

 | Congenital Heart Disease |  |
DOI: 10.32604/CHD.2020.013032
ARTICLE
Abnormal Coronary Anatomy in Patients with Transposition of the Great Arteries and Atrial Switch: A Predictor of Serious Cardiac Adverse Events?
1Hôpital Cardiologique Louis Pradel, 59 boulevard Pinel, 69500, Bron, Hospices Civils de Lyon, Lyon, France
2Montreal Heart Institute, Montreal, H1T 1C8, Canada
3Nationwide Children’s Hospital, Columbus, OH 43205, USA
*Corresponding Author: Francis Bessiere. Email: francis.bessiere@chu-lyon.fr
Received: 23 July 2020; Accepted: 20 August 2020
Abstract: Sudden cardiac death and heart failure are well known long-term complications after atrial switch for D-transposition of the great arteries (D-TGA). Right systemic ventricular dysfunction is common and myocardial ischemia has been implicated as a putative mechanism for sudden death, with coronary anomalies prevalent in 30% of cases. We sought to assess an association between adverse events and coronary anomalies in patients with D-TGA and atrial switch surgery. An observational study was conducted in 3 tertiary centers (Montreal Heart Institute, Canada, Nationwide Children’s hospital, Chicago, USA and Hopital cardiologique Louis Pradel de Lyon, France). Adults with D-TGA and atrial switch surgery qualified for inclusion if they had a major adverse cardiovascular event (MACE), i.e., ventricular arrhythmia, sudden cardiac death, heart failure, cardiac transplantation, or cardiovascular death. The prevalence of coronary anomalies was compared to historical controls. Forty-five patients were included. Twenty-one (46.7%) patients experienced a ventricular arrhythmia and 35 (77.8%) suffered from symptomatic heart failure and/or severe right ventricular dysfunction. Twelve patients (26.7%) had congenitally abnormal coronary arteries. There was no difference in the prevalence of coronary anomalies between the cohort with a MACE and a pooled population of 647 historical controls with D-TGA (28.7%, p = 0.89). In conclusion, the prevalence of congenital coronary anomalies is not higher in patients with D-TGA and atrial switch surgery who had adverse cardiovascular events. It could be hypothesized that ischemic complications in this patient population are more likely to be related to a supply-demand mismatch of the distal microvasculature rather than proximal coronary anomalies.
Keywords: D-TGA; coronary anomalies; atrial switch; mustard; senning; cardiac arrhythmia; cardiac failure; sudden death
D-transposition of the great arteries (D-TGA) accounts for 4–7% of all congenital heart defects (CHD). The incidence of this cyanogenic heart disease is 3 per 10 000 births [1–4]. Atrial switch repair (either the Mustard or Senning procedure) was the surgical technique performed from the 1960s to 1990s, until adoption of the arterial switch procedure [5–8].
Long-term complications after atrial switch procedure include baffle obstruction, residual shunt, atrial tachyarrhythmias, sinus node dysfunction, tricuspid regurgitation and heart failure due to dysfunction of the systemic right ventricle [9–11]. Gelatt et al. showed in 1997 that survival rates after the Mustard procedure were 89% at 5 years and 76% at 20 years, while in 2005 Dos et al. [12,13] reported 5.1% mortality up at 16.7 years of follow-up. Supraventricular arrhythmias and cardiac failure were identified as significant independent factors associated with late mortality.
D-TGA with atrial switch repair is among the CHD lesions with the highest risk of sudden cardiac death (SCD), afflicting 2.4 to 15% of patients [14]. SCD represents the most common cause of mortality in this population (28 to 42.8%), with an incidence of 4.5 events per 1000 patient-years. The main cause of SCD is presumed arrhythmia (73–83%) [15,16]. Selecting appropriate candidates for primary prevention implantable cardioverter-defibrillators (ICD) is a challenge. An expert consensus statement issued a weak recommendation (Class IIB) indicating that primary prevention ICDs may be considered in patients with an impaired systemic right ventricular ejection fraction, particularly when additional risk factors are present such as ventricular arrhythmias, unexplained syncope, New York Heart Association (NYHA) functional class II or III symptoms, QRS duration ≥140 ms, or severe systemic atrioventricular (AV) valve regurgitation [17].
Several studies have highlighted myocardial perfusion anomalies by functional imaging such as scintigraphy or photon emission computed tomography [18]. Moreover, 80% of SCDs occur during exercise in this patient population [19,20]. In 2016, Khairy [21] proposed the myocardial ischemia hypothesis: in the context of rapid heart rates, an impaired stroke volume response due to poor atrial transport can provoke ischemia-related malignant ventricular arrhythmias. This impairment might be exacerbated by stress-related sinus tachycardia or rapidly conducting atrial arrhythmias. In addition, an inefficient coronary circulation is a likely contributing factor due to a single right coronary artery that typically irrigates a pressure-loaded systemic right ventricle that has an increased myocardial oxygen demand and decreased perfusion pressure. In 2018, Chaix et al. [22] confirmed subendocardial ischemic scars of the systemic right ventricle at autopsy in patients who died suddenly and had no evidence of coronary atherosclerosis.
Coronary abnormalities are prevalent in about 30% of patients with D-TGA. In 1988, the Marie Lannelongue classification proposed four categories: Type I, normal anatomy, found in 68%; Type II, abnormal path, in 7.5%; Type III, anterior, posterior, or double loop, in 24%; and Type IV, complex anomalies, in 0.5% [23–26]. In 2018, the modified Leiden convention described 7 types of coronary patterns in 77 patients with D-TGA. Among these patients, 59 (76.6%) had normal coronary anatomy, 1 had an interarterial course and 4 had a single ostium [27].
It has yet to be determined whether the coronary artery pattern is associated with adverse outcomes in patients with D-TGA and Mustard or Senning baffles. We, therefore, sought to evaluate the impact of the native coronary distribution on long-term cardiac outcomes.
We conducted a retrospective study in 3 tertiary centers (Montreal Heart Institute, Canada Nationwide Children’s hospital, Chicago, USA and Hopital cardiologique Louis Pradel de Lyon, France). In order to calculate the prevalence of coronary anomalies in patients with D-TGA and atrial switch surgery who experienced a major adverse cardiovascular event (MACE), adults (≥18 years of age) with D-TGA and Mustard or Senning surgery were recruited. Patients were required to have experienced at least one MACE as defined by: ventricular arrhythmias, resuscitated or not resuscitated SCD, myocardial infarction, heart failure (right ventricular ejection fraction (RVEF) <35% with symptoms of cardiac failure), cardiac transplantation, or cardiovascular related death. Patients with congenitally corrected TGA or an arterial switch operation were excluded.
2.2 Population Characteristics
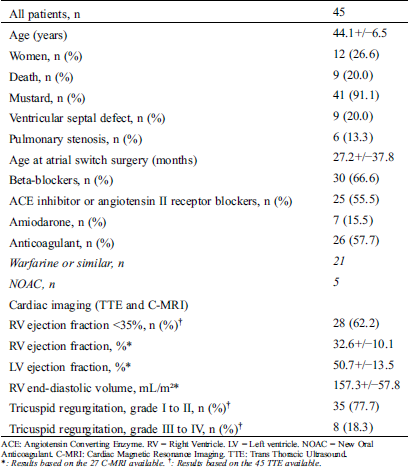
Baseline clinical data (Tab. 1) from the first follow-up visit in adulthood retrieved from medical charts included type of intervention (Mustard or Senning), age at the time of surgery, and accompanying congenital heart defects (e.g., presence of a pulmonary stenosis and/or a ventricular septal defect) [28]. Data were likewise collected on pharmacological therapy, imaging metrics from echocardiography and cardiac magnetic resonance imaging (MRI), pulmonary hypertension, arrhythmias, interventions (including pacemakers, ICDs, and catheter ablation procedures), and MACE, as defined above. The study was conducted in accordance with the International Council of Harmonization Tripartite Guidelines for Good Clinical Practice and was approved by the institutional review board of each participating center.
Table 1: Population characteristics
Electrocardiogram (ECG) gated Computed Tomography (CT) angiography was performed acquired using a Brilliance 64 or Iquon scanners (Philips). Coronary reconstruction by MRI or coronary angiography were used if a CT angiography was not available. Experienced senior imagers reviewed all CT/MRI studies in both centers. The analysis included coronary dominance, i.e., the artery that vascularizes the diaphragmatic wall and the lower part of the interventricular septum. Secondly, coronary abnormalities were described using the Marie Lannelongue and modified Leiden Convention classification schemes. Single sinus coronary arteries, single coronary arteries, loops and interarterial courses were reported.
The prevalence of coronary anomalies in the combined population of patients with D-TGA and MACE was compared to historical controls from existing published data reported in the modern era of newborns undergoing arterial switch surgery.
Continuous variables are summarized as mean ± standard deviation or median and inter-quartile range (IQR; 25th, 75th percentile), and categorical variables by frequency and percentage. Statistical analyses were performed using Statview software 5.0 (SAS Institute Inc, Cary NC). Comparisons used Student’s t test or Mann–Whitney–Wilcoxon test, when appropriate, for continuous variables. For comparison of categorical parameters, a Chi-2 test (>5 patients per group) or Fisher Exact test (<5 patients) was used. A two-tailed p value < 0.05 was considered to indicated statistical significance.
A total of 45 adults with D-TGA, atrial switch surgery, and a MACE were included: 23 patients from the Louis Pradel Cardiovascular Hospital (51.1%), 3 from the Nationwide Children’s hospital (6.6%) and 19 patients from the Montreal Heart Institute (42.2%). Patient baseline characteristics are summarized in (Tab. 1).
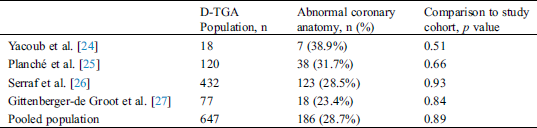
A coronary anomaly was present in 12 of 45 (26.6%) patients. Eleven (24.4%) had at least one pathological coronary loop and one (2.2%) had an inter-arterial course. The coronary arterial pattern is shown in (Tabs. 2 and 3) and (Figs. 1 and 2), according to the Marie Lannelongue and modified Leiden classification schemes, respectively. Single sinus coronary arteries were diagnosed in 6 (13.3%) patients and single coronary arteries in 4 (8.9%) patients. Right coronary dominance was observed in 91.1% of the cases. The pooled comparison between the distribution of coronary anomalies in the study population compared to published series individually and collectively (N = 647) showed no significant difference (Tab. 4) (26.6% vs. 28.7%, p = 0.89).
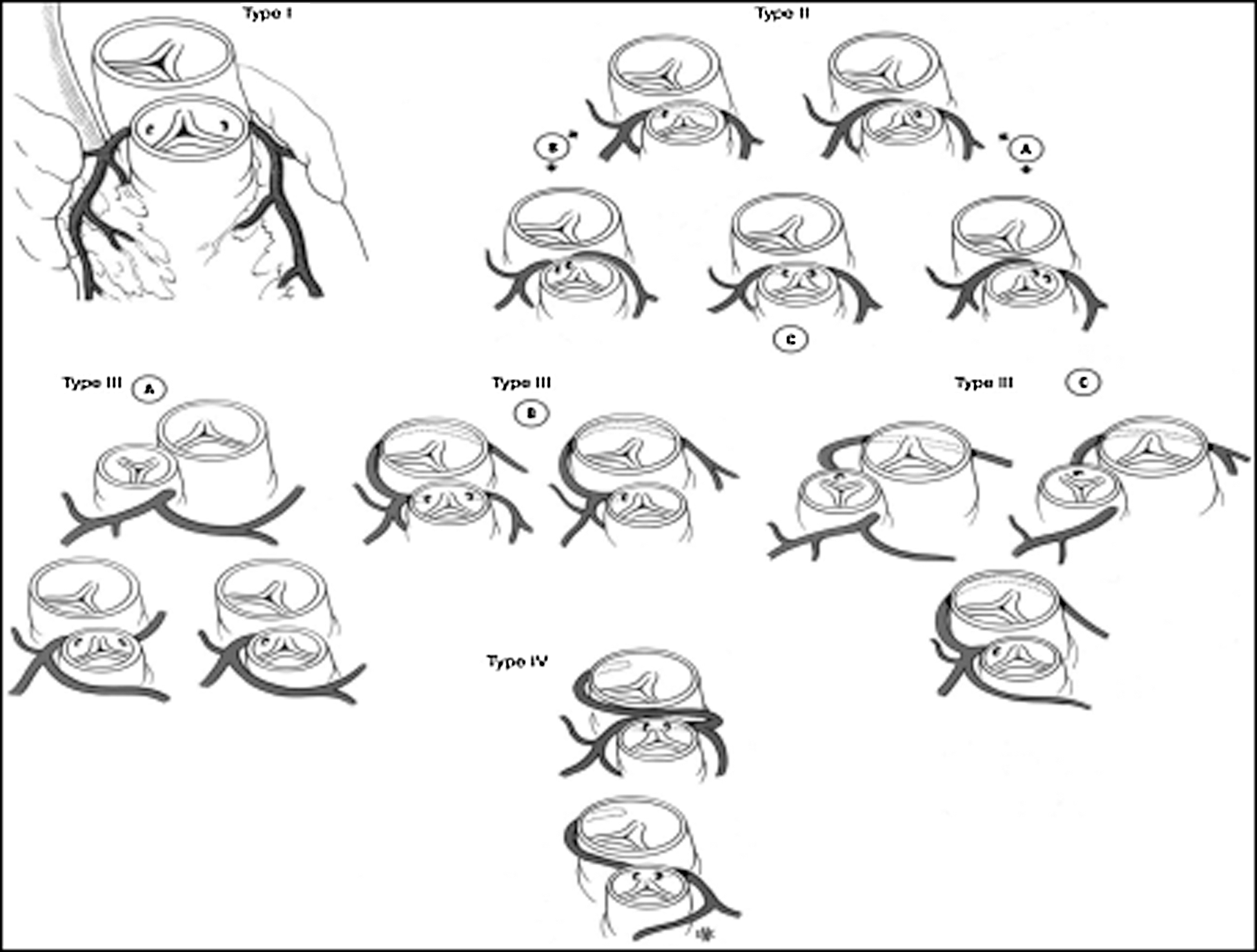
Figure 1: Coronary artery patterns seen in TGA according to Marie Lannelongue classification. (Type I: normal anatomy; Type II: abnormal path; Type III: loop; Type IV: complex anatomy)
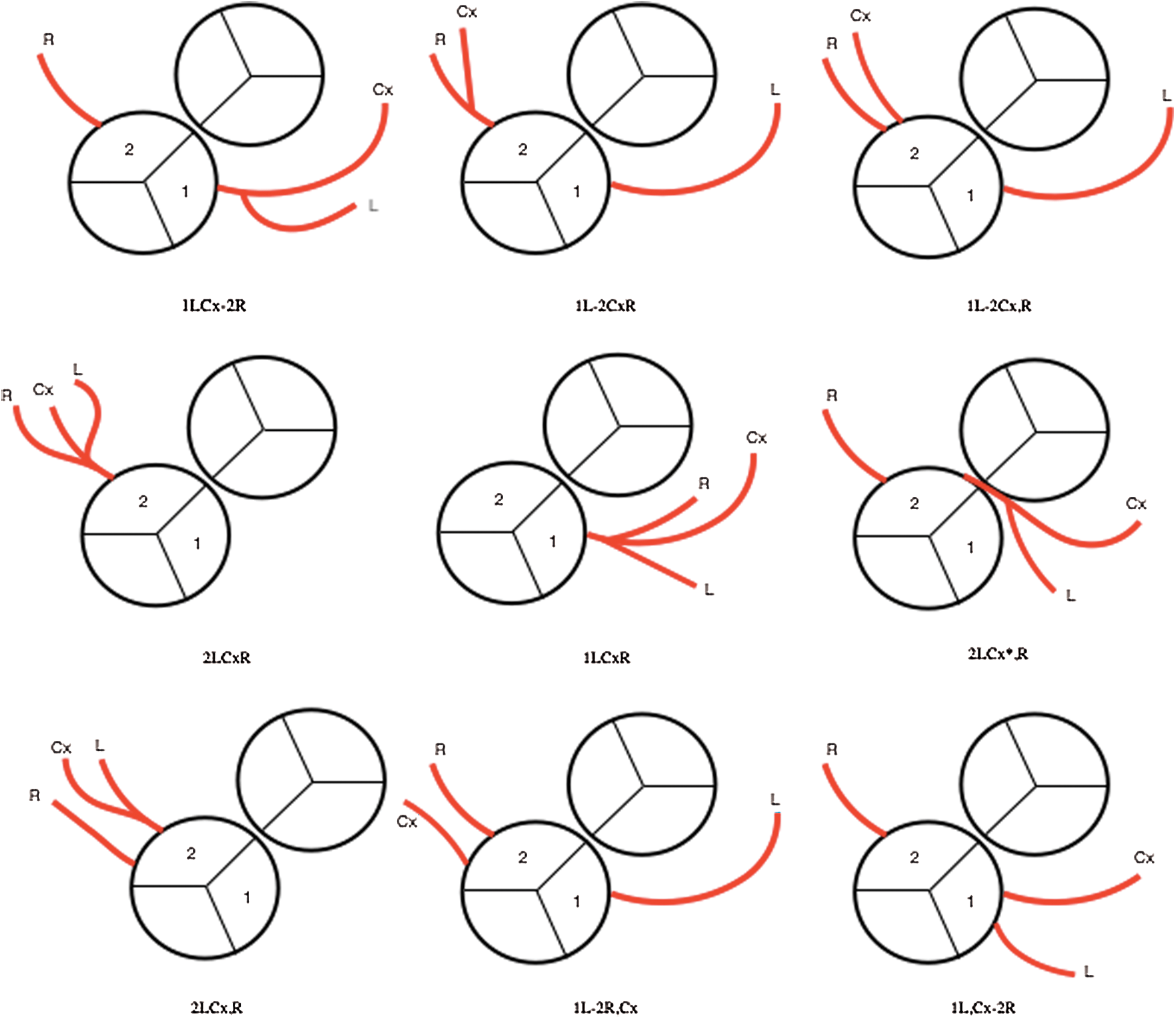
Figure 2: Coronary artery patterns seen in our cohort according to modified Leiden classification
Table 2: Comparison of the coronary anatomy according to Marie Lannelongue classification with the literature

Table 3: Comparison of coronary anatomy according to modified Leiden classification with the literature
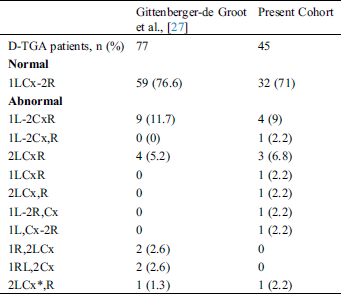
Table 4: Comparison of the prevalence of abnormal coronary anatomy in published studies and pooled population with our cohort
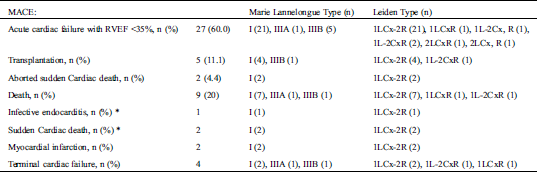
Over a median follow-up of 44.1 ± 6.5 years, ventricular arrhythmias were documented in 21 (46.6%) patients. Fifteen (33.3%) received an ICD (13 in primary prevention, age at implantation 39.6+/−6). Two patients suffered from resuscitated sudden cardiac death. Nine (20.0%) patients died (mean age 41.6+/−8.7 years) including 2 from sudden cardiac deaths, 1 from an infective endocarditis, 4 from cardiogenic shocks related to terminal cardiac failure, and 2 from myocardial infarctions. There were no other myocardial infarctions documented. Thirty-five patients (77.7%) suffered from symptomatic heart failure and severe right ventricular dysfunction (<35%) and 5 patients (11.1%) were transplanted at a mean age of 32.4+/−10.0 years. Thirteen (28.8%) patients suffered from both arrhythmic and heart failure events (Tab. 5).
Table 5: MACE during the follow-up and indications on coronary anomalies based on Marie Lannelongue classification and modified Leiden classification
Thirty-four patients (75.5%) had supra ventricular arrhythmias and 16 patients underwent ablation. Conduction disorders were observed in 53.3% of cases: 10 AV block and 14 sinus node dysfunction.
Among them, 11 (45.8%) received a permanent pacemaker and 9 (37.5%) an ICD while 4 (16%) did not receive any device. Thirty patients (66.6%) were prescribed beta-blockers therapy.
We hypothesized that patients with D-TGA, atrial switch surgery, and MACE would have a higher prevalence of coronary anomalies. Our hypothesis was refuted by this study. The prevalence of coronary anomalies in patients with MACE was similar to an unselected population of historical controls with D-TGA. The breakdown of coronary anomalies was likewise similar to published reports, be they using the Marie Lannelongue classification or modified Leiden classification. Inter-arterial and intramural courses seemed to be less represented in our cohort although this specific anomaly is thought to impart the highest risk for myocardial ischemia and sudden death [29,30]. Since patients with this anomaly might die before reaching adult age, they may not have been captured by our cohort. The coronary dominance was predominantly right (91.1% of the cases), i.e., higher than in the general population (80%). Although underlying reasons remain to be investigated, it could be hypothesized that the systemic position of the RV favors development of the right coronary artery blood flow and circulation.
Although ischemia is probably involved in the pathogenesis of RV dysfunction and of other related complications [22] it appears that the coronary artery branching pattern is not a major determinant of outcomes. Hypertrophy of the RV and a mismatch between increased oxygen demand and supply could predispose to RV dysfunction [21] Subendocardial ischemia may primarily be due to a distal and not proximal altered myocardial perfusion pattern. Cardiac CT and MRI angiography were used to assess the coronary anatomy. While these imaging tests provide accurate accounts of coronary branching patterns, they cannot assess distal vascularization. Therefore, no reliable information about the distal coronary artery blood flow was provided.
There is some evidence that beta-blockers could protect against SCD in this patient population. Khairy et al. [31] showed a reduction of appropriate shocks with beta-blockers in D-TGA patients with atrial switch implanted with an ICD in a multicenter study. Beta-blockers could be effective in reducing the ventricular response rate during supraventricular arrhythmias and increase the diastolic filling time [32]. This could potentially improve the myocardial supply-demand mismatch (24). In our cohort, only 30 (66.6%) patients were treated with beta-blockers. As suggested by the expert consensus statement, it could be reasonable to liberalize the use of beta-blockers in patients with Mustard/Senning baffles, particularly in those with high risk features (e.g., poor systemic right ventricular ejection fraction, ventricular arrhythmias, syncope, NYHA class III and IV, QRS duration ≥140 ms, or severe systemic atrioventricular valve regurgitation) [17]. Sinus dysfunction and atrioventricular block were frequent, with <50% of patients free from a clinically relevant conduction disorder. Almost half of the patients had a pacemaker. In patients without pacemakers, conduction disorders may compromise tolerability of beta-blockers.
Supraventricular arrhythmias were highly prevalent (75.5%) in the patient cohort. Supraventricular tachycardia is recognized as a risk factor for severe complications. Detection of self-terminating arrhythmias could be facilitated by the high rate of pacemaker implantation in this population. Catheter ablation was performed in about a third of our cohort. Early medical and interventional treatment should be encouraged since supraventricular arrhythmias can be trigger systemic right ventricular failure, ventricular arrhythmias, and SCD. The provocative finding that patients who underwent catheter ablation had better survival merits further study.
Although there was no trend towards a higher prevalence of coronary anomalies in patients with MACE, this retrospective study was underpowered to assess individual components of MACE. A subgroup analysis according to the different coronary artery anomalies would have been of interest but the limited number of cases in each subgroup precluded this analysis. Most patients were included because of heart failure as opposed to ventricular arrhythmias. It remains possible that coronary abnormalities may impact malignant arrhythmic events. However, there was no signal indicating a higher prevalence of coronary anomalies in patients with abnormal loops or an inter-arterial course. Moreover, the prevalence of coronary abnormalities in the subgroup of patients who died from a cardiovascular cause or underwent heart transplantation was similar to the entire cohort.
In conclusion, the present study did not show a higher proportion of coronary anomalies in patients with D-TGA and Mustard or Senning baffles who had adverse cardiovascular events compared to general published series of patients with D-TGA. No link was found between abnormal coronary artery branching patterns and major cardiac events.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Marelli, A. J., Ionescu-Ittu, R., Mackie, A. S., Guo, L., Dendukuri, N. et al. (2014). Lifetime prevalence of congenital heart disease in the general population from 2000 to 2010 clinical perspective. Circulation, 130(9), 749–756. DOI 10.1161/CIRCULATIONAHA.113.008396.
2. Khoshnood, B., Lelong, N., Houyel, L., Thieulin, A. C., Jouannic, J. M. et al. (2012). Prevalence, timing of diagnosis and mortality of newborns with congenital heart defects, a population-based study. Heart, 98(22), 1667–1673. DOI 10.1136/heartjnl-2012-302543.
3. Šamánek, M., Slavík, Z., Zbořilová, B., Hroboňová, V., Voříšková, M. et al. (1989). Prevalence, treatment, and outcome of heart disease in live-born children: a prospective analysis of 91,823 live-born children. Pediatric Cardiology, 10(4), 205–211. DOI 10.1007/BF02083294.
4. Campbell, M. (1973). Incidence of cardiac malformations at birth and later, and neonatal mortality. British Heart Journal, 35(2), 189–200. DOI 10.1136/hrt.35.2.189.
5. Mustard, W. T. (1964). Successful two-stage correction of transposition of the great vessels. Surgery, 55, 469–472.
6. Senning, A. (1959). Surgical correction of transposition of the great vessels. Surgery, 45(6), 966–980.
7. Warnes, C. A. (2006). Transposition of the great arteries. Circulation, 114(24), 2699–2709. DOI 10.1161/CIRCULATIONAHA.105.592352.
8. Martins, P., Castela, E. (2008). Transposition of the great arteries. Orphanet Journal of Rare Diseases, 3(1), 1–10. DOI 10.1186/1750-1172-3-27.
9. Khairy, P., Landzberg, M. J., Lambert, J. (2004). Long-term outcomes after the atrial switch for surgical correction of transposition, a meta-analysis comparing the Mustard and Senning procedures. Cardiology in the Young, 14(3), 284–292. DOI 10.1017/S1047951104003063.
10. Buch, J., Wennevold, A., Jacobsen, J., Hvid-Jacobsen, K., Lauridsen, P. (1988). Long-term follow-up of right ventricular function after Mustard operation for transposition of the great arteries. Scandinavian Journal of Thoracic and Cardiovascular Surgery, 22(3), 197–202. DOI 10.3109/14017438809106062.
11. Couperus, L. E., Vliegen, H. W., Zandstra, T. E., Kiès, P., Jongbloed, M. R. et al. (2019). Long-term outcome after atrial correction for transposition of the great arteries. Heart, 105(10), 790–796. DOI 10.1136/heartjnl-2018-313647.
12. Gelatt, M., Hamilton, R. M., McCrindle, B. W., Connelly, M., Davis, A. et al. (1997). Arrhythmia and mortality after the mustard procedure: a 30-year single-center experience. Journal of the American College of Cardiology, 29(1), 194–201. DOI 10.1016/S0735-1097(96)00424-X.
13. Dos, L., Teruel, L., Ferreira, I., Rodriguez-Larrea, J., Miro, L. et al. (2005). Late outcome of Senning and Mustard procedures for correction of transposition of the great arteries. Heart (British Cardiac Society), 91(5), 652–656. DOI 10.1136/hrt.2003.029769.
14. Yap, S. C., Harris, L. (2014). Sudden cardiac death in adults with congenital heart disease. Expert Review of Cardiovascular Therapy, 7(12), 1605–1620. DOI 10.1586/erc.09.153.
15. Deal, B. J. (2010). Late arrhythmias after surgery for transposition of the great arteries. World Journal for Pediatric and Congenital Heart Surgery, 2(1), 32–36. DOI 10.1177/2150135110386251.
16. Silka, M. J., Hardy, B. G., Menashe, V. D., Morris, C. D. (1998). A population-based prospective evaluation of risk of sudden cardiac death after operation for common congenital heart defects. Journal of the American College of Cardiology, 32(1), 245–251. DOI 10.1016/S0735-1097(98)00187-9.
17. Khairy, P., Hare, G. F., Balaji, S., Berul, C. I., Cecchin, F. et al. (2014). PACES/HRS expert consensus statement on the recognition and management of arrhythmias in adult congenital heart disease developed in partnership between the pediatric and congenital electrophysiology society (PACES) and the heart rhythm society (HRS). Endorsed by the governing bodies of PACES, HRS, the American college of cardiology (ACCthe american heart association (AHAthe european heart rhythm association (EHRAthe Canadian heart rhythm society (CHRSand the International society for adult congenital heart disease (ISACHD). Canadian Journal of Cardiology, 30(10), e1–63. DOI 10.1016/j.cjca.2014.09.002.
18. Kammeraad, J. A., van Deurzen, C. H., Sreeram, N., Bink-Boelkens, M. T., Ottenkamp, J. et al. (2004). Predictors of sudden cardiac death after mustard or senning repair for transposition of the great arteries. Journal of the American College of Cardiology, 44(5), 1095–1102. DOI 10.1016/j.jacc.2004.05.073.
19. Millane, T., Bernard, E. J., Jaeggi, E., Howman-Giles, R. B., Uren, R. F. et al. (2000). Role of ischemia and infarction in late right ventricular dysfunction after atrial repair of transposition of the great arteries. Journal of the American College of Cardiology, 35(6), 1661–1668. DOI 10.1016/S0735-1097(00)00585-4.
20. Labbé, L., Douard, H., Barat, J. L., Broustet, J. P., Bordenave, L. et al. (1997). Altération de la viabilité myocardique et dysfonction ventriculaire systémique après intervention de Senning [Alteration of myocardial viability and systemic ventricular dysfunction after Senning procedure]. Archives des Maladies du Coeur et Des Vaisseaux, 90(5), 631–637.
21. Khairy, P. (2017). Sudden cardiac death in transposition of the great arteries with a Mustard or Senning baffle, the myocardial ischemia hypothesis. Current Opinion in Cardiology, 32(1), 101–107. DOI 10.1097/HCO.0000000000000353.
22. Chaix, M. A., Chergui, M., Leduc, C., Khairy, P. (2019). Sudden death in transposition of the great arteries with atrial switch surgery: autopsy evidence of acute myocardial ischemia despite normal coronary arteries. International Journal of Cardiology, 288, 65–67. DOI 10.1016/j.ijcard.2019.02.026.
23. Shaher, R. (1963). The coronary circulation in complete transposition of the great vessels. British Heart Journal, 25(4), 481–488. DOI 10.1136/hrt.25.4.481.
24. Yacoub, M., Radley-Smith, R. (1978). Anatomy of the coronary arteries in transposition of the great arteries and methods for their transfer in anatomical correction. Thorax, 33(4), 418–424. DOI 10.1136/thx.33.4.418.
25. Planché, C., Bruniaux, J., Lacour-Gayet, F., Kachaner, J., Binet, J. et al. (1988). Switch operation for transposition of the great arteries in neonates. A study of 120 patients. Journal of Thoracic and Cardiovascular Surgery, 96(3), 354–363. DOI 10.1016/S0022-5223(19)35229-8.
26. Serraf, A., Lacour-Gayet, F., Bruniaux, J., Touchot, A., Losay, J. et al. (1993). Anatomic correction of transposition of the great arteries in neonates. Journal of the American College of Cardiology, 22(1), 193–200. DOI 10.1016/0735-1097(93)90834-N.
27. Gittenberger-de Groot, A. C., Koenraadt, W., Bartelings, M. M., Bökenkamp, R., DeRuiter, M. C. et al. (2018). Coding of coronary arterial origin and branching in congenital heart disease: the modified Leiden Convention. Journal of Thoracic and Cardiovascular Surgery, 156(6), 2260–2269. DOI 10.1016/j.jtcvs.2018.08.009.
28. Chaix, M. A., Dore, A., Mercier, L. A., Mongeon, F. P., Marcotte, F. et al. (2017). Late onset postcapillary pulmonary hypertension in patients with transposition of the great arteries and mustard or senning baffles. Journal of the American Heart Association, 6(10), e006481. DOI 10.1161/JAHA.117.006481.
29. Finocchiaro, G., Behr, E. R., Tanzarella, G., Papadakis, M., Malhotra, A. et al. (2019). Anomalous coronary artery origin and sudden cardiac death: clinical and pathological insights from a national pathology registry. JACC: Clinical Electrophysiology, 5(4), 516–522. DOI 10.1016/j.jacep.2018.11.015.
30. Hill, S. F., Sheppard, M. N. (2014). A silent cause of sudden cardiac death especially in sport: congenital coronary artery anomalies. British Journal of Sports Medicine, 48(15), 1151–1156. DOI 10.1136/bjsports-2013-092195.
31. Khairy, P., Harris, L., Landzberg, M. J., Fernandes, S. M., Barlow, A. et al. (2008). Sudden death and defibrillators in transposition of the great arteries with intra-atrial baffles: a multicenter study. Circulation: Arrhythmia and Electrophysiology, 1(4), 250–257. DOI 10.1161/CIRCEP.108.776120.
32. Bouallal, R., Godart, F., Francart, C., Richard, A., Foucher-Hossein, C. et al. (2010). Interest of β-blockers in patients with right ventricular systemic dysfunction. Cardiology in the Young, 20(6), 615–619. DOI 10.1017/S1047951110000764.
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |