DOI:10.32604/CHD.2021.013051

| Congenital Heart Disease DOI:10.32604/CHD.2021.013051 |  |
| Article |
Effect of Exercise-Based Cardiac Rehabilitation on Cardiorespiratory Fitness in Adults with Congenital Heart Disease
1Department of Cardiology, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland
2Department for Neuroscience and Movement Science, University of Fribourg, Fribourg, Switzerland
3Center for Congenital Heart Disease, Bern University Hospital, University of Bern, Bern, Switzerland
4Department of Cardiology, University Hospital Zurich, University of Zurich, Zurich, Switzerland
5Department of Cardiology, University Hospital Basel, University of Basel, Basel, Switzerland
*Corresponding Author: Prisca Eser. Email: Prisca.eser@insel.ch
Received: 24 July 2020; Accepted: 24 September 2020
Abstract: Background: The purpose of this study was to investigate whether patients with adult congenital heart disease (ACHD) benefit from exercise-based cardiac rehabilitation (CR) short- and long-term with regard to improvement of cardiorespiratory fitness. Methods: Cardiopulmonary exercise tests (CPET) completed by ACHD patients between January 2000 and October 2019 were analysed retrospectively. Linear mixed models were performed for peak oxygen consumption (VO2) with patients as random effect and age, sex, disease classification, preceding surgery (≤3 months) and preceding CR (≤4 weeks for short term and >4 weeks for long term) as fixed effects. Results: 1056 CPETs of 311 ACHD patients with simple (7), moderate (188) or great (116) complexity heart defects were analysed. The 59 patients who completed a CR (median age 27 yrs, 38% females) increased peak VO2 from before to after CR by a median of 2.7 (IQR –0.6 to 5.5) ml/kg/min. However, in the multivariate mixed model, peak VO2 was non-significantly increased short-term after CR (β 0.8, 95%CI –0.7 to 2.4), not maintained long-term after CR (β 0.0, 95%CI –1.7 to 1.6) but significantly reduced after surgery (β –5.1, 95%CI –7.1 to –3.1). The 20 CR patients after surgery increased their peak VO2 by 6.2 (IQR 3.6–9.5) ml/kg/min, while the 39 CR patients without preceding surgery increased it by 0.9 (IQR –1.5 to 3.1) ml/kg/min. Conclusions: The increase in peak VO2 with CR was mainly due to recovery from surgical intervention. The small independent benefit from CR was not maintained long-term, highlighting the potential to improve current CR concepts in ACHD populations.
Keywords: Grown-ups with congenital heart disease; exercise training; cardiac rehabilitation; surgical intervention; peak oxygen consumption; cardiorespiratory fitness
Almost one percent of all newborns are born with a congenital heart disease (CHD) [1]. Diagnoses vary from simple over moderate to great complexity. Due to the improved medical care, life expectancy in these patients has grown over the last decades, which has led to a growing population of adults with CHD (ACHD) patients [2,3].
Most patients with CHD are relatively sedentary [4]. Exercise intolerance is a major problem for ACHD patients, significantly restricting their quality of life [5,6], and increasing their risk for severe cardiac complications including arrhythmia and heart failure [7]. Furthermore, it may increase their risk for late complications such as heart failure and sudden cardiac death [8–10]. Consequently, incorporation of physical exercise into the daily routine is also recommended for ACHD patients [11].
Exercise capacity varies significantly across the spectrum of ACHD patients [7]. The lowest oxygen consumption (peak VO2) as measure of cardiorespiratory fitness was found in patients with complex heart disease (e.g., univentricular circulation, Eisenmenger syndrome). At the other end of the spectrum, patients with simple heart defects were found to have the highest peak VO2. However, cardiorespiratory fitness was still significantly reduced when compared with normal healthy people [12].
A number of studies have assessed the effect of exercise training interventions [13–17] or cardiac rehabilitation (CR) [18–22] on exercise capacity in ACHD patients. Most of these studies have shown favourable short-term effects. However, only one study has addressed long-term (3 years) maintenance of this benefit and has found that the short-term (10 weeks) beneficial effects of CR in patients with a systemic right ventricle did not persist over a three-year follow-up period [23]. Given this lack of evidence with regard to long-term benefits of exercise-based CR on exercise capacity in ACHD patients, we retrospectively examined data of our out-patient ACHD clinic. ACHD patients at our clinic perform cardiopulmonary exercise tests at regular 2–3 yearly intervals. Some of them have completed CR at some stage either because of physical deconditioning or after open chest surgery. The aim of this study was to assess if cardiorespiratory fitness improved short and long-term after CR and whether this effect was independent of the recovery process from preceding surgical intervention.
This study was a retrospective, single-centre study performed at a large tertiary referral centre. The study complied with the Declaration of Helsinki and the local Ethics Committee approved the research protocol. All patients provided informed consent either for the SACHER registry [24] or for the Insel hospital general informed consent.
Patients who were clinically followed-up at the Center for Congenital Heart Disease of the University Hospital Bern and performed cardiopulmonary exercise tests (CPET) at the Centre for Preventive Cardiology and Sports Medicine of the University Hospital of Bern between beginning of January 2000 and end of October 2019 were assessed for inclusion. Inclusion criteria were adults with congenital heart disease (minimal age 18 years) and completion of at least two CPETs within the inclusion period. Severity of CHD was rated according to ACHD AP classification guidelines [25] into mild, moderate and severe.
Cardiopulmonary exercise testing (CPET) was performed in ACHD patients as routine clinical work-up at usually 2- or 3-year intervals. During instable medical periods, CPET exams were performed more frequently. CPETs were always performed before enrolment in CR as well as after completion of CR.
CPETs were performed on a cycle ergometer and gas exchange was measured using a spirometry system (Jaeger Oxycon Pro, ViaSys Healthcare GmbH). Ramp protocols were selected for every patient individually in order to achieve voluntary exhaustion within 8–12 min. Patients were encouraged to maintain revolutions per minute (rpm) constant at between 60 and 70 rpm. The ramp test was terminated when the patient reached exhaustion and stopped cycling (or could not maintain 60 rpm) despite verbal encouragement to continue. Peak VO2 was defined as the highest VO2 averaged over 8 breathing cycles.
Primary outcome was peak VO2 relative to body weight. In addition, the predicted peak VO2 was calculated according to Wasserman [26].
Exercise-based CR consisted usually of 36 90-min training sessions, 2–3 times per week, lasting for 12–18 weeks. Patients trained on a cycling ergometer for 38 min during each session after which they performed resistance, agility, balance, stretching or relaxation training. Cycling training was mostly performed as continuous moderate intensity training, some fitter patients also performed some sessions as high-intensity interval training (HIIT) after an initial phase of continuous training. Workload at the beginning of CR was set at 50–60% of peak Watt reached at the CPET before the start of CR. After the first training session, training intensity was adjusted by Borg scale (targeting 13 to 14). HIIT training protocol started with 10 min warm-up at the intensity of 25% peak Watt. The main HIIT phase consisted of 4 × 4 cycles, each consisting of 4 min at a load of 80% peak Watt and 3 min at 25% of peak Watt. Perceived exertion was aimed at 15 to 16 on the Borg scale.
2.5 Data Handling and Statistical Analysis
All data were analysed with R (Version 3.6.1, 2019). Clinical data and CPET data were merged from patients with available consent and subsequently encoded. We further reduced the data set to patients who had data from at least 2 CPETs during the period of January 2000 and October 2019. For patients who completed a CR in this time period, data from CR were also retrieved. If patients completed more than one CR in the observation period, only data from the first CR were used. In this case, only CPETs before the first CR including data from follow-up CPETs before initiation of second CR were used. Baseline data are described as frequencies or means with standard deviations (or medians with interquartile ranges) as appropriate. A categorical variable to indicate whether the CPET was performed after a CR was defined and coded as follow: 0, no CR prior to CPET; 1, CPET 0–4 weeks after completion of CR; 2, CPETs performed >4 weeks following completion of CR.
To account for the confounding variable of preceding surgical intervention, we defined a binary categorical variable. If patients underwent an open chest surgery within 3 months prior to the CPET or a minimally invasive (catheter-based) intervention within 1 month prior to CPET, the surgery variable was coded as 1, otherwise as 0.
Linear mixed models were performed for the primary outcome peak VO2, with patients included as random effect. The following factors were included as fixed effects: Age, sex, disease classification, preceding surgery (categorical variable) and preceding CR (categorical variable).
A total of 696 patients were assessed for eligibility. Reasons for excluding 385 patients are listed in (Fig. 1). 1052 CPETs of 311 ACHD patients (38% female) including 59 patients with CR and 252 without CR were included in the analyses (Fig. 1). Patient characteristics are summarized in (Tab. 1). Median peak VO2 of the whole population was 25.6 ml/kg/min (IQR 20.2–32.5 ml/kg/min). A frequency plot of peak VO2 relative to body weight as well as of predicted separately for the 196 patients with mild or moderate defects and 116 with complex CHD is shown in the appendix (Appendix A, Supplement Fig. 1).
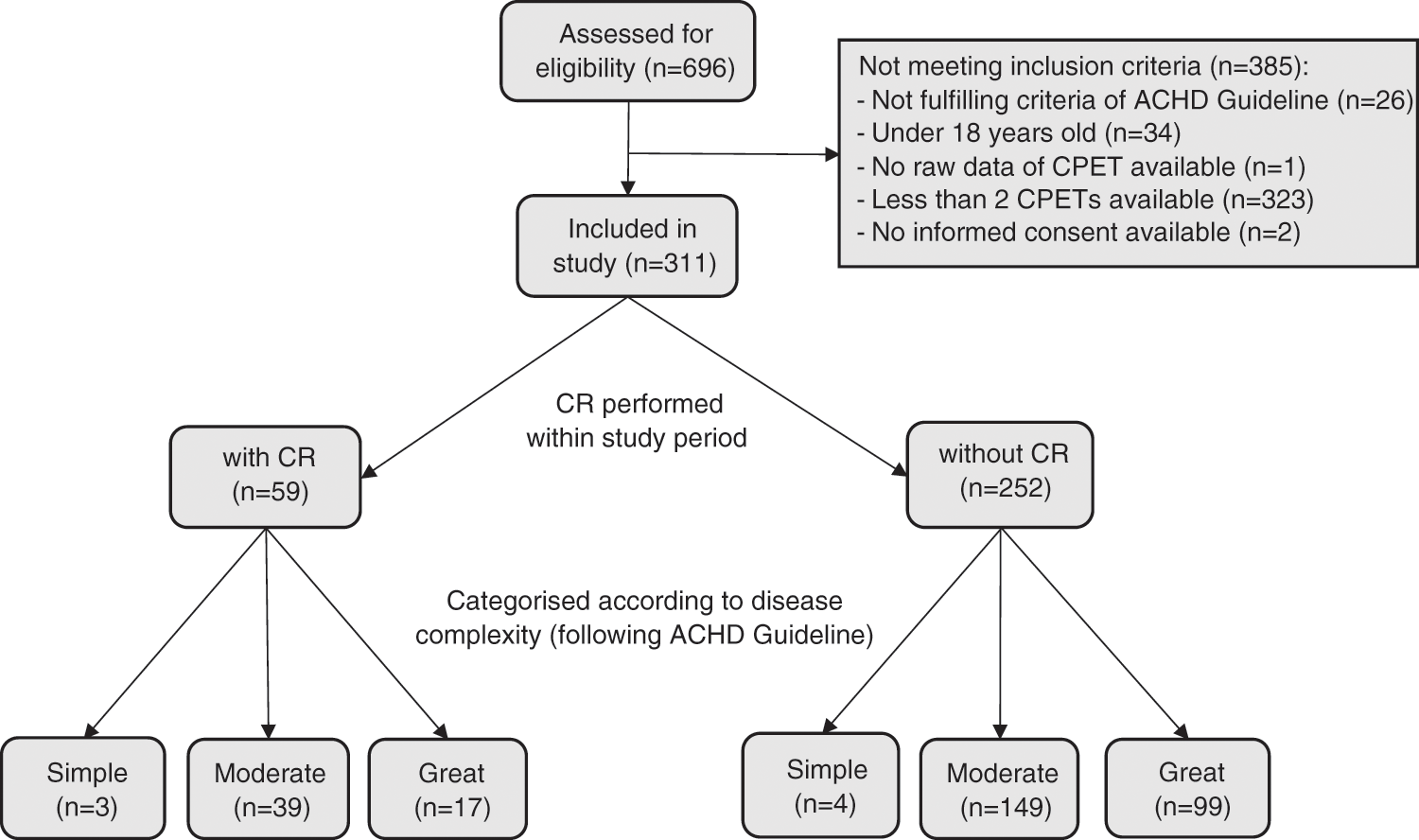
Figure 1: Patient flow. ACHD, Adults with congenital heart disease; CPET, cardiopulmonary exercise test; CR, cardiac rehabilitation
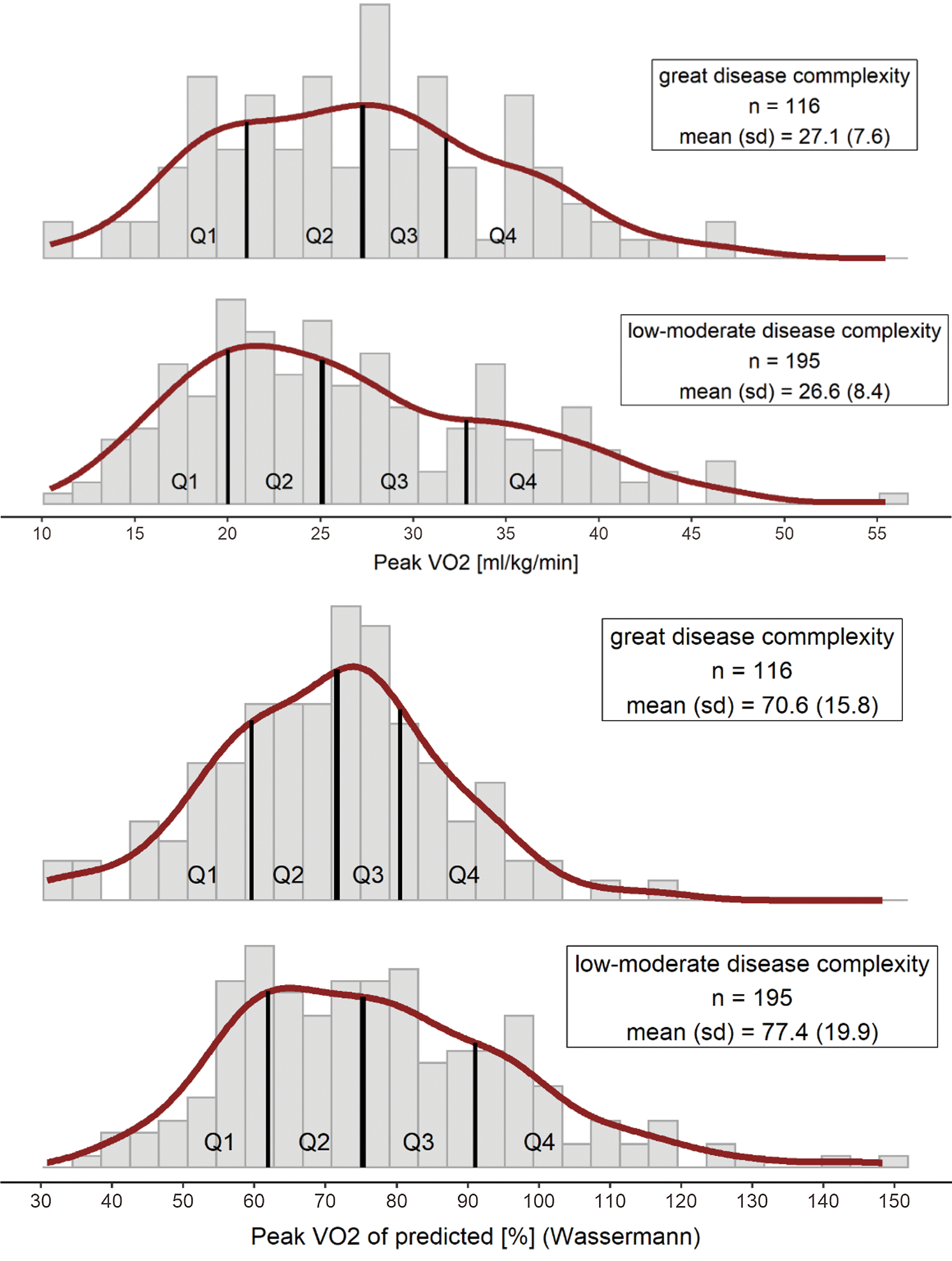
Supplement Figure 1: Histogram of peak VO2 in units relative to body weight (a) and of predicted values according to Wasserman [26] (b) for patients with great (top panels) versus mild and moderate (bottom panels) disease complexity
Table 1: Patient characteristics indicated as median (interquartile range) or number of patients (%)
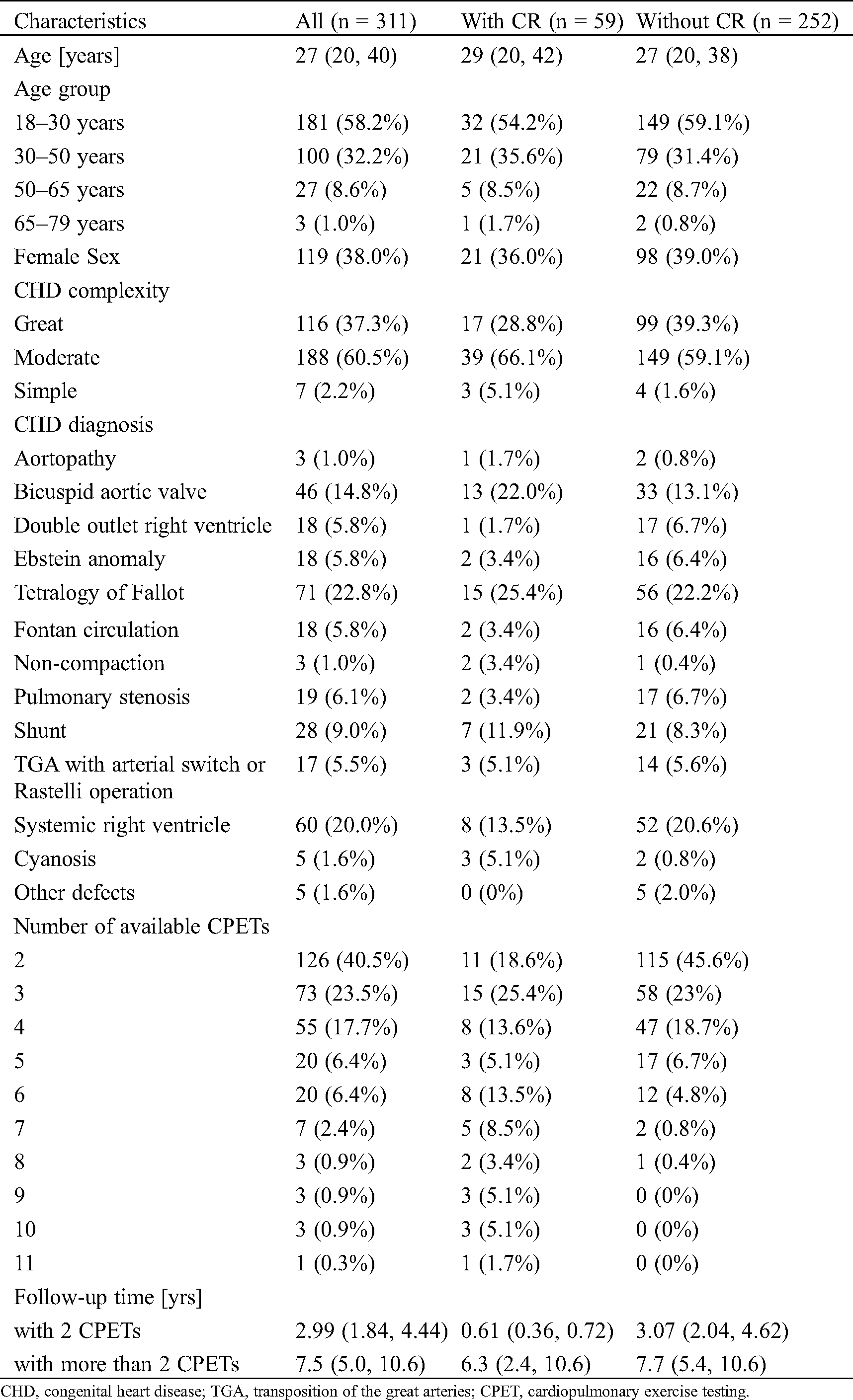
CHD, congenital heart disease; TGA, transposition of the great arteries; CPET, cardiopulmonary exercise testing.
Fig. 2 shows the output of the linear mixed model. Peak VO2 [ml/kg/min] was non-significantly increased short term after CR (β 0.8, 95% Confidence Interval (CI) –0.7; 2.4) and not increased long term after CR (β 0.0, 95%CI –1.7; 1.6). Peak VO2 was significantly reduced by age, female sex, complex CHD and preceding surgery.
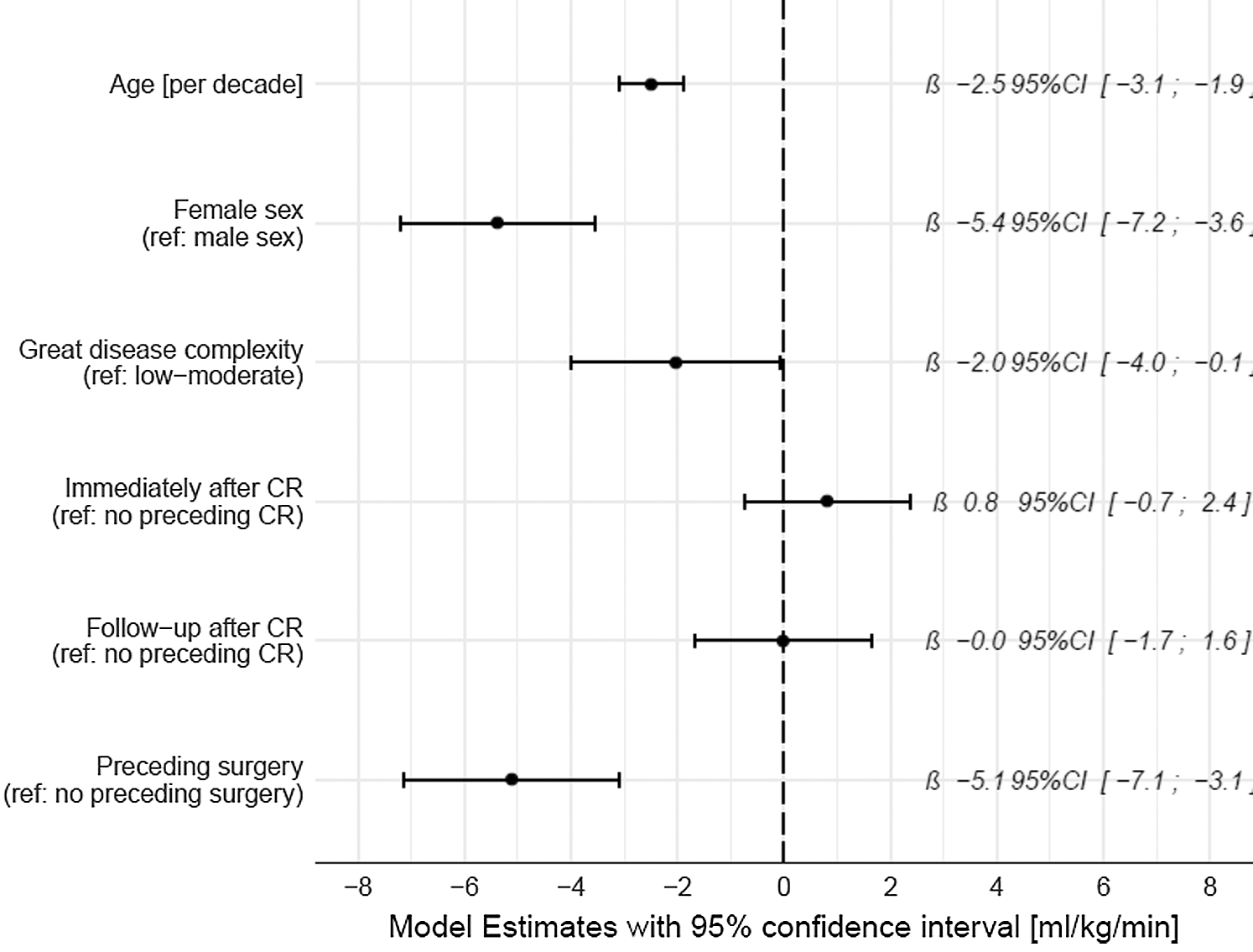
Figure 2: Coefficient plot of the linear mixed model for peak VO2 [ml/kg/min]. Patients were entered into the models as random effects. CR, cardiac rehabilitation
The 59 patients who completed CR attended a median of 24 (IQR 18–29) training sessions at a median work load of 47 (39, 50) Watts over a duration of 15 (12, 19) weeks (CR data was not available in eight of the CR patients). In the CR patients, peak VO2 increased from before to after CR by a median of 2.7 (IQR –0.6 to 5.5) ml/kg/min (Fig. 2). However, this increase was strongly mediated by preceding cardiac surgery, with 20 patients after cardiac surgical intervention showing a median increase of 6.2 (IQR 3.6–9.5) ml/kg/min, while 39 patients without surgical intervention had a median increase of 0.9 (IQR –1.5 to 3.1) ml/kg/min (Fig. 3). In an additional linear model for change in peak VO2 [ml/kg/min] for the 59 CR patients (adjusted for age, sex, disease complexity, preceding surgery, rehabilitation duration and baseline peak VO2), preceding cardiac surgery was the strongest independent predictor for improvements in peak VO2 (β 5.29, 95%CI 2.75–7.82, Tab. 2).
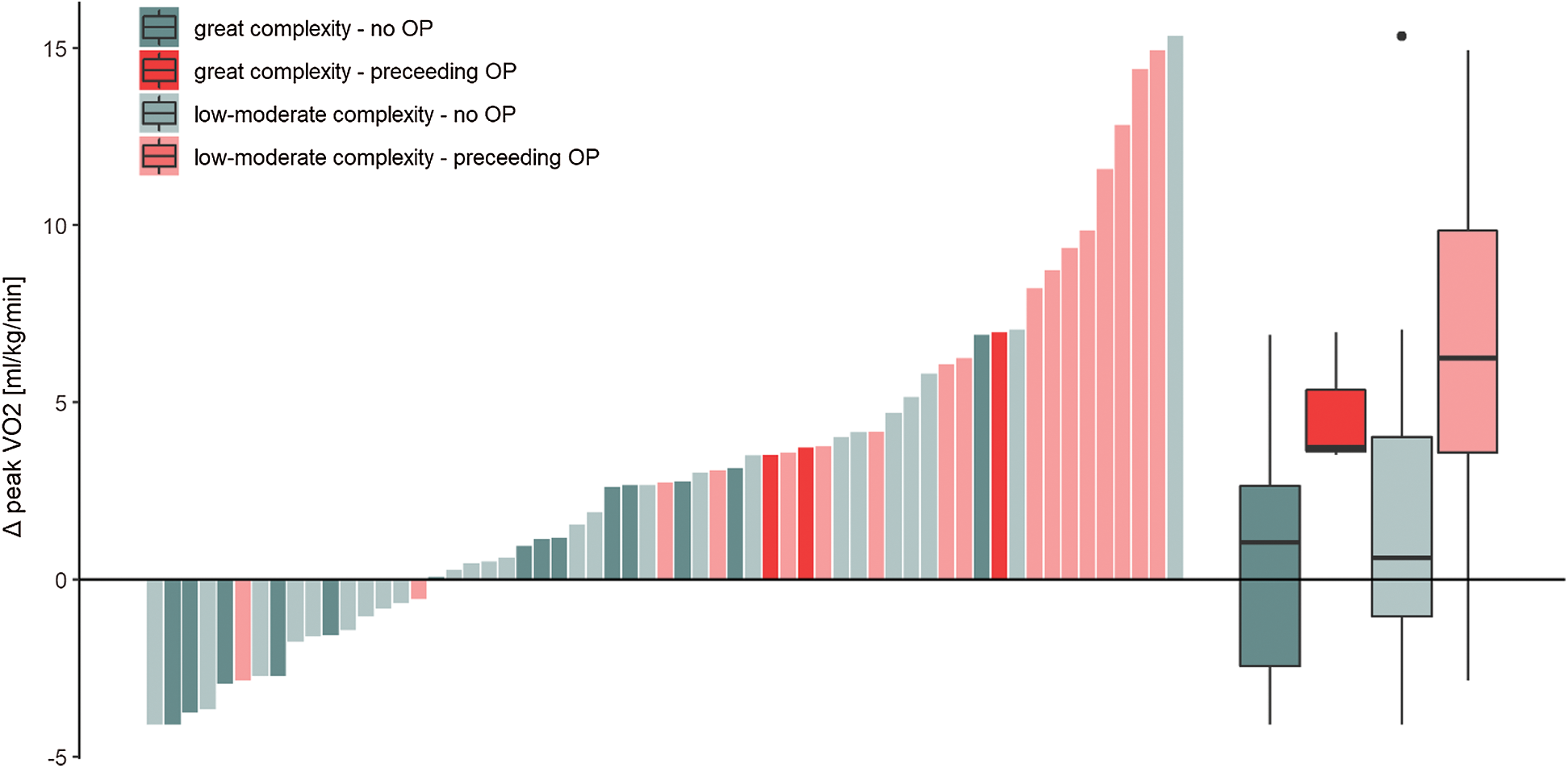
Figure 3: Individual changes (bars) from before to after CR in peak VO2 [ml/kg/min] of patient who completed CR. Patients who underwent an intervention prior to cardiac rehabilitation are depicted in red, while those who did not have surgical intervention within 6 months prior to CR are shown in green. OP, surgical procedure
Table 2: Linear model for change in peak VO2 in a subset of patients undergoing cardiac rehabilitation
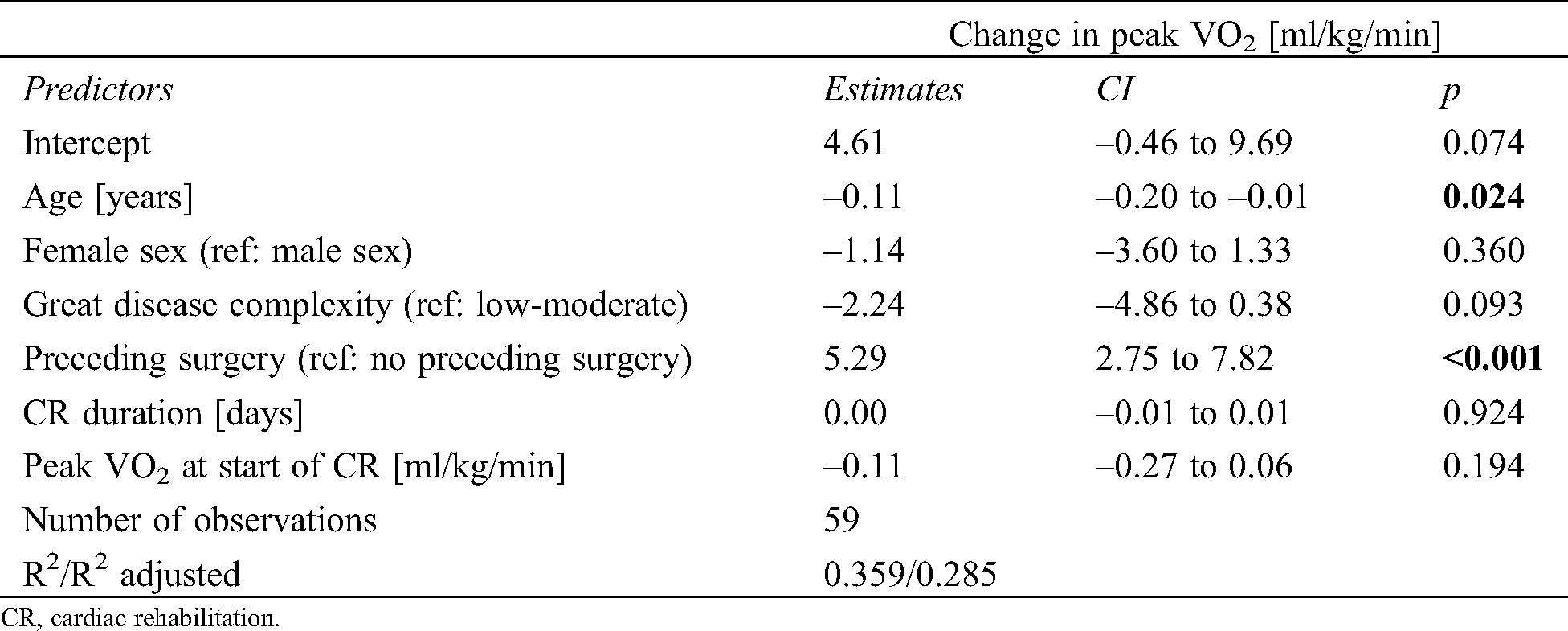
CR, cardiac rehabilitation.
Cardiorespiratory fitness in patients who did not complete CR after cardiac surgery (n = 7) also increased by a median of 5.8 (IQR 4.7–7.8) ml/kg/min at the next follow-up CPET.
In this retrospective study cardiorespiratory fitness was assessed in ACHD patients at several time points over a median of 3 years in patients with two and median of 7.5 years in patients with more than two CPETs. We found that cardiac surgical interventions had a large effect on peak VO2 (–5.1 ml/kg/min) but CR only had a non-significant and minor independent effect short-term (0.8 ml/kg/min), which was lost long-term. CR was often performed following surgical interventions, however, the recovery in peak VO2 which was measured after completion of CR was also found in patients not performing CR.
Our results confirm the findings of van der Bom et al. [23] who followed up patients enrolled in a randomized controlled exercise intervention study by Winter et al. [16]. This study included 28 patients with a systemic right ventricle into a 10-week exercise intervention with thrice-weekly 42-min interval training sessions. Patients of the exercise group improved their peak VO2 3.4 (95% CI 0.2–6.7) ml/kg/min more than the control group not enrolled into an exercise program [16]. Three years later, 22 patients of the exercise group and 18 patients of the control group were assessed again, at which time there was no difference between the groups anymore [23].
To our knowledge, this is the first study who assessed the independent effects of both, CR and surgical interventions in a clinical cohort of ACHD patients. Surgical or minimally invasive interventions are often the trigger for cardiologists to recommend an ambulatory CR and the motivation for patients to regain their physical fitness. Our study results imply that most of the 5.1 ml/kg/min recovery in peak VO2 after surgical interventions also occurred in patients not completing CR and that there was only a small independent gain due to CR. Previous studies have consistently found improvement of exercise capacity with exercise training interventions, mostly in the form of exercise-based CR. While some studies were designed as longitudinal studies without a control group [20,27], other studies compared patients completing CR to those who chose not to attend CR [18]. A few randomized controlled trials have also been performed [15–17,22], however, some of these randomized trials included fewer than 10 patients per group [14,28].
Most exercise intervention studies explicitly excluded patients with preceding surgical interventions. Compared to the median improvement in peak VO2 of 0.8 ml/kg/min in our patients without preceding surgical interventions, these studies found larger benefits with improvements of 1.6–1.8 ml/kg/min [13,14,17], or even 2.2–4.0 ml/kg/min [16,18,28]. The study performed by Winter et al. [16] achieved a mean improvement of 2.2 ml/kg/min by a 10-week programme of high-intensity exercise training thrice weekly. However, it was not clearly stated whether patients with preceding surgery were excluded. A comparable improvement of 2.2 ml/kg/min was found in a pilot study on nine patients randomized to exercise-based 12-week CR [28]. The third study reaching markedly higher improvements in cardiorespiratory fitness (4.0 ml/kg/min) compared to our study was a study on 31 patients who chose to complete a 4-week exercise-based CR with daily exercise sessions [18]. Importantly, the increase of a mean respiratory exchange ratio (RER) of 0.94 at baseline to 1.3 after CR indicates that part of the 4 ml/kg/min improvement was due to a higher exertion level at the follow-up testing. In our 39 patients who completed CR without preceding cardiac surgery, median RER was 1.15 both before and after CR.
The only study who found a comparable improvement to our study (1 ml/kg/min) was a study with congenital heart disease-associated pulmonary arterial hypertension patients performing a 3-week exercise-based CR with 90 min of exercise 7 days/week [20]. However, their patients had a mean peak VO2 at CR start of 11.4 ml/kg/min, which was much lower than the median exercise capacity of 25.6 ml/kg/min in our patients.
Many studies found significantly improved exercise capacity in the exercise group, but not the control group, however, these studies did not test whether the improvements in the exercise group were significantly greater than those in the control group [14,15,17,19,27,28]. Namely, in all of these studies cardiorespiratory fitness also increased by trend in the control group, often by about half the increase of the control group. The smaller (non-significant) increase found in the control group gives an indication on the size of familiarization effect with the testing procedure. Non-significant improvements found in the control group were between 0.3–0.9 ml/kg/min in some studies [14,15,17,28], but were zero [18] or even negative (–0.5 and –1.9 ml/kg/min) in other studies [16,22]. We found only one previous study that took non-CR related changes in exercise capacity into account and found a significant difference between the changes in cardiorespiratory fitness between the exercise and control group [22]. Their increase in peak VO2 after a 12-week home training was 1.8 ml/kg/min, which was about double the increase found in our study, however, it was not explicitly stated that they excluded patients with preceding cardiac surgery.
Our results on long-term changes in peak VO2 are in agreement with the only previous study in ACHD patients who assessed long-term effects of CR on peak VO2 and who found no persistent effect at three years follow-up [23]. Sustenance of gains in peak VO2 and long-term adherence to increased levels of physical activity have been found to be a challenge also in various other cardiac populations after cardiac rehabilitation [29,30].
The main limitation of this study is its retrospective design. Data was gathered over a duration of almost twenty years, therefore, changes in equipment and software took place during this time. However, this is unlikely to have introduced a systematic error, as CRs and surgical interventions were distributed over the whole time period. Further, this study focussed on the improvement of cardiorespiratory fitness, which is only one of several outcomes that may be improved by CR. Patient reported outcomes, such as quality of life also reflect physical function and may be more clinically relevant [5].
The main strength of this study was the longitudinal design in a clinical setting with standardized CPET with comparable levels of exertion, where patients had multiple assessments before and after CR but also after surgical interventions. This allowed a valid comparison of the independent effects of CR and surgical interventions, as CR is indicated and often performed after a surgical intervention to regain fitness and physical function. In our clinical setting, CR was feasible and safe, there were no major complication during training session with only occasional minor complications, such as dizziness and/or nausea, upon which the training session was interrupted or terminated.
Our study on retrospective data from a clinical setting showed that there was a small and insignificant increase in exercise capacity from CR when adjusted for preceding surgery, age and sex. Preceding surgery greatly reduced exercise capacity and mediated improvements in patients undergoing CR. Subsequent recovery was also seen in patients after cardiac surgery not completing CR. We found no long-term effect of CR on exercise capacity, highlighting the potential to improve current concepts of CR in the ACHD population. We recommend a personalised approach that offers comprehensive and individually tailored CR programmes [31] who identify facilitators and barriers to physical exercise and take these into account when formulating long-term physical activity goals to improve long-term adherence.
Author Contributions: PE, TG, TM, KW, CD, MS, MG, DT and MW contributed to the conception or design of the work. TG and CB contributed to the acquisition of the data. PE, TM, TG and CB performed the analyses. PE drafted the manuscript. All authors critically revised the manuscript, gave final approval and agree to be accountable for all aspects of work ensuring integrity and accuracy.
Data Sharing: Data sharing of anonymized data may be possible upon request to the corresponding author.
Funding Statement: This project was awarded a contribution from the Swiss Heart Foundation.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. van der Linde, D.,Konings, E. E., Slager, M. A., Witsenburg, M., Helbing, W. A. et al. (2011). Birth prevalence of congenital heart disease worldwide: A systematic review and meta-analysis. Journal of the American College of Cardiology, 58(21), 2241–2247. DOI 10.1016/j.jacc.2011.08.025. [Google Scholar] [CrossRef]
2. Ladouceur, M., Iserin, L., Cohen, S., Legendre, A., Boudjemline, Y. et al. (2013). Key issues of daily life in adults with congenital heart disease. Archives of Cardiovascular Diseases, 106(6–7), 404–412. DOI 10.1016/j.acvd.2013.02.004. [Google Scholar] [CrossRef]
3. Marelli, A. J., Mackie, A. S., Ionescu-Ittu, R., Rahme, E., Pilote, L. (2007). Congenital heart disease in the general population: Changing prevalence and age distribution. Circulation, 115(2), 163–672. DOI 10.1161/CIRCULATIONAHA.106.627224. [Google Scholar] [CrossRef]
4. Bay, A., Sandberg, C., Thilen, U., Wadell, K., Johansson, B. (2018). Exercise self-efficacy in adults with congenital heart disease. International Journal of Cardiology Heart & Vasculature, 18, 7–11. [Google Scholar]
5. Moons, P., Luyckx, K., Thomet, C., Budts, W., Enomoto, J. et al. (2020). Physical functioning, mental health, and quality of life in different congenital heart defects: Comparative analysis in 3538 patients from 15 countries. The Canadian Journal of Cardiology. AS0828-282X(20)30317-2. Advance online publication. [Google Scholar]
6. Rometsch, S., Greutmann, M., Latal, B., Bernaschina, I., Knirsch, W. et al. (2019). Predictors of quality of life in young adults with congenital heart disease. European Heart Journal Quality of Care & Clinical Outcomes, 5(2), 161–168. DOI 10.1093/ehjqcco/qcy046. [Google Scholar] [CrossRef]
7. Diller, G. P., Dimopoulos, K., Okonko, D., Li, W., Babu-Narayan, S. V. et al. (2005). Exercise intolerance in adult congenital heart disease: Comparative severity, correlates, and prognostic implication. Circulation, 112(6), 828–835. DOI 10.1161/CIRCULATIONAHA.104.529800. [Google Scholar] [CrossRef]
8. Oechslin, E. N., Harrison, D. A., Connelly, M. S., Webb, G. D., Siu, S. C. (2000). Mode of death in adults with congenital heart disease. American Journal of Cardiology, 86(10), 1111–1116. DOI 10.1016/S0002-9149(00)01169-3. [Google Scholar] [CrossRef]
9. Nieminen, H. P., Jokinen, E. V., Sairanen, H. I. (2007). Causes of late deaths after pediatric cardiac surgery: A population-based study. Journal of the American College of Cardiology, 50(13), 1263–1271. DOI 10.1016/j.jacc.2007.05.040.
10. Verheugt, C. L., Uiterwaal, C. S., van der Velde, E. T.,Meijboom, F. J., Pieper, P. G. et al. (2010). Mortality in adult congenital heart disease. European Heart Journal, 31(10), 1220–1229. DOI 10.1093/eurheartj/ehq032. [Google Scholar] [CrossRef]
11. Longmuir, P. E., Brothers, J. A., de Ferranti, S. D.,Hayman, L. L., Van Hare, G. F. et al. (2013). Promotion of physical activity for children and adults with congenital heart disease: A scientific statement from the American Heart Association. Circulation, 127(21), 2147–2159. DOI 10.1161/CIR.0b013e318293688f. [Google Scholar] [CrossRef]
12. Kempny, A., Dimopoulos, K., Uebing, A., Moceri, P., Swan, L. et al. (2012). Reference values for exercise limitations among adults with congenital heart disease. Relation to activities of daily life—Single centre experience and review of published data. European Heart Journal, 33(11), 1386–1396. DOI 10.1093/eurheartj/ehr461. [Google Scholar] [CrossRef]
13. Westhoff-Bleck, M., Treptau, J., Löffler, F., Widder, J. (2015). Exercise training in adults with complex congenital heart disease. Annals of Sports Medicine and Research, 2(6), 1037. [Google Scholar]
14. Novakovic, M., Prokselj, K., Rajkovic, U., Vizintin Cuderman, T., Jansa Trontelj, K. et al. (2018). Exercise training in adults with repaired tetralogy of Fallot: A randomized controlled pilot study of continuous versus interval training. International Journal of Cardiology, 255, 37–44. DOI 10.1016/j.ijcard.2017.12.105. [Google Scholar] [CrossRef]
15. Duppen, N., Etnel, J. R., Spaans, L., Takken, T., van den Berg-Emons, R. J. et al. (2015). Does exercise training improve cardiopulmonary fitness and daily physical activity in children and young adults with corrected tetralogy of Fallot or Fontan circulation? A randomized controlled trial. American Heart Journal, 170(3), 606–614. DOI 10.1016/j.ahj.2015.06.018. [Google Scholar] [CrossRef]
16. Winter, M. M., van der Bom, T.,de Vries, L. C.,Balducci, A., Bouma, B. J. et al. (2012). Exercise training improves exercise capacity in adult patients with a systemic right ventricle: A randomized clinical trial. European Heart Journal, 33(11), 1378–1385. DOI 10.1093/eurheartj/ehr396. [Google Scholar] [CrossRef]
17. van Dissel, A. C.,Blok, I. M., Hooglugt, J. Q., de Haan, F. H.,Jorstad, H. T. et al. (2019). Safety and effectiveness of home-based, self-selected exercise training in symptomatic adults with congenital heart disease: A prospective, randomised, controlled trial. International Journal of Cardiology, 278, 59–64. DOI 10.1016/j.ijcard.2018.12.042. [Google Scholar] [CrossRef]
18. Gierat-Haponiuk, K., Haponiuk, I., Szalewska, D., Chojnicki, M., Jaworski, R. et al. (2015). Effect of complex cardiac rehabilitation on physical activity and quality of life during long-term follow-up after surgical correction of congenital heart disease. Kardiologia Polska, 73(4), 267–273. DOI 10.5603/KP.a2014.0206. [Google Scholar] [CrossRef]
19. Fredriksen, P. M., Kahrs, N., Blaasvaer, S., Sigurdsen, E., Gundersen, O. et al. (2000). Effect of physical training in children and adolescents with congenital heart disease. Cardiology in the Young, 10(2), 107–114. DOI 10.1017/S1047951100006557. [Google Scholar] [CrossRef]
20. Becker-Grunig, T., Klose, H., Ehlken, N., Lichtblau, M., Nagel, C. et al. (2013). Efficacy of exercise training in pulmonary arterial hypertension associated with congenital heart disease. International Journal of Cardiology, 168(1), 375–381. DOI 10.1016/j.ijcard.2012.09.036. [Google Scholar] [CrossRef]
21. Duppen, N., Takken, T., Hopman, M. T., ten Harkel, A. D.,Dulfer, K. et al. (2013). Systematic review of the effects of physical exercise training programmes in children and young adults with congenital heart disease. International Journal of Cardiology, 168(3), 1779–1787. DOI 10.1016/j.ijcard.2013.05.086.
22. Westhoff-Bleck, M., Schieffer, B., Tegtbur, U., Meyer, G. P., Hoy, L. et al. (2013). Aerobic training in adults after atrial switch procedure for transposition of the great arteries improves exercise capacity without impairing systemic right ventricular function. International Journal of Cardiology, 170(1), 24–29. DOI 10.1016/j.ijcard.2013.10.009. [Google Scholar] [CrossRef]
23. van der Bom, T.,Winter, M. M., Knaake, J. L., Cervi, E., de Vries, L. S. et al. (2015). Long-term benefits of exercise training in patients with a systemic right ventricle. International Journal of Cardiology, 179, 105–111. DOI 10.1016/j.ijcard.2014.10.042. [Google Scholar] [CrossRef]
24. Tobler, D., Schwerzmann, M., Bouchardy, J., Engel, R., Stambach, D. et al. (2017). Swiss adult congenital heart disease registry (SACHER)—Rationale, design and first results. Swiss Medical Weekly, 147, w14519. [Google Scholar]
25. Stout, K. K., Daniels, C. J., Aboulhosn, J. A., Bozkurt, B., Broberg, C. S. et al. 2018, 2019. AHA/ACC guideline for the management of adults with congenital heart disease: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Journal of the American College of Cardiology, 73(12), e81–e192. DOI 10.1016/j.jacc.2018.08.1029. [Google Scholar] [CrossRef]
26. Wasserman, K., Hansen, J., Sue, D. (2004). Principles of exercise testing and interpretation. 4th ed. Philadelphia: Lippincott Williams and Wilkins. [Google Scholar]
27. Dua, J. S., Cooper, A. R., Fox, K. R., Graham Stuart, A. (2010). Exercise training in adults with congenital heart disease: Feasibility and benefits. International Journal of Cardiology, 138(2), 196–205. DOI 10.1016/j.ijcard.2009.01.038. [Google Scholar] [CrossRef]
28. Therrien, J., Fredriksen, P., Walker, M., Granton, J., Reid, G. J. et al. (2003). A pilot study of exercise training in adult patients with repaired tetralogy of Fallot. The Canadian Journal of Cardiology, 19(6), 685–689. [Google Scholar]
29. Moholdt, T., Aamot, I. L., Granøien, I., Gjerde, L., Myklebust, G. et al. (2011). Long-term follow-up after cardiac rehabilitation: A randomized study of usual care exercise training versus aerobic interval training after myocardial infarction. International Journal of Cardiology, 152(3), 388–390. DOI 10.1016/j.ijcard.2011.08.025. [Google Scholar] [CrossRef]
30. Ramadi, A., Haennel, R. G., Stone, J. A., Arena, R., Threlfall, T. G. et al. (2015). The sustainability of exercise capacity changes in home versus center-based cardiac rehabilitation. Journal of Cardiopulmonary Rehabilitation and Prevention, 35(1), 21–28. DOI 10.1097/HCR.0000000000000084. [Google Scholar] [CrossRef]
31. Ambrosetti, M., Abreu, A., Corra, U., Davos, C. H., Hansen, D. et al. (2020). Secondary prevention through comprehensive cardiovascular rehabilitation: From knowledge to implementation. 2020 update. A position paper from the secondary prevention and rehabilitation section of the European association of preventive cardiology. European Journal of Preventive Cardiology. DOI 10.1177/2047487320913379. [Google Scholar] [CrossRef]
Appendix A

Supplement Figure 1: Histogram of peak VO2 in units relative to body weight (a) and of predicted values according to Wasserman [26] (b) for patients with great (top panels) versus mild and moderate (bottom panels) disease complexity
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |