DOI:10.32604/CHD.2021.013462

| Congenital Heart Disease DOI:10.32604/CHD.2021.013462 |  |
| Article |
The Clinical Application Value of Selective Unifocalization in the Treatment of Severe Pulmonary Artery Atresia with Ventricular Septal Defect
1Pediatric Cardiac Surgery Center, National Center for Cardiovascular Disease and Fuwai Hospital, Chinese Academy of Medical Sciences, Peking Union Medical College, Beijing, China
2Department of Cardiac Surgery, Yunnan Fuwai Cardiovascular Hospital, Kunming, China
3Medical Nutrition Center, National Center for Cardiovascular Disease and Fuwai Hospital, Chinese Academy of Medical Sciences, Peking Union Medical College, Beijing, China
*Corresponding Author: Qiang Wang. Email: wq.cory@163.com; Xiang Li. Email: dawei1966@126.com
#All three authors contributed equally to this article
Received: 07 August 2020; Accepted: 29 October 2020
Abstract: Background: This study aims to explore the efficacy of selective unifocalization (UF) for major aortopulmonary collateral arteries (MAPCAs) unifocalization in children with pulmonary atresia with ventricular septal defect (PA/VSD). Methods: A retrospective analysis of 13 patients with PA/VSD/MAPCAs who underwent surgery from June 2017 to December 2019. Sex, age, preoperative cardiovascular angiography test results and McGoon ratio were collected. The properties of the collateral arteries were evaluated by angiography, and selective UF for the “dendritic” MAPCAs and ligation of MAPCAs demonstrating distortion and resistance. Results: A total of 13 severe patients underwent one-stage repair, of which 1 case underwent ventricular septal fenestration and died after 2 weeks of ECMO support. The median age was 37 months, and a median weight was 14.0 kg. A right ventricular to aortic systolic pressure ratio (pRV/pAo) of no more than 0.5 was achieved in 12 living patients. Conclusion: Selective unifocalization based on MAPCAs morphology can achieved a good outcome at the early stage. This surgical concept might be provided a novel insight into treatment for some of the subgroups presenting with this complex form of PA/VSD/MAPCAs.
Keywords: Pulmonary atresia; major aortopulmonary collateral arteries; selective unifocalization
Abbreviations
| UF | unifocalization |
| MAPCAs | major aortopulmonary collateral arteries |
| PA/VSD | pulmonary atresia and ventricular septal defect |
| VBJVC | valved bovine jugular vein conduit |
| GTVC | Gore-Tex valved conduit |
| ACCT | aortic cross-clamp time |
| CPB | cardiopulmonary bypass |
| MRA | magnetic resonance angiography |
| VT | ventilation |
| ICU | intensive care unit |
| TRICKS | time resolved imaging of contrast kinetics |
Severe pulmonary atresia and ventricular septal defect (PA/VSD) occurred in children is a congenital heart disease with complex anatomical form. The incidence rate is about 0.1‰, and the 20-year natural survival rate is only 20% [1,2], its pathological anatomy Similar to Tetralogy of Fallot, the difference is that there is no hemodynamic continuity between the right ventricle and the pulmonary artery or branches of the pulmonary artery. Due to the extremely poor development of native pulmonary arteries, it is necessary to rely on Major Aorto-pulmonary Collateral Artery (MAPCAs) to provide blood to involve in “blood gas exchange” for the pulmonary capillary network [3,4]. Unifocalization and rehabilitation respectively focus on the mobilization of collateral arteries and the growth of native pulmonary vessels [5]. Many groups have begun to have pursued the concept of midline unifocalizaiton [6]. International reports suggest that one-stage radical surgery can improve postoperative efficacy and reduce mortality compared with staging. The primary radical cure rate for unifocalization operation (UF) of MAPCAs is 85% [6]. The latest reported UF mortality rate in China is up to 40% [7]. This is because the core problem of poor pulmonary vascular bed development has not been well resolved. This study will assist us to re-recognize the function of different forms of MAPCA from the perspective of anatomic morphology, propose a treatment strategy for selective collateral UF based on morphology, and further investigate its safety and effectiveness, so as to provide new diagnosis and treatment direction for these severe patients.
This research was approved by the Institutional Review Board at Fuwai Hospital (No. 2020-1318) which is an efficient ethic committee in China to monitor biomedical research. Signed consents from all participants were obtained.
This study retrospectively analyzed 13 consecutive patients with PA/VSD/MAPCAs who met the enrollment criteria from June 2017 to December 2019, and all were treated by the same operator. Inclusion criteria: (1) Children diagnosed with severe (Types III or IV) MAPCAs/VSD/PA by preoperative cardiac catheterization, echocardiography and cardiac CTA. Diagnostic criteria refer to “Chinese Expert Consensus for Surgical Treatment of Pulmonary Atresia Combined with Ventricular Septal Defect” [4]. Exclusion criteria: mild patients (Types I or II) and those with other serious intracardiac malformations, such as complete pulmonary ectopic drainage, complete endocardial pad defect, and pulmonary vein stenosis.
2.3 Surgical Intervention and Observation Indicators
Collect preoperative cardiac color Doppler ultrasound, cardiovascular angiography and cardiac CTA imaging data, describe the number and anatomical distribution of MAPCAs, clarify the origin, size and morphology of MAPCAs, and evaluate the existence of native pulmonary artery and its development. According to the characteristics of MAPCAs, they are divided into “dendritic” distribution and “occlusive” distribution (see Fig. 1 for details). Based on this feature, it is divided into “functional collaterals” involving in blood gas exchange and non-functional collaterals. Functional MAPCAs were unifocalized during surgery, while non-functional collaterals were ligated during surgery. Fresh autologous pericardial patch was used to widen the opening following UF procedure, and Valved Bovine Jugular Vein Conduit (VBJVC) or Gore-Tex Valved Conduit (GTVC) was used for connection between RVOT and neo-pulmonary artery (neo-PA). We determined whether to close the ventricular septal defect or conduct VSD fenestration based on pRV/pAo and cardiac function status. Aortic cross-clamp time (ACCT), cardiopulmonary bypass (CPB) time, ICU duration, (ventilation) VT duration, postoperative stay length, and Neo-PA McGoon were collected. During the follow-up, echocardiography and electrocardiography were routinely performed.
Data (normally distributed) of all the patients were displayed by mean ± standard deviation, t test was used. Non-normally distributed measurement data are expressed as median (range: min-max), and count data are expressed as cases (%). The statistical analyses were done by using the SPSS on Windows (SPSS Inc., Chicago, IL, USA). A P value less than 0.05 was considered statistically significant.
3.1 Basic Characteristics of 13 Children
A total of 13 children were enrolled, including 5 cases of type III pulmonary artery atresia and 8 cases of type IV pulmonary artery atresia. There were 9 males (69.2%) and 4 females. A median age was 37 months (range: 6–228 months) and a median weight was 14.0 kg (range: 5.0–49.0 kg). The mean number of MAPCA was 2.53 ± 1.05, and the average preoperative McGoon index was 1.56 ± 0.51. Twelve of these thirteen patients underwent one-stage unifocalization and ventricular septal defect closure except one undergoing septal fenestration. The demographics and clinical characteristics of the patients are shown in Tab. 1.
Table 1: Patient clinical characteristics
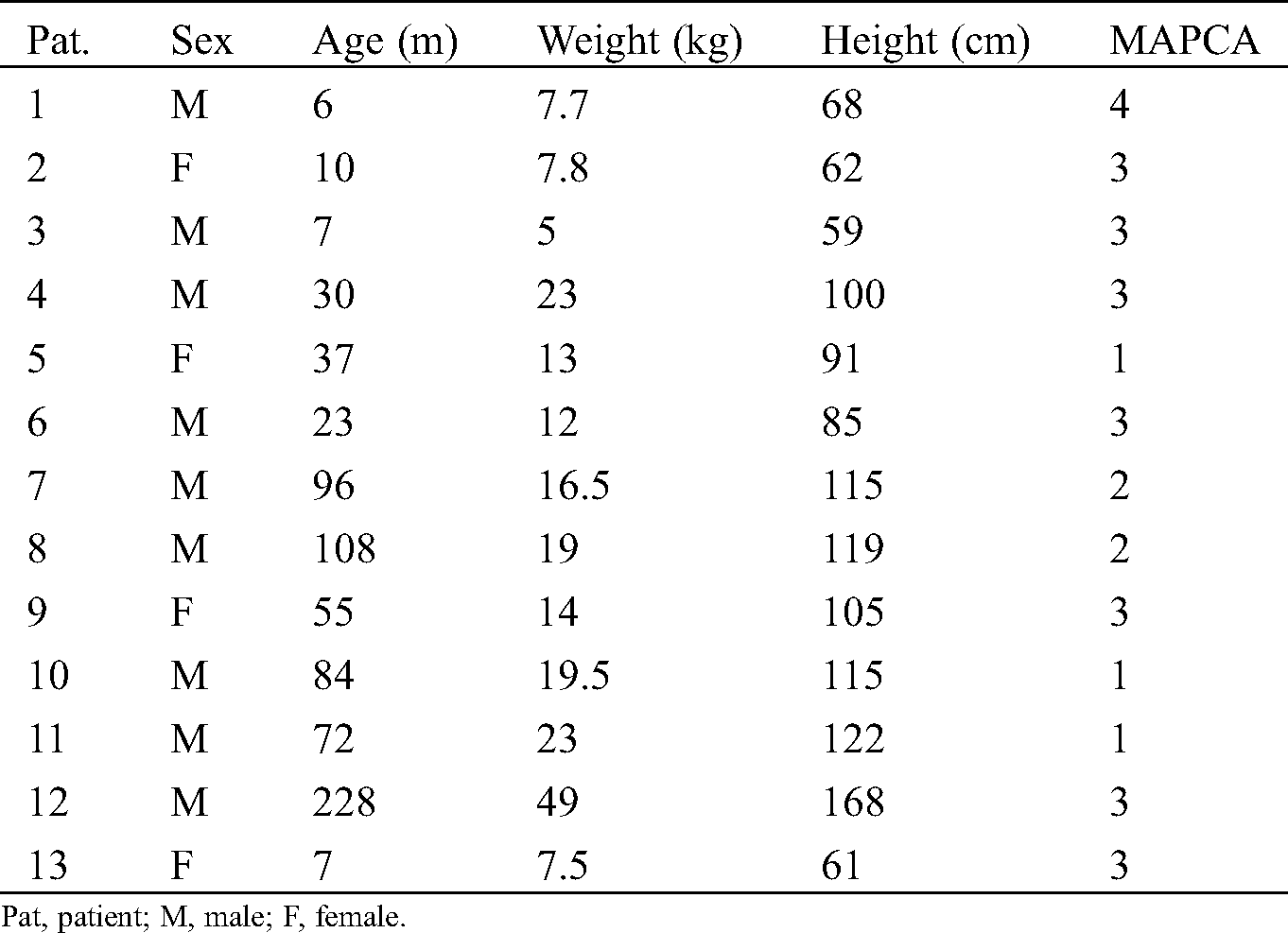
The median CPB time was 341 min (range: 205–1685 min), and median ACCT was 174 min (range: 71–447 min). The mean perioperative pRV/pAo was 0.47 ± 0.19 except that of 0.98 in one child after septal fenestration. The median ICU duration was 12 days (range: 3–58 days), and the median VT duration was 16 h (range: 21–1127 h). A case of pulmonary collateral closure was performed due to excessive lung congestion after the operation, and the situation improved. another patient had necrosis of the right upper lobe after 7 days of operation, and improved after the right upper lobe resection; Moreover, one patient developed increased pulmonary resistance after operation and died after 2 weeks of ineffective ECMO maintenance treatment. The remaining children had no long-term complications during follow-up of 11.2 (range: 6–30 months). These data are shown in Tab. 2.
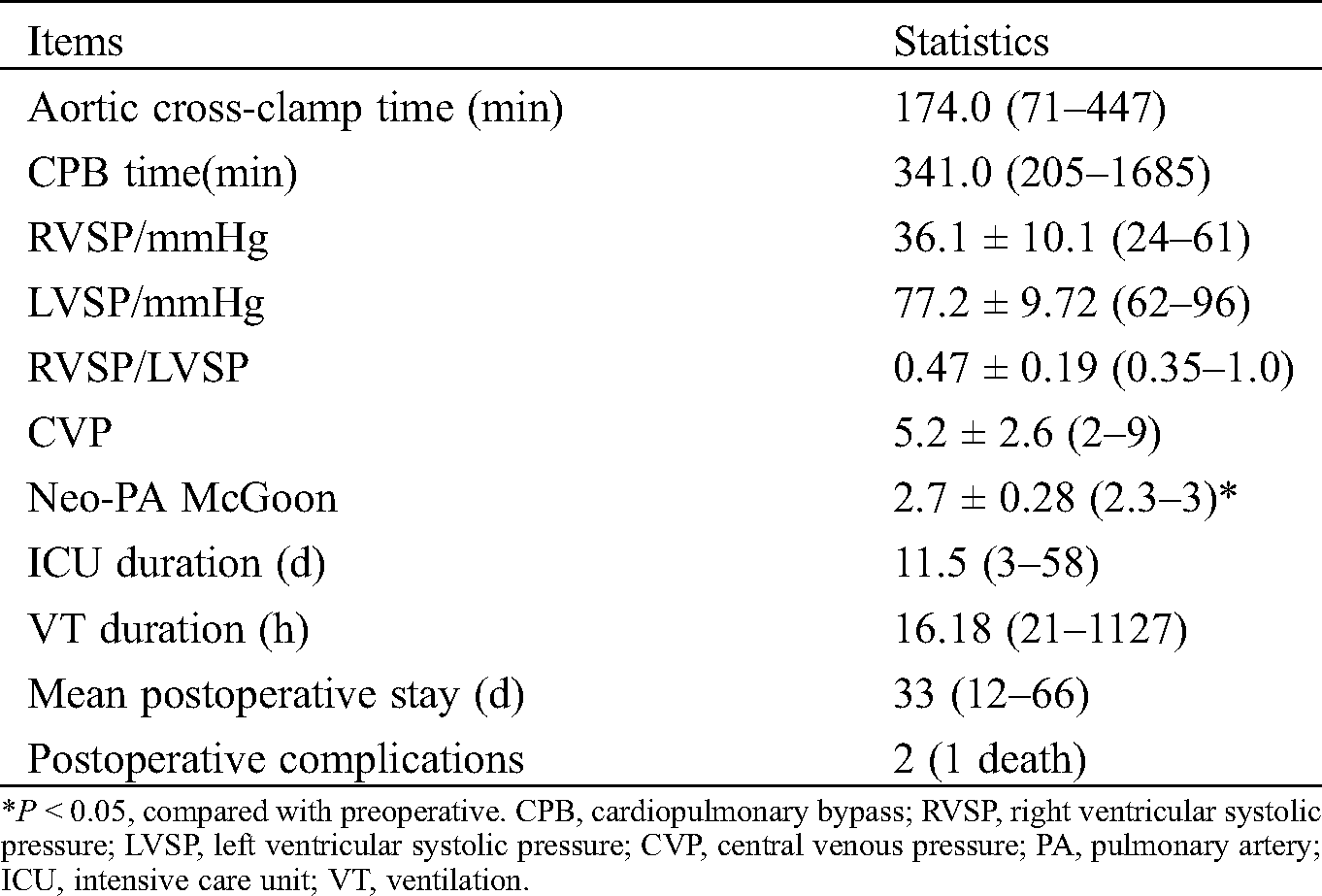
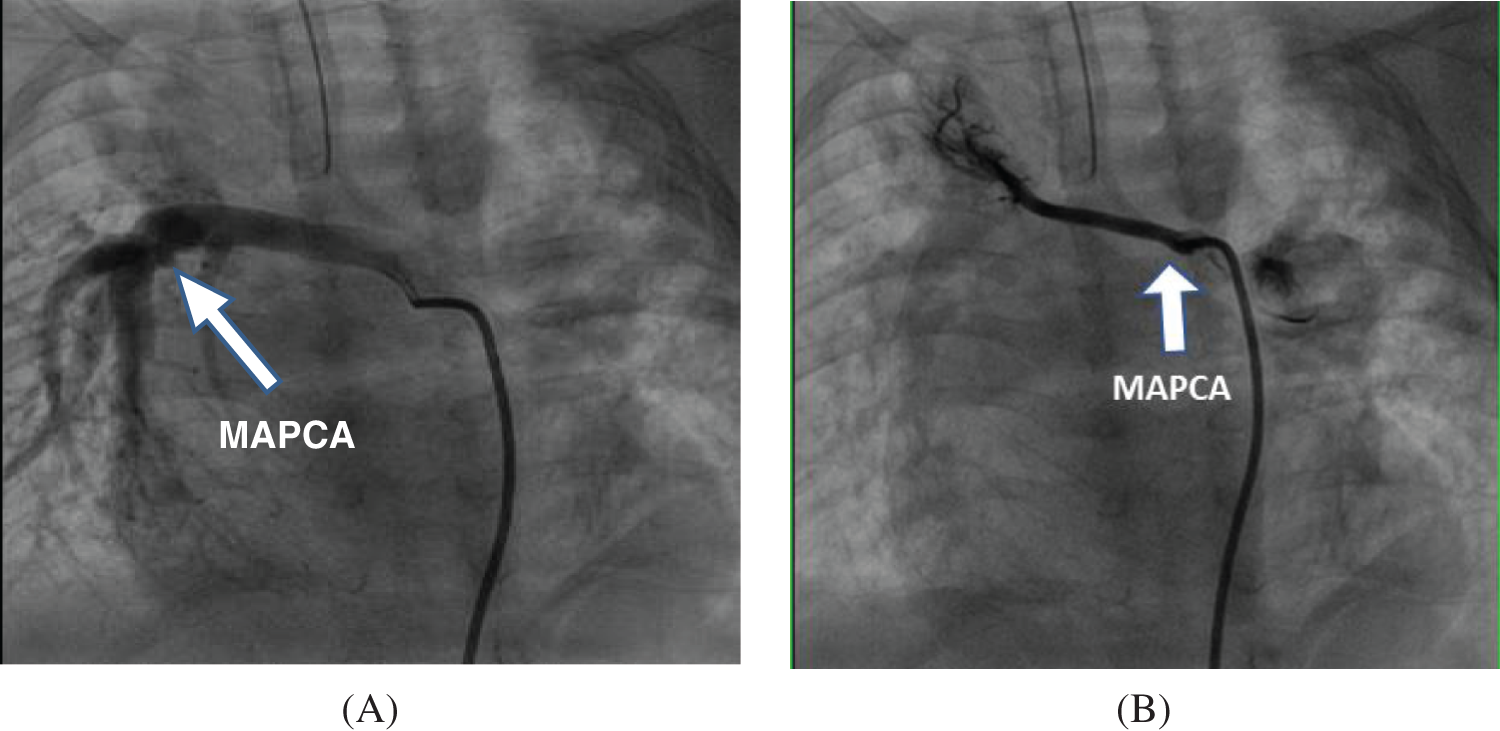
Figure 1: Comparison of preoperative angiography results of different types of MAPCAs (A) “Dendritic”—The functional MAPCAs from the descending aorta are distributed in a “dendritic” shape, forming the lower right pulmonary vascular bed and participating in its “blood gas exchange” for intraoperative fusion; (B) “Occlusive”—The right subclavian artery MAPCAs are non-functional, distributed in an “occluded” state, do not participate in “blood gas exchange”, and undergo ligation during surgery
Among the 13 patients included in this study, 5 were type III pulmonary atresia and 8 were type IV, all of which were severe cases. The postoperative RVSP/LVSP was 0.47 ± 0.19, which was no different from the staged surgery reported in the literature [8]. Compared with conventional surgical methods, the “Selective unifocalization” proposed by us did provide the possibility of one-stage repair for subgroup of severe PA. Whether reconstruction of the native pulmonary artery (Rehabilitation) or unifocalization of MAPCA, the key is to rebuild and restore a complete pulmonary vascular bed as much as possible [9]. Based on the results of preoperative angiography, this study proposes “functional” pulmonary collaterals, which are unifocalized during the operation, and ligation of non-functional tortuous collaterals without obvious blood-gas exchange. Importantly, the key to the success of selective UF surgery is to accurately determine the functional MAPCA, that is, to find the collateral vessels involved in blood gas exchange. Normally, the anterior blood flow of the pulmonary artery is stable and the original vascular tissue develops into the bronchial artery. If the pulmonary artery is atresia and the forward blood flow disappears, the original tissue that should have developed into the bronchial artery develops into MAPCAs [10]. However, it cannot be concluded that MAPCAs and bronchial arteries are the same tissues [11]. MAPCAs should be accurately classified according to whether they participate in blood gas exchange function [12]. Generally, their morphology is more similar to nature pulmonary blood vessels. Different from other literature [13], We proposed that pulmonary collaterals with “blood gas exchange” should be evaluated based on the characteristics of the morphology and distribution of blood vessels in the lung. That is, the “dendritic” distribution is more similar to the nature pulmonary blood vessels, which are functional MAPCA, and the “occlusive” distribution belongs to non-functional collaterals that do not participate in blood gas exchange. The former undergoes unifocalization during surgery, and the latter should be ligated.
In 1980, Haworth et al. [14] first proposed “Unifocalization” based on extensive research on the structural characteristics of MAPCA. With the deepening of understanding of disease anatomy and pathophysiology, we have seen an improvement in the outcomes for children affected by this disease [1]. It has been usual to classify the surgical treatment of PA/VSD into two strategies—unifocalization and rehabilitation. The “combine strategy” put forward by the Melbourne group, which has also been recognized by many researchers [1,15]. At present, there are still many children with severe PA who need staged treatment. The main reason is that they have poor pulmonary vascular development. Direct one-stage repair will cause high RV pressure. As a result, many children have died during the staging process or eventually missed the time for radical treatment due to poor development of the distal pulmonary vascular bed [16]. Literature reports [17] have confirmed that compared with staged surgery, the long-term mortality rate of one-stage unifocalization surgery combined with right ventricular outflow tract reconstruction and ventricular septal defect repair surgery has not significantly increased, and the high cost of staged surgery is avoided. However, the current indications are harsh, and are only suitable for those with better pulmonary artery development and MAPCA accounts for less than 30% of the blood supply lung segment. Against this backdrop, we can safely and effectively perform selective UF and achieve one-stage repair, which is a breakthrough and our strength.
This study preliminarily verified the safety and effectiveness of selective unifocalization, and provide some detailed surgical management strategies and some useful information for some of the subgroups presenting with this complex and heterogeneous form of PA/VSD/MAPCAs. In this study, a 19-year-old patient who underwent surgical treatment showed increased refractory pulmonary resistance postoperatively, and eventually died after 2-week ECMO support. This is mainly because of long-term exposure of high arterial pressure and resulting in irreversible changes in pulmonary vascular bed.
Notably, the optimal age for surgery is still controversial. Bauser-Heaton et al. [18] reported a repair rate of 88% at a median age of 8.6 months. The median age of the patients we included was greater than this study. Another study is described by Ishibashi et al. [19] obtaining an 80% repair rate at a mean age of 8.1 years. The selection of the best surgical age is a meaningful exploration in the future. In terms of imaging evaluation, the research of Romeih et al. [20,21] investigated the role of contrast enhanced magnetic resonance angiography (MRA) to evaluate the pulmonary blood supply and the anatomy of the pulmonary arteries and compared this with cardiac catheterization in children with PA. The result shows that time resolved imaging of contrast kinetics (TRICKS) MR angiography is a safe and accurate non-invasive technique to evaluate the pulmonary artery morphology and the sources of pulmonary blood supply in children with PA. The application of imaging techniques to determine the changes in the pulmonary vascular bed may be a further research direction for the UF.
This study preliminarily verified that selective unifocalization based on MAPCAs morphology can achieved a good outcome at the early stage, which will increase the rate of disease treatment and might be provided a novel insight into treatment for the PA/VSD/MAPCAs.
Ethical Statement: This study was reviewed and approved by the Medical Ethics Review Committee of Fuwai Hospital (No. 2020-1318).
Funding Statement: This study was supported by the Clinical and Translational Medicine Research Fund (2019XK320050) of the Basic Research Service Fund of the Central Public Welfare Research Institute of the Chinese Academy of Medical Sciences.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Presnell, L. B., Blankenship, A., Cheatham, S. L., Owens, G. E., Staveski, S. L. (2015). An overview of pulmonary atresia and major aortopulmonary collateral arteries. World Journal for Pediatric & Congenital Heart Surgery, 6(4), 630–639. DOI 10.1177/2150135115598559. [Google Scholar] [CrossRef]
2. Leonard, H., Derrick, G., O’Sullivan, J., Wren, C. (2000). Natural and unnatural history of pulmonary atresia. Heart (British Cardiac Society), 84(5), 499–503. DOI 10.1136/heart.84.5.499. [Google Scholar] [CrossRef]
3. Shimazaki, Y., Maehara, T., Blackstone, E. H., Kirklin, J. W., Bargeron, L. M. Jr. (1988). The structure of the pulmonary circulation in tetralogy of Fallot with pulmonary atresia. A quantitative cineangiographic study. Journal of Thoracic and Cardiovascular Surgery, 95(6), 1048–1058. DOI 10.1016/S0022-5223(19)35674-0. [Google Scholar] [CrossRef]
4. Tchervenkov, C. I., Roy, N. (2000). Congenital heart surgery nomenclature and database project: Pulmonary atresia—Ventricular septal defect. Annals of Thoracic Surgery, 69(4 Suppl), S97–S105. DOI 10.1016/S0003-4975(99)01285-0. [Google Scholar] [CrossRef]
5. D’Udekem, Y., Alphonso, N., Nørgaard, M. A., Cochrane, A. D., Grigg, L. E. et al. (2005). Pulmonary atresia with ventricular septal defects and major aortopulmonary collateral arteries: Unifocalization brings no long-term benefits. Journal of Thoracic and Cardiovascular Surgery, 130(6), 1496–1502. DOI 10.1016/j.jtcvs.2005.07.034. [Google Scholar] [CrossRef]
6. Mainwaring, R. D., Patrick, W. L., Roth, S. J., Kamra, K., Wise-Faberowski, L. et al. (2018). Surgical algorithm and results for repair of pulmonary atresia with ventricular septal defect and major aortopulmonary collaterals. Journal of Thoracic and Cardiovascular Surgery, 156(3), 1194–1204. DOI 10.1016/j.jtcvs.2018.03.153. [Google Scholar] [CrossRef]
7. Bo, S., Li, X. F., Yuan, F., Song, Z. J., Zheng, J. et al. (2010). Unifocalization single-source surgery for pulmonary atresia with ventricular septal defect and important main pulmonary artery collateral vessels. Journal of Clinical Pediatric Surgery, 09(6), 410–412. [Google Scholar]
8. Soquet, J., Barron, D. J., d’Udekem, Y. (2019). A review of the management of pulmonary atresia, ventricular septal defect, and major aortopulmonary collateral arteries. Annals of Thoracic Surgery, 108(2), 601–612. DOI 10.1016/j.athoracsur.2019.01.046. [Google Scholar] [CrossRef]
9. Malhotra, S. P., Hanley, F. L. (2009). Surgical management of pulmonary atresia with ventricular septal defect and major aortopulmonary collaterals: A protocol-based approach. Seminars in Thoracic and Cardiovascular Surgery: Pediatric Cardiac Surgery Annual, 145–151. DOI 10.1053/j.pcsu.2009.01.017. [Google Scholar] [CrossRef]
10. Nørgaard, M. A., Alphonso, N., Cochrane, A. D., Menahem, S., Brizard, C. P. et al. (2006). Major aorto-pulmonary collateral arteries of patients with pulmonary atresia and ventricular septal defect are dilated bronchial arteries. European Journal of Cardio-Thoracic Surgery: Official Journal of the European Association for Cardio-Thoracic Surgery, 29(5), 653–658. DOI 10.1016/j.ejcts.2005.12.054. [Google Scholar] [CrossRef]
11. Hanley, F. L. (2006). MAPCAs, bronchials, monkeys, and men. European Journal of Cardio-Thoracic Surgery: Official Journal of the European Association For Cardio-Thoracic Surgery, 29(5), 643–644. DOI 10.1016/j.ejcts.2006.02.031. [Google Scholar] [CrossRef]
12. Egbe, A. C., Crestanello, J., Dearani, J. A., Osman, K., Banala, K. et al. (2019). Long-term outcomes in adults with repaired tetralogy of Fallot and pulmonary atresia. Cardiology in the Young, 29(8), 1078–1081. DOI 10.1017/S1047951119001598. [Google Scholar] [CrossRef]
13. Greenleaf, C. E., Urencio, J. M., Salazar, J. D., Dodge-Khatami, A. (2016). Hypoplastic left heart syndrome: Current perspectives. Translational Pediatrics, 5(3), 142–147. DOI 10.21037/tp.2016.05.04. [Google Scholar] [CrossRef]
14. Haworth, S. G., Macartney, F. J. (1980). Growth and development of pulmonary circulation in pulmonary atresia with ventricular septal defect and major aortopulmonary collateral arteries. British Heart Journal, 44(1), 14–24. DOI 10.1136/hrt.44.1.14. [Google Scholar] [CrossRef]
15. Korun, O., Yurdakök, O., Dedemoğlu, M., Yücel, İ. K., Çelebi, A. et al. (2019). Midline one-stage complete unifocalization early outcomes from a single center. Anatolian Journal of Cardiology, 22(3), 125–131. [Google Scholar]
16. Fouilloux, V., Bonello, B., Kammache, I., Fraisse, A., Macé, L. et al. (2012). Management of patients with pulmonary atresia, ventricular septal defect, hypoplastic pulmonary arteries and major aorto-pulmonary collaterals: Focus on the strategy of rehabilitation of the native pulmonary arteries. Archives of Cardiovascular Diseases, 105(12), 666–675. DOI 10.1016/j.acvd.2012.08.003. [Google Scholar] [CrossRef]
17. Mainwaring, R. D., Patrick, W. L., Roth, S. J., Kamra, K., Wise-Faberowski, L. et al. (2018). Surgical algorithm and results for repair of pulmonary atresia with ventricular septal defect and major aortopulmonary collaterals. Journal of Thoracic and Cardiovascular Surgery, 156(3), 1194–1204. DOI 10.1016/j.jtcvs.2018.03.153. [Google Scholar] [CrossRef]
18. Bauser-Heaton, H., Borquez, A., Han, B., Ladd, M., Asija, R. et al. (2017). Programmatic approach to management of tetralogy of Fallot with major aortopulmonary collateral arteries: A 15-year experience with 458 patients. Circulation: Cardiovascular Interventions, 10(4), e004952. DOI 10.1161/CIRCINTERVENTIONS.116.004952. [Google Scholar] [CrossRef]
19. Ishibashi, N., Shin’oka, T., Ishiyama, M., Sakamoto, T., Kurosawa, H. (2007). Clinical results of staged repair with complete unifocalization for pulmonary atresia with ventricular septal defect and major aortopulmonary collateral arteries. European Journal of Cardio-Thoracic Surgery: Official Journal of the European Association For Cardio-Thoracic Surgery, 32(2), 202–208. DOI 10.1016/j.ejcts.2007.04.022. [Google Scholar] [CrossRef]
20. Razek, A. A., Gaballa, G., Megahed, A. S., Elmogy, E. (2013). Time resolved imaging of contrast kinetics (TRICKS) MR angiography of arteriovenous malformations of head and neck. European Journal of Radiology, 82(11), 1885–1891. DOI 10.1016/j.ejrad.2013.07.007. [Google Scholar] [CrossRef]
21. Romeih, S., Al-Sheshtawy, F., Salama, M., Blom, N. A., Abdel-Razek, A. et al. (2012). Comparison of contrast enhanced magnetic resonance angiography with invasive cardiac catheterization for evaluation of children with pulmonary atresia. Heart International, 7(2), e9. DOI 10.4081/hi.2012.e9. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |