 Congenital Heart Disease
Congenital Heart Disease
 Congenital Heart Disease Congenital Heart Disease |  |
DOI: 10.32604/CHD.2021.013724
ARTICLE
Changes in Reverse Cardiac Remodeling after Percutaneous Atrial Septal Defect Closure in Children and Adults
1Department of Pediatrics, Chungnam National University Sejong Hospital, Chungnam National University School of Medicine, Sejong, 30099, Korea
2Department of Pediatrics, Chungnam National University Hospital, Chungnam National University School of Medicine, Daejeon, 35105, Korea
*Corresponding Author: Hong Ryang Kil. Email: gilhongr@gmail.com
Received: 18 August 2020; Accepted: 20 October 2020
Abstract: Background: The influence of the timing of transcatheter atrial septal defect (ASD) closure on ventricular remodeling at 6 months after ASD closure is unclear. This study investigated changes in cardiac remodeling after transcatheter closure of large ASDs according to patient age at the time of the procedure. Methods: In this study, 41 children and 43 adults underwent percutaneous closure of a large ASD. Cardiac remodeling was assessed by two-dimensional echocardiography and electrocardiography before and at 6 months after ASD closure. Results: The age of the children and adults were 2.8 ± 3.1 and 50.0 ± 15.6 years, respectively. The Qp/Qs ratio of all patients was 2.24 ± 0.67. The right atrial (RA) maximal dimension and right ventricular (RV) transverse diameter were significantly decreased and the left ventricular (LV) dimension was significantly increased at 6 months after ASD closure. However, the difference in RA and RV dimension changes between the groups was not statistically significant. The difference in left atrial (LA) dimension changes between the groups was also not statistically significant, but the LV dimension significantly increased in children compared with that in adults (P = 0.018). The RV/LV ratio was decreased after ASD closure, and a significant difference was found in the RV/LV ratio changes between the groups. In ECG, the PR interval was significantly more decreased in adults than in children (P = 0.003). Conclusions: In conclusion, the LV diameter was significantly more increased in children than in adults at 6 months after percutaneous ASD closure. Thus, cardiac remodeling after percutaneous ASD closure varies in children and adults.
Keywords: Atrial septal defect; ventricular remodeling
Atrial septal defect (ASD) accounts for approximately 10% of congenital heart diseases [1]. It can be diagnosed earlier in childhood as well as later in adulthood because of the asymptomatic characteristics of ASD [2]. Percutaneous transcatheter closure of ASD has been regarded as a gold standard treatment option because it is considered to be a safe and effective treatment, except for special cases that require surgical repair [3]. Usually, the timing of transcatheter ASD closure in children varies according to intuitional policies [4]. However, in the recent trend of transcatheter ASD closure, the timing of treatment is earlier [5]. Early treatment in large ASD cases is important because complications, such as pulmonary hypertension, arrhythmia, and chronic right ventricular (RV) heart failure, could occur and would require a larger device when closure is performed later [2,6].
Right heart volume overload through a significant left-to-right shunt in ASD is improved after ASD closure. RV remodeling after transcatheter ASD closure showed a significant decrease in RV end-diastolic dimension (RVEDD) at 6 months after ASD closure. Moreover, left ventricular (LV) remodeling after ASD closure was shown to increase the LV end-diastolic dimension (LVEDD) [7,8]. Thus, we speculated whether the timing of transcatheter ASD closure influenced ventricular remodeling at 6 months after ASD closure. This study investigated changes in cardiac remodeling after transcatheter closure of large ASDs according to patient age at the time of the procedure.
2.1 Patients and Data Collection
This retrospective study reviewed the charts of 89 patients with secundum-type ASD who underwent percutaneous ASD closure in the pediatric cardiology and cardiology of Chungnam National University Hospital from January 2014 to December 2018. Our Institutional Review Board approved the retrospective design.
The inclusion criterion was large secundum ASD with a significant left-to-right shunt, showing right heart volume overload with a Qp/Qs ratio >1.5. The exclusion criteria were cases that needed surgical repair because of failed percutaneous ASD closure, cases above moderate pulmonary hypertension, cases with a combined arrhythmia that was difficult to analyze on ECG, and cases with other congenital heart diseases combined with ASD. Of the 89 patients, five were excluded as they either required device embolization, had severe pulmonary hypertension with pulmonary vascular resistance exceeding 8 Wood units, or were lost to follow-up. Finally, 84 patients were analyzed in this study.
We divided the patients into two groups: children (<18 years) and adults (≥18 years). We investigated the clinical and hemodynamic characteristics, echocardiographic data, and electrocardiographic data before and after the procedure. In addition, we analyzed the percentage change in cardiac remodeling according to time and the percentage change in electrocardiographic parameters between the two groups.
2.2 Echocardiography and Electrocardiography
Before the procedure and 6 months after the ASD procedure, all patients were evaluated using transthoracic echocardiography (TTE). We used available ultrasound machines (GE Vivid 7, GE Vingmed Ultrasound AS, Horten, Norway) with 6-MHz and 3-MHz transducers. In uncooperativechildren, the procedure was performed by echocardiographic evaluation under sedation, if needed. For reproducibility of the echocardiographic measurements, at least three images in the 4-chamber view were analyzed. RV and LV dimensions were measured as the transverse diameters within one-third of the distance below the tricuspid valve annulus towards the ventricular apices at the end-diastole phase by two-dimensional mode in the 4-chamber view [9]. The right atrium (RA) and left atrium (LA) minor axes were measured transversally at the end-systole phase in the 4-chamber view [10].
All values were indexed for body surface area (BSA). RVEDD, LVEDD, RA, and LA diameters were measured in the 4-chamber view before and at 6-months follow-up. Percent change was calculated by the following formula: (value after ASD closure—value before ASD closure)/value before ASD closure × 100.
We also checked the standard 12-derivation ECG at a rate of 25 mm/s and a calibration of 1 mV/cm for all patients at baseline and at 6 months after ASD closure. The P-wave, PR interval, and QRS duration were analyzed and the QT (QTc) interval and tallest R wave in the V1 lead was corrected in all patients.
For all children, ASD closure was performed using an Amplatzer septal occluder (St. Jude Medical, St. Paul, MN, USA) under general anesthesia and transesophageal echocardiography (TEE). In the adult group, we did not apply sedation; we used intracardiac echocardiography to guide the procedure and used an Amplatzer septal occluder or Occlutech septal occluder (Occlutech GmbH, Jena, Germany), as selected by the operator. A hemodynamic study, which included oxygen saturation in the chamber and vessels and pressures in the pulmonary artery, LV, and aorta, was conducted. The defect size was determined on a fluoroscopic image by balloon sizing, and the appropriate device size was selected. Successful deployment of the device was confirmed based on the presence or absence of a significant leakage by TEE or intracardiac echocardiography. During device closure, 100 IU/kg of heparin and prophylactic antibiotics were administered to all patients, and a low dose of aspirin was prescribed until 6 months after device closure.
All data were analyzed using SPSS version 21 for Windows (IBM Corp., Armonk, NY, USA). Continuous variables are expressed as mean value ± standard deviation for normally distributed data. Categorical variables are presented as counts with percentages. Independent t-test was used to compare continuous variables, while the Chi square test was used for categorial variables. Paired t-test was used to compare the change at 6 months in each group. A p-value <0.05 was considered statistically significant.
For the total cohort, the majority of patients were women (n = 56, 66%), and the age at the time of the procedure was 27.0 years ± 2.8 years (range, 1–82). The ASD diameter of all patients measured by TEE before ASD closure was 13.2 mm ± 5.7 mm (range, 6–30). The ASD diameter measured by catheter balloon sizing was 17.4 mm ± 5.5 mm, and the device diameter in ASD closure was 18.6 mm ± 7.6 mm (range, 6 mm–36 mm). The Qp/Qs ratio was 2.2 ± 0.6, and the pulmonary artery pressure measured before ASD closure was 21.3 mmHg ± 7.5 mmHg. The systolic arterial pressure was 106.4 mmHg ± 21.5 mmHg, and the ratio of the systolic pulmonary artery pressure to the systolic arterial pressure was 0.29 ± 0.10.
In Tab. 1, we demonstrated the clinical and hemodynamic characteristics of the children (n = 41) and adults (n = 43). The average age of the children was 2.8 years ± 3.1 years and that of the adults was 50.0 years ± 15.6 years. No difference was found in sex distribution, which means that most of the patients in the two groups were female. The mean weight of the children was 13.6 kg ± 11.5 kg and that of the adults was 62.6 kg ± 0.5 kg. The age, weight, and BSA were naturally different in each group. The ASD diameter at TTE in children and adults was also different, at 11.2 mm ± 3.6 mm and 21.3 mm ± 5.5 mm, respectively. The ASD diameter by BSA showed a significant difference. The ASD device size was larger in adults than in children, and the Qp/Qs ratio in each group showed no significant difference. Although the pulmonary artery pressure was higher in adults than in children, no significant difference was found in the ratio of the systolic pulmonary artery pressure to the systolic arterial pressure.
Table 1: Clinical and hemodynamic characteristics
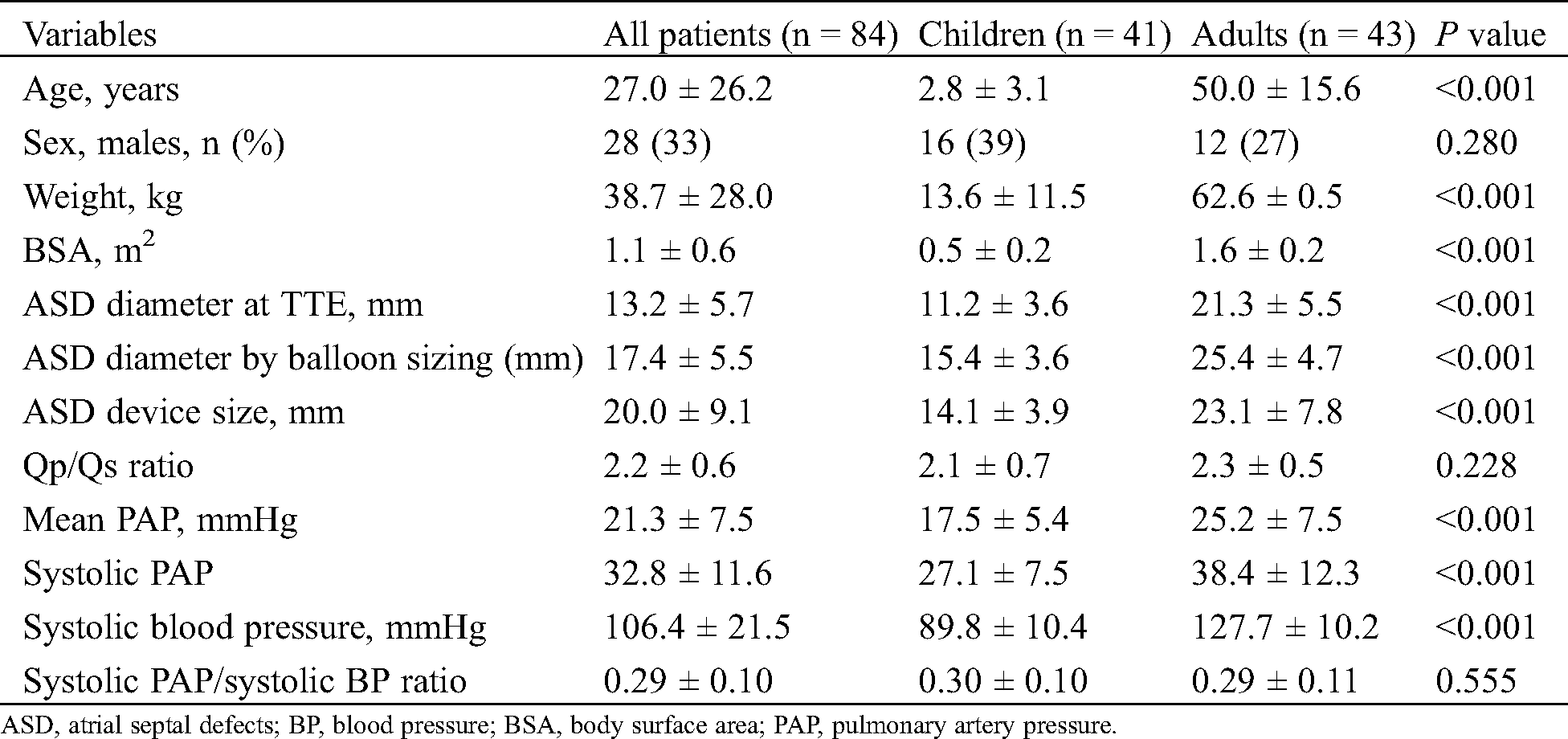
Comparing the timing of the procedure, significant decreases were found in the indexed RA diameter (36.0 mm/m2 ± 18.0 mm/m2 to 33.4 mm/m2 ± 18.3 mm/m2; P < 0.001) and the indexed RVEDD (35.2 ± 13.3 to 30.5 ± 12.3; P < 0.001). The indexed LA diameters before and after 6 months were 27.6 ± 12.8 and 28.4 ± 13.4, respectively. No significant change was found in the LA maximal diameter (P = 0.116). A significant increase in the indexed LVEDD was observed at 6 months compared with that before ASD closure (29.9 ± 12.1 to 33.8 ± 14.6; P < 0.001) (Fig. 1).
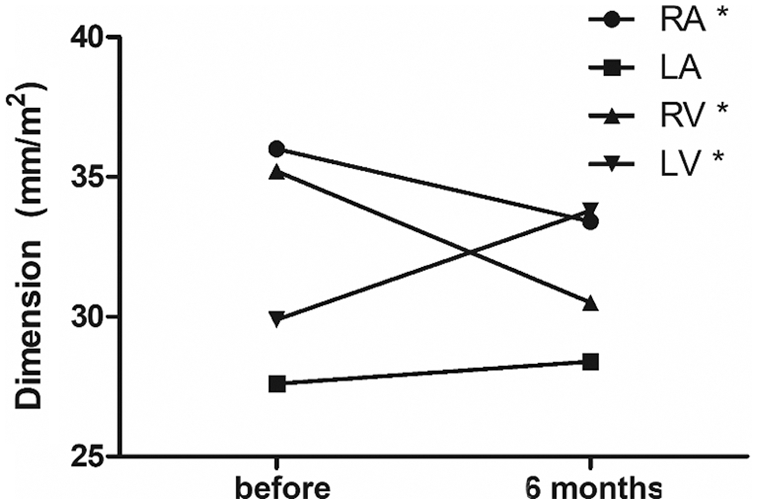
Figure 1: Changes in echocardiographic parameters in all patients. LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle
Tab. 2 shows the echocardiographic data of the two groups, namely, indexed RVEDD, LVEDD, RV/LV ratio, RA diameter, and LA diameter (i.e., divided by the BSA), which were described according to the timing, and their percentage changes were analyzed. Before ASD closure, significant differences were found between children and adults for indexed RVEDD (46.9 ± 9.3 vs. 24.5 ± 3.6; P < 0.001), indexed LVEDD (40.4 ± 8.8 vs. 20.1 ± 3.7; P < 0.001), indexed RA diameter (52.9 ± 10.7 vs. 25.5 ± 3.8; P < 0.001), and indexed LA diameter (38.5 ± 9.2 vs. 21.3 ± 3.0; P < 0.001). However, no difference in RV/LV ratio was found between the two groups before ASD closure (children vs. adults, 1.1 ± 0.1 vs. 1.2 ± 0.2, P = 0.104). At 6 months after ASD closure, significant differences were also observed between children and adults for indexed RVEDD (41.4 ± 8.5 vs. 20.4 ± 3.2; P < 0.001), indexed LVEDD (47.0 ± 9.4 vs. 21.6 ± 3.5; P < 0.001), indexed RA diameter (49.9 ± 9.7 vs. 22.8 ± 3.5; P < 0.001), and indexed LA diameter (40.5 ± 8.8 vs. 21.4 ± 3.3; P <0.001). No difference in RV/LV ratio was noted between the two groups after ASD closure (children vs. adults, 0.88 ± 0.14 vs. 0.96 ± 0.19; P = 0.057). The percentage change from before to after ASD closure for RVEDD in children and adults was −11.3% ± 9.7% and −15.8% ± 13.3%, respectively (P = 0.097). No significant differences were found in the changes in RA diameter (children vs. adults, −6.2% ± 9.6% vs. −9.9% ± 11.1%, P = 0.145), LA diameter (5.6 ± 17.1 vs. 0.9 ± 13.1; P = 0.187), and RV/LV ratio (−23.8% ± 10.9% vs. −20.6% ± 18.7%; P = 0.345) between the two groups. However, in LVEDD, the percentage change in children was significantly higher than that in adults (17.8 ± 14.1 vs. 9.0 ± 17.9; P = 0.018) (Tab. 2, Fig. 2).
Table 2: Echocardiographic data before and after ASD closure
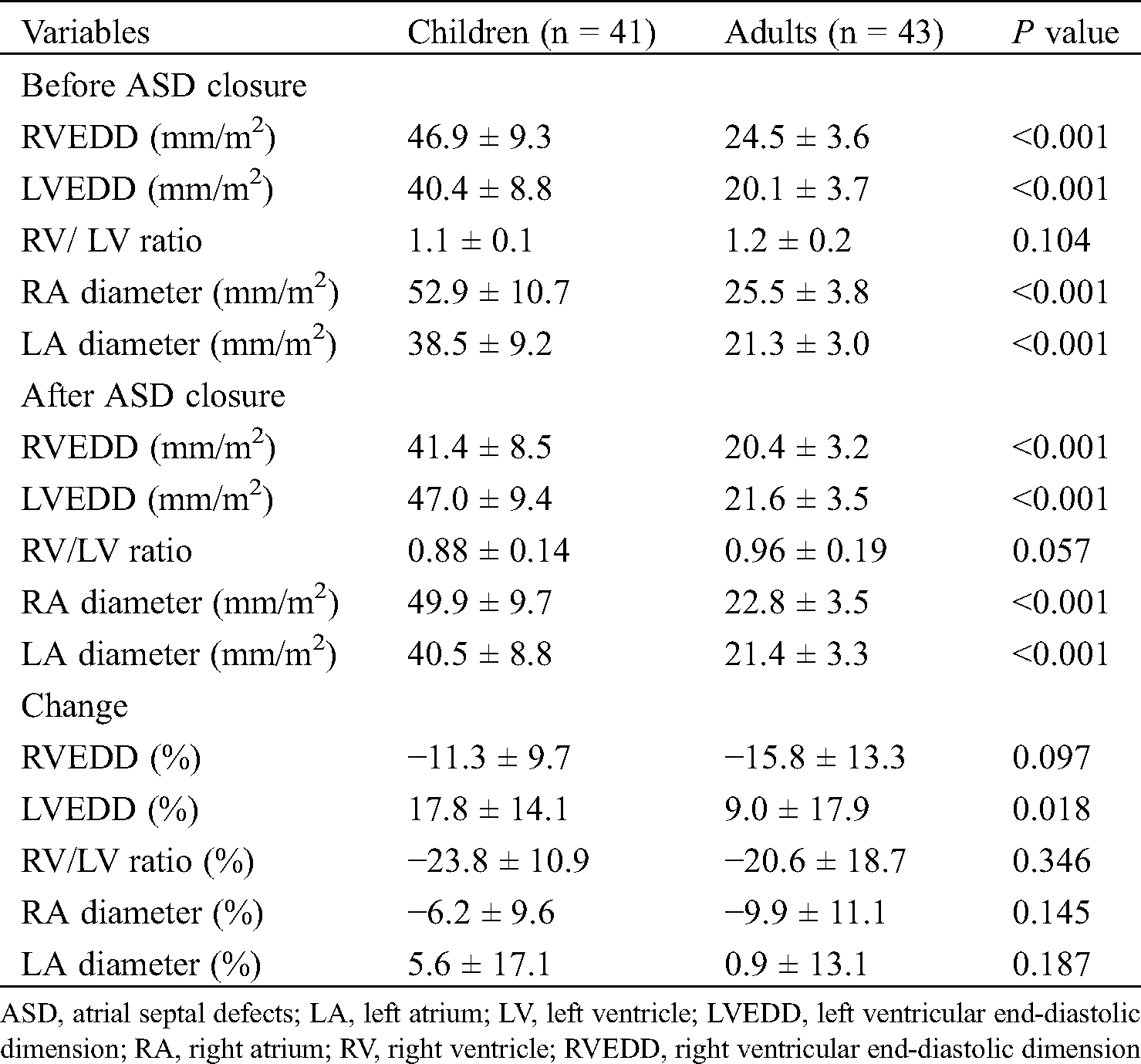
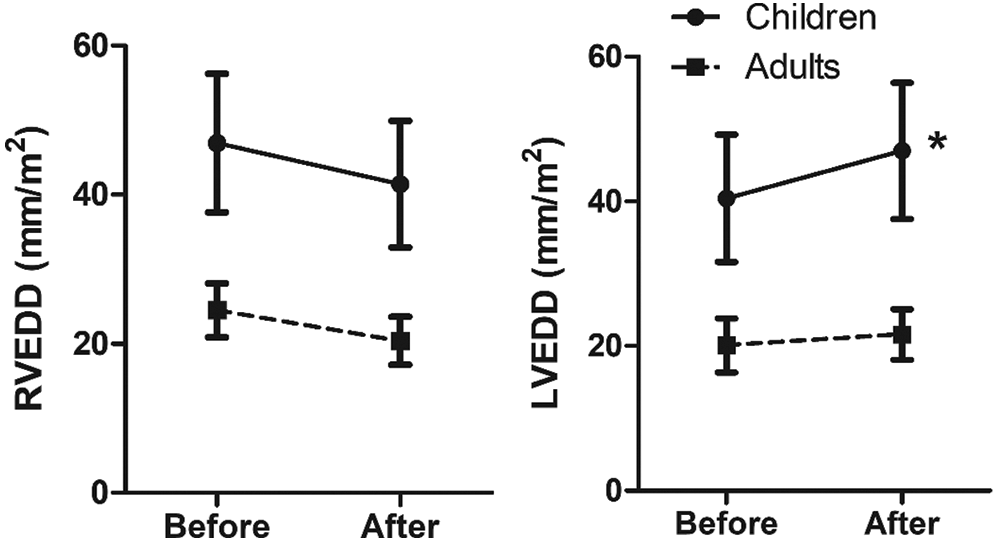
Figure 2: Comparison of size of ventricles before and at 6 months after ASD closure. RVEDD, right ventricular end-diastolic dimension; LVEDD, left ventricular end-diastolic dimension; * significant increase
In the ECG analyses of all patients, significant decreases from before to 6 months after ASD closure were observed in heart rate (93 bpm ± 26 bpm to 85 bpm ± 22 bpm; P = 0.001), PR interval (159 ms ± 34 ms to 151 ms ± 25 ms; P = 0.001), QRS duration (91 ms ± 18 ms to 89 ms ± 16 ms; P = 0.025), QTc interval (446 ms ± 28 ms to 435 ms ± 27 ms; P < 0.001), and R wave in V1 (0.75 mV ± 0.60 mV to 0.45 mV ± 0.34 mV; P < 0.001).
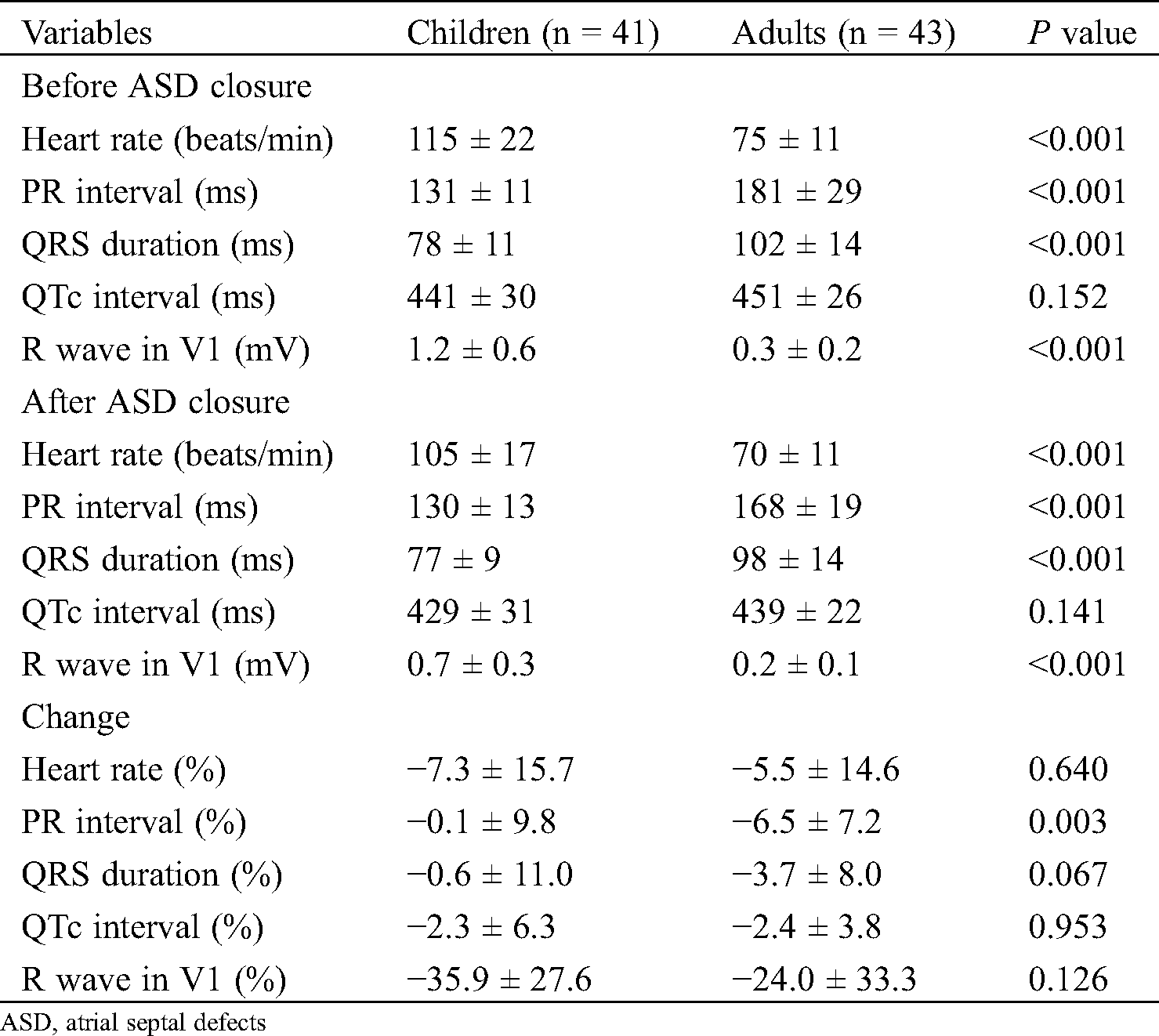
In Tab. 3, we analyzed electrocardiographic data before and after ASD closure in the two groups. The heart rate, PR interval, QRS duration, and R wave in V1 were significantly different between the two groups at baseline, except the QTc interval which had the same pattern after ASD closure. The percentage change in the heart rate in children and adults was −7.3% ± 15.7% and −5.5% ± 14.6%, respectively (P = 0.640). The percentage change in the QRS duration in children and adults was −0.6% ± 11.0% and −3.7% ± 8.0%, respectively (P = 0.067). The percent change in the QTc interval in children and adults was −2.3 ms ± 6.3 ms and −2.4 ms ± 3.8 ms, respectively (P = 0.953). Moreover, no significant reduction in R wave was found in V1 in children versus adults (children vs. adult, −35.9% ± 27.6% vs. −24.0% ± 33.3%, P = 0.126). However, a significant reduction was observed for the PR interval in the adults (−6.5% ± 7.2%) compared with children (−0.1% ± 9.8%; P = 0.003).
Table 3: Electrocardiographic data before and after ASD closure
We evaluated atrial and ventricular dimensions and other electrocardiographic parameters in children and adults who underwent transcatheter closure of secundum-type ASD for 6 months. We identified that the right heart volume tended to decrease, and the left heart dimension increased in all age groups. This study interestingly showed that the LV dimension in children had a significantly greater increase than in adults at 6 months after transcatheter ASD closure. Nonetheless, no significant differences were found in the changes in RVEDD, RA diameter, and LA diameter between children and adults.
In all patients, the right heart dimension decreased, and LV diameter increased at 6 months after transcatheter ASD closure. Consistent with our study, many studies reported on cardiac remodeling after transcatheter ASD closure. Right heart volume reduction has been previously established in many studies from the early to intermediate term [6,7,11]. A study reported that cardiac remodeling occurred earlier, even within 24 h after transcatheter ASD closure [12]. Santoro et al. [13] showed that RV volume decreased by 14% at 24 h and 19% at 1 month after ASD closure, regardless of the age and magnitude of the pre-closure cardiac overload. Moreover, the left heart volume has been known to increase at follow-up evaluation in other studies [13,14]. LV remodeling occurred earlier and a striking pattern was seen after shunt disappearance, eventually resulting in RV/LV ratio normalization [12]. This could be explained by ventricular interdependence, normalization of the ventricular septum because of RV volume reduction, increase in blood flow through the mitral valve, or the fact that LV volume was normalized and LV filling was increased after eliminating the ASD shunt [6,7].
In this study, the children were younger than those in another study that compared children and adults who underwent transcatheter ASD closure. In this study, the age of the children was 2.8 ± 3.1 years. Santoro et al. [15] reported the change in RV and LV dimensions at 6 months after transcatheter ASD closure by comparing asymptomatic patients younger than 16 years (median 8 years) and asymptomatic adults (median 38 years). Another study comparing children and adults by Vijarnsorn et al. [14] where subjects were divided into groups according to age, that is, children (below 18 years old), adults, and older adults, also showed no difference in ventricular remodeling according to the age group. Du et al. [12] reported that the reversion of RV volume overload was faster in younger patients with a relatively milder dilated RV than in older patients with a more dilated RV. Conversely, Pascotto et al. [16] showed that the larger amount of RV volume overload correlated with larger remodeling capacity after ASD closure. The percentage increase in LV end-diastolic volume correlated with RV volume overload, suggesting an important effect of the shunt relief on the stroke volume increase.
Considering the changes in LV diastolic function after transcatheter ASD closure, Gomez et al. [17] demonstrated no change in tissue Doppler early diastolic peak annular velocity (Ea) with changes in LV load after ASD closure but showed a significant increase in the mitral inflow peak E-wave, thereby indicating that the E/Ea ratio was more load dependent. Lange et al. demonstrated that Ea velocity was decreased in adults and that long-standing RV volume overload and LV myocardial property might cause the inability of the LV to adapt to an increased preload. However, Giardini et al. [18] showed that the Ea velocity in children was improved after ASD closure. The variations in the results could be caused by differences in age, amount of ASD shunt, and myocardium condition. Masutani et al. [19] also showed that changes in LV filling pressure were different between younger and older patients. In adults, the elevation of the LV filling pressure could be explained by myocardial stiffness after transcatheter ASD closure. On the contrary, the left ventricle of children, especially younger patients, might be more compliant to adapt to the increased flow to the left ventricle without accompanying increased LV filling pressure. In our study, LVEDD increased by 17.8% of the mean value in children and 9.0% of the mean value in adults after 6 months; this finding could be explained by the difference in LV myocardial stiffness. The results of this study indicated that a large amount of ASD shunts in young children could affect LV volume, and the LV end-diastolic volume might influence stroke volume. This finding implies that an early referral would be needed from the physician for transcatheter ASD closure in children if the patient has a significant RV volume overload and LV volume shrinkage regardless of the symptoms. One study argued that the timing of complete remodeling could be different depending on the patient’s age and that there is a decrease in RV dimension leveled off at 6 months after ASD closure in children and in older adults, but continued for 1 year in a middle-aged adult group; in our study, we did not subdivide adult patients into middle-aged or older adults and follow-up was only for 6 months [14].
In the ECG analyses, reductions in the heart rate, PR interval, QRS duration, QTc interval, and R wave in V1 was observed after ASD closure, which might be related to the improvement of symptoms, right heart volume loading, reversible right bundle branch block, and complex electrical remodeling. Prolonged PR interval, QRS duration, and QTc interval following a volume overload might reflect a mechanoelectrical phenomenon associated with RV volume overload. Roushdy et al. [20] also showed results consistent with ours, that is, a decrease in thePR interval and QTc interval. On the contrary, Kaya et al. [7] showed that the PR interval was not decreased after ASD closure, but they enrolled children and young adults, and Santoro et al. [13] presented no significant change in electrocardiographic intervals. Another study reported significant decreases in atrial and atrioventricular node refractory periods and increased atrial conduction velocity through an electrophysiologic study after surgical ASD closure [21]. Our study demonstrated that the PR interval in children was not influenced by ASD closure, because the PR interval was at a nearly normal level before ASD closure, but the PR interval in adults was significantly prolonged before and then significantly decreased after ASD closure. Chronic atrial geometrical changes in ASD in adults were associated with atrial electrical remodeling by an altered atrial effective refractory period, showing a prolonged P-wave duration [22]. Incomplete right heart electrical remodeling was reported at the mid-term follow-up regardless of geometrical remodeling [13]. Although this study did not evaluate the change in P dispersion after ASD closure as recently investigated in other studies [7,22], the difference in the PR interval change by age suggested the advantages of early intervention of transcatheter ASD closure.
This study had some limitations. First, this study had small patient numbers, a retrospective study design, and a short-term follow-up of 6 months. Second, the pulmonary arterial pressure of the adult group was mildly elevated, but there was no statistically significant difference. Third, echocardiographic measurement could have limited accuracy and reproducibility compared with other imaging modalities. Fourth, we did not measure functions of the RV and LV. Finally, other ECG parameters, like P dispersion or QT dispersion, could not be obtained from our data.
In conclusion, the LV diameter was significantly more increased in children than in adults 6 months after percutaneous ASD closure. Thus, cardiac remodeling after percutaneous ASD closure could differ between children and adults.
Funding Statement: The author(s) received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflict of interest to report regarding the present study.
1. Samanek, M., Slavik, Z., Zborilova, B., Hrobonova, V., Voriskova, M. et al. (1989). Prevalence, treatment, and outcome of heart disease in live-born children: A prospective analysis of 91,823 live-born children. Pediatric Cardiology, 10(4), 205–211. DOI 10.1007/BF02083294. [Google Scholar] [CrossRef]
2. Gatzoulis, M. A., Redington, A. N., Somerville, J., Shore, D. F. (1996). Should atrial septal defects in adults be closed? Annals of Thoracic Surgery, 61(2), 657–659. DOI 10.1016/0003-4975(95)01043-2. [Google Scholar] [CrossRef]
3. Du, Z. D., Hijazi, Z. M., Kleinman, C. S., Silverman, N. H., Larntz, K. (2002). Comparison between transcatheter and surgical closure of secundum atrial septal defect in children and adults: Results of a multicenter nonrandomized trial. Journal of the American College of Cardiology, 39(11), 1836–1844. DOI 10.1016/S0735-1097(02)01862-4. [Google Scholar] [CrossRef]
4. Fischer, G., Stieh, J., Uebing, A., Hoffmann, U., Morf, G. et al. (2003). Experience with transcatheter closure of secundum atrial septal defects using the Amplatzer septal occluder: A single centre study in 236 consecutive patients. Heart (British Cardiac Society), 89(2), 199–204. DOI 10.1136/heart.89.2.199. [Google Scholar] [CrossRef]
5. Houeijeh, A., Hascoet, S., Bouvaist, H., Hadeed, K., Petit, J. et al. (2018). Transcatheter closure of large atrial septal defects (ASDs) in symptomatic children with device/weight ratio ≥1.5. International Journal of Cardiology, 267, 84–87. DOI 10.1016/j.ijcard.2018.05.069. [Google Scholar] [CrossRef]
6. Pascotto, M., Santoro, G., Cerrato, F., Caputo, S., Bigazzi, M. C. et al. (2006). Time-course of cardiac remodeling following transcatheter closure of atrial septal defect. International Journal of Cardiology, 112(3), 348–352. DOI 10.1016/j.ijcard.2005.10.008. [Google Scholar] [CrossRef]
7. Kaya, M. G., Baykan, A., Dogan, A., Inanc, T., Gunebakmaz, O. et al. (2010). Intermediate-term effects of transcatheter secundum atrial septal defect closure on cardiac remodeling in children and adults. Pediatric Cardiology, 31(4), 474–482. DOI 10.1007/s00246-009-9623-y. [Google Scholar] [CrossRef]
8. Balci, K. G., Balci, M. M., Aksoy, M. M., Yilmaz, S., Ayturk, M. et al. (2015). Remodeling process in right and left ventricle after percutaneous atrial septal defect closure in adult patients. Archives of Turkey Society of Cardiology, 43, 250–258. [Google Scholar]
9. Shiller, N. B., Shah, P. M., Crawford, M., DeMaria, A., Devereux, R. et al. (1989). Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American society of echocardiography committee on standards, subcommittee on quantitation of two-dimensional echocardiograms. Journal of the American Society of Echocardiography, 2(5), 358–367. DOI 10.1016/S0894-7317(89)80014-8. [Google Scholar] [CrossRef]
10. Lopez, L., Colan, S. D., Frommelt, P. C., Ensing, G. J., Kendall, K. et al. (2010). Recommendations for quantification methods during the performance of a pediatric echocardiogram: A report from the pediatric measurements writing group of the American society of echocardiography pediatric and congenital heart disease council. Journal of the American Society of Echocardiography, 23(5), 465–495. DOI 10.1016/j.echo.2010.03.019. [Google Scholar] [CrossRef]
11. Omeish, A., Hijazi, Z. M. (2001). Transcatheter closure of atrial septal defects in children & adults using the Amplatzer Septal Occluder. Journal of Interventional Cardiology, 14(1), 37–44. DOI 10.1111/j.1540-8183.2001.tb00709.x. [Google Scholar] [CrossRef]
12. Du, Z. D., Cao, Q. L., Koenig, P., Heitschmidt, M., Hijazi, Z. M. (2001). Speed of normalization of right ventricular volume overload after transcatheter closure of atrial septal defect in children and adults. American Journal of Cardiology, 88(12), 1450–1453. DOI 10.1016/S0002-9149(01)02135-X. [Google Scholar] [CrossRef]
13. Santoro, G., Pascotto, M., Sarubbi, B., Cappelli Bigazzi, M., Calvanese, R. et al. (2004). Early electrical and geometric changes after percutaneous closure of large atrial septal defect. American Journal of Cardiology, 93(7), 876–880. DOI 10.1016/j.amjcard.2003.12.027. [Google Scholar] [CrossRef]
14. Vijarnsorn, C., Durongpisitkul, K., Chanthong, P., Chungsomprasong, P., Soongswang, J. et al. (2012). Beneficial effects of transcatheter closure of atrial septal defects not only in young adults. Journal of Interventional Cardiology, 25(4), 382–390. DOI 10.1111/j.1540-8183.2012.00723.x. [Google Scholar] [CrossRef]
15. Santoro, G., Pascotto, M., Caputo, S., Cerrato, F., Cappelli Bigazzi, M. et al. (2006). Similar cardiac remodelling after transcatheter atrial septal defect closure in children and young adults. Heart (British Cardiac Society), 92(7), 958–962. DOI 10.1136/hrt.2005.070169. [Google Scholar] [CrossRef]
16. Pascotto, M., Santoro, G., Caso, P., Cerrato, F., Caso, I. et al. (2005). Global and regional left ventricular function in patients undergoing transcatheter closure of secundum atrial septal defect. American Journal of Cardiology, 96(3), 439–442. DOI 10.1016/j.amjcard.2005.03.096. [Google Scholar] [CrossRef]
17. Gomez, C. A., Ludomirsky, A., Ensing, G. J., Rocchini, A. P. (2005). Effect of acute changes in load on left ventricular diastolic function during device closure of atrial septal defects. American Journal of Cardiology, 95(5), 686–688. DOI 10.1016/j.amjcard.2004.10.052. [Google Scholar] [CrossRef]
18. Giardini, A., Moore, P., Brook, M., Stratton, V., Tacy, T. (2005). Effect of transcatheter atrial septal defect closure in children on left ventricular diastolic function. American Journal of Cardiology, 95(10), 1255–1257. DOI 10.1016/j.amjcard.2005.01.062. [Google Scholar] [CrossRef]
19. Masutani, S., Taketazu, M., Ishido, H., Iwamoto, Y., Yoshiba, S. et al. (2012). Effects of age on hemodynamic changes after transcatheter closure of atrial septal defect: Importance of ventricular diastolic function. Heart and Vessels, 27(1), 71–78. DOI 10.1007/s00380-011-0122-8. [Google Scholar] [CrossRef]
20. Roushdy, A. M., Attia, H., Nossir, H. (2018). Immediate and short-term effects of percutaneous atrial septal defect device closure on cardiac electrical remodeling in children. Egyptian Heart Journal: Official Bulletin of the Egyptian Society of Cardiology, 70, 243–247. [Google Scholar]
21. Karpawich, P. P., Antillon, J. R., Cappola, P. R., Agarwal, K. C. (1985). Pre- and postoperative electrophysiologic assessment of children with secundum atrial septal defect. American Journal of Cardiology, 55(5), 519–521. DOI 10.1016/0002-9149(85)90238-3. [Google Scholar] [CrossRef]
22. Fang, F., Luo, X. X., Lin, Q. S., Kwong, J. S., Zhang, Y. C. et al. (2013). Characterization of mid-term atrial geometrical and electrical remodeling following device closure of atrial septal defects in adults. International Journal of Cardiology, 168(1), 467–471. DOI 10.1016/j.ijcard.2012.09.119. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |