vs. 17%, p = 0.037) (Tab. 5).
Table 5: Distribution of non-clinical versus borderline/clinical emotional and behavioral problems by school teachers of children with Ebstein anomaly (age 8–18 years)
The present study shows that self-reported health-related quality of life is not significantly reduced in children and adolescents with EA, except for social functioning in children 8–12 years of age. Mellion et al. [10] of the Pediatric Cardiac Quality of Life Inventory Testing Study Consortium, compared HRQoL in a large group of pediatric patients (625 children, 513 adolescents) with CHD and healthy controls using the same PedsQL 4.0 instrument. The Total, Physical and Psychosocial Health Summary Scores were lower in the CHD group as compared with healthy controls. However, their patient cohort was divided into three severity categories; mild (no surgical or catheter-based intervention), biventricular CHD and single ventricle CHD. When looking at these subgroups, they found no significant differences between children and adolescents with mild CHD and healthy controls. In our EA cohort 20/38 patients did not have previous CHD surgery. Furthermore, the majority was classified as NYHA class I or II. Therefore, the majority of our cohort could be classified as mild and thus, overall, would align with the results from the Mellion group.
Literature about HRQoL of children with CHD, however, remains inconsistent. Reiner et al. [32] enrolled 514 patients (aged 7–17 years) with CHD who underwent regular outpatient visits. The age-adapted KINDL questionnaire was used for evaluation of HRQoL. After adjusting for age and sex, the HRQoL of the patients was significantly higher than healthy peers for the total HRQoL score, for self-esteem, family and everyday functioning. No differences between severity classes were seen. They concluded that young patients can cope well with their disease burden. Possible positively contributing factors include family environment, peers, social support, school and community. Furthermore, the authors emphasize that these patients do not know a life without cardiac disease and have learnt to develop coping strategies from the beginning of their life. It is well recognized that HRQoL is dependent upon finding a balance between body, mind and spirit and on establishing and maintaining a harmonious set of relationships within the person’s social context and external environment [33]. Therefore, even young patients with serious physical limitations can experience good or even excellent quality of life, whereas to most external observers these individuals seem to live a less desirable daily life.
One of the biggest limitations of most studies on HRQoL in CHD patients is their inclusion of heterogeneous groups of patients including left and right heart obstructions, isolated shunt defects, transposition of the great arteries, univentricular hearts and other heart defects. We consider our focus on EA a surplus value of this study, since results can now be better applied to this particular cardiac group, providing insight into disease-specific screening.
The only significant difference between young patients with EA and healthy peers was on their self-report as to social functioning. Several studies suggest that for children and adolescents with CHD, the identification of other children’s emotions, is impaired, although this could not be ascertained by statistical analyses [34–36]. We speculate this might negatively influence the children’s capability to making and keeping friendships. Furthermore, we speculate that feeling different and missing out on school makes them feel isolated from peers. Moreover, overprotection by parents and/or reduced exercise tolerance can result in less sports or leisured time activities/clubs with friends, and by consequence in reduced social skills due to limited social experiences. Adolescents might have developed coping strategies, while younger patients might not have been able to do so yet. Also, CHD patients are at risk for neurodevelopmental morbidity caused by biological risk factors as underlying syndromes or genetic/developmental disorders, the circulatory abnormalities specific to the heart defect and the medical and surgical therapies required [37]. These risk factors are modified by environmental risk and protective factors at home, school and work. Only a few studies have investigated the impact of neurodevelopmental outcome on HRQoL in the pediatric population. For children with a D-transposition of the great arteries and for children with Fontan palliation for hypoplastic left heart syndrome, a lower parent-reported psychosocial quality of life has been reported [38,39]. These findings suggest a role for neurodevelopmental impairment as cause for reduced psychosocial health.
It is concerning that in our population, five children self-reported distressingly low scores for psychosocial functioning. We examined their medical files carefully. In three patients, no concerns about the child’s psychosocial wellbeing were reported in their files. We contacted their treating physicians. They did not have any indication for concern before. Therefore, none of these patients underwent psychosocial screening or assessment by an experienced mental health professional up to now. The treating physician is now informed and this matter will be discussed with the patient and its parents. The other two patients faced surgery later that year. Undergoing cardiac surgery is a stressful event that may negatively influence the patients’ or parents’ perception on HRQoL [3,40–44]. Remarkably, in our study, a history of previous CHD surgery was not related to impaired HRQoL when compared to a non-surgical group or when compared to healthy controls. Due to the small subgroups, we did not take time since last surgery or the number of surgeries into account. To our knowledge, no previous studies have been conducted on HRQoL in post-surgical patients with EA.
An important parameter for severity of disease in EA is the severity of TR. Increased TR aggravates enlargement of both the right atrium and the atrialized and functional right ventricle and worsens right ventricular function. This could eventually lead to reduced functional class and impaired HRQoL. However, in this study, TR severity did not predict HRQoL. A possible explanation could be that in only five patients, severe TR was observed. Other patients with severe TR underwent previous TV repair, resulting in less regurgitation at last visit. Furthermore, our patients are young and often show maintained right ventricular function. In adult patients with EA and severe TR, the right ventricle starts failing more rapidly.
We found a poor agreement in HRQoL scores between children and parents. In pediatric health care, doctors often convey and obtain information primarily from parents during outpatient visits. This study emphasizes the need to listen carefully to both patients and parents. These results are consistent with the study of Patel et al. [45] who examined agreements and discrepancies between child and parent reports on quality of life in the pediatric cardiology population using the PedsQL 4.0 instrument as well [45]. Child scores were consistently 2–3 points lower than parent scores for all domains. Parent and child scores were moderately to poorly correlated for school functioning, psychosocial and total summary scores. Emotional quality of life had the lowest agreement. In our study, we found the lowest correlation in physical functioning. Physical health is easier to observe than psychosocial health and, therefore, we would have expected a better agreement between child and parent reports in this domain. In fact, other studies showed the greatest agreement for physical quality of life [8,46,47]. However, studies on agreement between self- and parent reports on HRQoL are inconclusive [48]. All stress the importance of taking both child and proxy reports into account, a conclusion our study confirms.
Parents of children with EA reported significantly more internalizing problems than parents from the general population. A protective parenting style, which is recognizable to most pediatricians, may be an important risk factor. Other studies have also reported more emotional and behavioral problems in children with CHD [2,5,12,49–52]. Most of these, however, included patients with more common types of CHD, such as tetralogy of Fallot or transposition of the great arteries. To our knowledge, no specific studies on behavioral problems in EA exist.
Although parents report behavioral problems, these problems are not self-reported by children and adolescents. Spijkerboer et al. [2] examined long-term behavioral and emotional problems in four cardiac diagnostic groups of children and adolescents after invasive treatment for congenital heart disease. They came to the same conclusion. Overall, parents of patients with CHD reported higher levels of problems compared to parents of healthy children, whereas patients themselves reported no long-term impairment compared to same-sex reference peers.
Teachers of children and adolescents with EA report fewer externalizing problems in class than teachers of healthy peers. We hypothesize that, due to a protective parenting style, children might enter adolescence later. Because of their medical history, patients might just want to be normal and, therefore, try not to stand out in class. They may lack the cardiorespiratory reserves to spend their energy on externalizing behavior. Anxiety might play a role as well.
For the practicing pediatric cardiologist, there needs to be recognition that children and adolescents with EA, despite an overall adequate HRQoL, are at a higher risk for emotional and behavioral problems than their healthy peers. Considering our results, we recommend that HRQoL and psychological assessment should be made a routine part of clinical visits in order to screen for less apparent psychosocial problems. Completing a self- and proxy-HRQoL questionnaire as the Generic Pediatric Quality of Life Inventory 4.0 can be helpful. Questionnaires could be sent by email to parents before routine clinical visits. Results should be uploaded to the patients’ medical files, and scores discussed with patients and their parents. If required, treatment strategies can be optimized, additional examination can be performed or further support can be offered by experienced health care workers such as psychologists or physiotherapists.
We are currently working hard on protocols to arrange systematic screening. The present situation is that before visiting the outpatient clinic, patients are asked to complete online HRQoL questionnaires. Results are evaluated by a dedicated team. In case of concerning results, the dedicated team informs the treating physician by mail and makes a note in the patient’s medical file about the results and their interpretation. Next, it is up to the treating physician to discuss these results during the patients’ visit. Based on these findings the treating physician might refer the patient to e.g., the psychologist. First results are positive: patients and parents appreciate the attention paid to this important part of health care. However, we are aware of the fact that a treating physician is neither educated nor experienced enough to discuss and interpret results of HRQoL questionnaires. Further adjustments to this system are being made.
We also believe that continuous assessment of the patients’ development in order to recognize impairment as soon as possible, is crucial. In our center, a dedicated team (including neonatologists, pediatric cardiologists, psychologists, pediatric physiotherapists and exercise physiologists) evaluates all severe CHD patients at a special CHD Lifespan dedicated outpatient clinic. Follow-up is performed just before reaching important milestones in their neurodevelopment, until the patient turns 18 years of age. Thereafter, age-attuned assessment for adults and elderly should be specified. Our final goals are improving the current treatment strategies and optimizing neurodevelopmental and health-related quality of life in our patients with CHD and their families.
This is the first psychosocial study to specifically target young EA patients. We acquired a substantial sample size, considering the prevalence of this rare CHD. The multicenter recruitment and the broad age range improved the generalizability of our results in the pediatric EA population. We also included a large group of fathers, which is fairly rare for psychosocial research [53,54].
Although the sample size is relatively large for EA, the number of patients limits extensive statistical analysis and decreases statistical power. Generally known problems with questionnaire studies are, that patients are sometimes not willing to show the truth or they prefer strategic behavior. An emotional resistance to evaluate oneself could exist and lack of motivation could lead to falsified results. To avoid such bias a big sample size is required.
A cardiac module of the PedsQL is available for evaluation of HRQoL, which we did not include in our study. This cardiac module would compare the results of our patients with EA to patients with other types of CHD, whereas the aim of the current study was to compare our results to healthy peers.
In this study we evaluated the impact of clinical factors on HRQoL. We did not look into environmental factors, which are known to exert strong influence on HRQoL, such as psychological distress, problematic family conditions and limited social support.
Overall, despite satisfactory HRQoL, children and adolescents with EA show emotional and behavioral problems. We recommend routine HRQoL and psychological assessment to screen for less apparent internalizing and psychosocial difficulties. In addition, during visits, the treating physician should carefully listen to both patients and parents, as their perspectives might differ substantially.
Acknowledgement: We are grateful to all patients and parents for taking the effort and time to complete all questionnaires. We thank Dr. P. Vart for his statistical assistance.
Funding Statement: The author(s) received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Koot, H. M. (2001). The study of quality of life: concepts and methods. Quality of life in child and adolescent illness. Sussex: Brunner-Routledge Publishers.
2. Spijkerboer, A. W., Utens, E. M., Bogers, A. J., Verhulst, F. C., Helbing, W. A. (2008). Long-term behavioural and emotional problems in four cardiac diagnostic groups of children and adolescents after invasive treatment for congenital heart disease. International Journal of Cardiology, 125(1), 66–73. DOI 10.1016/j.ijcard.2007.02.025.
3. Spijkerboer, A. W., Utens, E. M., De Koning, W. B., Bogers, A. J. Helbing, W. A. et al. (2006). Health-related quality of life in children and adolescents after invasive treatment for congenital heart disease. Quality of Life Research, 15(4), 663–673. DOI 10.1007/s11136-005-3692-z.
4. Latal, B., Helfricht, S., Fischer, J. E., Bauersfeld, U., Landolt, M. A. (2009). Psychological adjustment and quality of life in children and adolescents following open-heart surgery for congenital heart disease: A systematic review. BMC Pediatrics, 9(1), 6. DOI 10.1186/1471-2431-9-6.
5. Miatton, M., De Wolf, D., Francois, K., Thiery, E., Vingerhoets, G. (2007). Behavior and self-perception in children with a surgically corrected congenital heart disease. Journal of Developmental and Behavioral Pediatrics, 28(4), 294–301. DOI 10.1097/DBP.0b013e3180cabc3c.
6. Ladak, L. A., Hasan, B. S., Gullick, J., Gallagher, R. (2019). Health-related quality of life in congenital heart disease surgery in children and young adults: A systematic review and meta-analysis. Archives of Disease in Childhood, 104(4), 340–347. DOI 10.1136/archdischild-2017-313653.
7. Karsdorp, P. A., Everaerd, W., Kindt, M., Mulder, B. J. (2007). Psychological and cognitive functioning in children and adolescents with congenital heart disease: A meta-analysis. Journal of Pediatric Psychology, 32(5), 527–541. DOI 10.1093/jpepsy/jsl047.
8. Uzark, K., Jones, K., Slusher, J., Limbers, C. A. Burwinkle, T. M. et al. (2008). Quality of life in children with heart disease as perceived by children and parents. Pediatrics, 121(5), e1060–e1067. DOI 10.1542/peds.2006-3778.
9. Marino, B. S., Lipkin, P. H., Newburger, J. W., Peacock, G., Gerdes, M. et al. (2012). Neurodevelopmental outcomes in children with congenital heart disease: Evaluation and management: a scientific statement from the American Heart Association. Circulation, 126(9), 1143–1172. DOI 10.1161/CIR.0b013e318265ee8a.
10. Mellion, K., Uzark, K., Cassedy, A., Drotar, D., Wernovsky, G. et al. (2014). Health-related quality of life outcomes in children and adolescents with congenital heart disease. Journal of Pediatrics, 164(4), 781–788.e1. DOI 10.1016/j.jpeds.2013.11.066.
11. Spijkerboer, A. W., Utens, E. M., Bogers, A. J., Helbing, W. A., Verhulst, F. C. (2008). A historical comparison of long-term behavioral and emotional outcomes in children and adolescents after invasive treatment for congenital heart disease. Journal of Pediatric Surgery, 43(3), 534–539. DOI 10.1016/j.jpedsurg.2007.10.037.
12. Utens, E. M., Verhulst, F. C., Meijboom, F. J., Duivenvoorden, H. J., Erdman, R. A. et al. (1993). Behavioural and emotional problems in children and adolescents with congenital heart disease. Psychological Medicine, 23(2), 415–424. DOI 10.1017/S0033291700028518.
13. Achenbach, T. M., Edelbrock, C. (1983). Manual for the child behavior checklist and revised child behavior profile. Burlington, VT: University of Vermont.
14. Zomer, A. C., Vaartjes, I., Uiterwaal, C. S., van der Velde, E. T.,Sieswerda, G. J. et al. (2012). Social burden and lifestyle in adults with congenital heart disease. American Journal of Cardiology, 109(11), 1657–1663. DOI 10.1016/j.amjcard.2012.01.397.
15. Opic, P., Roos-Hesselink, J. W., Cuypers, J. A., Witsenburg, M., van den Bosch, A. et al. (2015). Psychosocial functioning of adults with congenital heart disease: outcomes of a 30–43 year longitudinal follow-up. Clinical Research in Cardiology, 104(5), 388–400. DOI 10.1007/s00392-014-0792-1.
16. Geerdink, L. M., Delhaas, T., Helbing, W. A., du Marchie Sarvaas, G. J. Ter Heide, H. et al. (2018). Paediatric Ebstein’s anomaly: how clinical presentation predicts mortality. Archives of Disease in Childhood, 103(9), 859–863. DOI 10.1136/archdischild-2017-313482.
17. Zoghbi, W. A., Adams, D., Bonow, R. O., Enriquez-Sarano, M., Foster, E. et al. (2017). Recommendations for noninvasive evaluation of native valvular regurgitation: A report from the American society of echocardiography developed in collaboration with the society for cardiovascular magnetic resonance. Journal of the American Society of Echocardiography, 30(4), 303–371. DOI 10.1016/j.echo.2017.01.007.
18. International Labour Organization. (2012). International standard classification of occupations: ISCO-08. Geneva: International Labour Organization.
19. Rickham, P. P. (1964). Human experimentation. Code of ethics of the world medical association. Declaration of Helsinki. British Medical Journal, 2(5402), 177.
20. Varni, J. W., Burwinkle, T. M., Seid, M., Skarr, D. (2003). The PedsQL 4.0 as a pediatric population health measure: feasibility, reliability, and validity. Ambulatory Pediatrics, 3(6), 329–341. DOI 10.1367/1539-4409(2003)003<0329:TPAAPP>2.0.CO;2.
21. Varni, J. W., Seid, M., Rode, C. A. (1999). The PedsQL: Measurement model for the pediatric quality of life inventory. Medical Care, 37(2), 126–139. DOI 10.1097/00005650-199902000-00003.
22. Varni, J. W., Seid, M., Kurtin, P. S. (2001). PedsQL 4.0: Reliability and validity of the pediatric quality of life inventory version 4.0 generic core scales in healthy and patient populations. Medical Care, 39(8), 800–812. DOI 10.1097/00005650-200108000-00006.
23. Engelen, V., Haentjens, M. M., Detmar, S. B., Koopman, H. M., Grootenhuis, M. A. (2009). Health related quality of life of Dutch children: Psychometric properties of the PedsQL in the Netherlands. BMC Pediatrics, 9(1), 68. DOI 10.1186/1471-2431-9-68.
24. Achenbach, T. M., Becker, A., Dopfner, M., Heiervang, E., Roessner, V. et al. (2008). Multicultural assessment of child and adolescent psychopathology with ASEBA and SDQ instruments: Research findings, applications, and future directions. Journal of Child Psychology and Psychiatry, 49(3), 251–275. DOI 10.1111/j.1469-7610.2007.01867.x.
25. Ivanova, M. Y., Dobrean, A., Dopfner, M., Erol, N., Fombonne, E. et al. (2007). Testing the 8-syndrome structure of the child behavior checklist in 30 societies. Journal of Clinical Child & Adolescent Psychology, 36(3), 405–417. DOI 10.1080/15374410701444363.
26. Ivanova, M. Y., Achenbach, T. M., Rescorla, L. A., Dumenci, L., Almqvist, F. et al. (2007). The generalizability of the youth self-report syndrome structure in 23 societies. Journal of Consulting and Clinical Psychology, 75(5), 729–738. DOI 10.1037/0022-006X.75.5.729.
27. Verhulst, F. C., Akkerhuis, G. W., Althaus, M. (1985). Mental health in Dutch children: (I). A cross-cultural comparison. Acta Psychiatrica Scandinavica, 323, 1–108.
28. Verhulst, F. C., Prince, J., Vervuurt-Poot, C., de Jong, J. (1989). Mental health in Dutch adolescents: Self-reported competencies and problems for ages 11-18. Acta Psychiatrica Scandinavica, 356, 1–48. DOI 10.1111/j.1600-0447.1989.tb03050.x.
29. Ang, R. P., Rescorla, L. A., Achenbach, T. M., Ooi, Y. P., Fung, D. S. et al. (2012). Examining the criterion validity of CBCL and TRF problem scales and items in a large Singapore sample. Child Psychiatry and Human Development, 43(1), 70–86. DOI 10.1007/s10578-011-0253-2.
30. American Psychiatric Association. 2013). (. Diagnostic and statistical manual of mental disorders, 5th ed., Washington, DC: American Psychiatric Association Publishing.
31. Achenbach, T. M., Rescorla, L. A. (2001). Manual for the ASEBA school-age forms & profiles. Burlington: Research Centre for Children, Youth and Families, University of Vermont.
32. Reiner, B., Oberhoffer, R., Ewert, P., Muller, J. (2019). Quality of life in young people with congenital heart disease is better than expected. Archives of Disease in Childhood, 104(2), 124–128. DOI 10.1136/archdischild-2017-314211.
33. Albrecht, G. L., Devlieger, P. J. (1999). The disability paradox: High quality of life against all odds. Social Science and Medicine, 48(8), 977–988. DOI 10.1016/S0277-9536(98)00411-0.
34. Bellinger, D. C., Rivkin, M. J., DeMaso, D., Robertson, R. L., Stopp, C. et al. (2015). Adolescents with tetralogy of Fallot: neuropsychological assessment and structural brain imaging. Cardiology in the Young, 25(2), 338–347. DOI 10.1017/S1047951114000031.
35. Calderon, J., Angeard, N., Pinabiaux, C., Bonnet, D., Jambaque, I. (2014). Facial expression recognition and emotion understanding in children after neonatal open-heart surgery for transposition of the great arteries. Developmental Medicine and Child Neurology, 56(6), 564–571. DOI 10.1111/dmcn.12381.
36. Sarrechia, I., Miatton, M., Francois, K., Gewillig, M., Meyns, B. et al. (2015). Neurodevelopmental outcome after surgery for acyanotic congenital heart disease. Research in Developmental Disabilities, 45-46, 58–68. DOI 10.1016/j.ridd.2015.07.004.
37. Marino, B. S., Lipkin, P. H., Newburger, J. W., Peacock, G., Gerdes, M. et al. (2012). Neurodevelopmental outcomes in children with congenital heart disease: Evaluation and management: A scientific statement from the American Heart Association. Circulation, 126(9),1143–1172.
38. Dunbar-Masterson, C., Wypij, D., Bellinger, D. C., Rappaport, L. A., Baker, A. L. et al. (2001). General health status of children with D-transposition of the great arteries after the arterial switch operation. Circulation, 104(1), I138–I142. DOI 10.1161/hc37t1.094782.
39. Williams, D. L., Gelijns, A. C., Moskowitz, A. J., Weinberg, A. D., Ng, J. H. et al. (2000). Hypoplastic left heart syndrome: Valuing the survival. Journal of Thoracic and Cardiovascular Surgery, 119(4), 720–731. DOI 10.1016/S0022-5223(00)70007-9.
40. Werner, H., Latal, B., Valsangiacomo Buechel, E., Beck, I., Landolt, M. A. (2014). Health-related quality of life after open-heart surgery. Journal of Pediatrics, 164(2), 254–258.e1. DOI 10.1016/j.jpeds.2013.10.022.
41. Uzark, K., Zak, V., Shrader, P., McCrindle, B. W., Radojewski, E. et al. (2016). Assessment of quality of life in young patients with single ventricle after the Fontan Operation. Journal of Pediatrics, 170, 166–172.e1. DOI 10.1016/j.jpeds.2015.11.016.
42. Garcia Guerra, G., Robertson, C. M., Alton, G. Y., Joffe, A. R., Dinu, I. A. et al. (2013). Quality of life 4 years after complex heart surgery in infancy. Journal of Thoracic and Cardiovascular Surgery, 145(2), 482–488.e2. DOI 10.1016/j.jtcvs.2012.03.050.
43. Landolt, M. A., Valsangiacomo Buechel, E. R., Latal, B. (2008). Health-related quality of life in children and adolescents after open-heart surgery. Journal of Pediatrics, 152(3), 349–355. DOI 10.1016/j.jpeds.2007.07.010.
44. Matsuda, M., Takemura, H., Yamashita, A., Matsuoka, Y., Sawa, T. et al. (2019). Post-surgical chronic pain and quality of life in children operated for congenital heart disease. Acta Anaesthesiologica Scandinavica, 63(6), 745–750. DOI 10.1111/aas.13346.
45. Patel, B. J., Lai, L., Goldfield, G., Sananes, R., Longmuir, P. E. (2017). Psychosocial health and quality of life among children with cardiac diagnoses: Agreement and discrepancies between parent and child reports. Cardiology in the Young, 27(4), 713–721. DOI 10.1017/S1047951116001141.
46. Berkes, A., Pataki, I., Kiss, M., Kemeny, C., Kardos, L. et al. (2010). Measuring health-related quality of life in Hungarian children with heart disease: Psychometric properties of the Hungarian version of the pediatric quality of life inventory 4.0 generic core scales and the cardiac module. Health and Quality of Life Outcomes, 8(1), 14. DOI 10.1186/1477-7525-8-14.
47. Eiser, C., Morse, R. (2001). Can parents rate their child’s health-related quality of life? Results of a systematic review. Quality of Life Research, 10(4), 347–357. DOI 10.1023/A:1012253723272.
48. Upton, P., Lawford, J., Eiser, C. (2008). Parent-child agreement across child health-related quality of life instruments: a review of the literature. Quality of Life Research, 17(6), 895–913. DOI 10.1007/s11136-008-9350-5.
49. McCusker, C. G., Doherty, N. N., Molloy, B., Casey, F., Rooney, N. et al. (2007). Determinants of neuropsychological and behavioural outcomes in early childhood survivors of congenital heart disease. Archives of Disease in Childhood, 92(2), 37–41.
50. Wright, M., Nolan, T. (1994). Impact of cyanotic heart disease on school performance. Archives of Disease in Childhood, 71(1), 64–70. DOI 10.1136/adc.71.1.64.
51. Hovels-Gurich, H. H., Konrad, K., Wiesner, M., Minkenberg, R., Herpertz-Dahlmann, B. et al. (2002). Long term behavioural outcome after neonatal arterial switch operation for transposition of the great arteries. Archives of Disease in Childhood, 87(6), 506–510. DOI 10.1136/adc.87.6.506.
52. Hovels-Gurich, H. H., Konrad, K., Skorzenski, D., Minkenberg, R., Herpertz-Dahlmann, B. et al. (2007). Long-term behavior and quality of life after corrective cardiac surgery in infancy for tetralogy of Fallot or ventricular septal defect. Pediatric Cardiology, 28(5), 346–354. DOI 10.1007/s00246-006-0123-z.
53. Phares, V., Lopez, E., Fields, S., Kamboukos, D., Duhig, A. M. (2005). Are fathers involved in pediatric psychology research and treatment? Journal of Pediatric Psychology, 30(8), 631–643. DOI 10.1093/jpepsy/jsi050.
54. Parent, J., Forehand, R., Pomerantz, H., Peisch, V., Seehuus, M. (2017). Father participation in child psychopathology research. Journal of Abnormal Child Psychology, 45(7), 1259–1270. DOI 10.1007/s10802-016-0254-5.
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |