 Congenital Heart Disease
Congenital Heart Disease
 Congenital Heart Disease Congenital Heart Disease |  |
DOI: 10.32604/CHD.2021.015050
ARTICLE
Screening Coarctation of Aorta with Clinical and Echocardiographic Profiles in Infants: A Pilot Study
1Department of Neonatology, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Guangzhou, China
2Department of Maternal-Fetal Cardiology, Guangdong Cardiovascular Institute, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Guangdong Province Key Laboratory of Structural Heart Disease, Guangzhou, China
3Department of Cardiac Surgery, Guangdong Cardiovascular Institute, Guangdong Provincial Key Laboratory of South China, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Guangzhou, China
*Corresponding Author: Yifei Wang. Email: yifei_wang_cn@163.com
Received: 18 November 2020; Accepted: 09 December 2020
Abstract: Aim: To determine the profiles of clinical features including four-limb blood pressure (BP), saturations of peripheral oxygen (SpO2), and echocardiographic features in infants with coarctation of aorta (CoA) to facilitate congenital heart diseases screening. Methods: The charts of infants with CoA were retrospectively reviewed. All in-hospital infants suspected of congenital heart diseases by clinical teams were prospectively measured of four limbs BPs and SpO2 in a regional cardiac transferring center during 2013 and 2019. Echocardiography as a gold standard test was followed within 2 days after suspicion. All infants were divided into non-significant CoA group or significant CoA group based on the difference of BPs between right arm and lower limbs. Predictors of non-significant CoA were determined with multivariable logistic regression. Results: One hundred thirty-three infants with CoA were identified. The BPs on upper limbs were higher than those on lower limbs (P = 0.001). No statistical difference in SpO2 was found between four limbs. Fifty-three (39.8%) infants presented with significant CoA. Thirty-four infants presented with low SpO2 and 26 of 34 presented with non-significant CoA. Small ascending aorta diameter [0.070 (95% CI: 0.005–0.136), P = 0.036] was an independent risk factors for non-significant CoA. Eighteen (13.5%) infants with CoA didn’t present with any of the BP difference, low SpO2, murmur, or weak femoral pulse. Conclusion: Less than half of the infants with CoA presented with a significant BP difference. Another one fifth presented with low SpO2. Small ascending aorta diameter was an independent factor for non-significant CoA.
Keywords: Congenital heart disease; coarctation of aorta; screening; blood pressure; saturations of peripheral oxygen
Coarctation of aorta (CoA) is one of the critical congenital heart diseases (CHD), who might need surgical intervention within 1 year after birth. It accounts for half of missed critical CHD infants [1]. Late detection of these patients, particularly following cardiovascular collapse, is associated with poor outcomes and neurological morbidity [2–4]. Although PxO2 screening was introduced to enhance CCHD screening rate, CoA is still not on the list due to its non-cyanotic profile. At 2 days of age, more than 60% of CHD with a ductus-dependent circulation had not been diagnosed [5]. It is also being missed by enhanced prenatal detection of outflow tract anomalies with fetal echocardiography [6,7].
Based on the anatomic and hemodynamic properties of CoA, four-limb blood pressure (BP) was tried to screen this group of patients. Patankar et al. [8] did a case-control study of four limb BPs at birth in 138 infants and 60% CoA infants were reported with no significant BP gradient. Boelke et al. [9] even identified no critical CoA by prospective BP screening in 10,012 infants. Besides four limb BPs and SpO2, evaluation of left ventricular cardiac output by femoral pulse palpation or peripheral perfusion index (PI) had also been tried as an approach of screening CoA with no positive results [10–14]. There is no effective way to identify these groups of patients, especially those presenting without differential BPs on four limbs.
As a regional cardiac transferring center, our hospital admitted hundreds of CHD infants every year. CoA is common among these infants. We initiated a two-step CoA improvement program to determine the optimal approach to facilitate CoA management, which includes step one as retrospectively reviewing and identifying the profiles of these infants, and step two as prospectively following up for years and improving CoA management strategy depending on their outcomes. This study is the first step to determine the clinical and echocardiographic features, including four limb BPs, SpO2, and murmurs, in these infants to try to increase the efficacy of CoA identification.
This study was approved by the Institutional Review Board at Guangdong Provincial People’s Hospital. As a routine procedure to newly admitted infants of suspected CHD by clinical teams, four limb BPs and SpO2 had been prospectively collected for more than 7 years at Guangdong Provincial People’s Hospital, a regional cardiac transferring center. The charts of infants with confirmed CoA admitted to our department between January 2013 and December 2019 were retrospectively reviewed. All infants were diagnosed by echocardiography or cardiac computer tomography within 2 days after admission. If the results were inconsistent, the post-operative diagnosis was retrieved.
Four limb BPs and SpO2 were measured at admission in infants suspected of CHD with abnormal physical examinations, abnormal prenatal or postnatal echocardiography finding, or low SpO2 by well-trained nurses with suitable cuffs (two-thirds of the length of infant’s arm or leg) and SpO2 sensors when they were quiet or sleepy. Low SpO2 was defined as SpO2 less than 95% at any limbs. Repeated measurements were performed if the results were questioned by clinical teams. All BPs and SpO2 were acquired by bedside monitors (Draeger Infinity Kappa or Infinity Delta), which were regularly quality-controlled by the Guangzhou Bureau of Quality and Technical Supervision.
As not all infants with CoA had BP gradient, in order to maximally distinguish CoA infants without BP gradient and to determine risk factors of CoA, these infants were divided into non-significant CoA group (also as CoA group) and significant CoA group based on four-limb BP gradient. Significant CoA was defined as having systolic BP on right arm at least 10 mm Hg higher than those on lower limbs. The cutoff of 10 mm Hg was based on Patankar et al. [8,15] and our previous study, both of which found that 10 mm Hg BP gradient was the best predictor of CoA with the optimal area under the ROC curve. Clinical variables including anthropometrics, demographics, four limb BPs and SpO2 at admission, reasons for echocardiography except for BP gradient were extracted. The diameter of coarctation isthmus and ascending aorta, ascending aorta/CoA ratio, maximal velocity at coarctation isthmus (Vmax), the relative position between CoA and patent ductal arteriosus (PDA) (preductal or postductal) were retrieved as aorta indices, diameter of PDA and PDA/CoA ratio, PDA/left pulmonary artery ratio, PDA/weight at surgery ratio, left atrium/aorta ratio as PDA indices for infants with PDA, left ventricular ejection fraction (EF) and fractional shortening (FS) as left ventricular indices, severe pulmonary hypertension (PH) as right ventricular indices. The estimation of systolic pulmonary arterial pressure (sPAP) was based on the peak velocity of the jet of tricuspid regurgitation (TR). The simplified Bernoulli equation and CW-Doppler was used to assess velocity within TR jet [RVSP = PASP = 4 × (TRV)2]. PH was categorized as none or mild if the estimated sPAP to systolic BP ratio was <0.5, moderate if the ratio was ≥0.5 but <1, and severe if sPAP ≥ systolic BP. In the absence of TR, PDA, or VSD, sPAP was estimated by assessing the end-systolic interventricular septal position at the papillary muscle level in short-axis view through the multiple acoustic windows. PH by septal position was categorized as follows: none or mild if the septum was rounded at end systole, moderate if the septum was flattened, and severe if the septum was bowed into the left ventricle at end systole. Z scores of the parameters were also calculated [16]. Other factors potentially influencing hemodynamics at admission were also included such as clinically confirmed sepsis, use of inotropes which might influence BP results and mechanical ventilation which might increase intrathoracic pressure and indirectly result in abnormal BP, and supplemental oxygen for PDA diameter and SpO2. Isolated CoA was defined as CoA without significant associated cardiac defects needing surgery [17].
Clinical characteristics between significant CoA vs. CoA groups were compared by chi-square tests for categorical variables or Student’s t-test for continuous variables following normal distribution. When expected cell counts were small (<5), Fisher’s exact test was used. Non-normal data were compared using a Wilcoxon rank-sum test. Univariate logistic regression and multivariable logistic regression were used to obtain estimates of the association between non-significant CoA and potential indices which were the predictors of non-significant BP gradient. Predictors that were significant at the univariable level (P < 0.20) and had a sufficient sample size were included in multivariable modeling. Multivariable logistic regression analysis was performed using stepwise selection to identify independent predictors of significant CoA. Results of the multivariable analysis were reported as odds ratio (OR) with 95% confidence intervals (CI). Statistical analyses were performed by SPSS version 21.0 (Armonk, NY, USA). Statistical significance was defined as a P < 0.05.
During the study period, 133 infants were identified as having CoA, 53 (39.8%) with significant CoA and 33 (25.6%) with low SpO2. Thirty-six were isolated CoA. The median age at diagnosis was 9 (1, 19) days of age, ranging from 1 h to 31 days of age. Gestational age was 38.5 ± 1.7 weeks and birth weight 2975 ± 712 grams. Four limb BPs and SpO2 were presented in Tab. 1. The BPs on upper limbs were higher than those on lower limbs (P = 0.001). There was no statistically significant difference in BPs between left and right extremity and no difference in SpO2 between four limbs (Tab. 1). BPs and SpO2 of infants with CoA or significant CoA were presented in Fig. 1. BPs and SpO2 of infants with PDA or without PDA were presented in Fig. 2. CoA infants without PDA (37/133, 27.8%) presented with higher BP on upper limbs than those with PDA (Fig. 2). Three infants with weak femoral pulses were noted, and one of them developed shock and was transferred to our hospital with prostaglandin E1 infusion.
Among 53 infants with significant CoA, 44 (83.0%) had surgical correction of cardiac anomaly with 1 dead of post-operative cardiac low output syndrome, 1 discharged without surgery and 8 withdrew with no further treatment due to poor prognosis or financial issues. Among 80 infants with non-significant CoA, 54 (67.5%) had cardiac surgery with 7 died of post-operative cardiac low output syndrome or severe sepsis, 12 (15.0%) discharged with no need of further intervention, 11 (13.7%) withdrew and 3 (3.8%) died of poor cardiac output, multiple organ dysfunction syndrome or severe bronchial anomaly respectively.
Table 1: The profiles of four limb blood pressures and saturation of peripheral oxygen in infants with coarctation of aorta
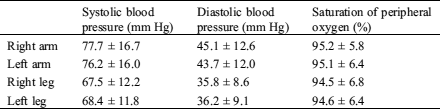

Figure 1: The profiles of four limb blood pressures and saturation of peripheral oxygen in infants with or without significant coarctation of aorta. BP indicates blood pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure; SpO2, saturation of peripheral oxygen; RA, right arm; LA, left arm; RL, right leg; LL, left leg. ** denotes P < 0.01, * P < 0.05

Figure 2: The profiles of four limb blood pressures and saturation of peripheral oxygen in coarctation of aorta infants with or without Patent Ductal Arteriosus. BP indicates blood pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure; SpO2, saturation of peripheral oxygen; RA, right arm; LA, left arm; RL, right leg; LL, left leg. ** denotes P < 0.01, * P < 0.05
Comparisons of baseline or echocardiographic characteristics of infants with CoA or significant CoA were listed in (Tab. 2). Provided with similar gestational age and birth weight, infants with significant CoA were older than CoA infants (6.6 vs. 16.5 days of age). No statistical significance was found between groups on reasons for echocardiography. But more infants in the CoA group were asymptomatic and were suspected of CHD prenatally. More than half of these infants in both groups presented with murmurs due to complicated cardiac defects. More infants in the CoA group presented with PDA (80.2% vs. 58.5%). There was no statistical difference between groups on aorta indices, PDA indices, left ventricular indices, and right ventricular indices, except for PDA, ascending aorta diameter and its derivative ascending aorta/CoA ratio.
Table 2: Baseline and echocardiographic characteristics of infants with or without significant coarctation of aorta at admission
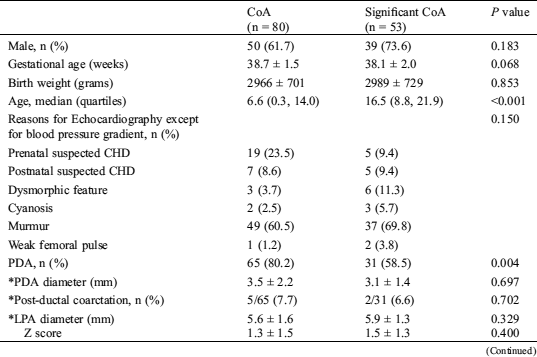

CoA, coarctation of aorta; CHD, congenital heart defects; EF, ejection fraction; FS, fractional shortening; PDA, patent ductal arteriosus; Vmax, maximal velocity at coarctation isthmus; *, calculated based on infants with PDA
To determine independent predictors of non-significant CoA, univariable and multivariable logistic regression analysis was performed. The univariable regression analysis showed that young age, low ascending aorta diameter, and PDA were associated with non-significant CoA (Tab. 2). In the multivariable regression analysis, ascending aorta diameter [0.070 (95% CI: 0.005–0.136), P = 0.036] was an independent risk factors for non-significant CoA.
In order to determine the special features among these infants, a thorough analysis was performed case by case (Fig. 3). Among 64 infants with significant BP difference between upper limbs and lower limbs, 53 had 10 mm Hg higher SBP on upper limbs than those on lower limbs and 11 with 10 mm Hg higher lower limb SBP than those on upper limbs. Forty-seven of 53 presented with higher SBP on both arms, 6 with higher SBP on right arm. Eight of 11 presented with low cardiac output, 3 complicated with aortic arch obstruction. Among the 8 with low cardiac output, 2 presented with poor left ventricular cardiac output. The other 6 with severe PH and significant right-to-left shunt at ductal level. Among 69 with no BP difference between upper and lower limbs, 56 presented with PDA (3.1 ± 2.5 mm). Ten of 13 without PDA presented with severe PH, 2 with poor left ventricular cardiac output, 1 with mild CoA (Vmax 2.1 m/s). Among 3 infants with a weak femoral pulse, 2 presented with significant BP gradient and the other with neither BP gradient nor low SpO2 (Fig. 3). Among 7 infants with postductal CoA, 2 presented with higher BP on upper limbs, 5 left without BP difference. All these infants presented with severe PH and poor cardiac output and 1 presented with sufficient collateral supply to descending aorta.
When 95% of SpO2 value was chosen as a cutoff, 31, 31, 34, and 34 infants failed the test respectively on right arm, left arm, right leg, and left leg. 10 infants presented with more than 5% of SpO2 gradient between right arm and lower limbs. Among them, 1 presented with SpO2 higher than 90% on four limbs (right arm 99% and right leg 92%). Three infants presented with more than 5% SpO2 gradient between right and left arm. Two of them also presented with significant BP gradients.

Figure 3: The distribution of infants with different positive features. BP indicates blood pressure; SpO2, saturation of peripheral oxygen
After extensive prenatal echocardiography and postnatal SpO2 screening, CoA is still the most probably being missed CHD [1,6,7,17–26]. This study tried to determine the profiles of the clinical and echocardiographic features in these infants. Fifty-three of 133 (39.8%) infants could be spotted out by BP gradient and 34/133 (25.6%) by SpO2. CoA infants of older age are more likely to present significant BP gradient, even with PDA. Infants without BP gradient might have CoA, if the ascending aorta is smaller than usual.
CoA is a narrowing of the descending aorta. This defect generally results in higher BP before the coarctation and lower BP after the coarctation [9,10,27]. BP gradient between right arm and lower limbs has been supposed to be a feature of CoA. The severity of BP gradient depends on the lumen of coarctation isthmus. Based on this principle, CHD screening with BP was performed [9]. Boelke et al. [9] retrospectively reviewed 10,012 asymptomatic infants and 12 infants failed the BP screening. Nine of 12 were excluded of CoA by echocardiogram and 3 did not finish echocardiography but presented well at 1 year old. The other one was lost to follow-up. But this study was performed in a region of advanced prenatal echocardiography screening and all infants with prenatal diagnosis of CHD or admitted to NICU were excluded. As reported, high antenatal detection rate of CHD could offset the efficacy of postnatal CHD screening [22,28]. No death records reviewed in Boelke’s study might underestimate the incidence of CoA. On the contrary, Patankar et al. [8] did a case-control study of four limb BPs in CoA/IAA infants and found out that ≥10 mm Hg gradient cutoff might be the best point of CoA BP screening. But only 40% patients had this BP gradient. Similar to Patankar’s study, 53/133 (39.8%) infants in this study could be screened out by the BP gradient. Notably, infants without BP gradient couldn’t be ruled out of CoA, especially when infants were at the first several days of age. Moreover, although coarctation between left common carotid artery and brachiocephalic trunk could result in a BP gradient between right arm and left arm, in this study only 6 cases presented with this kind of BP gradient. There was no difference in BPs between lower limbs. SBP gradient is more prominent than diastolic BP gradient. So SBP gradient between right arm and lower limbs might be the best choice of CoA screening in high-risk population.
Pulse oximetry testing had been adopted by many countries as a routine to screen cyanotic CCHD [20,29]. As a potentially non-cyanotic CHD, 60%–80% of the infants with CoA failed to be diagnosed during their early lives [11,17,18,27,30,31]. Hu et al. [32] screened 167,190 infants at 15 hospitals and 5 of 6 CoA infants were missed by SpO2 screening. Lannering et al. [17] reviewed 90 CoA infants. Among 19 screened by SpO2, 15 CoA infants were missed. Ewer et al. [1] missed 5/8. de-Wahl Granelli et al. [26] missed 7/13. Garg et al. [25] missed 14/15. All 4 CoA infants passed SpO2 screening in Koppel’s study, 1 prenatally diagnosed, 1 with murmur, and 2 missed and progressing to heart failure [33]. Reich et al. [34] identified 2 CoA, both with SpO2 ≥ 95%. In this study, 34/133 (25.6%) of infants with CoA presented with low SpO2. Ten of 34 infants also presented with SpO2 gradient. One presented with SpO2 higher than 90% on four limbs. Due to 8 failed infants also presenting with significant BP gradient in this study, SpO2 screening could find out 26 more cases, which added up to 79 cases among 133 CoA infants (Fig. 3).
Beside four limb BPs and SpO2, evaluation of left ventricular cardiac output by femoral pulse palpation or peripheral perfusion index (PI) had also been tried as an approach of screening CoA [10–14]. With no equipment required, femoral pulse palpation was introduced into clinical practice much earlier than PI which came from oximeters by measuring the ratio of infrared light signals from arterial blood vs. venous blood and tissues. There are two means to determine a weak femoral pulse. One is to directly palpate the femoral artery pulse and the other is to indirectly compare the difference in pulses between femoral artery and radial artery or carotid artery. Lannering et al. [17] retrospectively reviewed 90 CoA infants and 31 of 53 infants had weak femoral pulses. Patankar et al. [8] found out 21 of 46 infants with CoA/IAA had decreased femoral pulses. Hoke et al. [35] screened 2,876 newborns and screened out 4 CoA, 3 of them with decreased femoral pulses. Furthermore, Khammari Nystrom et al. [12] used femoral pulse palpation test to screen CoA or other left-sided obstructive heart anomalies in 118,592 newborn infants at Stockholm-Gottland. All infants were followed up until 1–2 years of age. Weak or absent femoral pulses were noted in 432 infants. Fifteen of 78 infants with CoA had a positive test at femoral pulse palpation (absent or weak femoral pulses) and 63 had a negative test (normal femoral pulses). The sensitivity for CoA increased greatly when examined from <12 h of age to ≥96 h of age. In this study, there were 3 infants identified with weak femoral pulse, which is much less than the former studies. The possible reason might be no routine femoral pulse evaluation and subjectiveness of femoral palpation results. No comparison of pulse palpation between upper limbs and lower limbs could also decrease the positive rate of femoral pulse screening. PI is a sensitive indicator of peripheral perfusion [10]. Several studies combined PI with SpO2 screening and successfully identified CoA infants with low PI who passed SpO2 screening [10,11,13,14]. Others combined physical examination with PI and reported no additional benefit from PI screening because these infants with low PI always had pathological murmur [36]. Jegatheesan et al. [10] even reported that some healthy or non-CCHD neonates had ‘low’ PI. The reason for the controversy might in part be no normal range of PI had been determined so far [37]. In our study, weak femoral pulse could add one more case into 79 cases screened out by BP gradient and SpO2. Therefore, it’s difficult to count on femoral pulse palpation and PI as a way to identify CoA if clinicians didn’t get well trained. But from a long perspective view, due to no substitute to identify CoA, femoral pulse palpation and PI might be an addition to BP screening in the future.
Most isolated CoA infants don’t present with murmurs, especially when postnatal pulmonary vascular resistance minimized left to right shunt or poor cardiac output presented [17,38,39]. But murmur is still a sign of CHD, which could also be used as an approach to screen CoA, especially when CoA coexists with other cardiac anomalies. Lannering et al. [17] found that 25% of CoA infants presented with murmur. In our study, 86/133 (64.7%) presented with murmurs, including 49/80 (61.3%) in non-significant CoA infants and 65/99 (65.7%) in non-hypoxemic infants. The possible reason might be the difference in the study population. We enrolled 36 isolated CoA infants and 97 CoA infants with other complex CHD. In summary, murmur could add 36 more cases into 79 cases screened out by BP gradient and SpO2 (Fig. 3).
Peak velocity at coarctation isthmus had been used as an indicator for CoA [40–42]. We also tried to determine the association between non-significant CoA with other indices. After multivariable logistic regression analysis, only a smaller ascending aorta diameter was an independent predictor for non-significant CoA. However, this study was dedicated to finding out special features to screen out CoA. If echocardiography were available, it’s hard to miss CoA under 2 Dimension or Doppler echocardiography. We also tried to determine the association between PDA and CoA profiles with no important results identified. With patency of ductal arteriosus, four limb BPs or SpO2 did not add more clue of CoA. Moreover, collateral circulation after coarctation is a cofounding factor. These patients may not progress after PDA closure and may not need further intervention. Dysmorphic features might be a sign of CHD, but with low sensitivity and specificity and limited value at identifying CoA [8].
There are some limitations. First, this is not a population-based study. No incidence or prevalence rate could be calculated. But 133 is a relatively large sample size and could in part represent the proportion of different CoA profiles. The ratio of gender is similar to other population-based studies, which indicates little choosing bias in this study. One notion is that the age of infants in the CoA group was 6.6 days of age, while those in the significant CoA group was 16.6 days of age. Some infants progressing to shock at home could be missed in this study. As in Ward’s study, the cardiovascular collapse happened at 8 to 12 days of age [43]. Second, no follow-up data might decay the value of this study. As Boelke et al. and Lannering et al. [9,17] presented, some CoA infants might not need to be intervened in their first several years of life. We do not have the ability to clarify this question in this study, although we are following up these infants. Third, BP by non-invasive measurement might be variable to lead to false positive or false negative results [44]. Some normal infants also present with BP gradient with no obvious reason [9]. This might be random effects or with some other potential diseases. Since some CoA could also be missed at their first echocardiographic examination [17], we cannot expect much more on BP gradient screening. Therefore, using BP gradient solely to perform a population-based screening of CoA might not be the best option. Focusing on high-risk populations, BP gradient may increase its sensitivity and specificity. Fourth, with neonatal BP properties of variation and increasing with aging, this research only studied BP gradient instead of specific figures. This decision was also supported by Patankar’s study, which showed that BP increased with increasing age or gestational age at birth, but the difference between arms and legs did not change with gestational age [8]. Fifth, some PDA-related echocardiographic parameters were not prospectively collected. This might devalue the importance of echocardiography on the hemodynamic index.
In summary, the profiles of four limb BPs and SpO2 in infants with CoA varies very much. The BP gradient between right arm and lower limbs is an approach to identify CoA. A combination of BP, SpO2, and physical examination of murmur and peripheral perfusion may increase the sensitivity of CoA detection. Given some CoA infants presented with normal BP and SpO2 [8,9,45], a population-based long-term follow-up is necessary to determine the outcomes of these infants, especially the most likely being missed population.
Author Contributions: Study concept/design: Ruikun Zou, Chengcheng Pang, Yifei Wang
Data Collection: Ruikun Zou, Chen Chen
Data Analysis: Ruikun Zou, Yunxia Sun, Chengcheng Pang
Data Interpretation: Ruikun Zou, Yunxia Sun, Jian Zhuang, Yifei Wang
Drafting Article: Ruikun Zou, Yifei Wang
Critical Revision/Approval of Article: Ruikun Zou, Yifei Wang, Chengcheng Pang, Yunxia Sun, Chen Chen, Jian Zhuang
Availability of Data and Materials: All data in this study is safely stored in the local computer server at Guangdong Provincial People’s Hospital and can be accessed by request.
Funding Statement: Disclosure of grants or other funding: The Natural Science Foundation of Guangdong Province [2020A1515010904]; the Science and Technology Planning Project of Guangdong Province [2019B020230003];
Conflict of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Ewer, A. K., Middleton, L. J., Furmston, A. T., Bhoyar, A., Daniels, J. P. et al. (2011). Pulse oximetry screening for congenital heart defects in newborn infants (PulseOxA test accuracy study. Lancet, 378(9793), 785–794. DOI 10.1016/S0140-6736(11)60753-8. [Google Scholar] [CrossRef]
2. Mahle, W. T., Newburger, J. W., Matherne, G. P., Smith, F. C., Hoke, T. R. et al. (2009). Role of pulse oximetry in examining newborns for congenital heart disease: A scientific statement from the American heart association and American academy of pediatrics. Circulation, 120(5), 447–458. DOI 10.1161/CIRCULATIONAHA.109.192576. [Google Scholar] [CrossRef]
3. Brown, K. L., Ridout, D., Hoskote, A., Verhulst, L., Ricci, M. et al. (2006). Delayed diagnosis of congenital heart disease worsens preoperative condition and outcome of surgery in neonates. Heart, 92(9), 1298–1302. DOI 10.1136/hrt.2005.078097. [Google Scholar] [CrossRef]
4. Dhandayuthapani, G., Chakrabarti, S., Ranasinghe, A., Hunt, L., Grant, D. et al. (2010). Short-term outcome of infants presenting to pediatric intensive care unit with new cardiac diagnoses. Congenital Heart Disease, 5(5), 444–449. DOI 10.1111/j.1747-0803.2010.00430.x. [Google Scholar] [CrossRef]
5. Mellander, M., Sunnegardh, J. (2006). Failure to diagnose critical heart malformations in newborns before discharge–An increasing problem? Acta Paediatrica, 95(4), 407–413. DOI 10.1080/08035250500541910. [Google Scholar] [CrossRef]
6. Mouledoux, J. H., Walsh, W. F. (2013). Evaluating the diagnostic gap: Statewide incidence of undiagnosed critical congenital heart disease before newborn screening with pulse oximetry. Pediatric Cardiology, 34(7), 1680–1686. DOI 10.1007/s00246-013-0697-1. [Google Scholar] [CrossRef]
7. Mouledoux, J., Guerra, S., Ballweg, J., Li, Y., Walsh, W. (2017). A novel, more efficient, staged approach for critical congenital heart disease screening. Journal of Perinatology, 37(3), 288–290. DOI 10.1038/jp.2016.204. [Google Scholar] [CrossRef]
8. Patankar, N., Fernandes, N., Kumar, K., Manja, V., Lakshminrusimha, S. (2016). Does measurement of four-limb blood pressures at birth improve detection of aortic arch anomalies? Journal of Perinatology, 36(5), 376–380. DOI 10.1038/jp.2015.203. [Google Scholar] [CrossRef]
9. Boelke, K. L., Hokanson, J. S. (2014). Blood pressure screening for critical congenital heart disease in neonates. Pediatric Cardiology, 35(8), 1349–1355. DOI 10.1007/s00246-014-0935-1. [Google Scholar] [CrossRef]
10. Jegatheesan, P., Nudelman, M., Goel, K., Song, D., Govindaswami, B. (2017). Perfusion index in healthy newborns during critical congenital heart disease screening at 24 hours: Retrospective observational study from the USA. BMJ Open, 7(12), e017580. DOI 10.1136/bmjopen-2017-017580. [Google Scholar] [CrossRef]
11. Siefkes, H., Kair, L., Tancredi, D. J., Vasquez, B., Garcia, L. et al. (2020). Oxygen saturation and perfusion index-based enhanced critical congenital heart disease screening. American Journal of Perinatology, 37(2), 158–165. DOI 10.1055/s-0039-1685445. [Google Scholar] [CrossRef]
12. Khammari Nystrom, F., Petersson, G., Stephansson, O., Johansson, S., Altman, M. (2020). Diagnostic values of the femoral pulse palpation test. Archives of Disease in Childhood–Fetal and Neonatal Edition, 105(4), 375–379. DOI 10.1136/archdischild-2019-317066. [Google Scholar] [CrossRef]
13. Schena, F., Picciolli, I., Agosti, M., Zuppa, A. A., Zuccotti, G. et al. (2017). Perfusion index and pulse oximetry screening for congenital heart defects. Journal of Pediatrics, 183, 74–79.e1. DOI 10.1016/j.jpeds.2016.12.076. [Google Scholar] [CrossRef]
14. Granelli, A., Ostman-Smith, I. (2007). Noninvasive peripheral perfusion index as a possible tool for screening for critical left heart obstruction. Acta Paediatrica, 96(10), 1455–1459. DOI 10.1111/j.1651-2227.2007.00439.x. [Google Scholar] [CrossRef]
15. Wang, Y., He, S., Sun, Y., Liang, S., Liu, Y. et al. (2017). Role of systolic blood pressure gradient of limb test in diagnosing neonatal aorta anomaly. Chinese Journal of Applied Clinical Pediatrics, 32(1), 22–25. [Google Scholar]
16. Pettersen, M. D., Du, W., Skeens, M. E., Humes, R. A. (2008). Regression equations for calculation of z scores of cardiac structures in a large cohort of healthy infants, children, and adolescents: An echocardiographic study. Journal of the American Society of Echocardiography, 21(8), 922–934. DOI 10.1016/j.echo.2008.02.006. [Google Scholar] [CrossRef]
17. Lannering, K., Bartos, M., Mellander, M. (2015). Late diagnosis of coarctation despite prenatal ultrasound and postnatal pulse oximetry. Pediatrics, 136(2), e406–e412. DOI 10.1542/peds.2015-1155. [Google Scholar] [CrossRef]
18. Peterson, C., Ailes, E., Riehle-Colarusso, T., Oster, M., Olney, R. et al. (2014). Late detection of critical congenital heart disease among US infants: Estimation of the potential impact of proposed universal screening using pulse oximetry. JAMA Pediatrics, 168(4), 361–370. DOI 10.1001/jamapediatrics.2013.4779. [Google Scholar] [CrossRef]
19. Thangaratinam, S., Brown, K., Zamora, J., Khan, K. S., Ewer, A. K. (2012). Pulse oximetry screening for critical congenital heart defects in asymptomatic newborn babies: A systematic review and meta-analysis. Lancet, 379(9835), 2459–2464. DOI 10.1016/S0140-6736(12)60107-X. [Google Scholar] [CrossRef]
20. Manzoni, P., Martin, G. R., Sanchez Luna, M., Mestrovic, J., Simeoni, U. et al. (2017). Pulse oximetry screening for critical congenital heart defects: A European consensus statement. Lancet Child & Adolescent Health, 1(2), 88–90. DOI 10.1016/S2352-4642(17)30066-4. [Google Scholar] [CrossRef]
21. Chew, C., Halliday, J. L., Riley, M. M., Penny, D. J. (2007). Population-based study of antenatal detection of congenital heart disease by ultrasound examination. Ultrasound in Obstetrics and Gynecology, 29(6), 619–624. DOI 10.1002/uog.4023. [Google Scholar] [CrossRef]
22. Cloete, E., Bloomfield, F. H., Cassells, S. A., de Laat, M. W. M.,Sadler, L. et al. (2019). Newborn pulse oximetry screening in the context of a high antenatal detection rate of critical congenital heart disease. Acta Paediatrica, 109(1), 93–99. DOI 10.1111/apa.14946. [Google Scholar] [CrossRef]
23. Marek, J., Tomek, V., Skovránek, J., Povysilová, V., Samánek, M. (2010). Prenatal ultrasound screening of congenital heart disease in an unselected national population: A 21-year experience. Heart, 97(2), 124–130. DOI 10.1136/hrt.2010.206623. [Google Scholar] [CrossRef]
24. Aamir, T., Kruse, L., Ezeakudo, O. (2007). Delayed diagnosis of critical congenital cardiovascular malformations (CCVM) and pulse oximetry screening of newborns. Acta Paediatrica, 96(8), 1146–1149. DOI 10.1111/j.1651-2227.2007.00389.x. [Google Scholar] [CrossRef]
25. Garg, L. F., Van Naarden Braun, K., Knapp, M. M., Anderson, T. M., Koppel, R. I. et al. (2013). Results from the New Jersey statewide critical congenital heart defects screening program. Pediatrics, 132(2), e314–e323. DOI 10.1542/peds.2013-0269. [Google Scholar] [CrossRef]
26. de-Wahl Granelli, A., Wennergren, M., Sandberg, K., Mellander, M., Bejlum, C. et al. (2009). Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: A Swedish prospective screening study in 39,821 newborns. BMJ, 338(jan08 2), a3037–a3037. DOI 10.1136/bmj.a3037. [Google Scholar] [CrossRef]
27. Rahiala, E., Tikanoja, T. (1997). Non-invasive blood pressure measurements and aortic blood flow velocity in neonates. Early Human Development, 49(2), 107–112. DOI 10.1016/S0378-3782(97)01883-5. [Google Scholar] [CrossRef]
28. Lytzen, R., Vejlstrup, N., Bjerre, J., Petersen, O. B., Leenskjold, S. et al. (2019). Mortality and morbidity of major congenital heart disease related to general prenatal screening for malformations. International Journal of Cardiology, 290, 93–99. DOI 10.1016/j.ijcard.2019.05.017. [Google Scholar] [CrossRef]
29. Glidewell, J., Grosse, S. D., Riehle-Colarusso, T., Pinto, N., Hudson, J. et al. (2019). Actions in support of newborn screening for critical congenital heart disease—United States, 2011-2018. MMWR. Morbidity and Mortality Weekly Report, 68(5), 107–111. DOI 10.15585/mmwr.mm6805a3. [Google Scholar] [CrossRef]
30. Diller, C. L., Kelleman, M. S., Kupke, K. G., Quary, S. C., Kochilas, L. K. et al. (2018). A modified algorithm for critical congenital heart disease screening using pulse oximetry. Pediatrics, 141(5), e20174065. DOI 10.1542/peds.2017-4065. [Google Scholar] [CrossRef]
31. Banait, N., Ward-Platt, M., Abu-Harb, M., Wyllie, J., Miller, N. et al. (2020). Pulse oximetry screening for critical congenital heart disease: A comparative study of cohorts over 11 years. Journal of Maternal-Fetal & Neonatal Medicine, 33(12), 2064–2068. DOI 10.1080/14767058.2018.1538348. [Google Scholar] [CrossRef]
32. Hu, X. J., Ma, X. J., Zhao, Q. M., Yan, W. L., Ge, X. L. et al. (2017). Pulse oximetry and auscultation for congenital heart disease detection. Pediatrics, 140(4), e20171154. DOI 10.1542/peds.2017-1154. [Google Scholar] [CrossRef]
33. Koppel, R. I., Druschel, C. M., Carter, T., Goldberg, B. E., Mehta, P. N. et al. (2003). Effectiveness of pulse oximetry screening for congenital heart disease in asymptomatic newborns. Pediatrics, 111(3), 451–455. DOI 10.1542/peds.111.3.451. [Google Scholar] [CrossRef]
34. Reich, J. D., Connolly, B., Bradley, G., Littman, S., Koeppel, W. et al. (2008). The reliability of a single pulse oximetry reading as a screening test for congenital heart disease in otherwise asymptomatic newborn infants. Pediatric Cardiology, 29(5), 885–889. DOI 10.1007/s00246-008-9214-3. [Google Scholar] [CrossRef]
35. Hoke, T. R., Donohue, P. K., Bawa, P. K., Mitchell, R. D., Pathak, A. et al. (2002). Oxygen saturation as a screening test for critical congenital heart disease: A preliminary study. Pediatric Cardiology, 23(4), 403–409. DOI 10.1007/s00246-002-1482-8. [Google Scholar] [CrossRef]
36. Uygur, O., Koroglu, O. A., Levent, E., Tosyali, M., Akisu, M. et al. (2019). The value of peripheral perfusion index measurements for early detection of critical cardiac defects. Pediatrics and Neonatology, 60(1), 68–73. DOI 10.1016/j.pedneo.2018.04.003. [Google Scholar] [CrossRef]
37. Searle, J., Thakkar, D. D., Banerjee, J. (2019). Does pulsatility index add value to newborn pulse oximetry screening for critical congenital heart disease? Archives of Disease in Childhood, 104(5), 504–506. DOI 10.1136/archdischild-2018-315891. [Google Scholar] [CrossRef]
38. Ainsworth, S., Wyllie, J. P., Wren, C. (1999). Prevalence and clinical significance of cardiac murmurs in neonates. Archives of Disease in Childhood–Fetal and Neonatal Edition, 80(1), F43–F45. DOI 10.1136/fn.80.1.F43. [Google Scholar] [CrossRef]
39. Singh, A., Desai, T., Miller, P., Rasiah, S. V. (2012). Benefits of predischarge echocardiography service for postnatal heart murmurs. Acta Paediatrica, 101(8), e333–e336. DOI 10.1111/j.1651-2227.2012.02687.x. [Google Scholar] [CrossRef]
40. Egbe, A. C., Reddy, Y. N. V., Obokata, M., Borlaug, B. A. (2020). Doppler-derived arterial load indices better reflect left ventricular afterload than systolic blood pressure in coarctation of aorta. Circulation Cardiovascular Imaging, 13(2), e009672. [Google Scholar]
41. Christopher, A. B., Apfel, A., Sun, T., Kreutzer, J., Ezon, D. S. (2018). Diastolic velocity half time is associated with aortic coarctation gradient at catheterization independent of echocardiographic and clinical blood pressure gradients. Congenital Heart Disease, 13(5), 713–720. DOI 10.1111/chd.12637. [Google Scholar] [CrossRef]
42. Boe, B. A., Norris, M. D., Zampi, J. D., Rocchini, A. P., Ensing, G. J. (2017). Temporal relationship between instantaneous pressure gradients and peak-to-peak systolic ejection gradient in congenital aortic stenosis. Congenital Heart Disease, 12(6), 733–739. DOI 10.1111/chd.12514. [Google Scholar] [CrossRef]
43. Ward, K. E., Pryor, R. W., Matson, J. R., Razook, J. D., Thompson, W. M. et al. (1990). Delayed detection of coarctation in infancy: Implications for timing of newborn follow-up. Pediatrics, 86(6), 972–976. [Google Scholar]
44. Crossland, D. S., Furness, J. C., Abu-Harb, M., Sadagopan, S. N., Wren, C. (2004). Variability of four limb blood pressure in normal neonates. Archives of Disease in Childhood–Fetal and Neonatal Edition, 89(4), F325–F327. DOI 10.1136/adc.2003.034322. [Google Scholar] [CrossRef]
45. Muppidi, S., Ngeny, G., Onwauayi, A. (2011). Coarctation of aorta with normal blood pressure. Journal of the National Medical Association, 103(2), 173–175. DOI 10.1016/S0027-9684(15)30268-6. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |