

 | Congenital Heart Disease |  |
DOI: 10.32604/CHD.2020.013058
ARTICLE
Late-Onset Pulmonary Hypertension After the Atrial Switch Procedure for Transposition of the Great Arteries
1Department of Pediatric Cardiology and Adult Congenital Cardiology, Tokyo Women’s Medical University, Tokyo, Japan
2Department of Cardiology, Tokyo Women’s Medical University, Tokyo, Japan
*Corresponding Author: Kei Inai. Email: inai.kei@twmu.ac.jp
Received: 24 July 2020; Accepted: 21 September 2020
Background: Pulmonary hypertension (PH) is one of the complications that can occur after the atrial switch procedure for transposition of the great arteries (TGA). This study aimed to assess the characteristics and prognosis of late-onset PH after the atrial switch procedure using catheterization data. Methods and Results: We retrospectively identified 40 patients with TGA after the atrial switch procedure that underwent catheterization between April 2007 and March 2020. Eligible patients were divided into two groups based on PH presence (PH group, n = 13 [33%]; non-PH group, n = 27 [67%]). Adverse events were defined as cardiac death and heart failure. Of the included patients, 63% were male, the mean (± standard deviation [SD]) age was 34.3 ± 8.8 years, and 43% had Mustard operations. During the follow-up period (mean ± SD, 4.3 ± 3.5 years), adverse events were significantly more common in the PH group than in the non-PH group (hazard ratio 4.5, 95% confidence interval 0.99-23.0, log-rank p = 0.032). There were five patients who underwent catheterization twice during the follow-up period. Two of the five patients who had post capillary PH had improved PH and New York Heart Association (NYHA) class due to appropriate early treatment initiation for heart failure, which included diuretics, angiotensin-converting-enzyme inhibitor/angiotensin II receptor blocker, and β-blockers. Conclusions: PH is very prevalent in adults with TGA who underwent the atrial switch procedure and is associated with an adverse prognosis. Early appropriate therapy might improve PH and NYHA class after the atrial switch procedure.
Keywords: Transposition of the great arteries; atrial switch operation; pulmonary hypertension
Transposition of the great arteries (TGA) is one of the most common congenital heart diseases (CHD) presenting at birth with cyanosis [1]. Unpalliated TGA has a 90% mortality rate within the first year of life [2]. Survival improved dramatically after the introduction of radical surgery in the form of atrial switch procedures by Drs Ake Senning in 1959 and William Mustard in 1964; this became standard practice in the 1970s [3,4]. The initial patients who underwent Mustard and Senning correction operations are now well into adulthood, making long-term follow-up possible. Prior studies reported that 60–80% of patients were alive 30 years after repair [5–7]. Complications encountered in adult life are well-characterized and include sinus node dysfunction, atrial arrhythmias, sudden death, systemic right ventricular (RV) dysfunction, tricuspid regurgitation, pulmonary hypertension (PH), and baffle obstructions and leaks [5–8].
PH has been documented in up to 7% of patients surviving to adulthood after the atrial switch procedure. After the development of PH, individuals have reduced exercise tolerance and quality of life [9]; additionally, PH has been associated with an adverse prognosis [9,10]. Although catheterization is required for the diagnosis of PH, there have been few studies that have investigated PH using catheterization in the long-term follow-up of these individuals [10]. We therefore conducted a retrospective cohort study of adults with TGA who underwent the atrial switch procedure to assess the prevalence, characteristics, and prognosis of PH using catheterization data.
This study was conducted in strict adherence with the principles of the Declaration of Helsinki and was approved by the Clinical Investigation Ethics Committee of Tokyo Women’s Medical University (5619). De-identified participant data will not be shared in public.
2.1 Study Design and Population
This was a retrospective cohort study that used inpatient data from Tokyo Women’s Medical University. All data were obtained from patients’ medical records. We retrospectively identified 40 patients older than 18 years of age with TGA who underwent the atrial switch procedure and who were involved in hemodynamic investigations using catheterization between April 2007 and March 2020 in our hospital. Indications for catheterization included decreased exercise tolerance, prior catheter ablation for tachyarrhythmia or implantation of a pacemaker for bradycardic arrhythmia with Adams-Stokes syndrome, or exacerbation of valve disease with echocardiography.
The following factors were included as baseline variables: age, sex, body mass index (BMI), body surface area, age at initiation of the atrial switch procedure, age at surgery, Mustard vs. Senning procedure, diabetes mellitus, dyslipidemia, hypertension, smoking, New York Heart Association (NYHA) class determined using the 6-minute walk test, prior history of heart failure and/or documented tachyarrhythmia by ECG, permanent pacemaker implantation, brain natriuretic peptide (BNP) at the catheterization, and the medication used at the catheterization. In an electrocardiogram, we evaluated the rhythm, heart rate, and QRS duration at catheterization.
All hemodynamic investigations were conducted under local anesthetics and spontaneous breathing. We examined the following hemodynamic parameters: invasive systolic and diastolic arterial pressure; systolic, diastolic, and mean pulmonary artery pressure (PAP); pulmonary capillary wedge pressure (PCWP); pulmonary vascular resistance (PVR); systemic RV and subpulmonary left ventricular (LV) end-diastolic pressures; systemic RV end-diastolic volume (EDV); systemic RV EDV index (EDVI); systemic RV ejection fraction (EF); tricuspid regurgitation; and leak and/or stenosis of systemic baffle.
PH was defined as an increase in mean PAP ≥25 mmHg at rest as determined in a previous hemodynamic study [11]. PH was classified as precapillary if the PCWP was ≤15 mmHg at rest or as postcapillary if the PCWP was >15 mmHg. Postcapillary PH was considered isolated if the diastolic pressure gradient (i.e., diastolic PAP minus mean PCWP) was <7 mmHg in combination with PVR ≤3 Wood units. Patients with a PCWP >15 mmHg and a diastolic pressure gradient ≥7 mmHg, PVR >3 Wood units, or a transpulmonary gradient (i.e., mean PAP minus mean PCWP) >15 mmHg were considered to have combined post- and precapillary PH [12]. We divided the eligible patients into two groups: patients with and without PH.
2.5 Adverse Events and Follow-up
Adverse events in this study were defined as cardiac death and heart failure. Heart failure was defined as hospitalization for heart failure or a pacemaker upgrade to cardiac resynchronization therapy (CRT). During the follow-up period, the continuation or discontinuation of medication for heart failure such as diuretics, angiotensin-converting-enzyme inhibitor (ACE-I)/angiotensin II receptor blocker (ARB), β-blocker, and anti-arrhythmic drugs was decided upon based on the discretion of each physician.
Five patients in the PH group underwent catheterization twice during the follow-up period. We compared the data on age, NYHA using the 6-minute walk test, hemodynamic investigations, and the medication usage between the first and second examinations.
Continuous variables were expressed as means ± standard deviations (SD) or medians and interquartile ranges (IQR) and were compared using Student’s t-tests or Wilcoxon’s rank-sum tests as appropriate. Categorical variables were expressed as numbers and proportions and were compared using Fisher’s exact tests. The Kaplan–Meier method was used to calculate event-free survival, which was compared using the log-rank test. All statistical analyses were performed with JMP Pro software version 13.1.0 (SAS Institute, Cary, NC, USA). Two-sided p-values < 0.05 were considered statistically significant.
Of the 40 patients included, the mean (± SD) age was 34.3 ± 8.8 years and the median (IQR) time from birth to the atrial switch procedure was 13.5 (7.3–32) months (Tab. 1). Seventeen (43%) and 23 (57%) patients underwent the Mustard and the Senning procedures, respectively. Twenty-seven (68%) patients had associated lesions at birth. The mean (± SD) PAP was 23 ± 13 mmHg, and systemic RV ejection fraction was 41 ± 9%.
Table 1: Baseline characteristics
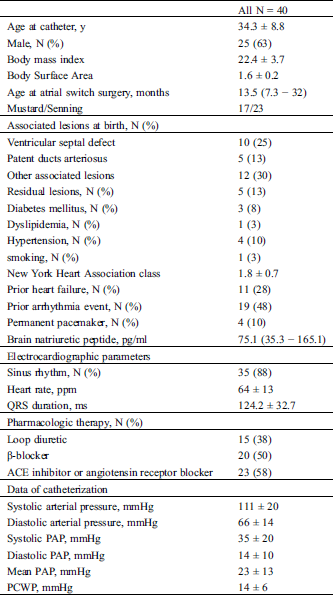
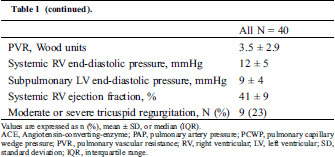
Overall, PH was detected in 13 (33%) patients. PH was isolated postcapillary, combined, and precapillary in seven, five, and one patient, respectively. Age at catheterization, age at initiation of the atrial switch procedure, time from surgery to catheterization, and the proportion of people who underwent the Mustard procedure did not significantly differ between the two groups (Tab. 2). The associated and residual lesions also did not differ between the two groups. The NYHA class and BNP in the PH group were significantly higher than those in the non-PH group.
Table 2: Clinical and demographic characteristics compared between the PH group and non-PH group
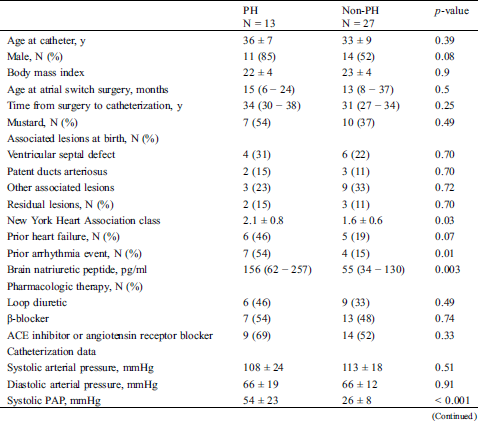
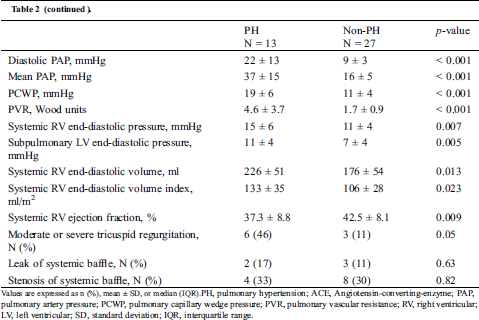
PH was diagnosed at an average (± SD) age of 36 ± 7 years and a median (IQR) of 34 (30–38) years after the atrial switch procedure. The mean (± SD) PAP was 37 ± 15 mmHg; PCWP, 19 ± 6 mmHg; and PVR, 4.6 ± 3.7 Woods units. Both of the ventricular end-diastolic pressures in the PH group were significantly higher than those in the non-PH group (systemic RV: 15 ± 6 mmHg vs. 11 ± 4 mmHg, p = 0.007; subpulmonary LV: 11 ± 4 vs. 7 ± 4 mmHg, p = 0.005). The systemic RV EDV in the PH group was significantly larger than that in the non-PH group (226 ± 51 mL vs. 176 ± 54 mL, p = 0.013), and the systemic RV ejection fraction in the PH group was significantly lower than that in the non-PH group (37.3 ± 8.8% vs. 42.5 ± 8.1%, p = 0.009).
4.3 Adverse Events and Follow-up
The mean (± SD) follow-up period after catheterization was 4.3 ± 3.5 years. During this period, 7 (18%) patients experienced adverse events; two patients died, four were hospitalized for heart failure, and one had his/her pacemaker upgraded to CRT. Patients in the PH group had a significantly higher rate of adverse events after catheterization than those in the non-PH group (hazard ratio, 4.5; 95% confidence interval, 0.99–23.0; log-rank p = 0.032; Fig. 1).
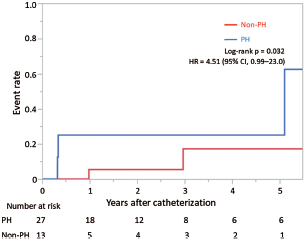
Figure 1: Kaplan–Meier curve displaying the occurrence of adverse events
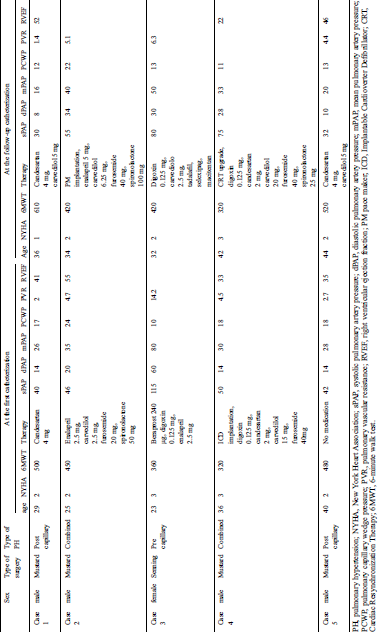
In the PH group, five patients underwent catheterization twice (Tab. 3). Cases 1 and 5 were classified as isolated postcapillary PH, cases 2 and 4 were classified as combined PH, and case 3 was classified as precapillary PH. Since patient 3 had a residual atrial septal defect, precapillary PH resulted from the left atrium to the right atrium shunt. The mean PAP of cases 1, 3, and 5 was lower in the second catheterization than in the first catheterization. Case 3 received higher doses of the pulmonary vasodilators and showed improved PH and exercise tolerance. Cases 1 and 5 were diagnosed with isolated postcapillary PH and had enough margin for standard heart failure therapy. Case 1 was started on a β-blocker, whereas case 5 was started on an ARB and β-blocker. During the second catheterization, both patients showed improvements in PH, the NYHA class, and the 6-minute walk test. The NYHA class of the three cases improved compared with that in the first catheterization. Case 2 was increased doses of ACE-I, β-blocker, furosemide, and spironolactone, and underwent PM implantation. Case 4 was increased dose of the β-blocker, was started on spironolactone, and received an upgrade to CRT. They did not show improvements in their PH and exercise tolerance. None of the five cases underwent intervention for the tricuspid valve regurgitation.
Table 3: Changes in the parameters between the first and follow-up catheterizations
In the present study, PH was detected in 33% of patients with TGA after undergoing the atrial switch procedure. Additionally, patients in the PH group had increased cardiac death and heart failure. Finally, improvements in PH and the NYHA class were achieved by the initiation of accurate therapy in the three of the five patients who underwent a second catheterization during the follow-up period. These three patients had isolated postcapillary or precapillary PH.
PH is a serious complication of atrial switch procedures documented in up to 7% of patients surviving to adulthood [9]. The main reason for the discrepant rate observed in our study and prior studies might result from differing inclusion criteria. Prior studies included all patients who underwent atrial switch procedures and survived to adulthood. Conversely, the present study only included patients who underwent catheterization. Another study that included patients who underwent catheterization after the atrial switch procedure reported that PH was detected in 54.5% of patients referred for catheterization [10]. The indications for catheterization in the previous study and our study were heart failure or worsening functional capacity. PH rates may be higher in patients with symptoms such as shortness of breath.
In the current study, patients in the PH group had an adverse prognosis; they had a higher number of prior arrhythmia events, BNP, and systemic RV EDV/EDVI, and a lower systemic RV ejection fraction. Additionally, PH was postcapillary in all cases except for one case. These findings demonstrated that PH in adulthood after the atrial switch procedure might result from RV heart failure. Prognosis in the PH group might be poor due to advanced RV heart failure. After the atrial switch procedure, systemic RV dysfunction can develop at any stage. However, it is almost uniformly present in older patients, thereby indicating that the anatomic RV is unable to sustain the systemic circulation over long follow-up periods [6,13]. A previous study reported that pulmonary stenosis can be helpful in supporting the RV function of patients with TGA who underwent atrial switch procedures [14]. Although we did not analyze pulmonary stenosis in the present study, the presence of pulmonary stenosis might have affected systemic RV function. Although systemic RV function in the non-PH group was also low (42.5 ± 8.1%), PH was not detected in the non-PH group. One reason for this might be the low levels of RV end-diastolic pressures and PCWP of the non-PH group. Low levels of RV end-diastolic pressures and PCWP indicate low afterload of the pulmonary circulation; therefore, PH would not have been detected in the non-PH group.
The management of PH in patients with TGA requiring atrial switch procedures remains unknown. Previous studies demonstrated that pulmonary vasodilators were effective for patients with precapillary PH after they underwent atrial switch procedures [8,15]. Conversely, because postcapillary PH might result from RV dysfunction, standard heart failure treatment might be effective. As for the pharmacological therapy for RV dysfunction, renin-angiotensin-aldosterone system blockers did not seem to convey benefits for RV function or exercise capacity [16,17]. In contrast, β-blockers may have a beneficial role in systemic RV [18]. The effectiveness of CRT for systemic RV has not been established. According to the European working group on CHD, CRT should be considered for patients with a NYHA class ≥II, impaired systemic RV function, and right bundle-branch block, and for patients who are already receiving LV pacing with systemic RV dyssynchrony and dysfunction [19]. Thus, among 13 patients in the present study with PH, six (46%) received an ACE-I or ARB, and seven (54%) received a β-blocker. One patient had his/her pace maker upgraded to CRT. Among the five patients who underwent a second catheterization during the follow-up period, patient 3 was diagnosed with precapillary PH. Subsequently, patient 3 received higher doses of the pulmonary vasodilators and showed improved PH and exercise tolerance. Patients 1 and 5 were diagnosed with isolated postcapillary PH and had enough margin for standard heart failure therapy. These patients received enhanced medical therapy and showed improvements in their PH and exercise tolerance. Patients 2 and 4 received full medications for heart failure at the first catheterization. Although the two patients underwent advanced heart failure therapy including CRT, their PH and exercise tolerance did not improve. Early interventions by medication might assist in improving PH and exercise tolerance among those with postcapillary PH due to systemic RV dysfunction. Although none of the patients underwent mechanical circulatory support and heart transplantation, the American Heart Association suggests that patients with systemic RV and advanced heart failure who do not respond to or are unsuitable for CRT and failing medical therapy should be referred to mechanical support or heart transplantation before other organ failure and PH occur [20].
To our knowledge, the number of patients who underwent catheterization after the atrial switch procedure is larger than that reported in previous studies. In our study, there were five patients who underwent a second catheterization during the follow-up period. As mentioned above, early interventions for systemic RV dysfunction via medications might assist in improving PH and exercise tolerance. Catheterization at an appropriate time is necessary for the early diagnosis of PH. Because a previous report and our study showed PH onset 20 years after the atrial switch procedure [10], it might be better to perform a catheterization 10 years after the atrial switch procedure and then follow-up every 5 years.
The present study had several limitations that merit mentioning here. First, this was a retrospective study based on a medical chart review. Second, because the number of cases was too small, adjustment or multivariable analysis could not perform in the present study. Third, the eligible patients were limited to those who had catheterization performed between April 2007 and March 2020. Although we have been performing catheterization on all patients in the remote period after the atrial switch procedure based on our department policy, patients who died before this period or who had catheterization performed outside of this period were excluded from the present study. Fourth, in this study, because 36/40 (90%) patients in this study have undergone catheterization before 2018, we did not use the current definition of PH that was presented at the 6th World Symposium on PH. The results of the present study may be different if the new definition of PH was used. Fifth, because the treatment protocol was determined by the responsible physician, the medication prescribed differed from patient to patient. Finally, the follow-up period of the present study was short, thus the long-term prognosis of patients with PH was not investigated.
PH is very prevalent in adults with TGA who underwent the atrial switch procedure and is associated with an adverse prognosis. Early therapy for systemic RV might improve PH and exercise tolerance after the atrial switch procedures.
Data sharing: The data underlying this article will be shared on reasonable request to the corresponding author.
Acknowledgement: We would like to thank Editage (www.editage.com) for English language editing.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Liebman, L., Cullum, L., Belloc, N. B. (1969). Natural history of transpositon of the great arteries. anatomy and birth and death characteristics. Circulation, 40(2), 237–262.
2. Levin, D. L., Paul, M. H., Muster, A. J., Newfeld, E. A., Waldman, J. D. (1977). D-Transposition of the great vessels in the Neonate. A clinical diagnosis. Archives of Internal Medicine, 137(10), 1421–1425. DOI 10.1001/archinte.1977.03630220061015.
3. Senning, A. (1959). Surgical correction of transposition of the great vessels. Surgery, 45(6), 966–980.
4. Mustard, W. T. (1964). Successful two-stage correction of transposition of the great vessels. Surgery, 55, 469–472.
5. Vejlstrup, N., Sorensen, K., Mattsson, E., Thilen, U., Kvidal, P. et al. (2015). Long-term outcome of mustard/senning correction for transposition of the great arteries in Sweden and Denmark. Circulation, 132(8), 633–638. DOI 10.1161/CIRCULATIONAHA.114.010770.
6. Cuypers, J. A., Eindhoven, J. A., Slager, M. A., Opic, P., Utens, E. M. et al. (2014). The natural and unnatural history of the Mustard procedure: Long-term outcome up to 40 years. European Heart Journal, 35(25), 1666–1674. DOI 10.1093/eurheartj/ehu102.
7. Couperus, L. E., Vliegen, H. W., Zandstra, T. E., Kies, P., Jongbloed, M. R. M. et al. (2019). Long-term outcome after atrial correction for transposition of the great arteries. Heart, 105(10), 790–796. DOI 10.1136/heartjnl-2018-313647.
8. Yehya, A., Lyle, T., Pernetz, M. A., McConnell, M. E., Kogon, B. et al. (2010). Pulmonary arterial hypertension in patients with prior atrial switch procedure for d-transposition of great arteries (dTGA). International Journal of Cardiology, 143(3), 271–275. DOI 10.1016/j.ijcard.2009.02.039.
9. Ebenroth, E. S., Hurwitz, R. A., Cordes, T. M. (2000). Late onset of pulmonary hypertension after successful mustard surgery for d-transposition of the great arteries. American Journal of Cardiology, 85(1), 127–130. DOI 10.1016/S0002-9149(99)00625-6.
10. Chaix, M. A., Dore, A., Mercier, L. A., Mongeon, F. P., Marcotte, F. et al. (2017). Late onset postcapillary pulmonary hypertension in patients with transposition of the great arteries and Mustard or Senning Baffles. Journal of the American Heart Association, 6(10), e006481. DOI 10.1161/JAHA.117.006481.
11. Hoeper, M. M., Bogaard, H. J., Condliffe, R., Frantz, R., Khanna, D. et al. (2013). Definitions and diagnosis of pulmonary hypertension. Journal of the American College of Cardiology, 62(25 Suppl), D42–D50. DOI 10.1016/j.jacc.2013.10.032.
12. Galie, N., Humbert, M., Vachiery, J. L., Gibbs, S., Lang, I. et al. (2016). 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: The joint task force for the diagnosis and treatment of pulmonary hypertension of the European society of cardiology (ESC) and the European respiratory society (ERSEndorsed by: Association for European paediatric and congenital cardiology (AEPCInternational society for heart and lung transplantation (ISHLT). European Heart Journal, 37(1), 67–119. DOI 10.1093/eurheartj/ehv317.
13. Roos-Hesselink, J. W., Meijboom, F. J., Spitaels, S. E., van Domburg, R., van Rijen, E. H. et al. (2004). Decline in ventricular function and clinical condition after Mustard repair for transposition of the great arteries (a prospective study of 22-29 years). European Heart Journal, 25(14), 1264–1270. DOI 10.1016/j.ehj.2004.03.009.
14. Warnes, C. A. (2006). Transposition of the great arteries. Circulation, 114(24), 2699–2709. DOI 10.1161/CIRCULATIONAHA.105.592352.
15. Cordina, R., Celermajer, D. (2010). Late-onset pulmonary arterial hypertension after a successful atrial or arterial switch procedure for transposition of the great arteries. Pediatric Cardiology, 31(2), 238–241. DOI 10.1007/s00246-009-9597-9.
16. van der Bom, T., Winter, M. M., Bouma, B. J., Groenink, M., Vliegen, H. W. et al. (2013). Effect of valsartan on systemic right ventricular function: A double-blind, randomized, placebo-controlled pilot trial. Circulation, 127(3), 322–330. DOI 10.1161/CIRCULATIONAHA.112.135392.
17. Dore, A., Houde, C., Chan, K. L., Ducharme, A., Khairy, P. et al. (2005). Angiotensin receptor blockade and exercise capacity in adults with systemic right ventricles: A multicenter, randomized, placebo-controlled clinical trial. Circulation, 112(16), 2411–2416. DOI 10.1161/CIRCULATIONAHA.105.543470.
18. Doughan, A. R., McConnell, M. E., Book, W. M. (2007). Effect of beta blockers (carvedilol or metoprolol XL) in patients with transposition of great arteries and dysfunction of the systemic right ventricle. American Journal of Cardiology, 99(5), 704–706. DOI 10.1016/j.amjcard.2006.10.025.
19. Budts, W., Roos-Hesselink, J., Radle-Hurst, T., Eicken, A., McDonagh, T. A. et al. (2016). Treatment of heart failure in adult congenital heart disease: A position paper of the working group of grown-up congenital heart disease and the heart failure association of the European society of cardiology. European Heart Journal, 37(18), 1419–1427. DOI 10.1093/eurheartj/ehv741.
20. Ross, H. J., Law, Y., Book, W. M., Broberg, C. S., Burchill, L. et al. (2016). Transplantation and mechanical circulatory support in congenital heart disease. Circulation, 133(8), 802–820. DOI 10.1161/CIR.0000000000000353.
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |