 Congenital Heart Disease
Congenital Heart Disease
 Congenital Heart Disease Congenital Heart Disease |  |
DOI: 10.32604/CHD.2021.014360
ARTICLE
Transcatheter Closure of a Right Pulmonary Artery to Left Atrium Fistula Using a Ventricular Septal Defect Occluder
1Department of Pediatric Cardiology, Shanghai Children’s Medical Center Affiliated to Shanghai Jiaotong University School of Medicine, Shanghai, 200127, China
2Department of Radiology Shanghai Children’s Medical Center Affiliated to Shanghai Jiaotong University School of Medicine, Shanghai, 200127, China
*Corresponding Authors: Jie Shen. Email: shenjie@scmc.com.cn; Fen Li. Email: lifenscmc@163.com
Received: 22 September 2020; Accepted: 17 November 2020
Abstract: Background: Communication between the right pulmonary artery (RPA) and left atrium (LA) is a rare cause of central cyanosis in pediatric patients. Case presentation: We describe a 3-year-old female patient with an oxygen saturation of 70% at admission. The echocardiogram indicated an abnormal color flow Doppler in the LA and she underwent standard cardiac catheterization. The angiography of pulmonary artery revealed a 7.4 mm × 7.6 mm fistula between the RPA and LA and achieved successful closure using ventricular septal defect occlusion. Conclusion: The fistula between pulmonary artery and left atrium is an extremely rare but treatable congenital defect. It should be considered in differential diagnosis of cyanosis in children.
Keywords: Transcatheter closure; fistula; ventricular septal defect occlude; central cyanosis
A right pulmonary artery to left atrium (RPA-LA) fistula is a rare congenital cardiovascular anomaly resulting in central cyanosis. It has been reported in fewer than 100 cases to date [1]. Here, we report a pediatric case of an RPA-LA fistula successfully treated by an interventional procedure.
A 3-year-old female patient was referred to our hospital and presented with a history of progressive central cyanosis starting at birth. Her oxygen saturation, measured at her extremities, was approximately 70%.
On admission, her temperature was 36.8°C, and her blood pressure was 90/60 mmHg. She presented with cyanosis in both lips and extremities with clubbing in the fingers and toes. Her heart rate was 118 bpm, which was within normal limits for her age, and no murmurs were heard on auscultation. Laboratory tests showed elevated hemoglobin at 19.7 g/dL, with normal white blood cell and platelet counts.
An electrocardiogram revealed sinus arrhythmia, and the electric axis deviated to the right. The echocardiogram showed a 7.8 mm × 7.2 mm secondary atrial septal defect (ASD) and a turbulent color flow Doppler in the LA, which made the shape of the LA, was “wired” (Fig. 1A). The left pulmonary artery diameter was 0.87 cm, while that of the right pulmonary artery (RPA) was 1.71 cm. The ultrasound confirmed that the pulmonary venous return and the left cardiac function were both normal. Computed tomography (CT) scan also revealed enlargement of the LA and ASD. The CT images displayed a fistula between the RPA and LA that was previously overlooked because of the lack of understanding of this rare disease (Fig. 1B).
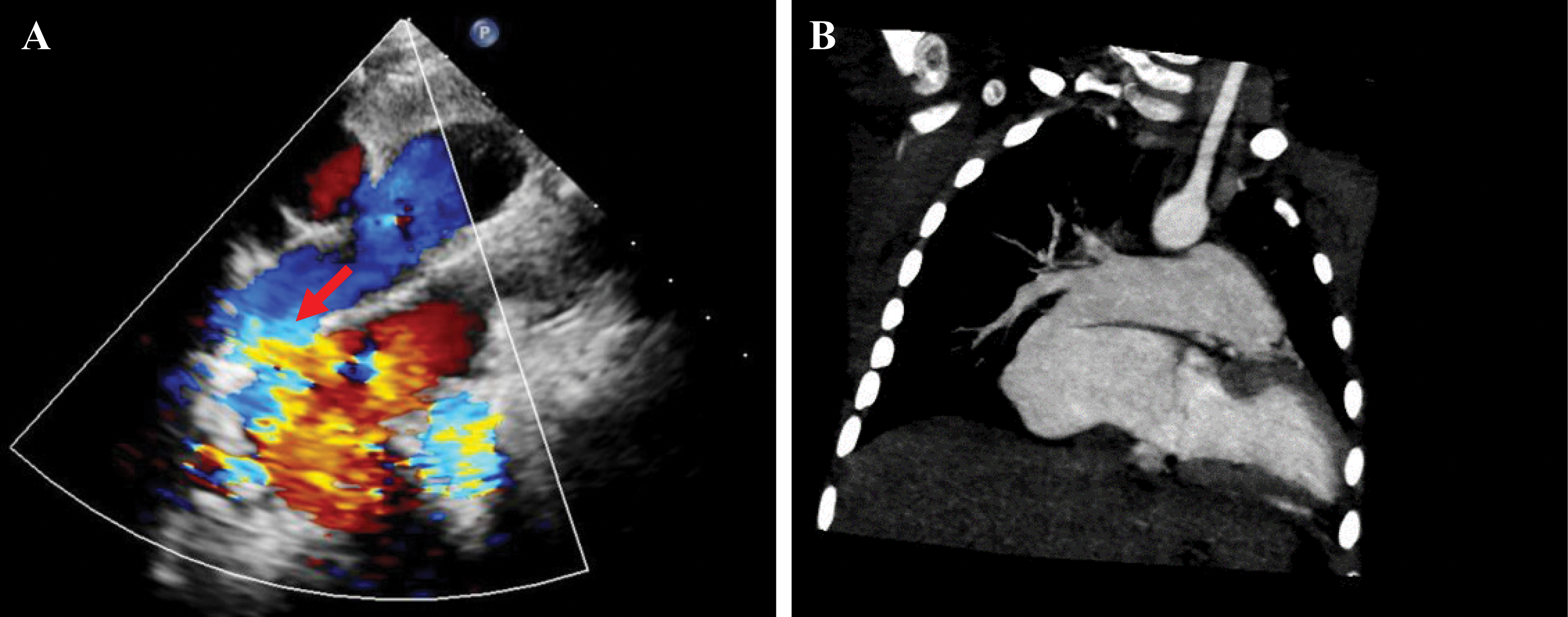
Figure 1: Echocardiography and CT scan showing the fistula between the RPA and LA; however, it is usually overlooked. (A) Echocardiography reveals communication between the RPA and LA. The red arrow indicates the fistula. (B) CT can also show the direct communication between the PA and LA
A standard cardiac catheterization was performed, and the right femoral artery and vein were punctured during the operation. The patient’s pulmonary artery pressure was 21/13/17 mmHg. Her blood oxygen level was 86.6% in the descending aorta with a fraction of inspired oxygen (FiO2) value of 40%. Pulmonary arterial trunk angiography showed a fistulous communication between the RPA and LA. The shunt was approximately 7.4 mm × 7.6 mm (diameter × length) (Fig. 2A). The RPA was expanded secondary to the shunt, and the left pulmonary artery appeared comparatively narrow. After the left femoral vein was punctured, we chose an 8F long sheath and established the path from the right femoral vein to the inferior vena cava and to the right atrium through the atrial septal defect. The path continued to the LA fistula and ended in the right inferior pulmonary artery (Fig. 2B). After that, a VSDO-I08 Occluder (Shanghai Push Medical Device Technology Co., Ltd.) was inserted to the narrowest area of the fistula. Her percutaneous oxygen saturation immediately increased from 89% to 99%. Then, the 5F pigtail catheter was inserted from the left femoral vein to the pulmonary artery to confirm the pulmonary venous return was not influenced by the occluder. A second pulmonary arterial trunk angiograph showed no residual shunting of the fistula or change in pulmonary venous return. There was no atrial-level shunting after transcatheter device closure was completed. We released the occluder, and echocardiography confirmed proper placement with no residential shunting (Fig. 2C). Further, there was no shunting observed at the atrial septal defect. Her blood oxygen saturation increased to 99.9% in the descending aorta postoperatively. The next day, echocardiography confirmed that the occluder was in the proper position, and no shunting had developed at the fistula (Fig. 2D).
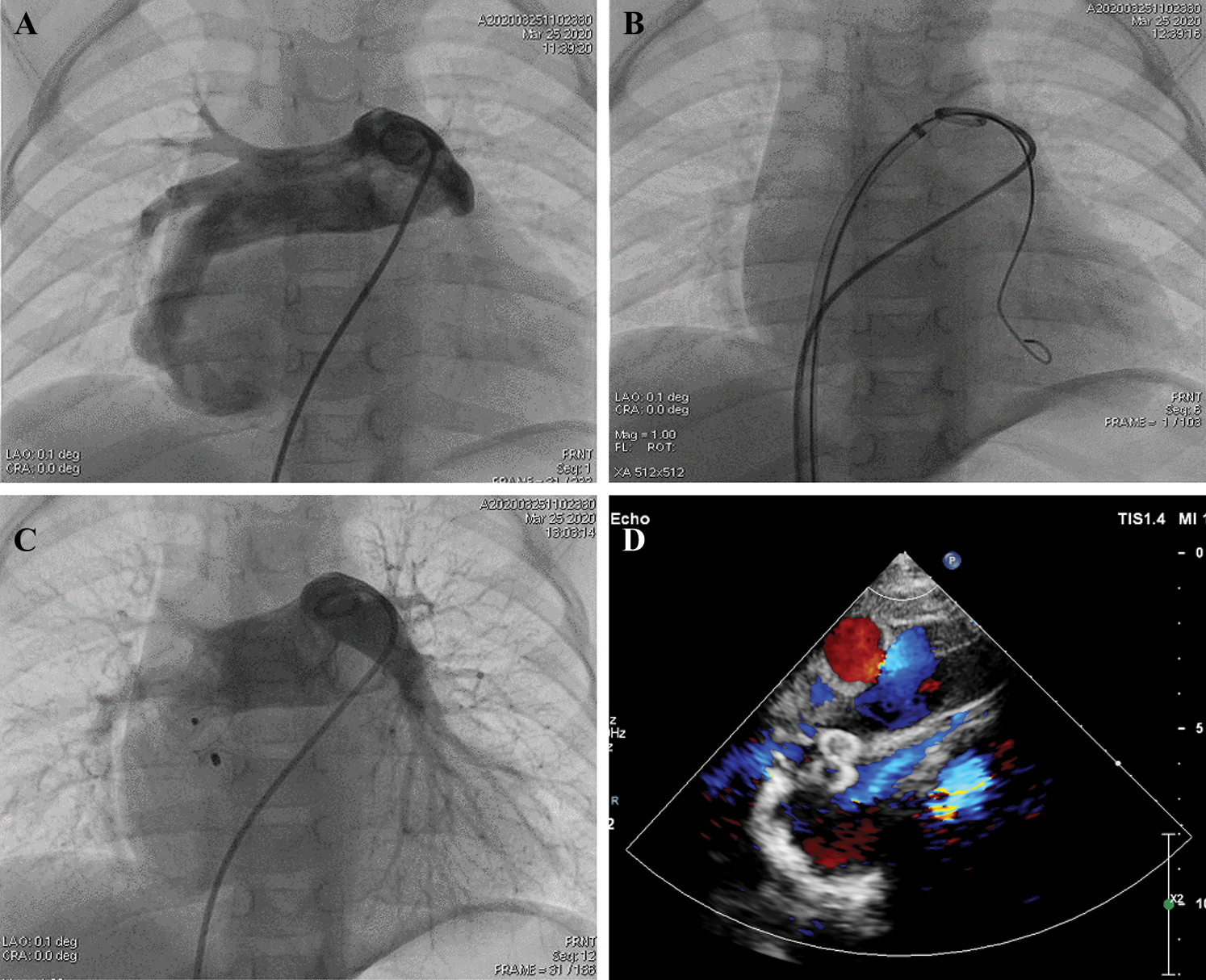
Figure 2: The procedure of transcatheter closure using a ventricular septal defect occluder. (A) Pulmonary arterial trunk angiography showing a fistulous communication between the RPA and LA. (B) The path from the right femoral vein to the inferior vena cava to the right atrium through the atrial septal defect to the LA to the fistula, ending in the right inferior pulmonary artery. (C) The VSD Occluder is inserted into the narrowest site of the fistula without affecting the pulmonary venous return. (D) An echocardiograph after the procedure confirms that the occluder was at the proper position and that there was no residual shunting
Direct communication of the RPA and LA is a rare cause of cyanosis in children. Most cases of direct communication were congenital defects, and only a few cases developed secondary to trauma [1].
The most common symptoms include cyanosis and clubbing. Older children and adults may develop dyspnea, hemoptysis, or encephalopyosis [2]. Many cases were not detected by transthoracic echocardiography [3]. This can be caused by an unclear view of the fistula in routine views, and the lack of knowledge on this type of malformation. In our case, we found a turbulent color flow Doppler in the LA but did not interpret it as direct communication.
Surgical treatment of ligation has been the standard procedure for treating RPA-LA [4,5]. Moreover, in the past 20 years, a series of successful transcatheter device closures were reported, even in neonates [6–11]. Individualized management should be considered based on patients’ ages and weights, in addition to the shape, length, and anatomic classification of the fistula. In our patient, the ASD made the venovenous railroad easily established, and the fistula was in a tubular form, which made transcatheter closure the most advantageous procedure. Various devices, including ductal, atrial, or ventricular septal defect occluders can be used in fistulas [12]. We believe transcatheter closure is safe and effective for fistulas with a particular morphology.
Congenital fistula between pulmonary artery and left atrium is a rare cause of cyanosis in children. The patients may present progressive cyanosis and developed symptoms of hypoxia. Echocardiogram and CT scan may discover the abnormal fistula, and the cardiac catheterization is the golden diagnosis. The direct communication can be treated by both surgery and transcatheter device depending on the length, position and shape of the fistula.
Ethics Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The approval number is SCMCIRB-W2020036.
Data Sharing: None.
Funding Statement: This work was supported by a grant from the National Science Foundation of China [Grant No. 81700286].
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Luo, G. H., Ma, W. G., Huang, L. J., Yan, J., Zhu, X. D. (2011). Surgical and transcatheter closure of right pulmonary artery to left atrial fistula. Journal of Cardiac Surgery, 26(2), 130–134. DOI 10.1111/j.1540-8191.2010.01204.x. [Google Scholar] [CrossRef]
2. Küçükosmanoglu, O., Poyrazoglu, H., Topçuoglu, M. S., Erdem, S., Erman, T. et al. (2006). Right pulmonary artery--left atrial communication presenting with brain abscess: A case report. Turkish Journal of Pediatrics, 48, 373–375. [Google Scholar]
3. Sarkar, A., Chandra, G. S. N., Majhi, B., Mandal, S., Pande, A. et al. (2013). Rare variant of pulmonary arteriovenous fistula: Direct communication between right pulmonary artery and left atrium associated with aortopulmonary collaterals. Journal of the American College of Cardiology, 62(9), 859. DOI 10.1016/j.jacc.2013.02.100. [Google Scholar] [CrossRef]
4. Yakut, K., Varan, B., Ozkan, M. (2018). A rare cause of cyanosis in newborns: Arteriovenous fistula between the right pulmonary artery and the left atrium and its treatment. Cardiology in the Young, 28(4), 605–607. DOI 10.1017/S1047951117002669. [Google Scholar] [CrossRef]
5. Krishnamoorthy, K. M., Rao, S. (2001). Pulmonary artery to left atrial fistula. European Journal of Cardio-Thoracic Surgery, 20(5), 1052–1053. DOI 10.1016/S1010-7940(01)00883-1. [Google Scholar] [CrossRef]
6. Srivastava, A., Radha, A. S. (2012). Right pulmonary artery to left atrium fistula: Transcatheter closure without conventional venovenous loop. Journal of Invasive Cardiology, 24(7), E145–147. [Google Scholar]
7. Zanchetta, M., Rigatelli, G., Pedon, L., Zennaro, M. Maiolino, P. et al. (2003). Transcatheter Amplatzer duct occluder closure of direct right pulmonary to left atrium communication. Catheterization and Cardiovascular Interventions, 58(1), 107–110. DOI 10.1002/ccd.10383. [Google Scholar] [CrossRef]
8. Uthaman, B., Al-Qbandi, M., Abushaban, L., Rathinasamy, J. (2007). Transcatheter closure of large pulmonary arteriovenous fistula including pulmonary artery to left atrial fistula with Amplatzer septal occluder. Catheterization and Cardiovascular Interventions, 70(3), 422–428. DOI 10.1002/ccd.21163. [Google Scholar] [CrossRef]
9. Ergül, Y., Nişli, K., Aydoğan, U. (2011). Transcatheter closure of a fistula between the right pulmonary artery and left atrium using the Amplatzer septal occluder. Turk Kardiyoloji Dernegi arsivi: Turk Kardiyoloji Derneginin yayin organidir, 39(3), 231–234. DOI 10.5543/tkda.2011.01201. [Google Scholar] [CrossRef]
10. Slack, M. C., Jedeikin, R., Jones, J. S. (2000). Transcatheter coil closure of a right pulmonary artery to left atrial fistula in an ill neonate. Catheterization and Cardiovascular Interventions, 50(3), 330–333. DOI 10.1002/1522-726X(200007)50:3<330::AID-CCD12>3.0.CO;2-T. [Google Scholar] [CrossRef]
11. Akhtar, M. J., Rosengart, R. M., Salem, M. M. (2013). Transcatheter closure of a giant communication between the right pulmonary artery and left atrium in a neonate. Catheterization and Cardiovascular Interventions, 82(1), 159–162. DOI 10.1002/ccd.24887. [Google Scholar] [CrossRef]
12. Bennati, E., Ciliberti, P., Curione, D., Guccione, P., Secinaro, A. (2018). Major right pulmonary artery to left atrium fistula successfully treated with Amplatzer muscular ventricular septal defect occluder. European Heart Journal Cardiovascular Imaging, 19(11), 1286. DOI 10.1093/ehjci/jey118. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |