

 | Congenital Heart Disease |  |
DOI: 10.32604/CHD.2021.014373
ARTICLE
A Systematic Approach to Pulmonary Valve Replacement in the Current Era
1The Pediatric Heart Institute, Joe DiMaggio Children’s Hospital, Memorial Regional Hospital-Memorial Healthcare System, Hollywood, Florida, USA
2Division of Congenital Cardiothoracic Surgery, The Pediatric Heart Institute, Joe DiMaggio Children’s Hospital, Florida, USA
*Corresponding Author: R. Allen Ligon. Email: allenligon@gmail.com
Received: 22 September 2020; Accepted: 23 October 2020
Abstract: Background: Pulmonary valve replacement (PVR) can be accomplished via surgical, transcatheter, or hybrid approaches. There are inherent advantages to transcatheter PVR and hybrid PVR without cardiopulmonary bypass. We review the methods and results of a standardized institutional approach to PVR. Methods: Retrospective review of all PVR cases between February 2017 and February 2020. Hybrid PVR entailed off-pump RVOT plication with percutaneous transcatheter PVR. Results: Primary transcatheter PVR was attempted in 37, hybrid PVR was performed in 11, and on-pump surgical PVR was performed in 9. Median age at PVR was 27 years (6–65). Primary transcatheter PVR was successful in 35/37 (2 converted to surgical). Standard surgical PVR was utilized for positive coronary compression testing (n = 4), stent/valve system migration (n = 2), or patient preference (n = 3). In the hybrid group mean RVOT diameter was 34 mm (32–38). Median length of stay was 1 day for transcatheter PVR, 5 for surgical, and 3 for hybrid (p = 0.02). Median follow-up was 1.5 years. Re-interventions were one balloon valve dilation in a transcatheter PVR, and one valve dilation with subsequent transcatheter valve-in-valve PVR in the surgical cohort. One hybrid patient expired 11 months post procedure. Conclusions: A systematic approach to PVR utilizing all approaches in pre-defined order of preference leads to consistent outcomes in a wide variety of anatomic configurations. Transcatheter PVR may be accomplished in the majority of patients. When necessary, hybrid off-pump RVOT plication with transcatheter PVR avoids the need for cardiopulmonary bypass.
Keywords: Transcatheter pulmonary valve replacement; adult congenital heart disease; congenital heart disease
Right ventricular outflow tract (RVOT) reconstruction is a common operation performed in patients with congenital heart disease (CHD), often requiring a series of reoperations during the lifetime of the patient due to conduit or valvular failure [1–4]. Surgical RVOT revision can be performed with a low mortality and morbidity, but implanted valves have a limited functional lifespan, commonly less than 10 years. Furthermore, successive surgical pulmonary valve replacement (PVR) procedures may have progressively increased risk of complications [5].
Transcatheter PVR has become a widely accepted alternative to surgical PVR utilizing cardiopulmonary bypass (CPB) [6,7]. In suitable patients, transcatheter PVR avoids the need for CPB and a surgical incision, and is routinely performed with only an overnight stay in the hospital. Thus, there is a very strong patient preference for this approach in suitable patients, and medium-term outcomes have proven to be comparable to the traditional surgical alternative [6,8–12]. Currently available transcatheter valves approved in the U.S. for use in this setting include the Melody transcatheter pulmonary valve (Medtronic, Minneapolis, MN) and the Edwards Sapien transcatheter heart valve system (Edwards Lifesciences, Irvine, CA). Numerous studies have demonstrated efficacy of the Melody valve for transcatheter PVR in RVOTs smaller than 24 mm and Sapien valve for RVOTs smaller than 30 mm [9,12–15]. Unfortunately, a large portion of patients requiring PVR have RVOT diameters still too large for these currently approved balloon expandable valve systems.
In order to overcome this limitation, a few centers have reported on the concept of a hybrid transcatheter-surgical approach to reduce the RVOT diameter off of CPB. This methodology entails limited dissection and surgical RVOT/pulmonary artery (PA) plication to allow subsequent implantation of transcatheter balloon expandable systems [16–19]. Data is however, limited in regards to this hybrid approach. Since February 2017, our institution has offered an off-pump hybrid procedure as an additional approach for PVR in patients with an RVOT too large for available transcatheter valves. The objective of this study is to outline our institutional systematic approach to PVR, which includes this hybrid approach in preference to traditional surgical PVR in suitable patients.
We performed an institutional retrospective chart review of all PVR cases and patient follow up evaluations from February 2017 to February 2020. Transthoracic echocardiograms were performed on all patient’s pre-procedure, before hospital discharge, and at routine visits postoperatively to evaluate valve function. Other pre-procedure workup included cardiac magnetic resonance imaging (MRI) if clinically indicated and feasible. In those patients unable to undergo cardiac MRI (e.g., -non-compatible pacemaker), a cardiac computed tomographic scan and pulmonary perfusion scan was performed. Indications for PVR included patient clinical symptomatology in addition to objective measures obtained during cardiopulmonary exercise testing, echocardiographic, and cardiac MRI data. Echocardiographic measures of importance included stenosis defined by a transvalvular peak instantaneous pressure gradient greater than 40 mmHg (or mean gradient greater than 30 mmHg), presence of diminished right ventricular (RV) function, or an RV pressure greater than 75% of systemic pressure (2/3 systemic if symptomatic). Parameters for valve regurgitation by cardiac MRI included grade 1/mild (<25%), grade 2/moderate (20–35%) or grade 3/severe (greater than 35%) regurgitant fraction and/or evidence of progressive RV dilation (having reached an indexed RV end-diastolic volume greater than 150 mL/m2 or indexed RV end-systolic volume greater than 75 mL/m2). Subgroups within the 3 cohorts were created based on primary indication for PVR (stenosis versus insufficiency), to avoid skewing of data variables based on different indications. All patients underwent cardiac catheterization for assessment of coronary artery disease if over 35 years of age, and transcatheter RVOT balloon sizing with coronary compression testing prior to index intervention. Transcatheter PVR was performed during the same catheterization in suitable patients.
All patients who underwent PVR were instructed to maintain excellent dental hygiene and to observe antibiotic prophylaxis precautions at appropriate times for prevention of infective endocarditis. Medical therapy with low-dose aspirin was recommended indefinitely after the index procedure in all patients. This study was approved by the Institutional Review Board at Memorial Healthcare Systems in Hollywood, FL. Statistical analysis included categorical variables reported as absolute numbers and percentages. Continuous variables are expressed as mean ± standard deviation or as the median value (range) if there was not a normal distribution of values. Patient groups (transcatheter, surgery, and hybrid PVR) were further divided into two groups based on their primary indication of stenosis or insufficiency for accurate data comparison. Comparison of continuous variables was performed using the two-tailed Student test for paired data and comparison of discrete variables was done with the Fisher exact test. A one-way ANOVA test was utilized to search for statistically significant differences between the 3 independent groups. For all tests, a p-value of less than 0.05 was considered statistically significant.
2.1 Percutaneous Transcatheter Approach
Percutaneous transcatheter PVR was performed in a cardiac catheterization laboratory under general anesthesia. Biplane angiography of the RVOT was performed to assess native diameters and length of the RVOT. Balloon sizing was performed in the RVOT or “landing zone” (whether native RVOT, surgical conduit or bioprosthetic valve), making special note of the diameter of the waist in the balloon as well as simultaneous coronary compression testing via an aortogram. Pre-stenting of the RVOT was performed when there was felt to be a significant risk of extrinsic compression of the valve, or in some cases of heavy conduit calcification. The Melody transcatheter pulmonary valve (Medtronic, Minneapolis, MN) was preferentially utilized for RVOT diameters less than 24 mm, unless it was felt that the RVOT needed significant additional structural support. In Melody PVR patients, they underwent pre-stenting of the RVOT prior to valve placement for system stability. The Edwards Sapien transcatheter heart valve system (Edwards Lifesciences, Irvine, CA) was preferentially utilized in RVOT diameters greater than 24 mm, or if it was felt that the RVOT was subject to significant risk of compression. The appropriate valve delivery system was advanced into the RVOT over a stiff guidewire that was anchored in a peripheral pulmonary artery branch. The valve was deployed by filling the balloon to the manufacturer-recommended pressure or volume. Post-dilatation was performed with an ultra-high pressure non-compliant balloon in warranted cases to improve final valve-stent system expansion. Valve function was assessed with angiography and transthoracic echocardiography.
The appropriate valve size was determined by standardized valve sizing charts-based on the patient’s body weight and body surface area. The larger end of the recommended size range was favored in an attempt to use the largest valve technically possible. All procedures were performed via a redo median sternotomy. All patients underwent atrial cannulation for venous return with ascending aortic arterial inflow. If the operation required access to the right side of the heart (in addition to RVOT reconstruction, such as to include tricuspid valve annuloplasty or repair or pulmonary arterioplasty), bi-caval cannulation was used and the aorta was not cross-clamped unless a shunt was present.
The native pulmonary valve and proximal pulmonary trunk were incised cranio-caudally and a variety of materials were used to cover the valve if necessary, including extracellular matrix porcine xenograft (CorMatrix, Alpharetta, Georgia, USA), or PhotoFix-treated bovine pericardium (CryoLife, Inc, Kennesaw, GA) or synthetic material (Gore-Tex; W. L. Gore & Associates, Flagstaff, AZ). The new-generation of stented Carpentier-Edwards Perimount bovine pericardial aortic prosthesis (Magna, Edwards Lifesciences, Irvine, CA, USA) was inserted in all cases.
Hybrid PVR entailed off-pump RVOT-PA plication with subsequent transcatheter PVR occurring during the same procedure. All procedures were performed in a hybrid catheterization suite under general anesthesia. A femoral venous catheter was advanced to a distal PA branch. Full median sternotomy was then performed to expose the main PA, with minimal dissection of the heart and aorta. RVOT angiography was performed to obtain a roadmap of the anatomy with the open chest. An approximate measurement of the main PA was made to assist in determining the appropriate extent of plication (Fig. 1). Main PA/RVOT plication was performed with multiple pledgeted horizontal mattress sutures to provide an elongated, waist-like reduction of the selected landing zone. If there was calcification of a preexisting RVOT patch, a mattress suture, usually 4.0 Prolene (Ethicon, Somerville, NJ), was used to provide focal narrowing of the RVOT to approximately the required minimal diameter for the planned transcatheter device. Circumferential dissection of the main PA was not required. Following completion of mPA/RVOT plication, repeat RVOT angiography and balloon sizing was performed to re-assess the anatomy of the landing zone and ensure that a diameter suitable for a transcatheter valve was achieved (Fig. 2). If the post-plication diameter was still too large for the valve, then further plication was performed. If clinically indicated for the valve system (e.g., - Melody PVR, small conduits/RVOT < 24 mm, etc.), the main PA was pre-stented to create a landing zone for the valve construct and delivered via either femoral vein. In one patient, the delivery system could not be advanced from the femoral approach to the landing zone due to alteration in angle following RV plication. The valve was successfully delivered via a direct PA insertion. After deployment of a valve, the function of the valve was assessed by angiography and transthoracic echocardiography.
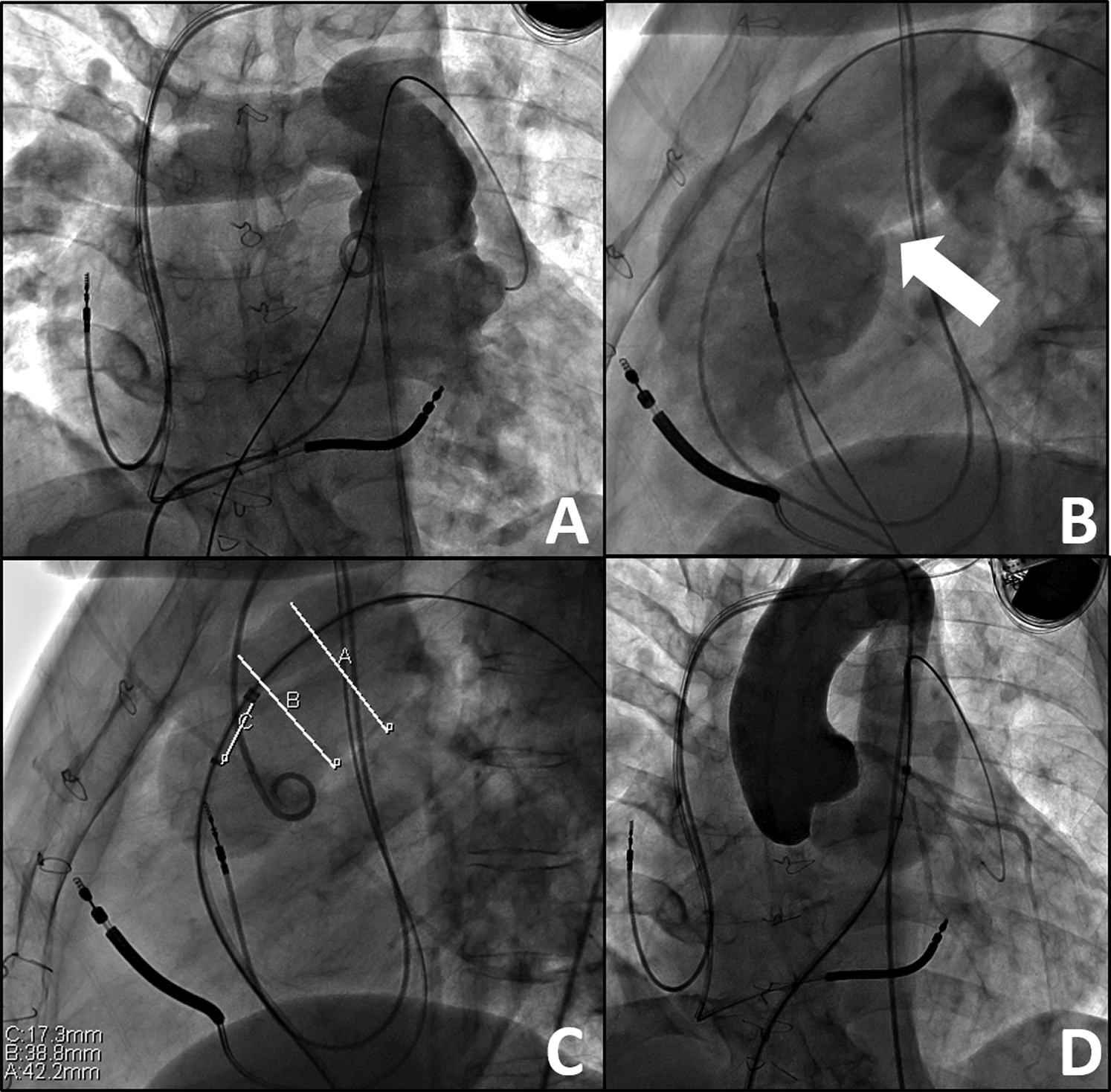
Figure 1: Baseline Angiography, prior to Hybrid Intervention. A) Anteroposterior view of a right ventriculogram with distal wire in the distal left pulmonary artery. B) Lateral view of the large right ventricular outflow tract with a landing zone (white arrow) too large for transcatheter valve placement. C) Balloon sizing of the right ventricular outflow tract demonstrating measurements too large for current transcatheter valve systems, narrowest portion being 38.8 mm during balloon sizing. D) Coronary artery compression testing via aortogram during balloon sizing of the right ventricular outflow tract–no concerns for coronary artery insult or pathology
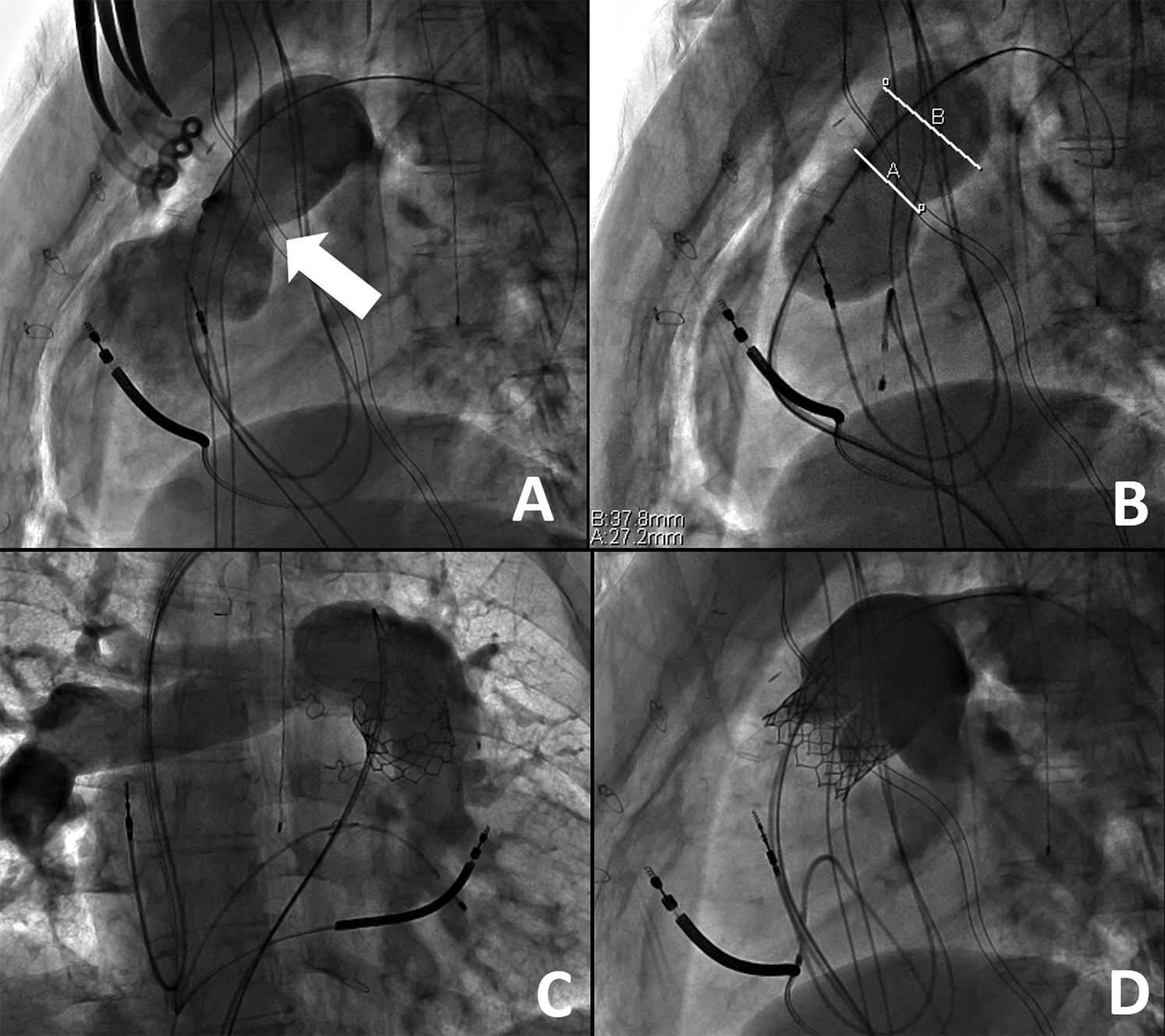
Figure 2: Right Ventricular Outflow Tract Rehabilitation via Hybrid Pulmonary Valve Replacement. A) Following surgical plication of the right ventricle, there is a new appearance to the right ventricular outflow tract on the lateral view with a new landing zone (white arrow). B) Upon balloon sizing, there are now measurements suitable for transcatheter pulmonary valve replacement as the narrowest portion measures about 27 mm. Following placement of a 29 mm Edwards Sapien transcatheter valve system (Edwards Lifesciences, Irvine, CA), there is a well seated valve in the anteroposterior (C) and lateral (D) views without any evidence for valve insufficiency
During the study period, 55 patients underwent PVR with the breakdown as outlined in Tab. 1. Primary transcatheter PVR was attempted in 37 patients (67%) and was successful in 35 patients (95%). Surgical PVR was performed in 9 patients (16%)-including the 2 failed transcatheter valve patients, 4 with positive coronary compression testing, and 3 who preferred the standard surgical approach over the newer hybrid approach. Finally, 11 patients (20%) underwent surgical RV plication then subsequent transcatheter PVR (“hybrid PVR”). All hybrid PVR patients had RVOT measurements too large for transcatheter PVR without RV plication, ranging from 32–38 mm on balloon sizing.
Table 1: Patients that underwent pulmonary valve replacement during study period. Constituting all those patients and follow-up evaluations from February 2017 to February 2020 (n = 55) performed at our institution
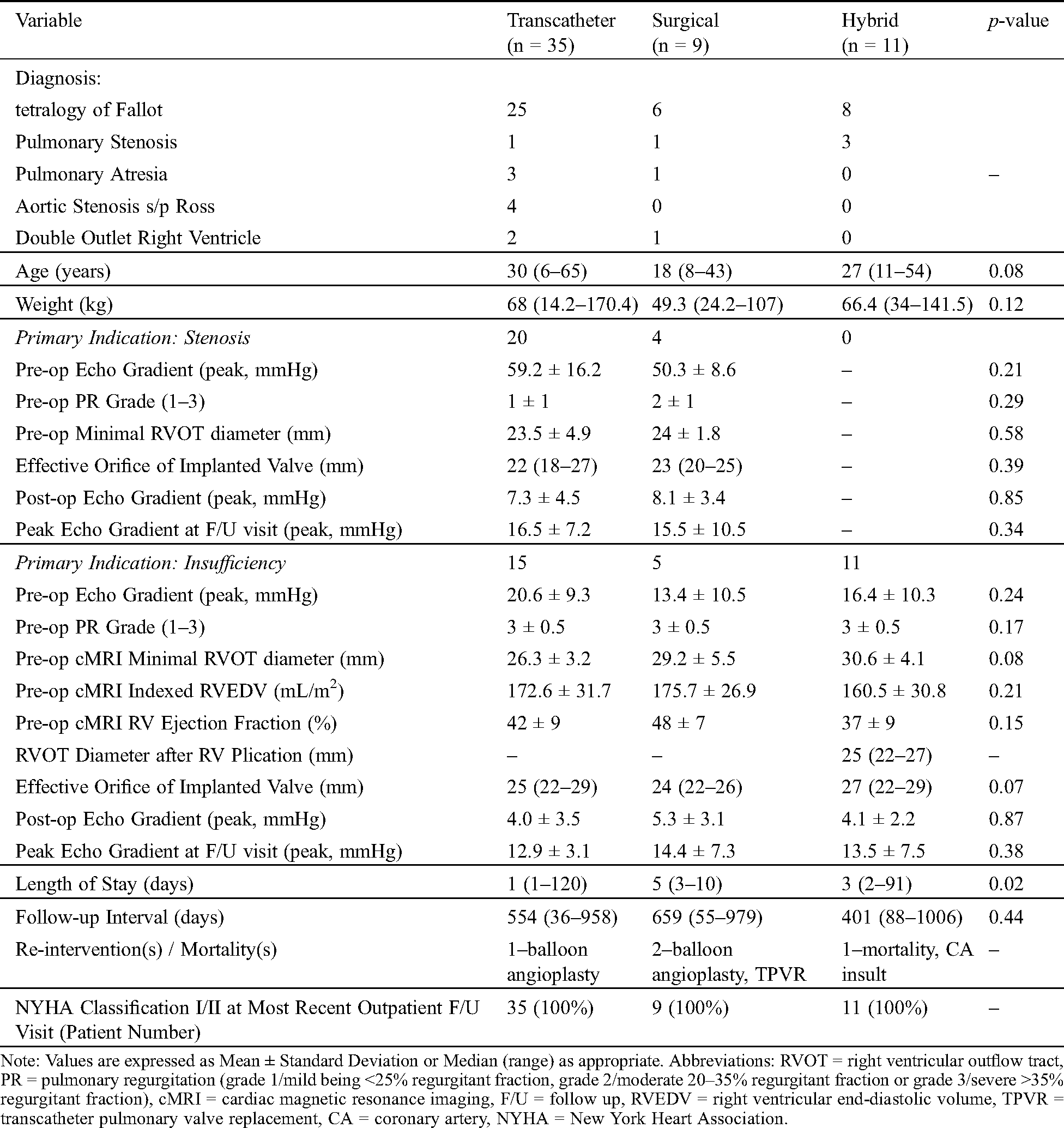
For the transcatheter PVR cohort (n = 35), the most common diagnosis was tetralogy of Fallot (n = 25) with a median patient age of 30 years (range, 6–65 years) and weight of 68 kg (14.2–170.4 kg). Within this transcatheter PVR cohort, there were 20 patients (57%) with the primary indication being stenosis. Pre-operatively, their mean RVOT gradient by echocardiogram was 59.2 ± 16.2 mmHg and the pulmonary insufficiency grade 2 ± 1. As measured on the pre-intervention cardiac MRI or computed tomography scan, the landing zone diameter was 23.5 ± 4.9 mm. Following transcatheter PVR, the median implanted effective orifice valve size (final, following any post-dilation) was 22 (18–27 mm) with either the Edwards Sapien (n = 5) or Medtronic Melody (n = 15) valve systems. The mean post-valve implantation peak RVOT gradient measured at the end of the catheterization was 7.3 ± 4.5 mmHg. The median outpatient follow-up interval for this subgroup was 585 days (93–958 days) and the peak RVOT echocardiographic gradient during that evaluation was 16.5 ± 7.2 mmHg.
The remaining 15 patients (43%) in the transcatheter cohort underwent PVR secondary to insufficiency as the primary indication. Pre-operatively, the mean RVOT gradient by echocardiogram was 20.6 ± 9.3 mmHg and the pulmonary insufficiency grade 3.5 ± 0.5. As measured on the pre-intervention cardiac MRI, the mean landing zone diameter was 26.3 ± 3.2 mm, the indexed RV end-diastolic volume 172.6 ± 31.7 mL/m2, and the right ventricular ejection fraction 42 ± 9%. Following transcatheter PVR, the median implanted effective orifice valve size (final, following any post-dilation) was 25 (22–29 mm) with either the Edwards Sapien (n = 10) or Medtronic Melody (n = 5) valve systems. The mean post-valve implantation peak RVOT gradient measured at the end of the catheterization was 4.0 ± 3.5 mmHg. The median outpatient follow-up interval for this subgroup was 427 days (36–930 days) and the peak RVOT echocardiographic gradient during that evaluation was 12.9 ± 3.1 mmHg.
The subsequent inpatient median length of stay for the entire transcatheter PVR cohort was 1 day (range 1–120 days), as 31 patients without significant co-morbidities were discharged the following day. Three patients required 3 days of inpatient stay for diuresis or heart failure management. Another patient (n = 1) required 21 days of inpatient stay due to respiratory issues. The final patient (n = 1) with inpatient stay (post-procedure) of 120 days was already admitted for heart failure management/failing physiology, felt unrelated to transcatheter PVR placement (cardiomyopathy present at time of index procedure). At the last documented outpatient evaluation, the median follow-up interval for the entire transcatheter PVR cohort was 554 days (36–958 days) and all patients (n = 35) reported New York Heart Association Classification System Class I-II. One patient underwent repeat intervention following transcatheter PVR, which included balloon valve dilation due to high gradient 183 days following implantation. There were no reported episodes of endocarditis and no known stent fractures.
For the surgical PVR cohort (n = 9), the most common diagnosis was tetralogy of Fallot (n = 6) with median patient age 18 years (range, 8–43 years) and weight 49.3 kg (24.2–107 kg). Surgical valve implantation performed on cardiopulmonary bypass was indicated for 4 patients due to positive coronary compression testing, 2 patients required removal of stent/valve system that had migrated during attempted transcatheter PVR, and 3 patients preferred to undergo surgical PVR over the newer hybrid approach. There were 4 patients (44%) in the surgical cohort that underwent PVR with stenosis as the primary indication. Pre-operatively, the mean RVOT gradient by echocardiogram was 50.3 ± 8.6 mmHg and the pulmonary insufficiency grade 2.5 ± 1. The mean landing zone diameter was 24 ± 1.8 mm as measured on the pre-intervention cardiac MRI or computed tomography scan. Following surgical PVR, the median implanted effective orifice valve size (final, following any post-dilation) was 23 mm (20–25 mm). The mean post-valve implantation peak RVOT gradient by echocardiogram was 8.1 ± 3.4 mmHg. The median outpatient follow-up interval for this subgroup was 624 days (55–821 days) and the peak RVOT echocardiographic gradient during that evaluation was 15.5 ± 10.5 mmHg.
The remaining 5 patients (56%) in the surgical cohort underwent PVR due to insufficiency as the primary indication. Pre-operatively, the mean RVOT gradient by echocardiogram was 13.4 ± 10.5 mmHg and the pulmonary insufficiency grade 3.5 ± 0.5. As measured on the pre-intervention cardiac MRI, the mean landing zone diameter was 29.2 ± 5.5 mm, the indexed RV end-diastolic volume 175.7 ± 26.9 mL/m2, and the right ventricular ejection fraction 48 ± 7%. Following surgical PVR, the median implanted effective orifice valve size was 24 (22–26 mm) and the post-valve implantation peak RVOT gradient by echocardiogram measured 5.3 ± 3.1 mmHg. The median outpatient follow-up interval for this subgroup was 659 days (433–979 days) and the peak RVOT echocardiographic gradient during that evaluation was 14.4 ± 7.3 mmHg.
The subsequent median inpatient length of stay for the entire surgical cohort was 5 days (range, 3–10 days). At the last documented outpatient evaluation, the median follow-up was 659 days (55–979 days) and all patients (n = 9) reported Class I–II within the New York Heart Association Classification system. One patient underwent subsequent transcatheter valve-in-valve PVR 413 days postop and another patient had balloon valve dilation 512 days postop–both secondary to a high RVOT gradient.
In terms of the hybrid PVR cohort (n = 11), the most common diagnosis was tetralogy of Fallot (n = 8) and all patients underwent PVR due to insufficiency as the primary indication. The median patient age was 27 years (range, 11–54 years) and weight 66.4 kg (34–141 kg). The RVOT gradient by echocardiogram prior to intervention was 16.4 ± 10.3 mmHg and the pulmonary insufficiency grade 4 ± 0.5 (range, 1–4). As measured on the pre-intervention cardiac MRI, the landing zone diameter was 30.6 ± 4.1 mm, the indexed RV end-diastolic volume 160.5 ± 30.8 mL/m2, and the right ventricular ejection fraction 37 ± 9%. The median landing zone diameter following RV plication was 25 mm (22–27 mm) and then subsequently the median implanted effective orifice valve size was 27 mm (range, 22–29 mm). The post-implantation peak gradient by echocardiogram was 4.1 ± 2.2 mmHg. One patient necessitated surgically guided direct trans-PA insertion of the transcatheter system, which required the usage of CPB. This was felt to be secondary to the change in RVOT configuration pre- and post-RV plication, creating difficulty in transfemoral delivery of the valve system. All other patients (n = 10) underwent TPVR placement from the femoral route following RV plication.
The subsequent median inpatient length of stay was 3 days (range, 2–91 days). Most patients were discharged within 3 days postoperatively (n = 9), but one patient stayed for 12 days secondary to respiratory failure, felt unrelated to the index procedure. One patient with multiple co-morbidities experienced right coronary artery injury in the index procedure during dissection for RV plication. This patient underwent saphenous vein graft to the right coronary artery and subsequently underwent completion of hybrid PVR, then remained inpatient for 91 days. She was re-admitted and then died 11 months post-index procedure secondary to multiple medical etiologies, but including left coronary artery occlusion with unclear relation to insult during RV plication. At the last documented outpatient evaluation, the cohort median follow-up was 401 days (range, 88–1006 days) and the peak echocardiographic RVOT gradient at that visit was 13.5 ± 7.5 mmHg. This cohort did not require any subsequent re-interventions (surgical nor transcatheter) during the study period.
With the wider use of three-dimensional imaging to more accurately measure RV size and function, there has been a trend toward valve implantation to reduce or eliminate pulmonary insufficiency prior to excessive RV dilation or decrease in RV function [14,20–23]. Surgically implanted pulmonary valves/homografts/conduits placed in infancy or childhood frequently require replacement in adolescents-young adults with numerous prior surgical interventions, thus making reoperation more complex [1,24]. Furthermore, patients undergoing surgical PVR with a bioprosthetic valve inevitably experience failure of the prosthetic valve in a time-dependent fashion, and younger patients may experience shorter freedom from valve dysfunction and/or reintervention [24,25].
The optimal prosthesis and PVR methodologies remain controversial. Historically, surgical PVR has been used in both children and adults with excellent results [2,3,26,27]. Transcatheter PVR can be performed as a primary procedure and/or later via valve-in-valve application. Furthermore, some surgical valves can be fractured then stretched for subsequent valve-in-valve transcatheter valve replacement [28,29]. These advantages plus the clear patient benefit of the less invasive percutaneous approach, has spurred an institutional preference for transcatheter-based intervention as the first-line of RVOT therapy with goal of placing the largest effective orifice valve system. Although the newer alternative of transcatheter PVR has proven safe and effective for RVOT treatment, this option has been limited by the size of currently available transcatheter valve systems [11,30,31]. Emerging options include self-expanding systems such as the Medtronic HarmonyTM valve (Medtronic, Minneapolis, MN) [32–34] and Edwards Alterra Adaptive PrestentTM (Edwards Lifesciences, Irvine, CA) [35,36]. However, these technologies are currently still undergoing clinical trial [32,33,35,37] and a large portion of patients fall out of inclusion criteria because of anatomic variations. The widespread application of self-expanding stent technologies to the vast spectrum of RVOT morphologies seen in postoperative CHD patients remains guarded [38,39]. Since February of 2017, our institution has offered an off-pump hybrid procedure as an additional approach for PVR in patients with an RVOT too large for available transcatheter valves. Surgical PVR is reserved as an option for patients in unique/rare situations such as positive coronary compression testing or adverse events following attempted transcatheter PVR.
Hybrid PVR is a practical and appealing alternative to surgical PVR when transcatheter PVR is not possible as it reduces the extent of dissection and eliminates the need for CPB as well as possible cardioplegic arrest, with a reduction in the need for repeated atrial cannulation [16,18]. Although this approach still requires a sternotomy, the extent of surgical dissection required is less than that needed for CPB [16,17,19]. For the adult CHD patient, well documented are the deleterious effects of repeat CPB events and vast concurrent morbidities of this population undergoing PVR [40–44]. Due to the inherent advantages of hybrid PVR, we transitioned to utilizing this methodology when transcatheter PVR is technically unable to be performed. It is notable that the length of stay following transcatheter PVR was the shortest given the majority (31/35; 89%) of patients were discharged one day following the index procedure. The hybrid PVR maintained a shorter hospital stay when compared to the surgical cohort as well. For patients undergoing PVR due to insufficiency, those that underwent a hybrid approach trended towards having larger effective orifice diameters of their implanted prostheses (p = 0.07) following palliative intervention - possibly allowing for additional future valve-in-valve interventions. Short-term outcomes (mean 1.5 years for all cohorts) demonstrate that the re-intervention number was highest in the surgical cohort (2/9; 22%) when compared to transcatheter PVR (1/35; 3%) and none in the hybrid cohort. Lastly, the hybrid approach has generally proven safe, but the possibility of coronary artery damage (as occurred in one patient) must be considered post-RV plication. Our institution now performs routine coronary angiography following RV plication. Lastly, alteration in the angle of the RVOT following RV plication resulted in the need for transcatheter valve delivery via the mPA on CPB in one patient.
Unfortunately, the majority of patients with a dysfunctional RVOT have outflow diameters that are too large for currently available balloon expandable valves [16,31,38,39]. Our approach to patients with a definitive or borderline large RVOT has now evolved to encompass the hybrid approach in planned management. All patients undergo a catheterization for definitive balloon sizing and coronary evaluation. If balloon sizing indicates that the patient is not a candidate for primary transcatheter pulmonary valve replacement at the initial catheterization, all relevant preparations and evaluations for a hybrid procedure are accomplished, and the patient is scheduled for a hybrid procedure. In our hybrid group, we found that plication of the RVOT/main PA could be easily and safely performed on the beating heart and avoiding CPB is possible in these patients. RV plication reshapes what is often a conical RVOT/PA trunk to a tubular form, which subsequently allows more secure seating of transcatheter valve system.
Before 2017, approximately one-quarter of the patients referred to our catheterization lab for evaluation of transcatheter PVR were not candidates due to technology limitations and were thus referred for surgical PVR. Collaboration between the cardiothoracic surgery and interventional cardiology teams laid the foundation for a practical and systematic approach to PVR in the CHD patient. This study summarized all PVR patient outcomes following the implementation of an institutional PVR algorithm which includes hybrid off-pump PVR-the first of its kind to do so. Transcatheter PVR may be accomplished in the majority of patients with RVOT dysfunction. In patients with unsuitably large outflow tract, a stepwise approach including initial catheterization for more detailed evaluation and preparation followed by hybrid off-pump plication (without CPB) with transcatheter PVR is safe and effective. Standard on-pump surgical PVR can then be reserved for patients at risk for coronary compression or following adverse events during transcatheter procedures.
Our study is limited by its retrospective nature from a single institution, recognizing that the surgical and transcatheter practices may vary widely from center to center. Statistical analyses of the patient cohorts proved difficult due to the limited subject size and heterogeneity of the subgroups. A separation into primary indication: stenosis versus insufficiency represented an attempt to distinguish the vast findings within the PVR patient and outline our systematic approach to this complex population. Further, the re-interventions in our short term follow up included a valve balloon angioplasty in the transcatheter cohort, a valve balloon angioplasty as well as a subsequent TPVR in the surgical cohort and none in the hybrid cohort. Although there have been no diagnosed episodes of endocarditis at the time of this publication, our follow up period remains short term (mean follow up 1.5 years) and all patients undergoing PVR by any technique remains at risk for endocarditis [13,45,46]. We aim to continue data collection with special attention to re-interventions and more long-term outcomes of PVR methodology.
Although technologies will continue to advance and our own institutional PVR algorithm may evolve in future years, our goal remains to benefit patients over a lifetime versus the first few years following palliative intervention. The outlined institutional model prioritizes transcatheter PVR and then employs hybrid PVR in order avoid CPB if at all possible and provides the largest valve effective orifice diameter following intervention.
Funding Statement: The author(s) received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Rodefeld, M. D., Ruzmetov, M., Turrentine, M. W., Brown, J. W. (2008). Reoperative right ventricular outflow tract conduit reconstruction: Risk analyses at follow up. Journal of Heart Valve Disease, 17(1), 119–126. [Google Scholar]
2. Brown, J. W., Ruzmetov, M., Rodefeld, M. D., Vijay, P., Darragh, R. K. (2006). Valved bovine jugular vein conduits for right ventricular outflow tract reconstruction in children: An attractive alternative to pulmonary homograft. Annals of Thoracic Surgery, 82(3), 909–916. DOI 10.1016/j.athoracsur.2006.03.008. [Google Scholar] [CrossRef]
3. Brown, J. W., Ruzmetov, M., Rodefeld, M. D., Vijay, P., Darragh, R. K. (2006). Valved bovine jugular vein conduits for right ventricular outflow tract reconstruction in children: An attractive alternative to pulmonary homograft. Annals of Thoracic Surgery, 82(3), 909–916. DOI 10.1016/j.athoracsur.2006.03.008. [Google Scholar] [CrossRef]
4. Lee, C., Kim, Y. M., Lee, C. H., Kwak, J. G., Park, C. S. et al. (2012). Outcomes of pulmonary valve replacement in 170 patients with chronic pulmonary regurgitation after relief of right ventricular outflow tract obstruction: Implications for optimal timing of pulmonary valve replacement. Journal of the American College of Cardiology, 60(11), 1005–1014. DOI 10.1016/j.jacc.2012.03.077. [Google Scholar] [CrossRef]
5. Shinkawa, T., Lu, C. K., Chipman, C., Tang, X., Gossett, J. M. et al. (2015). The midterm outcomes of bioprosthetic pulmonary valve replacement in children. Seminars in Thoracic and Cardiovascular Surgery, 27(3), 310–318. DOI 10.1053/j.semtcvs.2015.07.010. [Google Scholar] [CrossRef]
6. Caughron, H., Kim, D., Kamioka, N., Lerakis, S., Yousef, A. et al. (2018). Repeat pulmonary valve replacement: Similar intermediate-term outcomes with surgical and transcatheter procedures. JACC: Cardiovascular Interventions, 11(24), 2495–2503. DOI 10.1016/j.jcin.2018.07.042. [Google Scholar] [CrossRef]
7. Bonhoeffer, P., Boudjemline, Y., Saliba, Z., Merckx, J., Aggoun, Y. et al. (2000). Percutaneous replacement of pulmonary valve in a right-ventricle to pulmonary-artery prosthetic conduit with valve dysfunction. Lancet (London, England), 356(9239), 1403–1405. DOI 10.1016/S0140-6736(00)02844-0. [Google Scholar] [CrossRef]
8. McRae, M. E., Coleman, B., Atz, T. W., Kelechi, T. J. (2017). Patient outcomes after transcatheter and surgical pulmonary valve replacement for pulmonary regurgitation in patients with repaired tetralogy of Fallot: A quasi-meta-analysis. European Journal of Cardiovascular Nursing: Journal of the Working Group on Cardiovascular Nursing of the European Society of Cardiology, 16(6), 539–553. DOI 10.1177/1474515117696384. [Google Scholar] [CrossRef]
9. Fiszer, R., Dryżek, P., Szkutnik, M., Góreczny, S., Krawczuk, A. et al. (2017). Immediate and long-term outcomes of percutaneous transcatheter pulmonary valve implantation. Cardiology Journal, 24(6), 604–611. DOI 10.5603/CJ.a2017.0023. [Google Scholar] [CrossRef]
10. Zablah, J. E., Misra, N., Gruber, D., Kholwadwala, D., Epstein, S. (2017). Comparison of patients undergoing surgical versus transcatheter pulmonary valve replacement: Criteria for referral and mid-term outcome. Pediatric Cardiology, 38(3), 603–607. DOI 10.1007/s00246-016-1554-9. [Google Scholar] [CrossRef]
11. Sharma, V., Griffiths, E. R., Eckhauser, A. W., Gray, R. G., Martin, M. H. et al. (2018). Pulmonary valve replacement: A single-institution comparison of surgical and transcatheter valves. Annals of Thoracic Surgery, 106(3), 807–813. DOI 10.1016/j.athoracsur.2018.04.002. [Google Scholar] [CrossRef]
12. Cheatham, J. P., Hellenbrand, W. E., Zahn, E. M., Jones, T. K., Berman, D. P. et al. (2015). Clinical and hemodynamic outcomes up to 7 years after transcatheter pulmonary valve replacement in the US melody valve investigational device exemption trial. Circulation, 131(22), 1960–1970. DOI 10.1161/CIRCULATIONAHA.114.013588. [Google Scholar] [CrossRef]
13. Gillespie, M. J., McElhinney, D. B., Kreutzer, J., Hellenbrand, W. E., El-Said, H. et al. (2015). Transcatheter pulmonary valve replacement for right ventricular outflow tract conduit dysfunction after the ross procedure. Annals of Thoracic Surgery, 100(3), 996–1003. DOI 10.1016/j.athoracsur.2015.04.108. [Google Scholar] [CrossRef]
14. Cabalka, A. K., Asnes, J. D., Balzer, D. T., Cheatham, J. P., Gillespie, M. J. et al. (2018). Transcatheter pulmonary valve replacement using the melody valve for treatment of dysfunctional surgical bioprostheses: A multicenter study. Journal of Thoracic and Cardiovascular Surgery, 155(4), 1712–1724.e1. DOI 10.1016/j.jtcvs.2017.10.143. [Google Scholar] [CrossRef]
15. Wilson, W. M., Benson, L. N., Osten, M. D., Shah, A., Horlick, E. M. (2015). Transcatheter pulmonary valve replacement with the Edwards Sapien System: The Toronto experience. JACC: Cardiovascular Interventions, 8(14), 1819–1827. DOI 10.1016/j.jcin.2015.08.016. [Google Scholar] [CrossRef]
16. Sosnowski, C., Matella, T., Fogg, L., Ilbawi, M., Nagaraj, H. et al. (2016). Hybrid pulmonary artery plication followed by transcatheter pulmonary valve replacement: Comparison with surgical PVR. Catheterization and Cardiovascular Interventions: Official Journal of the Society for Cardiac Angiography & Interventions, 88(5), 804–810. DOI 10.1002/ccd.26620. [Google Scholar] [CrossRef]
17. Chen, Q., Turner, M., Caputo, M., Stoica, S., Marianeschi, S. et al. (2013). Pulmonary valve implantation using self-expanding tissue valve without cardiopulmonary bypass reduces operation time and blood product use. Journal of Thoracic and Cardiovascular Surgery, 145(4), 1040–1045. DOI 10.1016/j.jtcvs.2012.05.036. [Google Scholar] [CrossRef]
18. Porras, D., Gurvitz, M., Marshall, A. C., Emani, S. M. (2015). Hybrid approach for off-pump pulmonary valve replacement in patients with a dilated right ventricular outflow tract. Annals of Thoracic Surgery, 100(5), e99–e101. DOI 10.1016/j.athoracsur.2015.02.124. [Google Scholar] [CrossRef]
19. Boudjemline, Y., Schievano, S., Bonnet, C., Coats, L., Agnoletti, G. et al. (2005). Off-pump replacement of the pulmonary valve in large right ventricular outflow tracts: A hybrid approach. Journal of Thoracic and Cardiovascular Surgery, 129(4), 831–837. DOI 10.1016/j.jtcvs.2004.10.027. [Google Scholar] [CrossRef]
20. Martin, M. H., Shahanavaz, S., Peng, L. F., Asnes, J. D., Riley, M. et al. (2018). Percutaneous transcatheter pulmonary valve replacement in children weighing less than 20 kg. Catheterization and Cardiovascular Interventions: Official Journal of the Society for Cardiac Angiography & Interventions, 91(3), 485–494. DOI 10.1002/ccd.27432. [Google Scholar] [CrossRef]
21. Borik, S., Crean, A., Horlick, E., Osten, M., Lee, K. J. et al. (2015). Percutaneous pulmonary valve implantation: 5 years of follow-up: Does age influence outcomes? Circulation: Cardiovascular Interventions, 8(2), e001745. DOI 10.1161/CIRCINTERVENTIONS.114.001745. [Google Scholar] [CrossRef]
22. Pagourelias, E. D., Daraban, A. M., Mada, R. O., Duchenne, J., Mirea, O. et al. (2017). Right ventricular remodelling after transcatheter pulmonary valve implantation. Catheterization and Cardiovascular Interventions: Official Journal of the Society for Cardiac Angiography & Interventions, 90(3), 407–417. DOI 10.1002/ccd.26966. [Google Scholar] [CrossRef]
23. Li, W. F., Pollard, H., Karimi, M., Asnes, J. D., Hellenbrand, W. E. et al. (2018). Comparison of valvar and right ventricular function following transcatheter and surgical pulmonary valve replacement. Congenital Heart Disease, 13(1), 140–146. DOI 10.1111/chd.12544. [Google Scholar] [CrossRef]
24. Fiore, A. C., Rodefeld, M., Turrentine, M., Vijay, P., Reynolds, T. et al. (2008). Pulmonary valve replacement: A comparison of three biological valves. Annals of Thoracic Surgery, 85(5), 1712–1718. DOI 10.1016/j.athoracsur.2008.02.001. [Google Scholar] [CrossRef]
25. Dehaki, M. G., Al-Dairy, A., Rezaei, Y., Omrani, G., Jalali, A. H. et al. (2019). Mid-term outcomes of mechanical pulmonary valve replacement: A single-institutional experience of 396 patients. General Thoracic and Cardiovascular Surgery, 67(3), 289–296. DOI 10.1007/s11748-018-1012-0. [Google Scholar] [CrossRef]
26. Bibevski, S., Ruzmetov, M., Fortuna, R. S., Turrentine, M. W., Brown, J. W. et al. (2017). Performance of synergraft decellularized pulmonary allografts compared with standard cryopreserved allografts: Results from multiinstitutional data. Annals of Thoracic Surgery, 103(3), 869–874. DOI 10.1016/j.athoracsur.2016.07.068. [Google Scholar] [CrossRef]
27. Ruzmetov, M., Shah, J. J., Geiss, D. M., Fortuna, R. S. (2012). Decellularized versus standard cryopreserved valve allografts for right ventricular outflow tract reconstruction: A single-institution comparison. Journal of Thoracic and Cardiovascular Surgery, 143(3), 543–549. DOI 10.1016/j.jtcvs.2011.12.032. [Google Scholar] [CrossRef]
28. Shahanavaz, S., Asnes, J. D., Grohmann, J., Qureshi, A. M., Rome, J. J. et al. (2018). Intentional fracture of bioprosthetic valve frames in patients undergoing valve-in-valve transcatheter pulmonary valve replacement. Circulation: Cardiovascular Interventions, 11(8), e006453. DOI 10.1161/CIRCINTERVENTIONS.118.006453. [Google Scholar] [CrossRef]
29. Allen, K. B., Chhatriwalla, A. K., Cohen, D. J., Saxon, J. T., Aggarwal, S. et al. (2017). Bioprosthetic valve fracture to facilitate transcatheter valve-in-valve implantation. Annals of Thoracic Surgery, 104(5), 1501–1508. DOI 10.1016/j.athoracsur.2017.04.007. [Google Scholar] [CrossRef]
30. Alassas, K., Mohty, D., Clavel, M. A., Husain, A., Hijji, T. et al. (2018). Transcatheter versus surgical valve replacement for a failed pulmonary homograft in the Ross population. Journal of Thoracic and Cardiovascular Surgery, 155(4), 1434–1444. DOI 10.1016/j.jtcvs.2017.10.141. [Google Scholar] [CrossRef]
31. Lurz, P., Nordmeyer, J., Giardini, A., Khambadkone, S., Muthurangu, V. et al. (2011). Early versus late functional outcome after successful percutaneous pulmonary valve implantation: Are the acute effects of altered right ventricular loading all we can expect? Journal of the American College of Cardiology, 57(6), 724–731. DOI 10.1016/j.jacc.2010.07.056. [Google Scholar] [CrossRef]
32. Gillespie, M. J., Benson, L. N., Bergersen, L., Bacha, E. A., Cheatham, S. L. et al. (2017). Patient selection process for the harmony transcatheter pulmonary valve early feasibility study. American Journal of Cardiology, 120(8), 1387–1392. DOI 10.1016/j.amjcard.2017.07.034. [Google Scholar] [CrossRef]
33. Bergersen, L., Benson, L. N., Gillespie, M. J., Cheatham, S. L., Crean, A. M. et al. (2017). Harmony feasibility trial: Acute and short-term outcomes with a self-expanding transcatheter pulmonary valve. JACC: Cardiovascular Interventions, 10(17), 1763–1773. DOI 10.1016/j.jcin.2017.05.034. [Google Scholar] [CrossRef]
34. Schoonbeek, R. C., Takebayashi, S., Aoki, C., Shimaoka, T., Harris, M. A. et al. (2016). Implantation of the medtronic harmony transcatheter pulmonary valve improves right ventricular size and function in an ovine model of postoperative chronic pulmonary insufficiency. Circulation: Cardiovascular Interventions, 9(10), e003920. DOI 10.1161/CIRCINTERVENTIONS.116.003920. [Google Scholar] [CrossRef]
35. Zahn, E. M., Chang, J. C., Armer, D., Garg, R. (2018). First human implant of the Alterra Adaptive PrestentTM: A new self-expanding device designed to remodel the right ventricular outflow tract. Catheterization and Cardiovascular Interventions: Official Journal of the Society for Cardiac Angiography & Interventions, 91(6), 1125–1129. DOI 10.1002/ccd.27581. [Google Scholar] [CrossRef]
36. Balzer, D. (2019). Pulmonary valve replacement for tetralogy of fallot. Methodist DeBakey Cardiovascular Journal, 15(2), 122–132. [Google Scholar]
37. Zahn, E. M. (2019). Self-expanding pulmonary valves for large diameter right ventricular outflow tracts. Interventional Cardiology Clinics, 8(1), 73–80. DOI 10.1016/j.iccl.2018.08.003. [Google Scholar] [CrossRef]
38. Schievano, S., Coats, L., Migliavacca, F., Norman, W., Frigiola, A. et al. (2007). Variations in right ventricular outflow tract morphology following repair of congenital heart disease: Implications for percutaneous pulmonary valve implantation. Journal of Cardiovascular Magnetic Resonance: Official Journal of the Society for Cardiovascular Magnetic Resonance, 9(4), 687–695. DOI 10.1080/10976640601187596. [Google Scholar] [CrossRef]
39. Zaidi, S. J., Cossor, W., Singh, A., Maffesanti, F., Kawaji, K. et al. (2018). Three-dimensional analysis of regional right ventricular shape and function in repaired tetralogy of Fallot using cardiovascular magnetic resonance. Clinical Imaging, 52, 106–112. DOI 10.1016/j.clinimag.2018.07.007. [Google Scholar] [CrossRef]
40. Haapanen, H., Tsang, V., Kempny, A., Neijenhuis, R., Kennedy, F. et al. (2020). Grown-up congenital heart surgery in 1093 consecutive cases: A Hidden burden of early outcome. Annals of Thoracic Surgery, 110(5), 1667–1676. DOI 10.1016/j.athoracsur.2020.01.071. [Google Scholar] [CrossRef]
41. Mikhalkova, D., Novak, E., Cedars, A. (2016). Short-term costs and hospitalization rates in patients with adult congenital heart disease after pulmonic valve replacement. American Journal of Cardiology, 118(10), 1552–1557. DOI 10.1016/j.amjcard.2016.08.018. [Google Scholar] [CrossRef]
42. Ruckdeschel, E., Kay, J. D. (2014). Pulmonic regurgitation and management challenges in the adult with tetralogy of fallot. Current Treatment Options in Cardiovascular Medicine, 16(6), 314. DOI 10.1007/s11936-014-0314-5. [Google Scholar] [CrossRef]
43. Kogon, B. E., Rosenblum, J. M., Mori, M. (2015). Current readings: Issues surrounding pulmonary valve replacement in repaired tetralogy of fallot. Seminars in Thoracic and Cardiovascular Surgery, 27(1), 57–64. DOI 10.1053/j.semtcvs.2015.02.010. [Google Scholar] [CrossRef]
44. Zaragoza-Macias, E., Stout, K. K. (2013). Management of pulmonic regurgitation and right ventricular dysfunction in the adult with repaired tetralogy of fallot. Current Treatment Options in Cardiovascular Medicine, 15(5), 575–586. DOI 10.1007/s11936-013-0258-1. [Google Scholar] [CrossRef]
45. Ugaki, S., Rutledge, J., Al Aklabi, M., Ross, D. B., Adatia, I. et al. (2015). An increased incidence of conduit endocarditis in patients receiving bovine jugular vein grafts compared to cryopreserved homograft for right ventricular outflow reconstruction. Annals of Thoracic Surgery, 99(1), 140–146. DOI 10.1016/j.athoracsur.2014.08.034. [Google Scholar] [CrossRef]
46. McElhinney, D. B., Sondergaard, L., Armstrong, A. K., Bergersen, L., Padera, R. F. et al. (2018). Endocarditis after transcatheter pulmonary valve replacement. Journal of the American College of Cardiology, 72(22), 2717–2728. DOI 10.1016/j.jacc.2018.09.039. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |