DOI:10.32604/CHD.2021.013459

| Congenital Heart Disease DOI:10.32604/CHD.2021.013459 |  |
| Article |
Prevalence and Spectrum of Complex Congenital Heart Disease in the Neonatal Intensive Care Unit at High Altitude in China
1Department of Obstetrics and Gynecology, Xuanwu Hospital, Capital Medical University, Beijing, China
2Department of Neonatology, Women and Children’s Hospital of Qinghai Province, Xining, China
3Department of Echocardiography, Women and Children’s Hospital of Qinghai Province, Xining, China
4Intensive Care Unit, Capital Institute of Pediatrics, Beijing, China
5Clinical Physiology Laboratory, Institute of Pediatrics, Guangzhou Women and Children’s Medical Center, Guangzhou Medical University, Guangzhou, China
6Guangdong Provincial Key Laboratory of Research in Structural Birth Defect Disease, Guangzhou Women and Children’s Medical Center, Guangzhou Medical University, Guangzhou, China
7Clinical Physiology Laboratory, Institute of Pediatrics, Beijing, China
*Corresponding Authors: Jia Li. Email: jiali_beijing@126.com; Haiying Qi. Email: qhxnqhy@163.com
#The two authors had equal contribution
Received: 07 August 2020; Accepted: 09 October 2020
Abstract: Background: Previous studies from high altitudes have reported significantly higher prevalence of congenital heart disease (CHD), consisting almost solely of simple CHD. Little is known about the occurrence of complex CHD. Neonates with complex CHD are likely admitted to NICU. We examined the prevalence and spectrum of complex CHD in NICU in order to depict a truer picture of CHD at high altitude. Methods: We reviewed charts of 4,214 neonates admitted to NICU in Qinghai province (average altitude 3,000 m). Echocardiography was performed in 1,943 babies when CHD was suspected based on clinical examinations. Results: CHD was diagnosed in 1,093 (56.3% of echoed babies). Mild CHD in 96.8% (1058 babies). Moderate CHD in 0.8% (9) included 1 (0.1%) large secundum atrial septal defect, 3 (0.3%) moderate pulmonary stenosis, 2 (0.2%) aortic stenosis and 3 (0.3%) partial anomalous pulmonary venous connection. Severe CHD in 2.4% (26) included 6 (0.5%) complete atrioventricular septal defect, 5 (0.5%) complete transposition of the great arteries, 5 (0.5%) hypoplastic right heart, 3 (0.3%) hypoplastic left heart, 3 (0.3%) double outlet right ventricle, 3 (0.3%) tetralogy of Fallot, 2 (0.2%) truncus arteriosus, 2 (0.2%) total anomalous pulmonary venous connection, 2 (0.2%) severe aortic stenosis, 2 (0.2%) interrupted aortic arch and 2 (0.2%) severe pulmonary stenosis and 1 (0.1%) single-ventricle abnormality. At two-years follow-up in 737 (67.4%) patients, 18 (90%) with severe CHD and 38 (5.3%) with mild and moderate CHD died, and 15 underwent cardiac surgery with 1 early death. Conclusions: At high altitude, a wide spectrum of CHD exists, with many heretofore unreported complex CHD. There is urgent need for routine echocardiography and early interventions in newborns particularly in NICU.
Keywords: Congenital heart disease; neonatal intensive care unit; high altitude
The prevalence of congenital heart disease (CHD), according to data from low altitude regions, ranges between 4 and 10 of every 1,000 live birth, with severe CHD in 15 to 25%, which requires early interventions in the first year of life [1,2]. At high altitude, the prevalence of CHD is substantially higher. Almost all the CHDs previously reported are the simple forms with left to right shunt, with the predominance of secundum atrial septal defect (ASD) and patent ductus arteriosus (PDA). Notably, most of the previous studies were conducted in children older than 2 years of age [3–8]. Children with complex and severe CHD may most likely have died at younger age. This problem was partly overcome in our previous study using echocardiography screening for CHD in newborns in Qinghai province (average altitude 3,000 m) [7]. The study screened 1,002 consecutive asymptomatic babies and a small number of sick babies admitted to the NICU before the screening (n = 335). Only two severe CHD were found (one had the anomalous right superior pulmonary venous return to the right atrium and left inferior pulmonary venous stenosis; the other had complete transposition of the great arteries). Both died during the stay in the NICU. There was only one more study paid attention to critically ill in a small group of children (n = 383), and some more types of complex CHD were found (e.g., tetralogy of Fallot, double outlet right ventricle, etc.) with the incidence of 5.2% [5]. There were also other mild and moderate forms of CHD, including tricuspid, aortic and pulmonary valve abnormalities, etc. As such, the prevalence and spectrum of moderate and severe CHD at high altitudes remains largely unknown. Since neonates with moderate and severe CHD are likely to be admitted to the NICU, we, in the present study, retrospectively examined a larger population of NICU admissions in a period of two years in order to depict a truer picture of the prevalence and spectrum of CHD, with focus on moderate and severe CHD at high altitude.
The retrospective study was conducted in the NICU of Women and Children’s Hospital in Xining (2,260 meters), Qinghai province (average altitude 3,000 meters). All the patients admitted in 2015 and 2016 for any critical illness were included in the study. Their clinical charts were reviewed.
2.2 Demographic and Clinical Data
Demographic and clinical data included sex, gestation age, birth weight, admission age, mother’s age, ethnic, altitude of residence and discharge diagnosis (Tab. 1). According to the history of migrating to the plateau, ethnic was ordered as following: Tibetans, Hui, Salar and some other minorities such as Tu and Mongolian migrated, and Han [7].
2.3 Echocardiographic Diagnosis
Neonatologists carried out the initial clinical examination. The presence of an abnormal heart murmur, cyanosis, tachypnoea, feeding difficulty and other features of congestive cardiac failure were considered as positive clinical findings suggestive of CHD [9,10]. Suspected cases of CHD were further examined using transthoracic echocardiography. Two-dimensional imaging and color Doppler were performed by one technician, using a Hewlett-Packard-8500 ultrasound system equipped with a 2.5 MHz transthoracic transducer (Andover, MA, U.S.A.). CHD was classified into mild, moderate and severe types as proposed by Hoffman et al. [1].
Phone call follow-up was made in CHD patients in 2018 when the study was conducted in order to obtain the outcome data.
The patients’ demographics were analyzed using descriptive statistics. Birth weight range was classified into very or extremely low birth weight (<1,500 g), low birth weight (<2,500 g), normal birth weight (2,500–4,000 g) and macrosomia (≥4,000 g). The ethnic was classified according to historic order of migration. Altitude was divided according to altitude medicine classification whereby altitude ≥2,500 is considered to have medical significance. These variables were numbered 0, 1, or/and 2, respectively (Tab. 1). Differences in proportions between each demographic parameter and the presence of CHD were examined with Chi-square test. When the expected value in any of the tests was less than 5, the Fisher exact test was used. A 2-way analysis was performed in all comparisons. The Bonferroni modification was applied, and the alpha level and p values were corrected accordingly. The level of significance was set at 0.05. Data were analyzed using SPSS Statistics for Windows version 20.0 (Armonk, NY: IBM Corp., 2011).
Table 1: Number and frequency distribution (%) of CHD diagnosed by echocardiography across the demographic variables in 1943 neonates suspected of CHD based on clinical examinations in the NICU
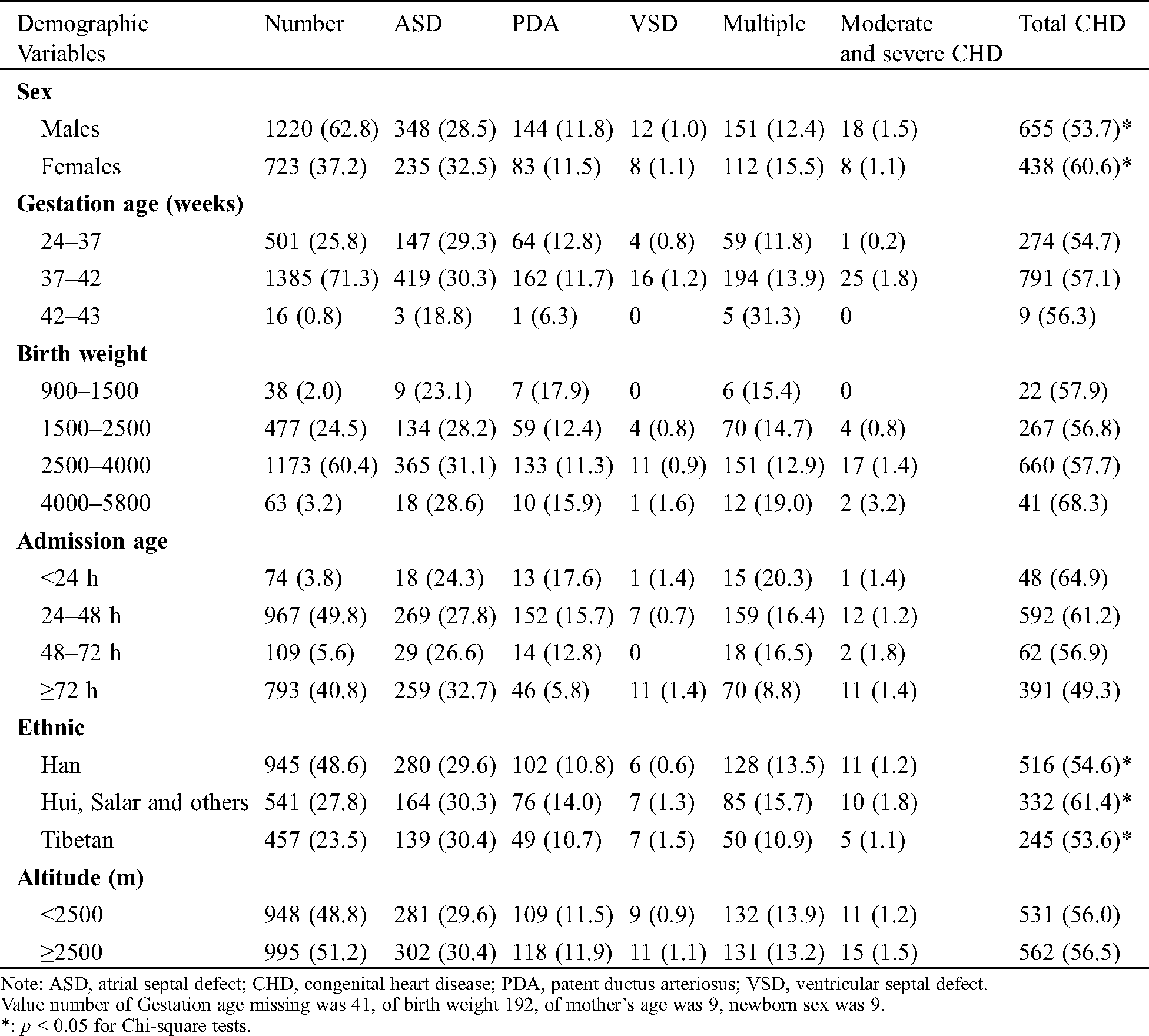
There were 4,214 NICU admissions in the two-year study period, primarily due to pneumonia (62.6%), asphyxia (13.2%) and hyperbilirubinemia (7.3%), none to cardiac malformations. Echocardiography was performed in 1,943 babies (46.1%) and CHD was diagnosed in 1,093 babies (56.3% of 1,943 echoed babies) (Tab. 2). Thus, the prevalence of CHD in the entire NICU population was estimated as 26%.
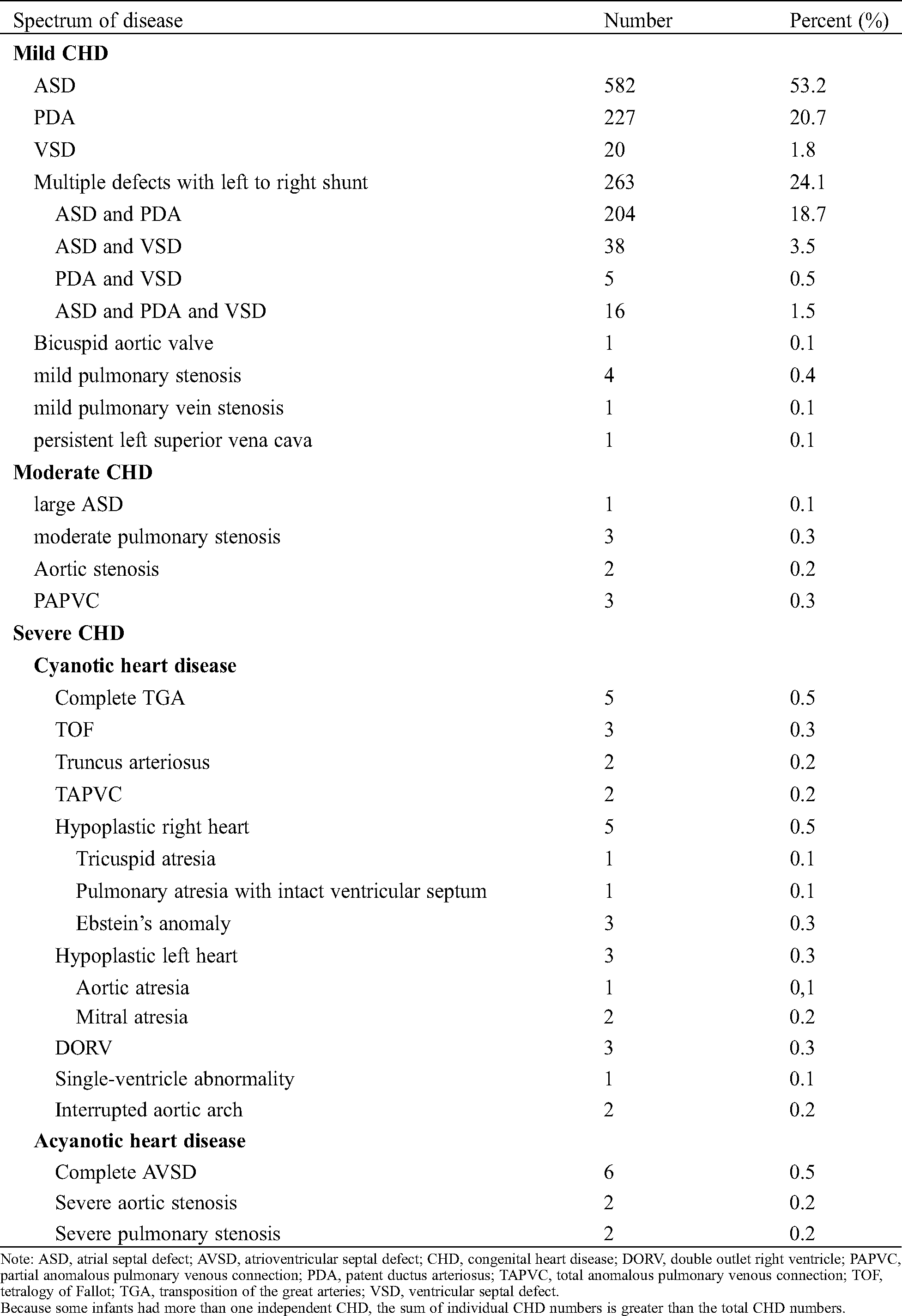
Among the 1,093 CHD babies, mild CHD accounted for 96.8% (1,058 cases), moderate CHD 0.8% (9 cases), and severe CHD 2.4% (26 cases). The distribution of CHD is shown in Tab. 2. The size of ASD was 3.0 ± 1.2 mm, PDA 2.4 ± 0.8 mm, VSD 4.3 ± 2.4 mm.
The prevalence of CHD in Hui was significantly higher than that in Han and Tibetan (p = 0.011 and 0.013, respectively), whereas there was no statistical difference between the Han and the Tibetans (p = 0.727). The prevalence of CHD in females was significantly higher than that in males (p = 0.003). No correlation was found between the prevalence and forms of CHD and gestation age, birth weight and altitudes (p > 0.1 for all) (Tab. 1).
At 2–3 years after the NICU discharge, phone call follow-up was made in total 737 patients (67.4%). Among them, 57 cases died and 680 cases survived. The survival rates of Tibetan, Hui, Salar and others and Han were 90.9%, 83.1% and 86.0%. No correlation was found for the survival of CHD babies between different ethnics (p > 0.05). Among 737 patients, including 717 patients with mild and moderate CHD in whom 38 (5.3%) died within 2–3 years of age; and 20 patients with severe CHD in whom 18 (90%) died within 2 months of age. Fifteen patients underwent cardiac surgery (3 patients with VSD, 4 with ASD, 1 with PDA, 3 with ASD and VSD, 1 with PDA and ASD, 1 with truncus arteriosus, ASD and VSD and 1 with complete transposition of the great arteries, ASD and PDA, 1 total anomalous pulmonary venous connection). The patient with anomalous pulmonary venous connection died early after operation, others survived and healthy.
Table 2: Frequency distribution of different forms of CHD in 1093 patients diagnosed by echocardiography
The present study examined the occurrence of complex CHD in the largest cohort in the NICU at high altitude. Since newborns with moderate or severe CHD are prone to have other serious illness and admitted to the NICU and subsequently die at young age at high altitude before the screening age in most of the previous studies, our data reveals a truer picture of CHD with the widest spectrum of moderate and severe types to date, in addition to the simple CHD as previously reported [3–8].
It has been well documented that the prevalence CHD is substantially higher at high altitude, consisting predominately of ASD and PDA. This figure is incomplete, largely resulted from the limitations of previous study design. All of them were conducted in children older than 2 years of age [3–6,8] except for ours [7]. In our previous study, we screened newborns for CHD used echocardiography also in Qinghai province (average altitude 3,000 m) including asymptomatic babies and a small number of 335 sick babies admitted to the NICU before the screening in 3–5 days after birth. Only 2 severe CHD patients (one had the anomalous right superior pulmonary venous return to the right atrium and left inferior pulmonary venous stenosis; another had complete transposition of the great arteries) were found, and both died during the stay in the NICU [7]. This does not represent our clinical observations at high altitude.
The occurrence of moderate and severe CHD at high altitude has been scarcely reported. The only series, to our knowledge, was reported by Zheng et al from a small number of children including critically ill in Tibet [5]. They found 5.2% patients with severe CHD (20 out of 383 CHD patients), including complete atrioventricular septal defect, severe pulmonary stenosis with ASD, coarctation of the aorta with VSD, tetralogy of Fallot, pulmonary atresia with VSD, hypoplastic right heart with partial anomalous pulmonary venous connection or Ebstein’s anomaly, double outlet right ventricle [5]. The incidence of severe CHD was lower as compared to that (13.7%) from low altitude 1,000–2,000 m in Yunnan province, which was attributed to the older age at screening (4–17 years) [5].
In our present study in the NICU population, more types of severe CHD were found including complete transposition of the great arteries, tetralogy of Fallot, double outlet right ventricle, complete atrioventricular septal defect, truncus arteriosus, total anomalous pulmonary venous connection, hypoplastic left heart, hypoplastic right heart with tricuspid atresia, pulmonary atresia with intact ventricular septum and Ebstein’s amonaly, interrupted aortic arch, single-ventricle abnormality, severe aortic or pulmonary stenosis. We also found more types of mild and moderate CHD, including large ASD, mild or moderate pulmonic stenosis, aortic stenosis, bicuspid aortic valve, mild pulmonary vein stenosis, partial anomalous pulmonary venous connection and persistent left superior vena cava. As such, this is the widest spectrum to date, consisting almost all types of severe, moderate and mild CHD described by Hoffman et al. [1].
In Zheng et al’s data, right ventricular outflow obstruction, e.g., tetralogy of Fallot, severe pulmonary valvular stenosis and pulmonary artery atresia, was more common than left heart obstructive lesions, e.g., aortic stenosis and coarctation of the aorta. They considered it consistent with the reports demonstrating more pulmonary outflow obstructions and fewer left ventricular outflow tract obstructions in Asian or Chinese populations [11,12]. This difference was not clearly shown in our data, which might be attributable to the particular NICU setting where patients were admitted duo to critical illness other than cardiac malformations.
According to data from low altitude regions, severe CHD occurs in about 15%–25% of all types of CHD [1,2]. A study from the NICU in plain area also using echocardiography screening for CHD found a higher prevalence of CHD of 1.5%, compromising 31% of severe CHD [13]. At high altitude, the substantially high prevalence of ASD and PDA makes the proportion of severe CHD much less, being 4% or 5% even when critically ill patients are included as in our present study and Zheng et al’s [5]. Recent studies report that the prevalence of complex and severe CHD has decreased over time as a result of implementation of fetal echocardiography and pregnancy termination by intervention [2,14,15]. Although fetal echocardiography screening has been carried out in large-scales in plateau provincial capitals, such as Xining of Qinghai Province, only a very small portion have received fetal screening in surrounding regions. In our cohort, about 70% patients did not have fetal echocardiography.
The strikingly high incidence of ASD and PDA has been strongly suggested due to a postnatal (i.e., altitude hypoxia) rather than a fetal teratogenic mechanism [3]. The wide spectrum of moderate and severe CHD as seen in the plain regions appears less likely due to the environmental factors related to altitude hypoxia than that of genetics. However, the etiology of CHD is complex involving genetic heterogeneity and environment interactions. The adaptation/maladaptation in CHD development at high altitude remains to be explored.
Death resulting from CHDs still remains the most common cause of infant mortality from birth defects [16]. This is particularly so at high altitude. In our patients, despite early diagnosis, 90% of severe CHD have died within 2 months after NICU discharge, and 5.3% with mild and moderate CHD died within 2–3 years old. Only 15 patients underwent cardiac surgery with one early death, and other patients survived. Despite fast development, the health concept and medical resources for the interventional and surgical treatment of CHD remain limited at high altitude worldwide.
Our study is subject to a number of limitations due to its retrospective nature. (1) Echocardiography was required only when CHD was suspected. As a result, more than half of the NICU babies did not have echocardiography. The incidence of CHD in the entire NICU was an estimate. (2) The hospital is in area where the main habitats are Hui, which may explain the higher incidence of CHD in Hui as compared to Han and Tibetans. This finding is different from our previous study screening the asymptomatic newborns showing the higher incidence of CHD in Han with the shortest period since migrating to high altitude as compared to Hui et al. [7]. (3) Recruitment protocol for echocardiography in the NICU is center-specific. It is possible that differences in recruitment may have introduced some selection bias. (4) Although the largest cohort with the widest spectrum of CHD, the distribution of the CHD types may not represent the figure at high altitude, not even in Qinghai province as the sample size is still fairly small for this matter. (5) Follow-up was not routinely made but only when the study was conducted at 2–3 years after their NICU discharge. As a result, only 67% cases were successfully followed up by phone call. (6) The study did not emphasize the impact of fetal echocardiography on the prevalence of major CHD. About 70% of the population did not have prenatal ultrasound screening. Any study on prevalence rate model comparing high- with low-altitude sites must consider these demographic and practice differences as potential confounding variables.
In the NICU population at high altitude, a wide spectrum of CHD exists, consisting of many heretofore unreported moderate and severe types of CHD. Data from our present study combined with our previous newborn screening using echocardiography provides a truer picture of the CHD occurrence at high altitude. Given the high prevalence and mortality, there is urgent need for the implementation of routine echocardiography in newborns particularly in those admitted to the NICU as well as early interventions to treat CHD at high altitude.
Authors’ Contributions: All authors contributed to the study conception and design. Material preparation and data collection were performed by Jingjing Li, Xiaorong Wang, Yuan Liu, Guodong Zhao, Ting Dai, Hong Chen and Haiyan Liao. Data analysis was performed by Jingjing Li, Haiying Qi and Jia Li. The first draft of the manuscript was written by Jing-Jing Li. And the final draft of the manuscript was written by Jingjing Li, Haiying Qi and Jia Li. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding Statement: The author(s) received no specific funding for this study.
Conflict of Interest: No financial or non-financial benefits have been received or will be received from any party related directly or indirectly to the subject of this article.
1. Hoffman, J. I., Kaplan, S. (2002). The incidence of congenital heart disease. Journal of the American College of Cardiology, 39(12), 1890–1900. DOI 10.1016/S0735-1097(02)01886-7. [Google Scholar] [CrossRef]
2. van der Linde, D., Konings, E. E., Slager, M. A., Witsenburg, M., Helbing, W. A. et al. (2011). Birth prevalence of congenital heart disease worldwide: A systematic review and meta-analysis. Journal of the American College of Cardiology, 58(21), 2241–2247. DOI 10.1016/j.jacc.2011.08.025. [Google Scholar] [CrossRef]
3. Miao, C. Y., Zuberbuhler, J. S., Zuberbuhler, J. R. (1988). Prevalence of congenital cardiac anomalies at high altitude. Journal of the American College of Cardiology, 12(1), 224–228. DOI 10.1016/0735-1097(88)90378-6. [Google Scholar] [CrossRef]
4. Chen, Q. H., Wang, X. Q., Qi, S. G. (2008). Cross-sectional study of congenital heart disease among Tibetan children aged from 4 to 18 years at different altitudes in Qinghai Province. Chinese Medical Journal, 121(24), 2469–2472. DOI 10.1097/00029330-200812020-00001.
5. Zheng, J. Y., Qiu, Y. G., Li, D. T., He, J. C., Chen, Y. et al. (2017). Prevalence and composition of CHD at different altitudes in Tibet: A cross-sectional study. Cardiology in the Young, 27(8), 1497–1503. DOI 10.1017/S1047951117000567. [Google Scholar] [CrossRef]
6. Marticorena, E., Penaloza, D., Severino, J., Hellriegel, K. (1962). Incidencia de la persistencia del conducto arterioso en las grandes alturas. In Memorias del IV Congreso Mundial de Cardiologia, 1A, 155, 159, Mexico. [Google Scholar]
7. Li, J. J., Liu, Y., Xie, S. Y., Zhao, G. D., Dai, T. et al. (2019). Newborn screening for congenital heart disease using echocardiography and follow-up at high altitude in China. International Journal of Cardiology, 274, 106–112. DOI 10.1016/j.ijcard.2018.08.102. [Google Scholar] [CrossRef]
8. Chun, H., Yue, Y., Wang, Y., Dawa, Z., Zhen, P. et al. (2019). High prevalence of congenital heart disease at high altitudes in Tibet. European Journal of Preventive Cardiology, 26(7), 756–759. DOI 10.1177/2047487318812502. [Google Scholar] [CrossRef]
9. de Lira Albuquerque, F. C.,Maia, E. T., de Figueiredo, V. L.,Mourato, F. A., da Silva Mattos, S. (2015). Clinical examination and pulse oximetry to detect congenital heart defects. International Journal of Cardiovascular Sciences, 28, 148–151. [Google Scholar]
10. Qi, H. Y., Ma, R. Y., Jiang, L. X., Li, S. P., Mai, S. et al. (2014). Anatomical and hemodynamic evaluations of the heart and pulmonary arterial pressure in healthy children residing at high altitude in China. International Journal of Cardiology, Heart & Vasculature, 7, 158–164. [Google Scholar]
11. Leung, M., Yung, T., Ng, Y., Wong, K., Lee, S. et al. (1996). Pattern of symptomatic congenital heart disease among oriental neonates—A decade’s experience. Cardiology in the Young, 6(4), 291–297. DOI 10.1017/S1047951100003917. [Google Scholar] [CrossRef]
12. Jacobs, E. G., Leung, M. P., Karlberg, J. (2000). Distribution of symptomatic congenital heart disease in Hong Kong. Pediatric Cardiology, 21(2), 148–157. DOI 10.1007/s002469910025. [Google Scholar] [CrossRef]
13. Hussain, S., Sabir, M. U., Afzal, M., Asghar, I. (2014). Incidence of congenital heart disease among neonates in a neonatal unit of a tertiary care hospital. The Journal of the Pakistan Medical Association, 64(2), 175–178. [Google Scholar]
14. Calzolari, E., Garani, G., Cocchi, G., Magnani, C., Rivieri, F. (2003). Congenital heart defects: 15 years of experience of the Emilia-Romagna Registry (Italy). European Journal of Epidemiology, 18(8), 773–780. DOI 10.1023/A:1025312603880. [Google Scholar] [CrossRef]
15. Egbe, A., Uppu, S., Lee, S., Stroustrup, A., Ho, D. et al. (2015). Temporal variation of birth prevalence of congenital heart disease in the United States. Congenital Heart Disease, 10(1), 43–50. DOI: 10.1111/chd.12176. [Google Scholar] [CrossRef]
16. Gilboa, S. M., Devine, O. J., Kucik, J. E., Oster, M. E., Riehle-Colarusso, T. et al. (2016). Congenital heart defects in the United States: Estimating the magnitude of the affected population in 2010. Circulation, 134(2), 101–109. DOI 10.1161/CIRCULATIONAHA.115.019307. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |