

 | Congenital Heart Disease |  |
DOI: 10.32604/CHD.2021.013793
ARTICLE
The Prognostic Value of Myocardial Deformation in Patients with Congenital Aortic Stenosis
Department of Cardiology, Erasmus Medical Centre, Rotterdam, The Netherlands
*Corresponding Author: Annemien E. van den Bosch. Email: a.e.vandenbosch@erasmusmc.nl
Received: 21 August 2020; Accepted: 12 October 2020
Abstract: Aims: To assess the prognostic value of left ventricular (LV) global longitudinal strain (GLS) and global longitudinal early diastolic strain rate (GLSre) with regard to cardiovascular events, as congenital aortic stenosis (AoS) is associated with significant mortality and morbidity but predictors for clinical outcome are scarce. Strain analysis provides a robust and reproducible method for early detection of LV dysfunction, which might be of prognostic value. Methods: This prospective study, included clinically stable patients with congenital AoS between 2011–2013. LV GLS and GLSre was performed in the apical 4, 3 and 2-chamber views using Tomtec software. The endpoint was a composite of death, heart failure, hospitalization, arrhythmia, thrombo-embolic events and re-intervention. Results: In total 138 patients were included (33[26–43] years, 86(62%) male), NYHA class I: 134(97%). Mean LV GLS was –15.3 ± 3.2%, GLSre 0.66 ± 0.18 s–1. Both correlated with NT-proBNP, LV volumes and ejection fraction (strongest LV GLS with LV EF: r –0.539, p < 0.001, strongest LV GLSre with age: r –0.376, p < 0.001). During median follow-up of 5.9[5.5–6.2] years, the endpoint occurred in 53(38%) patients: 4 patients died, 9 developed heart failure, 22 arrhythmias, 8 thrombo-embolic events and 35 re-interventions. Both LV GLS (standardized HR (sHR 0.62(95%CI 0.47–0.81) and GLSre (sHR 0.62(95%CI 0.47–0.83) were associated with the endpoint. Additional multivariable analysis showed that both GLS and GLSre were associated independent of left atrial volume, NT-proBNP and prior re-interventions. Conclusion: Left ventricular GLS and GLSre are reduced in adult patients with congenital AoS. Both markers are associated with adverse cardiac events and have clear clinical relevance.
Keywords: Speckle tracking echocardiography; congenital aortic stenosis; strain; prognosis
Congenital aortic stenosis is responsible for over 4% of all congenital heart defects [1]. Indeed, it is the most frequent indication for aortic valve replacement in young adults [2]. The last few decades research has been focused primarily on re-intervention free survival of different surgical techniques as well as balloon valvuloplasty [3–10]. There are however only a few studies that assess clinical endpoints such as heart failure or mortality [9,11].
The presence of an aortic stenosis gives rise to several hemodynamic and pathophysiological changes. An important derangement in aortic valve stenosis is the relative reduction of coronary blood flow to the hypertrophic left ventricle, which has an increased oxygen demand. This imbalance is enhanced by a reduction in diastolic filling period, resulting in an even more extreme imbalance between demand and supply. The remodeling and subendocardial ischemia results in changes in myocardial function, both systolic and diastolic, which can be assessed using strain analysis [12,13].
Previous work from our group reported on disease progression over time mainly focusing on progression of stenosis and aortic dilatation [14], and determined that left ventricular (LV) hypertrophy is associated with faster progression of stenosis [15]. However, to the best of our knowledge there are no studies evaluating myocardial deformation using speckle tracking echocardiography (STE). In this study consisting of adult patients with congenital aortic stenosis we performed a cross-sectional analysis, investigating myocardial function with STE derived variables, both systolic and diastolic, and detect possible correlations with baseline variables. In addition, the predictive value of myocardial function was prospectively investigated.
Patients with a congenital aortic stenosis were extracted from a prospective cohort of consecutively included clinically stable patients with adult congenital heart disease, between September 2011 and June 2014 at the outpatient clinic of our tertiary center. Inclusion criteria were ≥18 years of age, and a diagnosis of congenital aortic stenosis. This study protocol has been described previously [16], and was carried out according to the principles of the Declaration of Helsinki and approved by the local ethics committee. Written informed consent was obtained from every patient.
The study protocol included a questionnaire on medical history, a physical examination, 12-leads electrocardiogram, comprehensive echocardiogram and venous blood sampling (not fasting) on the same day. Hypertension was defined as systolic pressure above 140 mmHg or diastolic pressure above 90 mmHg.
Two-dimensional greyscale images were obtained in the left lateral decubitus position with an iE33 or EPIC7 ultrasound system (Philips medical systems, Best, the Netherlands) equipped with a transthoracic X5-1 matrix transducer (3040 elements, extended operating frequency range 1–5 MHz). Care was taken to retain a minimum framerate of 50 Hz. The studies were stored in digital imaging and communications in medicine (DICOM) format.
2.3 Echocardiographic Measurement
For all measurements the current guideline from the American of European Society of Cardiology were adhered to when performing measurements [17]. Diastolic function was assessed to the most recent guideline from the SE/EACVI, recommendation for the evaluation of LV diastolic function [18]. For ejection fraction, the method-of-disk summation technique was used, for LV mass, the linear method was used.
Speckle tracking analysis was performed with dedicated commercially available software (2D Cardiac Performance Analysis, Tomtec Imaging Systems, Unterschleissheim, Germany). By determining the end-systolic and end-diastolic frame and identifying the annulus and apex, the software semi-automatically detects the myocardial contours. This contour was visually checked and corrected if necessary. This was performed in the apical four-, three- and two-chamber view. The left ventricle was assessed according to the 17-segment model as stated by the guideline for echocardiographic chamber quantification [19]. LV global longitudinal strain (GLS and LV global longitudinal early diastolic strain rate (GLSre) were assessed. The latter was as the maximum strain rate during early diastole (Fig. 1). Measurements regarding STE were done according to the guidelines set by ASE/EAE consensus statement [20] and Taskforce to standardize deformation imaging [21].
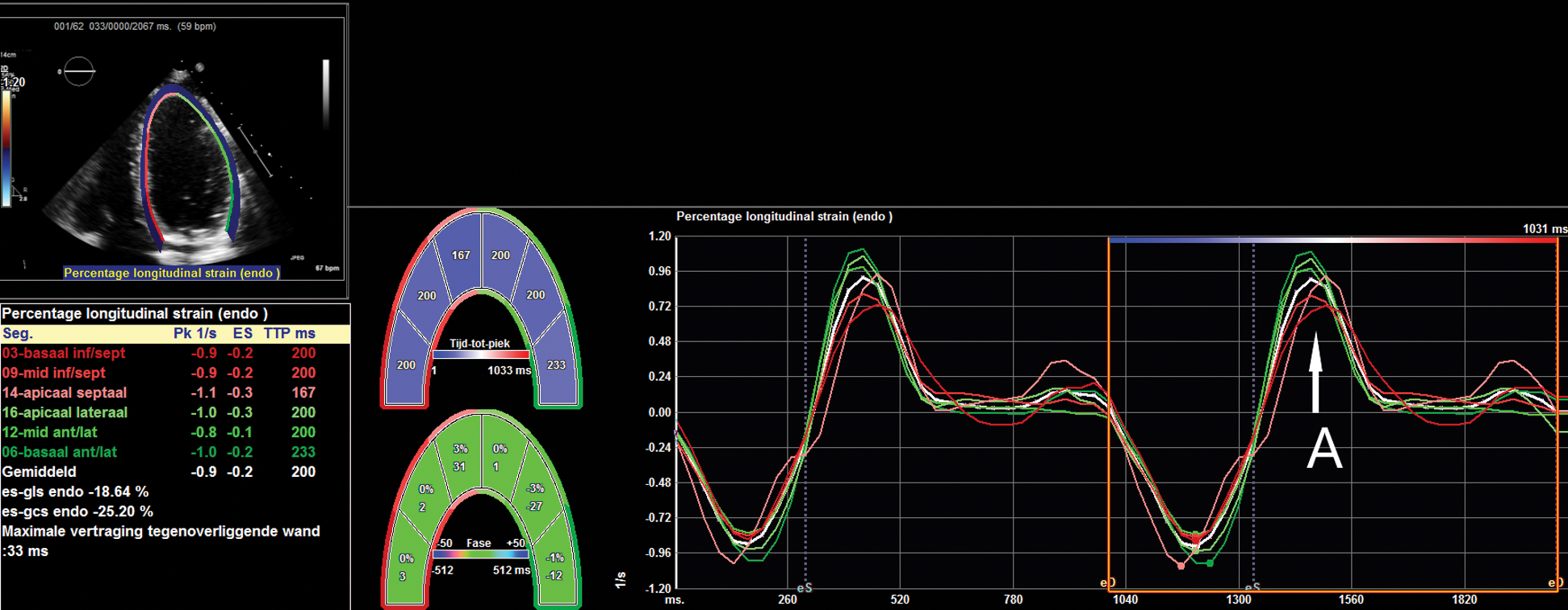
Figure 1: An example of strain analysis, where the apical four-chamber view has been used to analyze longitudinal strain and strain rate. The graph shows strain rate during the cardiac cycle; the peak at the arrow A represents the speed of early diastolic lengthening of the myocardium
The endpoint was a composite of death, heart failure, arrhythmia (both supraventricular and ventricular, had to be symptomatic and recorded or treated), hospitalization for cardiac reasons, thrombo-embolic events and re-interventions (both surgical and percutaneous). These endpoints were defined before any data analysis was performed. Each patient was regularly seen at the outpatient clinic and endpoints were manually checked on a yearly basis, while being blinded for clinical data. The Municipal Population Register was checked to obtain survival status. If patients did not experience any event, subjects were censored at the end of the follow-up period (01-01-2018). When patients did experience an event, they were censored for the rest of the follow-up time, but new events were still being registered. Every patient was treated to the physician’s discretion and in accordance with the ACHD guidelines [22].
Data distribution was checked using histograms and the Shapiro–Wilk test. Continuous data were presented as mean ± SD or median and interquartile range [IQ1–IQ2], as appropriate. Categorical data were presented as frequencies and percentages. The student’s T-test or Mann–Whitney-U test was used to assess differences between groups for continuous data, and the Chi-square test or Fisher’s exact test was used for categorical data as appropriate. Missing data regarding LV GLS and LV GLSre was handled with by imputation of the mean. Correlations were assessed for baseline characteristics and echocardiographic variables with both LV GLS and LV GLSre.
Patients were stratified into tertiles according to LV GLS and LV GLSre. Using the Kaplan–Meier method, cumulative endpoint-free survival estimates were calculated. The log-rank test was used to determine significant differences between groups. Cox proportional hazard ratios (HR) were calculated to determine possible associations between variables of interest and endpoints. These were standardized to make comparison easier. For both LV GLS and LV GLSre several bivariable Cox regression models were performed for the occurrence of the combined endpoints. Ion the first model NT-proBNP was added, in the second was left atrial (LA) volume and in the third with LV number of prior re-interventions. This resulted in 6 bivariable models; three with LV GLS and three with LV GLSre.
Statistical analysis was performed using IBM SPSS 24.0 (IBM Corp., Armonk, NY, USA). Tests were considered statistically significant when two-sided p-value was less than 0.05.
There were 138 patients included in the study: median age was 34.4 [25.5–42.8] years, of which 86(62.3%) were male. Baseline characteristics are presented in Tab. 1. The majority of patients were in NYHA class I: 134(97.1%) at baseline. Median BMI was 25.1 [22.6–27.6] kg/m2, 12(8.7%) patients had hypertension and 108(78.3%) had a bicuspid aortic valve. At the time of inclusion, 41(29.7%) patients had at least prior valvular intervention. The echocardiographic findings are presented in Tab. 2. Mean LV ejection fraction (EF) was 57.9 ± 7.0%.
Table 1: Baseline characteristics
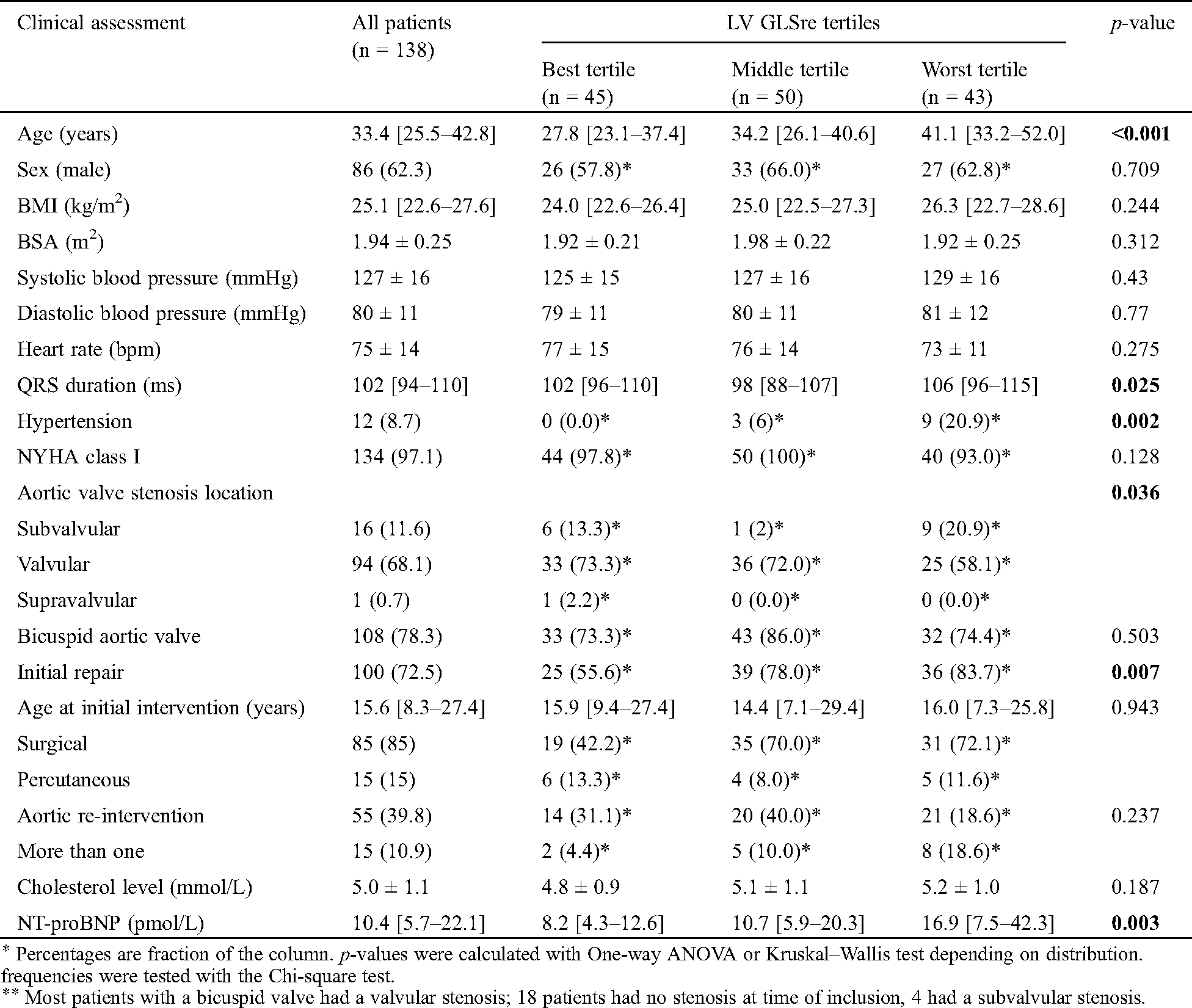
Table 2: Echocardiographic measurements

3.2 Strain Values and Associations with Baseline Variables
LV GLS was feasible in 134 (97.1%) and LV GLSre in 132 (95.7%) patients. Mean values were –15.3 ± 3.2% and 0.66 ± 0.18 s–1 for the entire study population.
Tab. 3 shows the correlations between baseline variables and both LV GLS and LV GLSre. For LV GLS, age, number of reinterventions and NT-proBNP were significantly correlated. The echocardiographic variables that correlated strongest with LV GLS were LV EF (r: 0.539, p < 0.001) and LV GLSre (r: 0.620, p < 0.001). Conventional diastolic parameters correlated with LV GLS: LA volume, A-wave and E/A-ratio, of which LA volume had the strongest correlation (r: –0.264, p < 0.01).
Table 3: Correlations with myocardial deformation
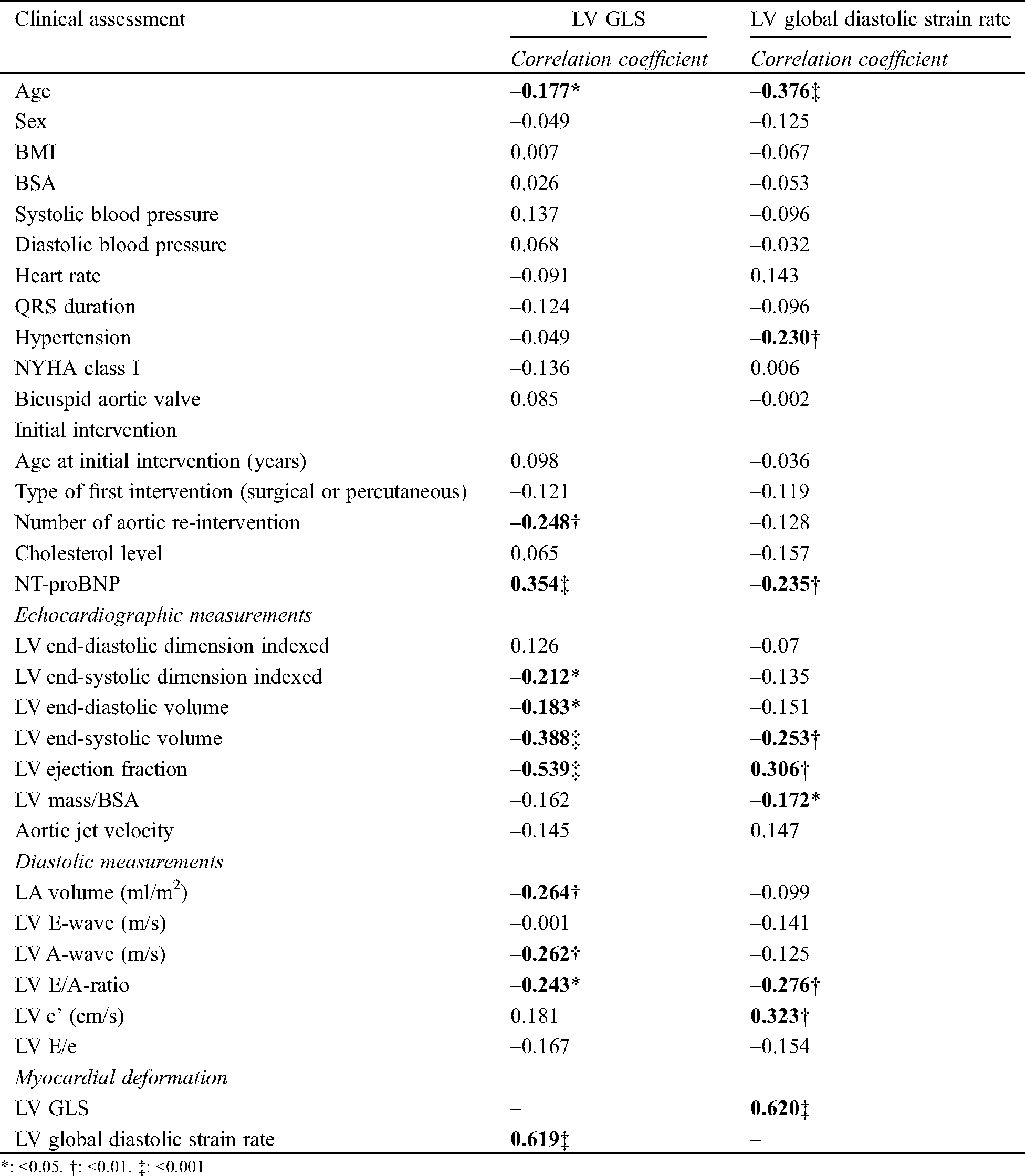
For LV GLSre, older age, presence of hypertension and higher NT-proBNP levels were correlated with lower LV GLSre values. A higher LV and mass correlated with lower LV GLSre values, and also with conventional diastolic parameters such as E/A-ratio and e’.
3.3 Prognostic Value of Myocardial Deformation
The composite endpoint occurred in 53 (38%) patients. During a median follow-up period of 5.9 [5.5–6.2] years. During that period, four patients died; 3 due to cardiac arrest and 1 presumed sudden cardiac death. Another 45 patients were hospitalized for a myriad of reasons (specified in supplemental Tab. 1). In total 22 patients experienced arrhythmias: 11 patients had supraventricular tachycardia’s, 10 patients suffered from ventricular arrhythmias, of which 5 were out-of-hospital-cardiac-arrests due to ventricular fibrillation. In total 8 patients had a thrombo-embolic event: 6 ischemic cerebral vascular events, 1 myocardial infarction and 1 superior mesenteric artery thrombus. In total 35 patients had a re-intervention during follow-up, 22 surgical and 16 percutaneous.
In Fig. 2, the cumulative event-free survival is depicted for LV GLS and LV GLSre. Both variables have been stratified into tertiles. Both variables show that decreased values are associated with a decreased event-free survival (LV GLS p: 0.048, LV GLSre p < 0.001).
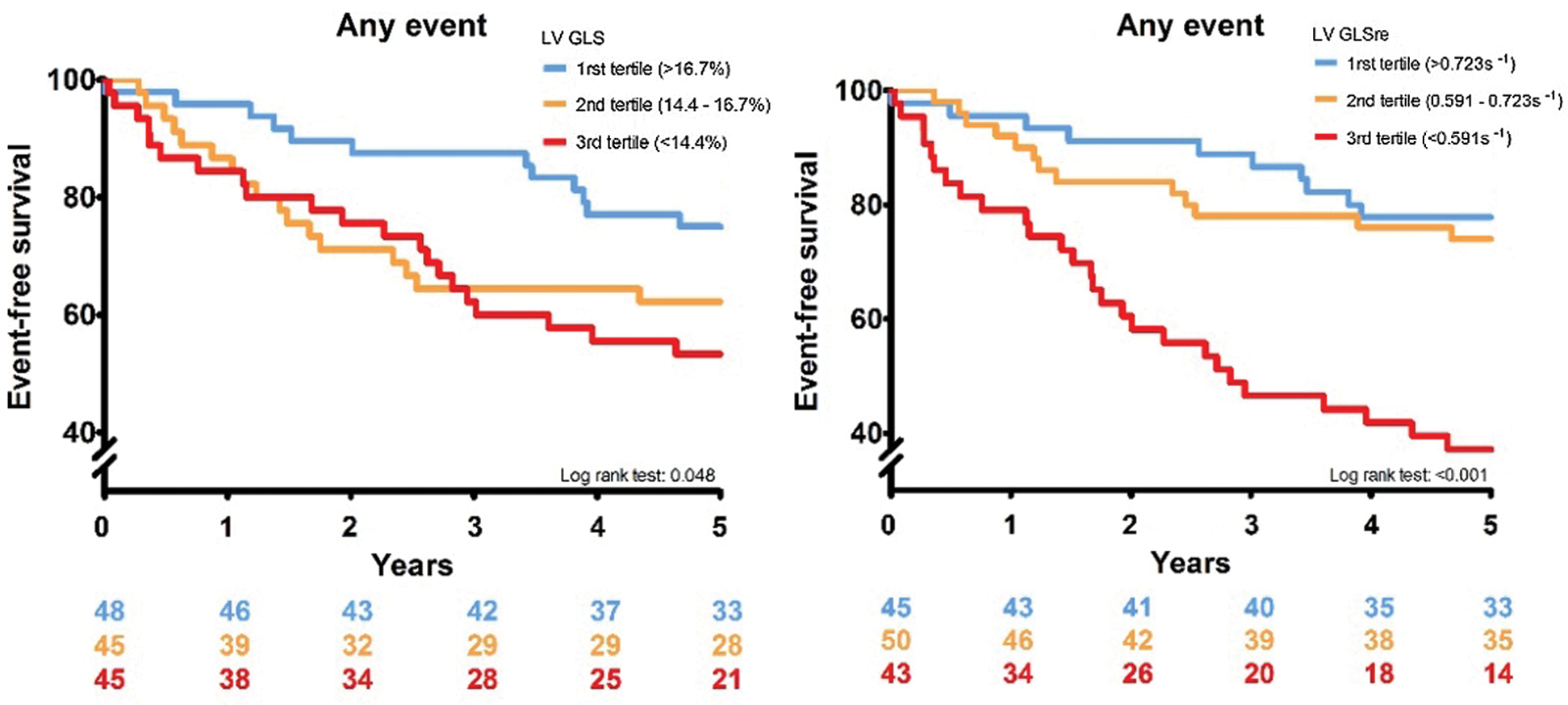
Figure 2: Two graphs showing the Kaplan–Meier curves for LV GLS an GLSre respectively. In the left panel LV GLS is stratified in tertiles, and in the right panel LV GLSre is stratified in tertile
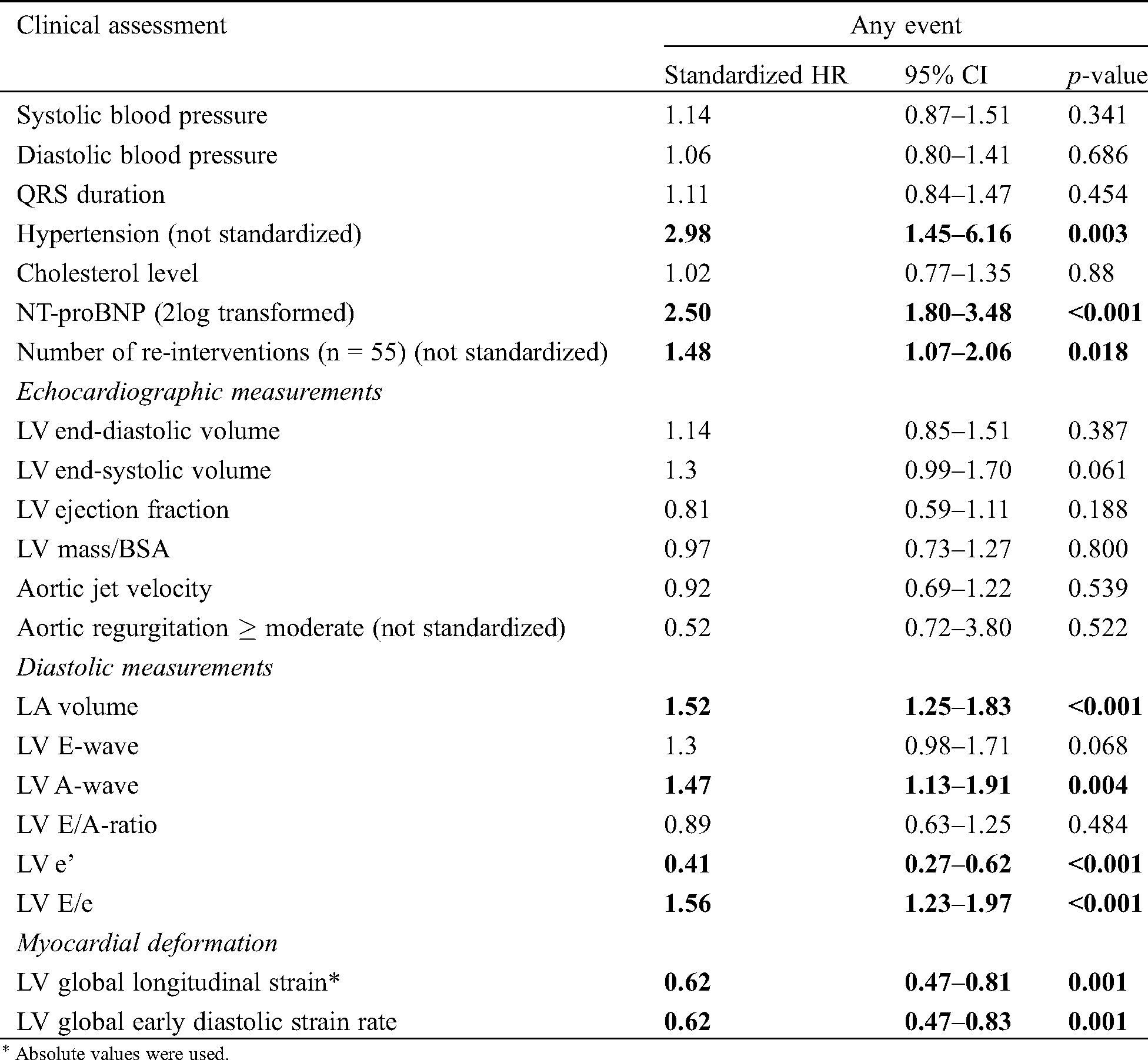
Tab. 4 shows univariable standardized hazard ratios (sHR) of baseline characteristics, conventional echocardiographic and STE derived variables for the combined endpoint. The presence of hypertension, elevated NT-proBNP levels and re-intervention prior to inclusion were associated with a higher risk for the occurrence of the combined endpoint. Notable is that of the conventional echocardiographic variables only LV diastolic variables were associated with the combined endpoint. Both LV GLS and LV GLSre were significantly associated with the combined endpoint (sHR 0.62 95%CI 0.47–0.81 and sHR 0.62 95%CI 0.47–0.83 respectively). Interestingly, LV mass and aortic jet velocity were not.
Three bivariable models were analyzed: 1. LV GLS combined with NT-proBNP and 2. LV GLS combined with LA volume and 3. LV GLS combined with the number of re-interventions prior to inclusion. The first model showed that both LV GLS and NT-proBNP were independently associated with the combined endpoint (sHR 0.76 95%CI 0.58–0.99 and sHR 2.28 95%CI 1.63–3.21 respectively). The second model with LA volume showed similar results: both were independently associated (LV GLS sHR 0.67 95%CI 0.51–0.89 and LA volume sHR 1.38 95%CI 1.14–1.68). LV GLS was independently associated with the endpoint of re-intervention, which was no longer significantly associated. The third model showed that LV GLS was significantly associated with the endpoint (LV GLS sHR 0.67 95%CI 0.50–0.88), independent of reintervention prior to inclusion, which was no longer associated with the endpoint.
For LV GLSre, we also analyzed three bivariable models. The first model revealed that LV GLSre and NT-proBNP were independently associated with the combined endpoint (LV GLSre sHR 0.73 95%CI 0.55–0.96, NT-proBNP sHR 2.35 95%CI 1.68–3.28, respectively). The second model showed again that LV GLSre and LA volume were independently associated with the combined endpoint (LV GLSre 0.62 85%CI 0.47–0.83, LA volume 1.48 95%CI 1.22–1.79). Lastly, the third showed that LV GLSre was significantly associated with the endpoint (sHR 0.66 95%CI 0.50–0.87) independently of re-intervention, which was no longer associated with the endpoint.
Table 4: Cox regression analysis
To our knowledge, this is the first study to investigate the prognostic value of left ventricular strain measurements in adult patients with a congenital aortic stenosis. We conclude that LV global longitudinal strain and global longitudinal early diastolic strain rate are reduced in these patients compared to healthy controls [23–25]. Hypertension correlated with reduced LV GLSre, and LV EF with LV GLS, though LV EF was normal whereas LV GLS was reduced, suggesting that LV GLS is better capable to detect systolic dysfunction.
Both LV GLS and GLSre contain prognostic value for risk-stratification in adult patients with congenital aortic stenosis. LV GLS and LV GLSre are associated with the composite endpoint, independently from variables such as NT-proBNP, left atrial volume or number of prior interventions.
4.1 Left Ventricular Function in Congenital Aortic Stenosis
There are several observations to be made from the cross-sectional data. First, volumetric assessment and ejection fraction of the left ventricle were predominantly good. On the other hand, the average LV GLS was reduced, as was LV GLSre. And although there was a strong correlation between LV GLS and LV EF, LV EF evidently failed to detect LV systolic dysfunction; conventional echocardiographic assessment approximates but does not fully describe the intricacies of left ventricular function.
The fact that LV systolic and diastolic function are interconnected and influence each other can be witnessed by a number of correlations found in this study; left ventricular GLS as a measure of systolic function was correlated with LV GLSre, E-wave, E/A-ratio and LA volume. Conversely, LV GLSre correlated LV end-systolic volume and EF.
Interestingly, the diagnosis hypertension at baseline correlated with reduced LV GLSre. It is known that prolonged pressure overload negatively influences diastolic function. Pressure overload induces LV hypertrophy, and indeed increased LV mass also correlated with reduced LV GLSre. We found that LV GLSre correlated well with conventional diastolic markers, however the results from the bivariate models suggest that LV GLSre provides additional prognostic information over LA volume alone, since LV GLSre was significantly associated with the endpoint, independently of LA volume.
4.2 Prognostic Value of Myocardial Deformation
This study shows that patients with congenital aortic stenosis have a high morbidity and mortality, therefore comprehensive risk-stratification and follow-up are imperative. We found that multiple re-interventions in childhood did correlate with reduced LV GLS, and LV GLS was associated with adverse cardiac events. This demonstrates the value of strain measurements in routine clinical follow-up. Severe aortic stenosis or rapid progression leads to LV hypertrophy and might cause reduced coronary flow [12,13] and has previously been linked to higher intervention rates [15]. Both hypertrophy and reduced coronary flow can induce fibrosis and may cause sub endocardial dysfunction. Indeed, the number of re-interventions prior to inclusion was associated with the combined endpoint. However multivariable analysis showed that it was no longer associated with the endpoint after including either LV GLS or GLSre. Myocardial deformation is a very sensitive way to assess LV function, systolic and diastolic. With strain analysis, new tools have come available for adequate risk-assessment. The results also suggest that more severely decreased LV GLS or GLSre seems to lead to a worse prognosis, considering the Kaplan–Meier curves in Fig. 2.
Interestingly, conventional LV parameters were unable to predict cardiovascular events in our study, most notably LV EF, LV mass and aortic jet velocity. And even though the aortic stenosis may no longer be present, the imbalance between oxygen demand and supply has already induced myocardial changes. These changes in combination with hypertension and hypertrophy, are most likely why LV GLSre and conventional diastolic parameters were predictors for adverse outcome.
Studies pertaining adult patients with a congenital aortic stenosis are relatively scarce, and studies investigating clinical outcome even more so. Van der Linde et al. [14] found that severity of the aortic stenosis is fairly stable over time but identified the presence of LV hypertrophy to be associated with disease progression. It is also one of the few studies which reported mortality rates (3 out of 414 patients, 0.7%, during a median follow-up of 4.1 years). In our study, LV mass was not associated with clinical events. This is most likely due to lower values of LV mass in our cohort: mean LV mass in our study was 90 ± 25.2 g/m2 against 106 ± 32.2 g/m2. However, LV mass was correlated with LV GLSre, and it is well known that increased LV mass is associated with impaired LV diastolic dysfunction [26,27]. But the added value of LV mass seems to be a limited in clinical decision making in these patients as it was not associated with clinical outcome in this study.
This study has identified LV GLS and GLSre as prognostic markers for clinical events in patients with congenital aortic stenosis. LV GLS and GLSre are measured in the same analysis, making them applicable for routine clinical use. Left ventricular GLS is more sensitive to detect systolic dysfunction than LV EF [21,28,29], and LV GLSre improves the detection of diastolic dysfunction [24]. In other words, both are more sensitive markers that provide benefit over conventional measurements and should therefore be included clinical evaluation when feasible.
These patients have a high risk for late cardiac complications. During a median follow-up of 6 years, 4(2.9%) patients died. Arrhythmias and re-intervention occurred much more frequently, underlining the need for adequate risk-stratification. Especially the incidence of ventricular arrhythmias is concerning: 5 patients had an out-of-hospital-cardiac-arrest and 2 patients developed ventricular tachycardia. These life-threatening complications should be prevented. Reduced strain indices may help in the identification of these patients.
Patients were included in a tertiary care center, possibly resulting in inclusion bias. On the other hand, care was taken to include clinically stable patients. The strain results presented here are based on software from Tomtec. Though several studies have concluded that differences between vendors are negligible [30,31], care should be taken when extrapolating these results to other vendors.
Myocardial deformation measurements can be used to assess the risk for late complications in patients with congenital aortic stenosis. Furthermore, both LV systolic and diastolic strain have incremental value over conventional echocardiographic measurements. The high rate of cardiovascular events further underlines the need for adequate risk-stratification; therefore, we recommend that LV strain analysis should be incorporated in the clinical assessment of these patients in routine practice.
Acknowledgement: All authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Data Sharing: Data is available upon reasonable request.
Funding Statement: This study was supported by a grant from the Erasmus Thorax Foundation.
Conflicts of Interest: The author declare that they have no conflicts of interest to report regarding the present study.
1. van der Linde, D.,Konings, E. E., Slager, M. A., Witsenburg, M., Helbing, W. A. et al. (2011). Birth prevalence of congenital heart disease worldwide, a systematic review and meta-analysis. Journal of American College of Cardiology, 58(21), 2241–2247. DOI 10.1016/j.jacc.2011.08.025. [Google Scholar] [CrossRef]
2. Puvimanasinghe, J. P., Steyerberg, E. W., Takkenberg, J. J., Eijkemans, M. J., van Herwerden, L. A. et al. (2001). Prognosis after aortic valve replacement with a bioprosthesis, predictions based on meta-analysis and microsimulation. Circulation, 103(11), 1535–1541. DOI 10.1161/01.CIR.103.11.1535. [Google Scholar] [CrossRef]
3. Jijeh, A., Ismail, M., Al-Bahanta, A., Alomrani, A., Tamimi, O. (2018). Percutaneous balloon dilatation for congenital aortic stenosis during infancy: A 15-year single-center experience. Annals of Pediatric Cardiology, 11(2), 143–147. DOI 10.4103/apc.APC_171_17. [Google Scholar] [CrossRef]
4. Padalino, M. A., Frigo, A. C., Comisso, M., Kostolny, M., Omeje, I. et al. (2017). Early and late outcomes after surgical repair of congenital supravalvular aortic stenosis: A European Congenital Heart Surgeons Association multicentric study. European Journal of Cardio-Thoracic Surgery, 52(4), 789–797. DOI 10.1093/ejcts/ezx245. [Google Scholar] [CrossRef]
5. Auld, B., Carrigan, L., Ward, C., Justo, R., Alphonso, N. et al. (2019). Balloon aortic valvuloplasty for congenital aortic stenosis: A 14-year single centre review. Heart, Lung & Circulation, 28(4), 632–636. DOI 10.1016/j.hlc.2018.02.014. [Google Scholar] [CrossRef]
6. Boe, B. A., Zampi, J. D., Kennedy, K. F., Jayaram, N., Porras, D. et al. (2017). Acute success of balloon aortic valvuloplasty in the current era: A national cardiovascular data registry study. JACC: Cardiovascular Interventions, 10(17), 1717–1726. DOI 10.1016/j.jcin.2017.08.001. [Google Scholar] [CrossRef]
7. Kallio, M., Rahkonen, O., Mattila, I., Pihkala, J. (2017). Congenital aortic stenosis: Treatment outcomes in a nationwide survey. Scandinavian Cardiovascular Journal, 51(5), 277–283. DOI 10.1080/14017431.2017.1355069. [Google Scholar] [CrossRef]
8. Awasthy, N., Garg, R., Radhakrishnan, S., Shrivastava, S. (2016). Long-term results of percutaneous balloon valvuloplasty of congenital aortic stenosis in adolescents and young adults. Indian Heart Journal, 68(5), 604–611. DOI 10.1016/j.ihj.2016.03.001. [Google Scholar] [CrossRef]
9. Sullivan, P. M., Rubio, A. E., Johnston, T. A., Jones, T. K. (2017). Long-term outcomes and re-interventions following balloon aortic valvuloplasty in pediatric patients with congenital aortic stenosis: A single-center study. Catheterization and Cardiovascular Interventions, 89(2), 288–296. DOI 10.1002/ccd.26722. [Google Scholar] [CrossRef]
10. Kramer, P., Absi, D., Hetzer, R., Photiadis, J., Berger, F. et al. (2014). Outcome of surgical correction of congenital supravalvular aortic stenosis with two- and three-sinus reconstruction techniques. Annals of Thoracic Surgery, 97(2), 634–640. DOI 10.1016/j.athoracsur.2013.09.083. [Google Scholar] [CrossRef]
11. Hörer, J., Belli, E., Roussin, R., LeBret, E., Ly, M. et al. (2018). Evaluation of the adult congenital heart surgery mortality score at two European centers. Annals of Thoracic Surgery, 105(5), 1441–1446. DOI 10.1016/j.athoracsur.2017.12.018. [Google Scholar] [CrossRef]
12. Rajappan, K., Rimoldi, O. E., Dutka, D. P., Ariff, B., Pennell, D. J. et al. (2002). Mechanisms of coronary microcirculatory dysfunction in patients with aortic stenosis and angiographically normal coronary arteries. Circulation, 105(4), 470–476. DOI 10.1161/hc0402.102931. [Google Scholar] [CrossRef]
13. Mahmod, M., Francis, J. M., Pal, N., Lewis, A., Dass, S. et al. (2014). Myocardial perfusion and oxygenation are impaired during stress in severe aortic stenosis and correlate with impaired energetics and subclinical left ventricular dysfunction. Journal of Cardiovascular Magnetic Resonance, 16(1), 29. DOI 10.1186/1532-429X-16-29. [Google Scholar] [CrossRef]
14. van der Linde, D.,Andrinopoulou, E. R., Oechslin, E. N., Budts, W., van Dijk, A. P. et al. (2013). Congenital valvular aortic stenosis in young adults: Predictors for rate of progression of stenosis and aortic dilatation. International Journal of Cardiology, 168(2), 863–870. DOI 10.1016/j.ijcard.2012.10.027. [Google Scholar] [CrossRef]
15. Yap, S. C., Kouwenhoven, G. C., Takkenberg, J. J., Galema, T. W., Meijboom, F. J. et al. (2007). Congenital aortic stenosis in adults: Rate of progression and predictors of clinical outcome. International Journal of Cardiology, 122(3), 224–231. DOI 10.1016/j.ijcard.2006.11.092. [Google Scholar] [CrossRef]
16. Eindhoven, J. A., van den Bosch, A. E.,Ruys, T. P., Opić, P., Cuypers, J. A. et al. (2013). N-terminal pro-B-type natriuretic peptide and its relationship with cardiac function in adults with congenital heart disease. Journal of the American College of Cardiology, 62(13), 1203–1212. DOI 10.1016/j.jacc.2013.07.019. [Google Scholar] [CrossRef]
17. Lang, R. M., Badano, L. P., Mor-Avi, V., Afilalo, J., Armstrong, A. et al. (2015). Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. European Heart Journal Cardiovascular Imaging, 16(3), 233–270. DOI 10.1093/ehjci/jev014. [Google Scholar] [CrossRef]
18. Nagueh, S. F., Smiseth, O. A., Appleton, C. P., Byrd III, B. F., Dokainish, H. (2016). Recommendations for the evaluation of left ventricular diastolic function by echocardiography: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. European Heart Journal Cardiovascular Imaging, 17(12), 1321–1360. DOI 10.1093/ehjci/jew082. [Google Scholar] [CrossRef]
19. Lang, R. M., Badano, L. P., Mor-Avi, V., Afilalo, J., Armstrong, A. et al. (2015). Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Journal of the American Society of Echocardiography, 28(1), 1–39.e14. DOI 10.1016/j.echo.2014.10.003. [Google Scholar] [CrossRef]
20. Mor-Avi, V., Lang, R. M., Badano, L. P., Belohlavek, M., Cardim, N. M. et al. (2011). Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese Society of Echocardiography. European Journal of Echocardiography, 12(3), 167–205. DOI 10.1093/ejechocard/jer021. [Google Scholar] [CrossRef]
21. Voigt, J. U., Pedrizzetti, G., Lysyansky, P., Marwick, T. H., Houle, H. et al. (2015). Definitions for a common standard for 2D speckle tracking echocardiography, consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. European Heart Journal Cardiovascular Imaging, 16(1), 1–11. DOI 10.1093/ehjci/jeu184. [Google Scholar] [CrossRef]
22. Baumgartner, H., Bonhoeffer, P., De Groot, N. M., de Haan, F., Deanfield, J. E. et al. (2010). ESC Guidelines for the management of grown-up congenital heart disease (new version 2010). European Heart Journal, 31(23), 2915–2957. DOI 10.1093/eurheartj/ehq249. [Google Scholar] [CrossRef]
23. Menting, M. E., McGhie, J. S., Koopman, L. P., Vletter, W. B., Helbing, W. A. et al. (2016). Normal myocardial strain values using 2D speckle tracking echocardiography in healthy adults aged 20 to 72 years. Echocardiography, 33(11), 1665–1675. DOI 10.1111/echo.13323. [Google Scholar] [CrossRef]
24. Morris, D. A., Takeuchi, M., Nakatani, S., Otsuji, Y., Belyavskiy, E. et al. (2018). Lower limit of normality and clinical relevance of left ventricular early diastolic strain rate for the detection of left ventricular diastolic dysfunction. European Heart Journal Cardiovascular Imaging, 19(8), 905–915. DOI 10.1093/ehjci/jex185. [Google Scholar] [CrossRef]
25. van Grootel, R. W. J.,Kauling, R. M., Menting, M. E., McGhie, J., Roos-Hesselink, J. W. et al. (2018). Influence of age and sex on left ventricular diastolic strain analysis. International Journal of Cardiovascular Imaging, 35(3), 491–498. DOI 10.1007/s10554-018-1480-4. [Google Scholar] [CrossRef]
26. Devereux, R. B., Wachtell, K., Gerdts, E., Boman, K., Nieminen, M. S. et al. (2004). Prognostic significance of left ventricular mass change during treatment of hypertension. JAMA, 292(19), 2350–2356. DOI 10.1001/jama.292.19.2350. [Google Scholar] [CrossRef]
27. Kupari, M., Turto, H., Lommi, J. (2005). Left ventricular hypertrophy in aortic valve stenosis, preventive or promotive of systolic dysfunction and heart failure? European Heart Journal, 26(17), 1790–1796. DOI 10.1093/eurheartj/ehi290. [Google Scholar] [CrossRef]
28. Menting, M. E., van Grootel, R. W., van den Bosch, A. E., Eindhoven, J. A., McGhie, J. S. et al. (2016). Quantitative assessment of systolic left ventricular function with speckle-tracking echocardiography in adult patients with repaired aortic coarctation. International Journal of Cardiovascular Imaging, 32(5), 777–787. DOI 10.1007/s10554-016-0838-8. [Google Scholar] [CrossRef]
29. Zhang, K. W., French, B., May Khan, A., Plappert, T., Fang, J. C. et al. (2014). Strain improves risk prediction beyond ejection fraction in chronic systolic heart failure. Journal of American Heart Association, 3(1), e000550. [Google Scholar]
30. Farsalinos, K. E., Daraban, A. M., Unlu, S., Thomas, J. D., Badano, L. P. et al. (2015). Head-to-head comparison of global longitudinal strain measurements among nine different vendors, The EACVI/ASE Inter-Vendor Comparison Study. Journal of the American Society of Echocardiography, 28(10), 1171–1181, e2. DOI 10.1016/j.echo.2015.06.011. [Google Scholar] [CrossRef]
31. Unlu, S., Mirea, O., Duchenne, J., Pagourelias, E. D., Bezy, S. et al. (2018). Comparison of feasibility, accuracy, and reproducibility of layer-specific global longitudinal strain measurements among five different vendors: A report from the EACVI-ASE Strain Standardization Task Force. Journal of American Society of Echocardiography, 31(3), 374–380e1. DOI 10.1016/j.echo.2017.11.008. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |