DOI:10.32604/CHD.2021.012526

| Congenital Heart Disease DOI:10.32604/CHD.2021.012526 |  |
| Article |
1Paediatric Cardiology Department, Royal Brompton Hospital, London, SW3 6NP, UK
2Paediatric Cardiology Department, Bambino Gesù Paediatric Hospital, Rome, 00165, Italy
3Cardiology Department, “Magna Graecia” University, Catanzaro, 88100, Italy
4Paediatric Cardiology Department, University of Padua, Padua, 35128, Italy
*Corresponding Author: Giovanni Di Salvo. Email: giodisal@yahoo.it
Received: 24 June 2020; Accepted: 09 October 2020
Abstract: Background: Still little is known about the impact on right ventricle function of the 2 main approaches to Norwood palliation in Hypoplastic left heart syndrome, the right ventricle-pulmonary artery shunt (RVPAS) and the modified Blalock-Taussig shunt (mBTS). Methods: The cohort included 27 patients with Hypoplastic left heart syndrome (10 in the mBTS group, 17 in the RVPAS group). Longitudinal strain, tricuspid annulus peak systolic excursion and fractional area change were evaluated before Norwood and in four different breakpoints in the steady-state after Norwood procedure (30-days, 90-days, 140-days and 200-days after Norwood). Results: No significant differences were found in all standard echocardiographic functional parameters between the two groups at any times. However, when we compared right ventricle function before and after Norwood procedure, longitudinal strain significantly improved in mBTS group compared to pre-surgical assessment (after 90-days: mBTS +27,35% ± 43,47 vs. RVPAS −8,20% ± 25,25, p = 0,03; after 200-days: mBTS +10.19% ± 36.58 vs. RVPAS −21.64% ± 30.43, p = 0.04). Conclusion: The mBTS group, which did not receive any ventriculotomy, showed a significant increase in right ventricle longitudinal strain during convalescence. These data support the use of longitudinal strain in Hypoplastic left heart syndrome evaluation and may suggest potential value in terms of cardiac mechanics in using mBTS, preserving the right ventricle integrity.
Keywords: Hypoplastic left heart syndrome; speckle tracking echocardiography; congenital heart disease
Almost four decades after its description [1], the Norwood procedure still represents the cornerstone of Hypoplastic left heart syndrome (HLHS) staged palliation. Very early in life, neonates with HLHS receive reconstruction of neo-aorta and connection of systemic and pulmonary circulations (Norwood stage I). After pulmonary vasculature maturation, the systemic venous return is redirected toward pulmonary arteries through two surgical stages performed at 4 to 6 months of age and 18 to 48 months of age [2].
Essentially, two are the techniques allowing the connection of systemic and pulmonary circulations in the first stage:
• The Modified Blalock–Taussig Shunt (mBTS), where a tube is placed from the innominate or subclavian artery to the pulmonary arteries to supply pulmonary blood flow. This tube provides a continuous systo-diastolic flow towards pulmonary circulation with a possible result in “coronary steal” during diastole.
• The Right Ventricle-to-Pulmonary Artery Conduit (RVPAS), where a valveless conduit is placed from the right ventricle (RV) to the pulmonary arteries to supply pulmonary blood flow. Possible complications are related to the right ventriculotomy and scar creation [3].
As a consequence of “coronary steal” elimination, it is thought RVPAS conducts to a very stable early post-operative course [4]. On the other side, RVPAS presupposes an antero-basal right ventriculotomy with the creation of ventricular scar and alteration in local contractility [5] and, even though RVPAS has demonstrated to be a better choice for the early post-operative transplantation-free survival, this approach lost its superiority on the long run [6]. Indeed, RVPAS seems to be associated with a lower ventricular function when compared to mBTS in some studies on longer follow-up after second surgical stage [7,8], but a direct correlation with RVPAS placement at the time of Norwood procedure is not demonstrated yet [8–10].
Evaluation of RV function is challenging due to no exact geometric model existing for volume measurements, with 2D and Doppler echocardiographic measurements modestly correlating with magnetic resonance estimates of ejection fraction [11,12]. Furthermore, classical functional echocardiographic parameters have failed to demonstrate RV function differences in early and mid-term follow up of patients who underwent RVPAS or mBTS [13].
Speckle-tracking echocardiography derived longitudinal strain (LS) has been demonstrated to be a reliable technique, allowing the assessment of RV deformation without geometric assumption and the influence of acute preload and afterload changes [14,15], with good correlation with cardiac magnetic resonance imaging-derived RV ejection fraction in patients with HLHS [9,16].
Different studies have focused on speckle tracking-analysis to study ventricular function in patients with HLHS [16–20], but just a few tried to demonstrate postoperative ventricular deformation differences in the two types of shunt [9].
In our study, we used speckle-tracking echocardiography over standard echocardiographic parameters to evaluate changes in RV function in HLHS patients after RVPAS and mBTS.
This is a retrospective observational case-series on 27 consecutive paediatric patients. We conducted a re-analysis of a retrospective study on patients who underwent Norwood palliation between June 2015 and April 2019 at Royal Brompton Hospital of London, United Kingdom.
We collected patients’ data between July 2018 and July 2019. Only patients with a diagnosis of “pure HLHS” were considered eligible for the study. Exclusion criteria were “HLHS variants” (e.g., critical aortic stenosis, double-outlet RV with mitral atresia, unbalanced atrioventricular septal defect, and heterotaxic syndrome; n = 6), and initial procedures different from Norwood operation. Patients were divided in two groups on the basis of Norwood surgical technique received: RVPAS group and mBTS group (Suppl. Fig. 1).

Figure 1: Box-and-whisker plots for comparison between RVPAS and mBTS groups of TAPSE (cm), FAC (%) and LS (%). RVPAS, right ventricle-pulmonary artery shunt; mBTS, modified Blalock-Taussig shunt; TAPSE, tricuspid annulus peak systolic excursion, FAC, fractional area change; LS, longitudinal strain. 1, pre-Norwood assessment; 2, 30-days post Norwood assessment; 3, 90-days post Norwood assessment; 4, 140-days post Norwood assessment; 5, 200-days post Norwood assessment

Supplement Figure 1: Flow diagram of patients’ selection and outcome. The number of echocardiographic available loop was included for each follow-up
Demographic, clinical management, and outcome data were obtained from patients’ medical recordings.
Informed consent was obtained from patients’ parents before the enrolment when data were collected retrospectively. The study protocol conformed the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the local research committee.
Five different follow-up time points were chosen for the study purpose: (1) A pre-Norwood assessment “baseline”; (2) A 30-days post-Norwood assessment; (3) A 90-days post-Norwood assessment; (4) A 140-days post-Norwood assessment; (5) A 200-days post-Norwood assessment.
The 90-days and 140-days post Norwood evaluations were done after the first but before the second surgical step (Glenn procedure); the 200-days post-Norwood assessment was done after the second surgical step.
Echocardiographic recordings were obtained using a GE E95 ultrasound machine (GE Healthcare, Wauwatosa, WI) and Philips EpiQ 7 ultrasound machine (Koninklijke Philips N.V., Amsterdam, The Netherlands) and, then, kept digitally for offline analyses. Uncompressed data were stored on an Xcelera platform (Koninklijke Philips N.V., Amsterdam, The Netherlands) and then analysed offline with TomTec system (TomTec Imaging System GMBH, Unterschleissheim, Germany) by one blinded physician.
From the apical window, we measured: tricuspid annulus peak systolic excursion (TAPSE), fractional area change (FAC), and peak LS.
TAPSE was measured as the difference between end-diastolic and end-systolic tricuspid annulus displacement. FAC was measured as the difference between end-diastolic and end-systolic RV area tracing manually the endocardial boundary in a focused apical four-chamber view; values were expressed in percentage.
The RV LS analysis was obtained from grayscale optimized images while in apical 4-chamber view. To be included in the analysis, the image quality had to have a frame rate >50 frames per second with a good definition of the RV wall. The region of interest was selected using three point-click method, with three points manually placed on each side of tricuspid anulus and at apex. The endocardium contour was semi-automatically traced, manually adjusted as needed, and then the LS was automatically evaluated by the software algorithm. A 6-segment model was used for the study purpose. Peak strain was defined as the highest strain value within the cardiac cycle [16,21].
Moreover, we evaluated variations in LS, FAC, and TAPSE between echocardiograms at 30-days, 90-days, 140-days, and 200-days post-Norwood and baseline, as follow:
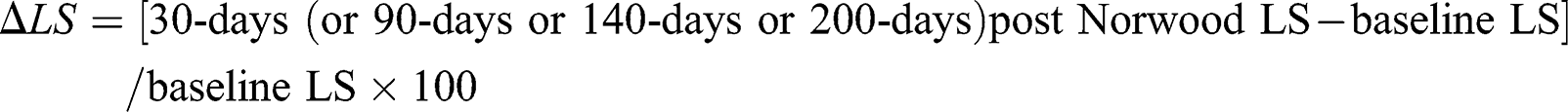
The same method was used for ∆FAC and ∆TAPSE.
Tricuspid regurgitation was classified from 0 to 3 as follow: 0 = none/trivial (single narrow jet), 1 = mild (multiple narrow jets), 2 = moderate (wide jet reaching the midportion of the right atrium), and 3 = severe (wide jet reaching the back wall of the right atrium). This method has been used in previous studies on HLHS patients by our group [20] and other authors [16,22].
Clinical and echocardiographic data were indicated as median and interquartile ranges or mean and standard deviation as appropriate. Normality was tested using the Shapiro-Wilk method. Comparisons were made with the Mann-Whitney U test or unpaired T-test, on MedCalc, version 18.11 (MedCalc Software, Mariakerke, Belgium). A p-value < 0.05 was considered statistically significant.
We measured TAPSE, FAC, and LS intra- and inter- observer variability in 10 randomly selected exams by using Bland-Altman scatterplot analysis. To evaluate intra-observer variability, the same observer who performs all the echocardiographic measurements reassessed the same parameters at least 4 weeks later to avoid recall bias. To evaluate inter-observer variability, another observer, independent and blinded to the original measurements, evaluated also the RV parameters. We calculated the relative mean absolute difference between intra- and inter- observer measurements and then performed regression testing for proportional bias. Finally, we calculated intraclass correlation coefficients.
In the time-study period, 27 patients (17/27 male patients, 63%) with HLHS fulfilled the enrolment criteria and were included in the study. Ten patients (37%) received mBTS, while 17 patients (63%) received RVPAS.
All patients (100%) early survived the Norwood stage 1 operation (RVPAS and mBTS). Three patients died before the second surgical stage: Two of them (2/17 patients, 12%) belonged RVPAS group, one of them (1/10 patients, 10%) belonged mBTS group. At the time of the second surgical stage, 21/24 patients were treated with a straightforward Glenn operation, while in one case Glenn operation was associated with tricuspid valve repair. The patient who received tricuspid valve repair belonged RVPAS group. Since this repair could influence longitudinal RV performance, we excluded the patient from the analysis at the fifth evaluation, after Glenn operation. There were no differences in terms of weight or age at the time of Norwood operation between the two groups. Patients’ demographic and clinical characteristics are summarized in Tab. 1.
Table 1: Patient’s demographic and clinical characteristics

Echocardiographic images’ frame rate was 65 ± 15 frames per second. Thirty-two echocardiograms (13/27 patients, 48%) showed a moderate or severe tricuspid regurgitation, but no significant difference was found between mBTS and RVPAS group. Tab. 2 and Fig. 1 present the population’s echocardiographic data.
Table 2: Patient’s echocardiographic data

RVPAS, right ventricle-pulmonary artery shunt; mBTS, modified Blalock-Taussig shunt; TR, tricuspid regurgitation; TAPSE, tricuspid annulus peak systolic excursion, FAC, fractional area change; LS, longitudinal strain, LSR, longitudinal strain rate; LV, left ventricle; RV, right ventricle; RV ED, right ventricle end-diastolic; RV ES, right ventricle end-systolic.
There were no differences in TAPSE, FAC, and LS between the RVPAS group and the mBTS group in all the echocardiographic time points (Fig. 2). At 90-days and 200-days post-Norwood assessment, the mBTS group showed a significant improvement in LS compared to pre-Norwood assessment (∆LS). This finding, albeit present, was no statistically significant at 30-days and 140-days post-Norwood assessment. At 200-days post Norwood evaluation mBTS group showed a significantly smaller RV compared to RVPAS group.
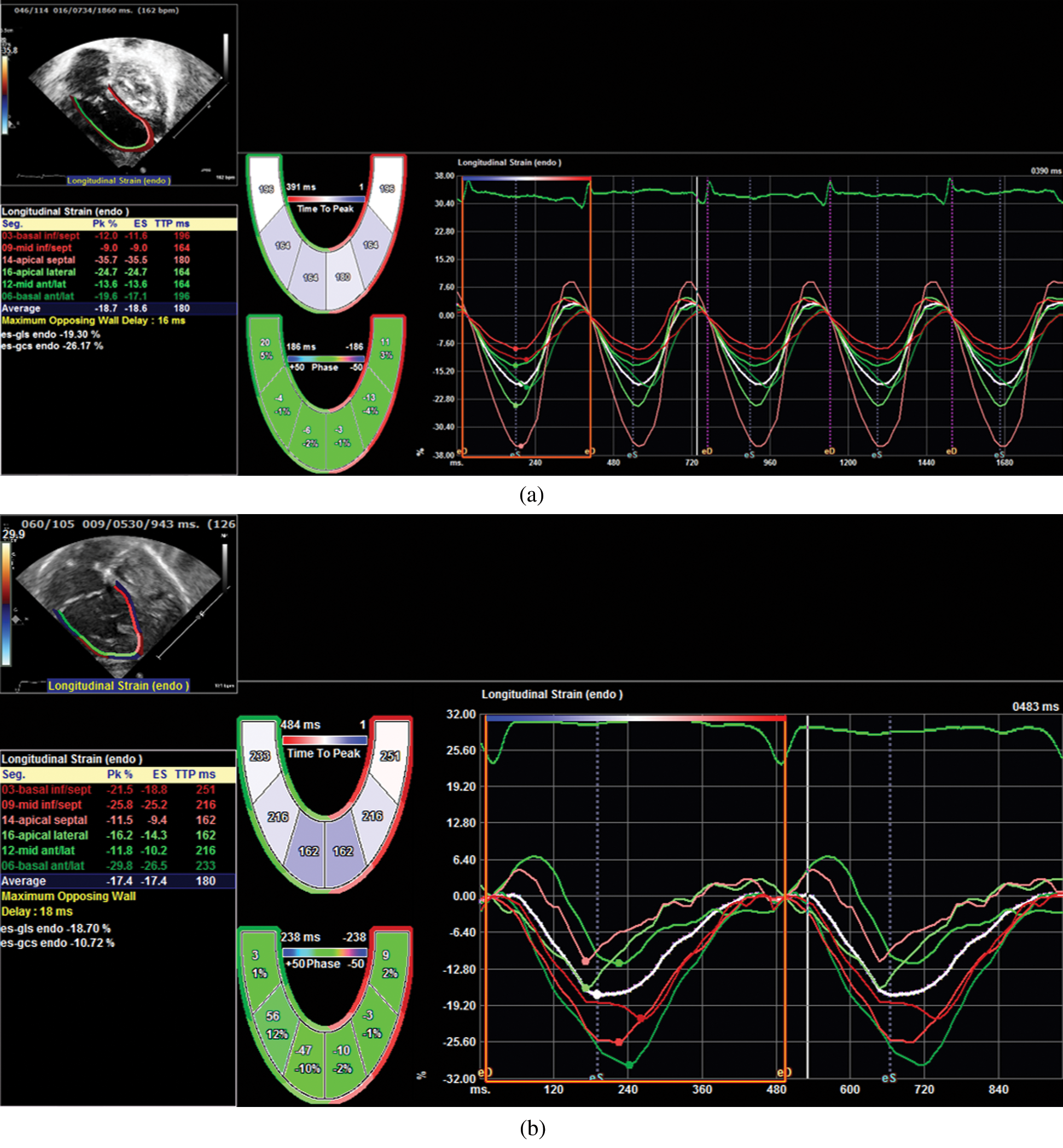
Figure 2: LS measurement at 90-days post Norwood evaluation. Image a shows LS in a patient treated with BTS. Image b shows LS in a patient treated with RVPAS
Inter-observer agreement of our data was very good for TAPSE and LS with an ICC of 0.84 and 0.85 respectively. Intra-observer reproducibility was also very good for TAPSE and LS with an ICC 0.95 and 0.91, respectively. FAC showed poor inter- and intra-observer reproducibility with an ICC of 0.55 and 0.52, respectively (Fig. 3).

Figure 3: Bland-Altman scatterplots showing intra- and interobserver agreement for TAPSE and longitudinal strain (LS). TAPSE, tricuspid annulus peak systolic excursion, LS, longitudinal strain
With this study, we want to describe the effect on cardiac mechanics of the two main initial approaches to HLHS palliation, the RVPAS and the mBTS [23]. Identifying patients with single ventricle initial dysfunction is crucial because RV damage correlates with intermediate and overall poor outcomes [24]. RV function, currently, remains best assessed by conductance catheterization [25], however, in patients with HLHS, studies with magnetic resonance imaging and conductance catheter have shown RV speckle tracking indices are relatively preload independent and correlates well with end-systolic elastance and ventricular contractility [16,26].
In our knowledge, our study is the first using LS over classical echocardiographic parameters to recognize variations over time in RV function early after the 2 main Norwood shunts. Very few studies used speckle tracking in this clinical setting [16–20] and even less compared ventricular function after the two types of Norwood shunt [9]. Indeed, Hill et al. [9] analysed speckle tracking differences at one specific time point, when the shunt was already taken down, at 14 months of age.
In line with the observations of Frommelt et al. [13], we did not find differences in standard functional echocardiographic parameters at any time in the early post-operative course. A straight evaluation of LS differences between the two groups revealed no statistically significant differences at any time points analysed. However, when we compared right ventricle myocardial deformation parameters before and after RVPAS/mBTS, we found an improvement of LS in the mBTS group. This amelioration was already present 30-days after mBTS but became statistically significant only 90-day after mBTS.
At 140-days post mBTS operation, this gain in LS was still present, but it lost its statistical significance. However, 200-days post mBTS, after all patients received Glenn operation (II stage), it was confirmed ∆LS statistical difference between the two groups. We speculated that the loss of statistical significance at 140-days post mBTS might be related to the small volume sample, but, also, to RV outset of dysfunction and be a red-flag for early second intervention.
On the other hand, RVPAS showed a constant, but not significant, reduction in LS in all post-Norwood assessments compared to pre-Norwood assessment.
No statistical differences were found between post-Norwood FAC and TAPSE and the baseline evaluation (∆FAC and ∆TAPSE) in the two groups. This finding is probably related to a greater sensibility [27,28] and reproducibility of LS compared to the other functional parameters (greater intra- and inter-observer reproducibility compared to FAC and greater interobserver reproducibility compared to TAPSE).
Notably, 200-days post Norwood, RV dimensions were significantly smaller in mBTS group compared to RVPAS group. This finding was related to the well-known RV volume overload due to the regurgitation from the valveless RVPAS [6].
Other authors previously reported that RVPAS ventriculotomy may be responsible of RV damage and this may cause the loss of survival benefits over mBTS with time [9]. Similarly, we hypothesize that RV intactness in the mBTS group may favour a precocious LS improvement and may be beneficial for cardiac mechanics over RVPAS.
Moreover, since we found this result only analysing echocardiographic differences between pre- and post-surgical times, our study suggests the noteworthy value of serial LS measurements in the assessment of patients with HLHS.
There were some limitations in this study. The main one was represented by the small population size. However, given the rarity of the condition, the monocentric character of the study, and the strict inclusion criteria, the volume sample was fairly substantial. The quality of images in some cases was not enough to allow speckle tracking analysis, as a result, some echocardiographic data were cut off from the study. However, considering the retrospective nature of the study and the complex cardiac anatomy of HLHS, these losses were random and very few with no prejudice on the analysis.
Acknowledgement: We would like to thank Dr Mario Panebianco, from Bambino Gesù Paediatric Hospital of Rome, for the assistance with this project.
Funding Statement: The author(s) received no specific funding for this study.
Conflicts of Interest:The authors declare that they have no conflicts of interest to report regarding the present study.
1. Norwood, W. I., Lang, P., Casteneda, A. R., Campbell, D. N. (1981). Experience with operations for hypoplastic left heart syndrome. Journal of Thoracic and Cardiovascular Surgery, 82(4), 511–519. DOI 10.1016/S0022-5223(19)39288-8. [Google Scholar] [CrossRef]
2. Ohye, R. G., Schranz, D., D’Udekem, Y. (2016). Current therapy for hypoplastic left heart syndrome and related single ventricle lesions. Circulation, 134(17), 1265–1279. DOI 10.1161/CIRCULATIONAHA.116.022816. [Google Scholar] [CrossRef]
3. Ohye, R. G., Devaney, E. J., Hirsch, J. C., Bove, E. L. (2007). The modified Blalock-Taussig shunt versus the right ventricle-to-pulmonary artery conduit for the Norwood procedure. Pediatric Cardiology, 28(2), 122–125. DOI 10.1007/s00246-006-1449-2. [Google Scholar] [CrossRef]
4. Sano, S., Ishino, K., Kawada, M., Arai, S., Kasahara, S. et al. (2003). Right ventricle-pulmonary artery shunt in first-stage palliation of hypoplastic left heart syndrome. Journal of Thoracic and Cardiovascular Surgery, 126(2), 504–510. DOI 10.1016/S0022-5223(02)73575-7. [Google Scholar] [CrossRef]
5. Menon, S. C., Minich, L. L., Casper, T. C., Puchalski, M. D., Hawkins, J. A. et al. (2011). Regional myocardial dysfunction following Norwood with right ventricle to pulmonary artery conduit in patients with hypoplastic left heart syndrome. Journal of the American Society of Echocardiography, 24(8), 826–833. DOI 10.1016/j.echo.2011.05.008. [Google Scholar] [CrossRef]
6. Ohye, R. G., Sleeper, L. A., Mahony, L., Newburger, J. W., Pearson, G. D. et al. (2010). Comparison of shunt types in the Norwood procedure for single-ventricle lesions. New England Journal of Medicine, 362(21), 1980–1992. DOI 10.1056/NEJMoa0912461. [Google Scholar] [CrossRef]
7. Sano, S., Huang, S. C., Kasahara, S., Yoshizumi, K., Kotani, Y. et al. (2009). Risk factors for mortality after the Norwood procedure using right ventricle to pulmonary artery shunt. Annals of Thoracic Surgery, 87(1), 178–186. DOI 10.1016/j.athoracsur.2008.08.027. [Google Scholar] [CrossRef]
8. Newburger, J. W., Sleeper, L. A., Frommelt, P. C., Pearson, G. D., Mahle, W. T. et al. (2014). Transplantation-free survival and interventions at 3 years in the single ventricle reconstruction trial. Circulation, 129(20), 2013–2020. DOI 10.1161/CIRCULATIONAHA.113.006191. [Google Scholar] [CrossRef]
9. Hill, G. D., Frommelt, P. C., Stelter, J., Campbell, M. J., Cohen, M. S. et al. (2015). Impact of initial Norwood shunt type on right ventricular deformation: The single ventricle reconstruction trial. Journal of the American Society of Echocardiography, 28(5), 517–521. DOI 10.1016/j.echo.2015.01.018. [Google Scholar] [CrossRef]
10. Barron, D. J. (2013). The Norwood procedure: In favor of the RV-PA conduit. Seminars in thoracic and cardiovascular surgery. Pediatric Cardiac Surgery Annual, 16(1), 52–58. [Google Scholar]
11. Margossian, R., Schwartz, M. L., Prakash, A., Wruck, L., Colan, S. D. et al. (2009). Comparison of echocardiographic and cardiac magnetic resonance imaging measurements of functional single ventricular volumes, mass, and ejection fraction (from the Pediatric Heart Network Fontan Cross-Sectional Study). American Journal of Cardiology, 104(3), 419–428. DOI 10.1016/j.amjcard.2009.03.058. [Google Scholar] [CrossRef]
12. Khattab, K., Schmidheiny, P., Wustmann, K., Wahl, A., Seiler, C. et al. (2013). Echocardiogram versus cardiac magnetic resonance imaging for assessing systolic function of subaortic right ventricle in adults with complete transposition of great arteries and previous atrial switch operation. American Journal of Cardiology, 111(6), 908–913. DOI 10.1016/j.amjcard.2012.11.044. [Google Scholar] [CrossRef]
13. Frommelt, P. C., Guey, L. T., Minich, L. L., Bhat, M., Bradley, T. J. et al. (2012). Does initial shunt type for the Norwood procedure affect echocardiographic measures of cardiac size and function during infancy? The single ventricle reconstruction trial. Circulation, 125(21), 2630–2638. DOI 10.1161/CIRCULATIONAHA.111.072694. [Google Scholar] [CrossRef]
14. Rudski, L. G., Lai, W. W., Afilalo, J., Hua, L., Handschumacher, M. D. et al. (2010). Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. Journal of the American Society of Echocardiography, 23(7), 685–788. DOI 10.1016/j.echo.2010.05.010. [Google Scholar] [CrossRef]
15. Zaidi, A., Knight, D. S., Augustine, D. X., Harkness, A., Oxborough, D. et al. (2020). Echocardiographic assessment of the right heart in adults: A practical guideline from the British Society of Echocardiography. Echo Research and Practice, 7(1), G19–G41. DOI 10.1530/ERP-19-0051. [Google Scholar] [CrossRef]
16. Lin, L. Q., Conway, J., Alvarez, S., Goot, B., Serrano-Lomelin, J. et al. (2018). Reduced right ventricular fractional area change, strain, and strain rate before bidirectional cavopulmonary anastomosis is associated with medium-term mortality for children with hypoplastic left heart syndrome. Journal of the American Society of Echocardiography, 31(7), 831–842. DOI 10.1016/j.echo.2018.02.001. [Google Scholar] [CrossRef]
17. Petko, C., Hoffmann, U., Möller, P., Scheewe, J., Kramer, H. H. et al. (2010). Assessment of ventricular function and dyssynchrony before and after stage 2 palliation of hypoplastic left heart syndrome using two-dimensional speckle tracking. Pediatric Cardiology, 31(7), 1037–1042. DOI 10.1007/s00246-010-9760-3.
18. Petko, C., Voges, I., Schlangen, J., Scheewe, J., Kramer, H. H. et al. (2011). Comparison of right ventricular deformation and dyssynchrony in patients with different subtypes of hypoplastic left heart syndrome after Fontan surgery using two-dimensional speckle tracking. Cardiology in the Young, 21(6), 677–683. DOI 10.1017/S1047951111000631.
19. Colquitt, J. L., Loar, R. W., Morris, S. A., Feagin, D. K., Sami, S. et al. (2019). Serial strain analysis identifies hypoplastic left heart syndrome infants at risk for cardiac morbidity and mortality: A pilot study. Journal of the American Society of Echocardiography, 32(5), 643–650. DOI 10.1016/j.echo.2019.01.006.
20. Borrelli, N., Di Salvo, G., Sabatino, J., Ibrahim, A., Avesani, M. et al. (2020). Serial changes in longitudinal strain are associated with outcome in children with hypoplastic left heart syndrome. International Journal of Cardiology, 317, 56–62. DOI 10.1016/j.ijcard.2020.03.085. [Google Scholar] [CrossRef]
21. Sabatino, J., Di Salvo, G., Krupickova, S., Fraisse, A., Prota, C. et al. (2019). Left ventricular twist mechanics to identify left ventricular noncompaction in childhood. Circulation Cardiovascular Imaging, 12(4), e007805. DOI 10.1161/CIRCIMAGING.118.007805. [Google Scholar] [CrossRef]
22. Ohye, R. G., Gomez, C. A., Goldberg, C. S., Graves, H. L., Devaney, E. J. et al. (2004). Tricuspid valve repair in hypoplastic left heart syndrome. Journal of Thoracic and Cardiovascular Surgery, 127(2), 465–472. DOI 10.1016/j.jtcvs.2003.07.053. [Google Scholar] [CrossRef]
23. Davies, R. R., Pizarro, C. (2015). Decision-making for surgery in the management of patients with univentricular heart. Frontiers in Pediatrics, 3, 61. DOI 10.3389/fped.2015.00061. [Google Scholar] [CrossRef]
24. Altmann, K., Printz, B. F., Solowiejczky, D. E., Gersony, W. M., Quaegebeur, J. et al. (2000). Two-dimensional echocardiographic assessment of right ventricular function as a predictor of outcome in hypoplastic left heart syndrome. American Journal of Cardiology, 86(9), 964–968. DOI 10.1016/S0002-9149(00)01131-0. [Google Scholar] [CrossRef]
25. Mertens, L. L., Friedberg, M. K. (2010). Imaging the right ventricle-current state of the art. Nature Reviews Cardiology, 7(10), 551–563. DOI 10.1038/nrcardio.2010.118. [Google Scholar] [CrossRef]
26. Schlangen, J., Petko, C., Hansen, J. H., Michel, M., Hart, C. et al. (2014). Two-dimensional global longitudinal strain rate is a preload independent index of systemic right ventricular contractility in hypoplastic left heart syndrome patients after Fontan operation. Circulation Cardiovascular Imaging, 7(6), 880–886. DOI 10.1161/CIRCIMAGING.114.002110. [Google Scholar] [CrossRef]
27. Grant, A. D., Smedira, N. G., Starling, R. C., Marwick, T. H. (2012). Independent and incremental role of quantitative right ventricular evaluation for the prediction of right ventricular failure after left ventricular assist device implantation. Journal of the American College of Cardiology, 60(6), 521–528. DOI 10.1016/j.jacc.2012.02.073. [Google Scholar] [CrossRef]
28. Lee, J. H., Park, J. H. (2018). Strain analysis of the right ventricle using two-dimensional echocardiography. Journal of Cardiovascular Imaging, 26(3), 111–124. DOI 10.4250/jcvi.2018.26.e11. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |