

 | Congenital Heart Disease |  |
DOI: 10.32604/CHD.2021.014495
ARTICLE
Lesion-based Patterns of Morbidity and Mortality in Hospitalized Adolescents with Congenital Heart Disease
1Cohen Children’s Medical Center, Zucker School of Medicine at Hofstra/Northwell, New York, USA
2Columbia University, New York, USA
3Helen B. Taussig Heart Center, Johns Hopkins Hospital, Baltimore, Maryland, USA
*Corresponding Author: Aparna Kulkarni. Email: akulkarni@northwell.edu
Received: 29 September 2020; Accepted: 22 December 2020
Abstract: Objective: The objective of this analysis is to describe the characteristics and morbidity during hospitalizations among adolescents with congenital heart disease (AdoCHD) from the Pediatric Health Information System (PHIS) database. Methods: The PHIS database was queried for all AdoCHD admissions aged 12–18 years (1/1/2004–12/31/2013). Major forms of CHD were identified by their International Classification of Diseases, ninth revision codes, further verified based on their secondary diagnosis and/or procedure codes. Patient characteristics, diagnoses, procedures and vital status were assessed. Results: In total, there were 4,267 adolescents admitted to 42 Children’s Hospitals, 58.3% were males, 24.6% single ventricle (SV) patients, 64.1% bi-ventricle (BV), and 11.3% could not be classified. They accounted for 8,512 hospitalizations (41,240 total hospital days), of which 31.6% were intensive care unit (ICU) stays. ICU stay was similar for the SV and BV patients with similar duration of mechanical ventilation between the two groups. Overall, the most common CHD among in-patients was tetralogy of Fallot (TOF, 36.4%). Larger proportion of the BV AdoCHD admissions were for elective surgical and electrophysiological procedures. There were 109 (2.5%) heart transplantations (1.3% SV vs. 0.6% BV) and 120 in-hospital deaths (2.8%) (1.1% SV vs. 1.3% BV). Hypoplastic left heart syndrome was the most common diagnosis in transplanted patients (46%) and those who died (28%); TOF (29%) was frequent in 91 (2.1%) patients who had cardiac arrests. Conclusions: Different hospitalization patterns exist for BV and SV AdoCHD. Recognizing this risk may encourage directing resources toward optimizing long-term care of CHD patients.
Keywords: Congenital heart defects; adolescents; hospitalizations; single ventricle; bi-ventricle
Improved post-operative survival has increased the prevalence of Congenital Heart Disease (CHD) [1–4]. Numerous publications have established the clinical course in infancy and in adult survivors of CHD (ACHD) and that adults are frequently hospitalized for reoperation, heart failure, and for treatment of arrhythmias [5–7]. Concurrently though, there is inadequate information regarding the clinical course in adolescents with CHD (AdoCHD). It is known that outpatient follow up in AdoCHD is sporadic, and between 11%–60% are lost to follow-up [8,9]. Heart transplantation data (Pediatric Heart Transplant Study and United Network of Organ Sharing) have indicated that listing for heart transplantation is frequent in AdoCHD, second only to rates seen in infants with CHD [10,11]. Arrhythmias and their surgical or catheter-based treatment are also believed to contribute to significant morbidities in AdoCHD [12]. This study therefore sought to describe the characteristics of hospitalizations in AdoCHD, and to compare characteristics and outcomes between those with single ventricle (SV) and bi-ventricle (BV) CHD from the Pediatric Health Information System (PHIS), an inpatient database. We hypothesized that outcomes of inpatient care in AdoCHD differ depending on whether heart disease is SV or BV.
This was an observational retrospective study to characterize inpatient AdoCHD admissions to tertiary care pediatric hospitals in the United States. Data for this study were obtained from the PHIS database, an administrative database that contains in-patient, emergency department, ambulatory surgery, and observational data from 45 not-for-profit, tertiary care pediatric hospitals in the United States. These hospitals are affiliated with the Children’s Hospital Association (Overland Park, KS). Data quality and reliability are assured through a joint effort between the Children’s Hospital Association and participating hospitals. For the purposes of external benchmarking, participating hospitals provide discharge /encounter data including demographics, diagnoses, and procedures. All data are de-identified at the time of data submission, and data are subjected to a number of reliability and validity checks through careful audits before being included in the database. Diagnoses and procedure data were submitted using the International Classification of Disease, 9th revision, Clinical Modification (ICD-9) codes. Patients in this database are assigned hospital-specific coded identifiers (coded medical record numbers) allowing for tracking of patients admitted multiple times to the same hospital.
The database was queried for all admissions that were coded for any form of CHD in children 12–18 years of age, admitted to a PHIS member institution for any reason from January 1, 2004 to December 31, 2013. Database entry switched to ICD-10 in 2014; therefore, data query ended at December 31, 2013 to avoid confusion from variation in diagnosis and procedure coding between the ICD-9 and ICD-10 versions. The person-years were calculated by the time of their entry into the database at first admission and included time between then and December 31, 2013. Demographic data regarding gender and race, presence of genetic syndromes such as Down and DiGeorge for each admission were extracted.
The data extraction and classification algorithm has been illustrated in Fig. 1. Hospitals that contributed to the database for 5 years or less were excluded from all analyses due to small numbers and incomplete data (19 patients were excluded from 2 hospitals). After interrogation of the extracted data, six major recurring CHD diagnosis codes associated with all admissions for every child were identified [745.3 (common ventricle–CV), 746.1 (congenital tricuspid atresia–TA), 746.7 (hypoplastic left heart syndrome–HLHS), 745.2 (tetralogy of Fallot–TOF), 745.1 (transposition of great vessels–TGV), 745.6 (endocardial cushion defects–ECD)]. The following ICD9 codes were categorized as SV: 745.3, 746.1 or 746.7 and ICD-9 codes 745.2, 745.1, or 745.6 were categorized as BV CHD. To ensure that patients were correctly classified each of the 6 main ICD-9 codes were then examined to assess their secondary codes; this included the remaining 5 codes and other CHD codes. The SV and BV categorizations were further refined using these secondary diagnoses, examples: CV (745.3) with ECD (745.6); TA (746.1) with TGV (745.1), these were categorized under the SV group. Patients with single CHD codes that are known to be distinctly associated with BV hearts such as ventricular septal defect (VSD) (745.4), ostium secundum type atrial septal defect (ASD) (745.5), pulmonary valve anomaly (PVA) (746.09), congenital atrioventricular valve insufficiency (AVI) (746.4), coarctation of aorta (CoA) (747.10), pulmonary artery anomaly (PAA) (747.3) were added to the BV CHD category. In addition, certain procedure codes that helped clarify SV vs. BV CHD were used for refining the CHD categories (for example, Mustard conduit repair 35.91 classified as BV CHD). Neonatal or infant procedure codes such as Norwood or Glenn/Hemi-Fontan were not available in the database query of adolescent patients. Patients with overlapping codes where the type of CHD could not be clearly defined as being SV or BV CHD were categorized into a third unclassifiable (UC) group and those with ill-defined codes were classified into a fourth neither (N) group, examples: TOF (745.2) with TGV (745.1) (UC), other congenital heart anomaly (746.89) (N), malposition of heart (746.87) (N), non-specific congenital heart anomaly (746.9) (N).
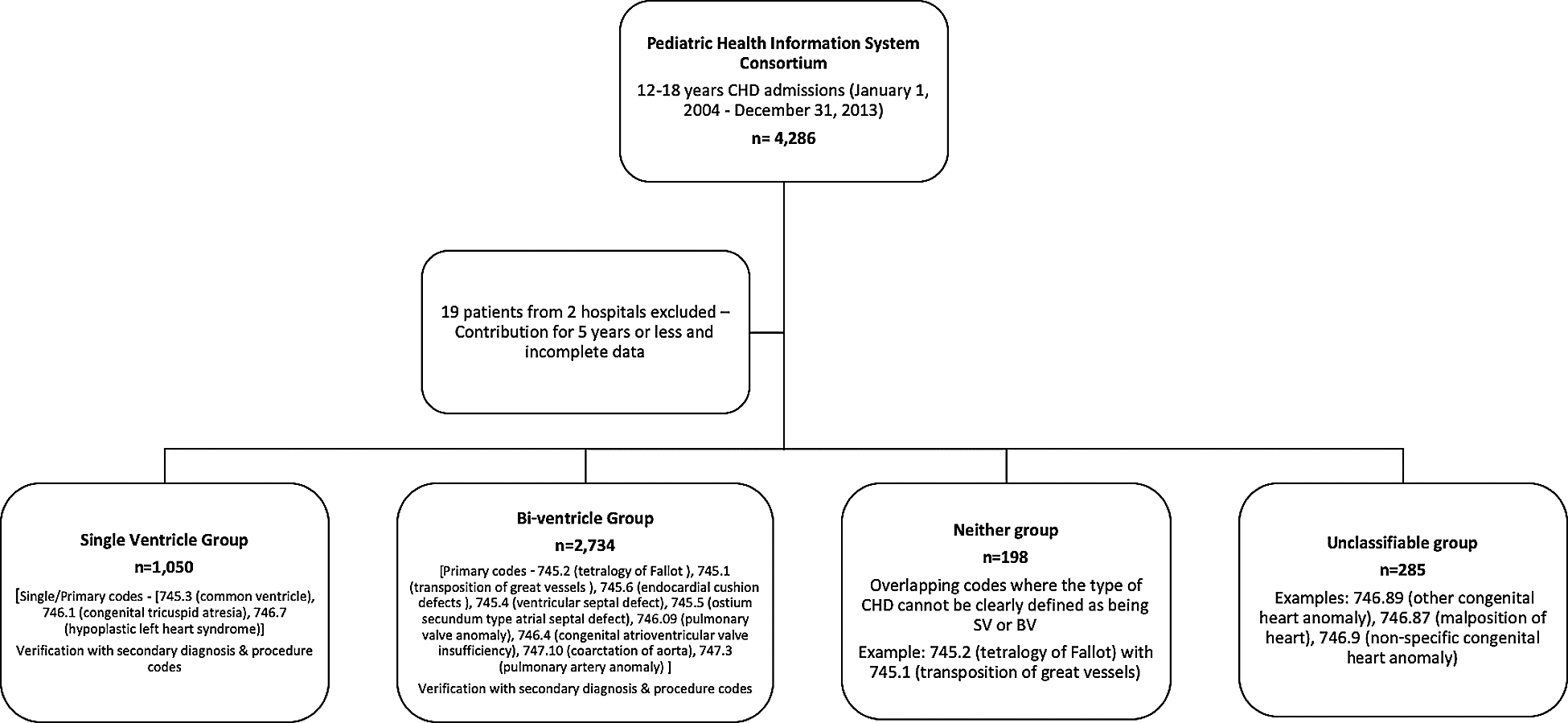
Figure 1: Data extraction and congenital heart disease category classification algorithm. International classification of disease–9th revision (ICD-9) Congenital Heart Disease (CHD) diagnosis codes: Single ventricle group–745.3–common ventricle, 746.1–congenital tricuspid atresia, 746.7–hypoplastic left heart syndrome; Bi-ventricle group–745.2–tetralogy of Fallot, 745.1–D-transposition of great vessels, 745.6–endocardial cushion defects, 745.4–ventricular septal defect, 745.5–ostium secundum type atrial septal defect, 746.09–pulmonary valve anomaly, 746.4–congenital atrioventricular valve insufficiency, 747.10– coarctation of aorta; Neither group–747.3–pulmonary artery anomaly, 746.89–other congenital heart anomaly, 746.87–malposition of heart, 746.9–non-specific congenital heart anomaly
The primary outcomes of interest were the number of hospitalizations per year, number of hospital-days per year, intensive care unit stay (ICU) days per year, mechanical ventilation days per year, number of episodes of care related to surgical procedures, overall incidence of clinically significant arrhythmias, cardiac arrest, pacemaker and defibrillator implantations, cardiac catheterizations, heart failure, renal dialysis, extracorporeal membrane oxygenator (ECMO) therapy, cardiac transplantation, and in-hospital deaths. Once patients were categorized into SV, BV, N or UC groups their demographics, hospitalizations patterns and procedures were again examined.
This study used de-identified data from administrative database and was therefore, exempt from institutional review board review. Data are available upon reasonable request for review from the corresponding author.
Data were described using standard summary statistics (Students t test for normally distributed data and Kruskall-Wallis test for skewed data), overall and stratified by lesion. Differences in means between groups were assessed using ANOVA. The “Enter” method for logistic regression was used to perform a multivariable analysis based on the following recurring and clinically significant variables: Age, race, sex, HLHS (dx 7467), CV (dx7453), ECD (dx code 74569), TOF (dx 7452), TGA (dx code 74510) and Down syndrome (dx code 7580) (DS). This was followed by a stepwise verification of the mortality prediction using sex, age, race and the specific diagnosis. Because all patient-level data were de-identified, data on out-of-hospital deaths were not available. All analyses were performed using STATA version 13.1 (StatCorp, College Station, Texas).
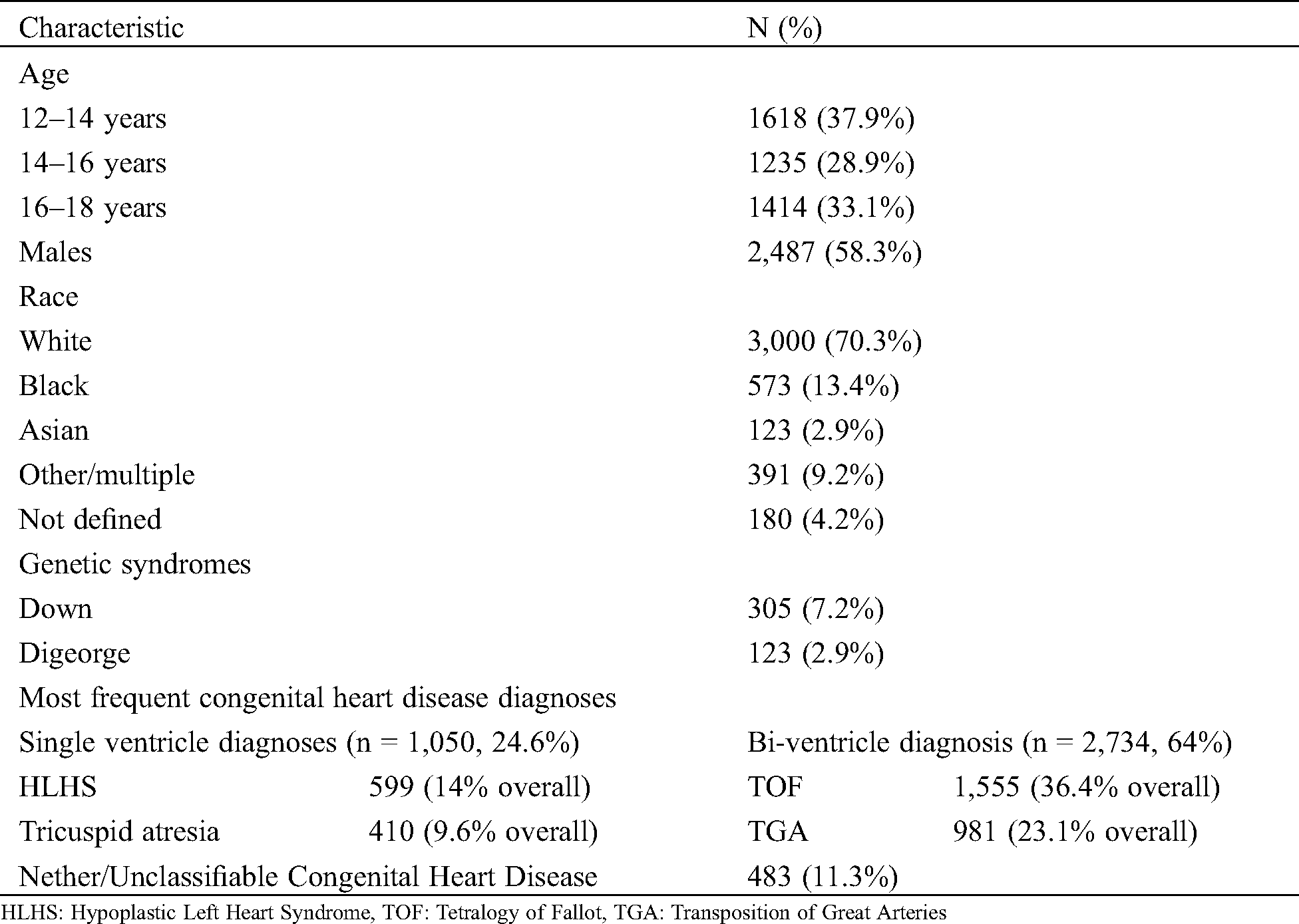
A total of 4,267 AdoCHD (aged 12–18 years) were admitted to 43 Children’s Hospitals (2 hospitals excluded). Baseline demographics are detailed in Tab. 1. Admissions did not vary by age. The majority of patients were males (58.3%) and white (70.3%). DS was coded in 7.1% and DiGeorge syndrome in 2.9% of patients. Among the 4,267 patients, 1050 patients (24.6%) had SV CHD, 2734 (64.1%) had BV CHD and 483 patients (11.3%, UC = 285, N = 198) were UC/N. TOF was the most frequent diagnosis among all patients, and HLHS was the most common in the SV CHD group (36%).
Table 1: Baseline demographics, n = 4,267 patients
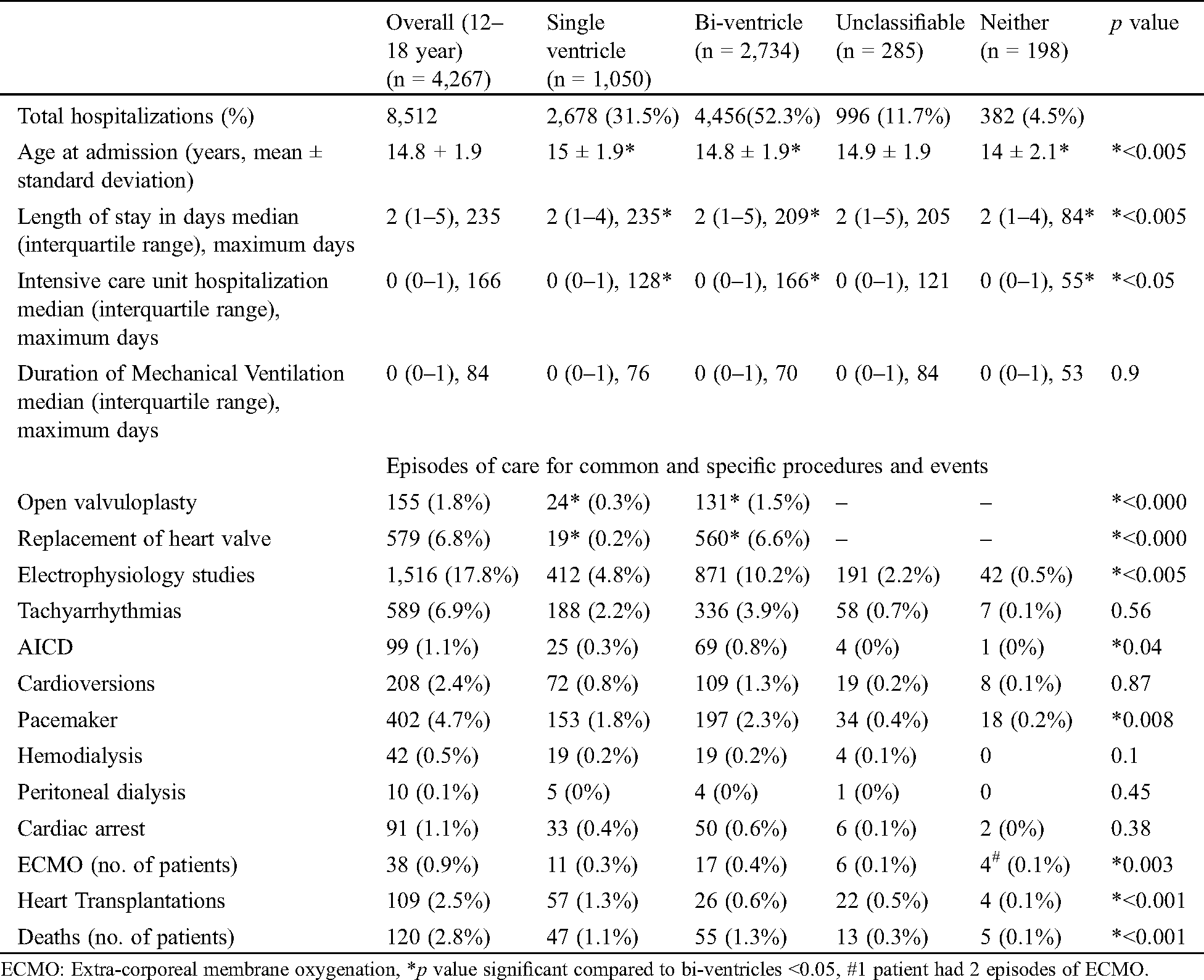
The 4,267 adolescents accounted for 8,512 hospitalizations over 41,240 total hospital days. Patterns of hospitalizations across the overall cohort, SV, BV and UC/N groups are detailed in Tab. 2. Of the 8,512 hospitalizations, 2,678 (31.5%) were for SV patients, 4,456 (52.3%) for BV and 1,378 (16.2%, UC = 996, N = 382) for UC/N. Mean age for adolescent admissions was slightly higher in the SV hospitalizations (mean 15.0 ± 1.9 years vs. 14.8 ± 1.9 years), however, not clinically significant. The median total hospital length of stay (LOS) was 2 days (Inter-quartile range (IQR) 2–5, max 235 days). A third of the 8,512 hospitalizations (2,690 hospitalizations, 31.6% ICU stays) were ICU admissions for at least 1 day, of which 6.2% of ICU stays (167 hospitalizations) were longer than two weeks; and 2.1% of ICU hospitalizations (56 admissions) were longer than one month. ICU stay was not clinically different for the SV patients than BVs (SV: Median 0 days (range 0–1, max 128 days) vs. BV: 0 days (0–1, max 166 days). The median duration of mechanical ventilation did not differ between the two groups. The most common CHD requiring ICU hospitalization was TOF (2,674 hospitalizations, 31.4%) followed by HLHS (1,867 hospitalization, 21.9%).
Table 2: Hospitalizations and related intensive care unit stays for overall, single ventricle, bi-ventricle, and unclassifiable groups of patients with episodes of care for significant procedures and events (total = 8512 hospitalizations)
4.2 Episodes of Care with Morbidity and Mortality
Review of the most frequent episodes of care showed that BV CHD patients had majority of the elective surgical interventions, including open valvuloplasties (0.3% SV vs. 1.5% BV, p < 0.005) and valve replacements (0.2% SV vs. 6.6% BV, p < 0.005). In addition, 79 TOF repairs, 6 total anomalous pulmonary venous repair, 1 truncus repair and 468 Mustard repair/revisions were performed.
Elective cardiac catheterization valvotomies (33 procedures–all BV), electrophysiological studies (EPS) (4.8% SV vs. 10.2% BV, p < 0.005), billing for automated implantable cardioverter-defibrillators (AICD) (0, 3% SV vs. 0.8% BV, p = 0.04), and elective pacemakers (1.8% SV vs. 2.3% BV, P = 0.008) were more frequent in BV patients. In the BV group, the most common diagnosis that had EPS (457/871) and AICD (39/69) was TOF.
Among all patients, 38 patients (0.9%) received ECMO support, 109 patients (2.5%) underwent HT and 120 died in the hospital (2.8%). There were 91 (1.1%) episodes of cardiac arrest. HLHS in the SV group was the most frequent diagnosis in the ECMO patients (n = 8, 24%), those who received HT (n = 50, 46%) and those who died (n = 33, 28%). TOF (n = 26, 29%) in the BV group was most frequent diagnosis associated with inpatient cardiac arrests (91, 1.1%).
Hemodialysis or peritoneal dialysis was required in 52 patients (1.2%). There was no significant difference in the number of episodes billed for tachyarrhythmias, cardioversions, dialysis or cardiac arrests between SV and BV groups.
A total of 532 patients (12.5%) were admitted with heart failure (ICD-9 codes: 428–428.9), of whom majority were SV [CV (n = 138, 26%), HLHS (n = 122, 23%), and TOF (n = 106, 20%) were the most frequent diagnosis]. Thirty-four patients (6.4%) with heart failure had Down syndrome and 7 (1.3%) had DiGeorge syndrome.
4.3 Risk of Inpatient Mortality
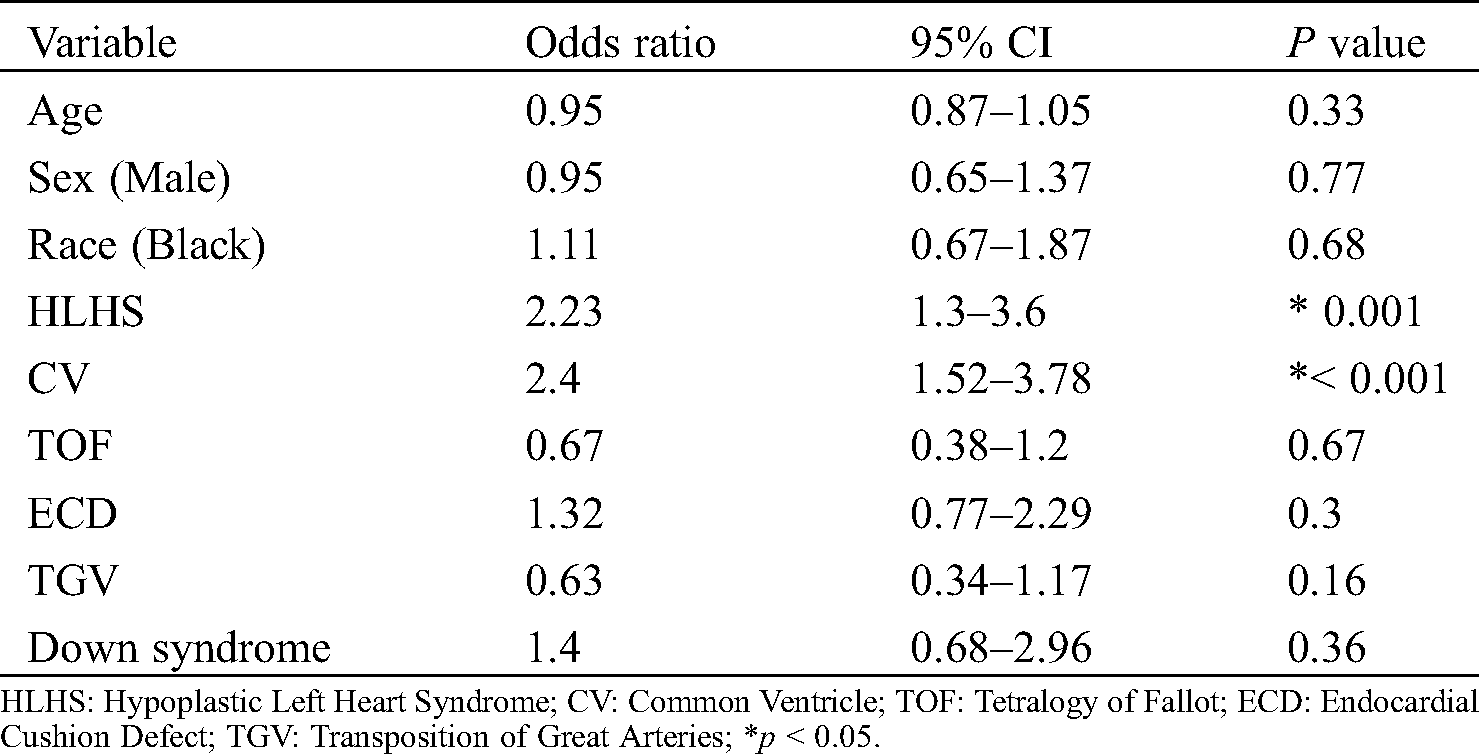
A multivariable analysis for risk for death among the recurring and clinically significant variables in the univariable analysis revealed that AdoCHD with HLHS (OR 2.38, CI 1.58–3.60) and CV (OR 2.47, CI 1.64–3.73) were at higher risk for death while in the hospital (Tab. 3). Black race and DS were not associated with a higher risk for mortality.
Table 3: Multivariable analysis of risk factors for death
In the present study, we characterized hospitalizations in a large population of adolescents with CHD in pediatric hospitals. We found that among AdoCHD hospitalizations, there was a greater proportion of patients with BV than SV. Hospitalized adolescents with BV CHD undergo a large proportion of the elective surgical and catheterization interventions, but SV CHD patients (most common HLHS and CV) are sicker and likely to die. While a larger proportion of the BV CHD (most commonly TOF) have electrophysiologic studies, defibrillator implants and pacemakers, other procedures including, ECMO, and transplantation are frequent among SV hospitalized patients.
The present investigation is one of the largest analyses in the AdoCHD population from a national multi-institutional database, with over 8,500 hospitalizations in adolescents accounting for over 41,000 hospital days. A similar analysis of the California hospital discharge database found similar patterns of hospitalization in adolescents, with decreasing rates as they transition into adulthood [13,14]. Interestingly, the median LOS in adolescents (2 days) is considerably shorter than a similar analysis of adults (5 days) from the PHIS database [15]. Greater co-morbidity burden in ACHD patients compared to AdoCHD likely accounts for their longer LOS.
In the present analysis, patients with SV generally and, CV and HLHS specifically were most likely to be admitted in heart failure, require ECMO and have had HT. Our results support previously published observations indicating poor outcomes in adolescent SV survivors. It is also likely that SV survivors, in particular those with HLHS experience heart failure earlier than their BV counterparts, as a consequence of the challenging Fontan-palliated physiology and presence of a systemic morphologic right ventricle. Conversely, this analysis showed that BV CHD patients have elective procedures including valve replacements and valvuloplasties. It is well known already that TOF patients undergo valve replacements as the RV dilates.
Concurrently, we found that patients with TOF were responsible for a large proportion of hospitalizations in the BV CHD group requiring EPS and ICDs. Similarly, TOF patients also had the majority of cardiac arrests. While this may be related to the known arrhythmogenic potential associated with TOF repair17, it might also be related to the high prevalence of TOF as a proportion of the total AdoCHD population. The inability to assess the numbers of individuals in the non-hospitalized population precludes more definitive statement regarding the risk of TOF as a lesion.
The recent focus on racial disparities have noted worse CHD outcomes in children born to black mothers compared to white [16]. Our analyses did not reveal an increased risk for mortality associated with black race in AdoCHD. This may be attributable to a survival bias, and that the socioeconomic disparities at birth are obscured by adolescence due to attrition of high-risk individuals. Similarly, in our multivariable analysis, we did not find a higher risk for death from DS. An analysis of adults from the National Inpatient Sample noted higher mortality in patients with DS and CHD than those without DS [7]. It is unclear what this difference may be attributable to, and perhaps speculative that it relates to higher comorbid conditions or merely the difference in the data source. Our data was derived specifically from pediatric hospitals which typically have better resources for DS specialized care to support the multiple comorbidities to which DS patients are subject. The National Inpatient Sample is derived from a mix of pediatric and adult hospitals with the majority of data coming from adult facilities.
Overall, AdoCHD have a significant clinical burden of morbidity and mortality during their inpatient hospitalizations. It is common experience that ACHD patients present for medical care only when symptomatic. By demonstrating the inpatient patterns in AdoCHD, this study emphasizes the need for standardized longitudinal follow-up of children into adolescence facilitated by the implementation of electronic medical records and enlistment in registries that may allow for early recognition of risk factors for morbidity and mortality in AdoCHD. Concurrently, establishment of transition care programs for these AdoCHD may decrease loss to follow up and thereby risk of hospitalization and associated morbidity in this at-risk population.
This analysis is derived from the PHIS administrative database and is subject to all the limitations of such databases. There is a lack of clinical detail and possibilities of billing and coding errors are inherent to all administrative databases. Since the data are de-identified, outcomes such as deaths outside of the network are not available and could not be corroborated. Clinical details are also not available. Primary and secondary diagnosis for admission could not be verified, thereby limiting the understanding for the reason for hospitalization. Where procedure codes were available, it was assumed that the hospitalization was related to that procedures. Multiple procedure codes were associated with hospitalizations for some patients. It is also likely that some patients may have been wrongly classified as SV or BV CHD or that some patients were hospitalized for non-cardiac indications. However, we were stringent in verification of the primary, secondary and procedure codes when available to categorize the patients appropriately into these classes, and precisely because of this have a proportion of patients that were unclassifiable or who fit neither category. The PHIS database is also solely an inpatient data repository, therefore, there is a selection bias for hospitalized patients in this analysis. The true proportion of AdoCHD that are hospitalized is impossible to determine at the present time in the absence of prevalence data of AdoCHD.
This study demonstrates that adolescents with CHD may have significant inpatient morbidity. Elective high-volume procedures such as valve surgeries and valve replacements account for the burden of the BV AdoCHD hospitalizations, in contrast to SV AdoCHD patients with high inpatient mortality and emergency care needs such as heart failure and ECMO.
Acknowledgement: We appreciate the statistical analytical support provided by Patricia Zybert MPH PhD (Teacher’s College, Columbia University, New York) and the data extraction from the PHIS database performed by Brett R Anderson, MD MBA MS (Children’s Hospital of New York Presbyterian, New York). We also thank Ari Cedars, MD (Helen B. Taussig Heart Center, Johns Hopkins Hospital, Baltimore, Maryland) for his edits and comments.
Data Sharing: Deidentified data may be shared by authors on request for a period within 6 months of publication with direct communication with corresponding author.
Funding Statement: The author(s) received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Nathan, M., Karamichalis, J., Liu, H., Gauvreau, K., Colan, S. et al. (2014). Technical performance scores are strongly associated with early mortality, postoperative adverse events, and intensive care unit length of stay–analysis of consecutive discharges for 2 years. Journal of Thoracic and Cardiovascular Surgery, 147(1), 389–396. DOI 10.1016/j.jtcvs.2013.07.044. [Google Scholar] [CrossRef]
2. Karamlou, T., Jacobs, M. L., Pasquali, S., He, X., Hill, K. et al. (2014). Surgeon and center volume influence on outcomes after arterial switch operation: Analysis of the STS congenital heart surgery database. Annals of Thoracic Surgery, 98(3), 904–911. DOI 10.1016/j.athoracsur.2014.04.093. [Google Scholar] [CrossRef]
3. St Louis, J. D., Jodhka, U., Jacobs, J. P., He, X., Hill, K. D. et al. (2014). Contemporary outcomes of complete atrioventricular septal defect repair: Analysis of the society of thoracic surgeons congenital heart surgery database. Journal of Thoracic and Cardiovascular Surgery, 148(6), 2526–2531. DOI 10.1016/j.jtcvs.2014.05.095. [Google Scholar] [CrossRef]
4. Anderson, B. R., Wallace, A. S., Hill, K. D., Gulack, B. C., Matsouaka, R. et al. (2017). Association of surgeon age and experience with congenital heart surgery outcomes. Circulation: Cardiovascular Quality and Outcomes, 10(7), 353. DOI 10.1161/CIRCOUTCOMES.117.003533. [Google Scholar] [CrossRef]
5. Verheugt, C. L., Uiterwaal, C. S., van der Velde, E. T., Meijboom, F. J., Pieper, P. G. et al. (2010). The emerging burden of hospital admissions of adults with Congenital Heart Disease. Heart (British Cardiac Society), 96(11), 872–878. DOI 10.1136/hrt.2009.185595. [Google Scholar] [CrossRef]
6. Zomer, A. C., Vaartjes, I., van der Velde, E. T., de Jong, H. M., Konings, T. C. et al. (2013). Heart failure admissions in adults with congenital heart disease; risk factors and prognosis. International Journal of Cardiology, 168(3), 2487–2493. DOI 10.1016/j.ijcard.2013.03.003. [Google Scholar] [CrossRef]
7. Baraona, F., Gurvitz, M., Landzberg, M. J., Opotowsky, A. R. (2013). Hospitalizations and mortality in the United States for adults with Down syndrome and Congenital Heart Disease. American Journal of Cardiology, 111(7), 1046–1051. DOI 10.1016/j.amjcard.2012.12.025. [Google Scholar] [CrossRef]
8. Mackie, A. S., Ionescu-Ittu, R., Therrien, J., Pilote, L., Abrahamowicz, M. et al. (2009). Children and adults with Congenital Heart Disease lost to follow-up: who and when? Circulation, 120(4), 302–309. DOI 10.1161/CIRCULATIONAHA.108.839464. [Google Scholar] [CrossRef]
9. Skogby, S., Moons, P., Johansson, B., Sunnegårdh, J., Christersson, C. et al. (2020). Outpatient volumes and medical staffing resources as predictors for continuity of follow-up care during transfer of adolescents with Congenital Heart Disease. International Journal of Cardiology, 310(22), 51–57. DOI 10.1016/j.ijcard.2020.01.016. [Google Scholar] [CrossRef]
10. Lamour, J. M., Kanter, K. R., Naftel, D. C., Chrisant, M. R., Morrow, W. R. et al. (2009). The effect of age, diagnosis, and previous surgery in children and adults undergoing heart transplantation for Congenital Heart Disease. Journal of the American College of Cardiology, 54(2), 160–165. DOI 10.1016/j.jacc.2009.04.020. [Google Scholar] [CrossRef]
11. Almond, C., Thiagarajan, R. R., Piercey, G. E., Gauvreau, K., Blume, E. D. et al. (2009). Waiting list mortality among children listed for heart transplantation in the United States. Circulation, 119(5), 717–727. DOI 10.1161/CIRCULATIONAHA.108.815712. [Google Scholar] [CrossRef]
12. Idorn, L., Juul, K., Jensen, A. S., Hanel, B., Nielsen, K. G. et al. (2013). Arrhythmia and exercise intolerance in Fontan patients: Current status and future burden. International Journal of Cardiology, 168(2), 1458–1465. DOI 10.1016/j.ijcard.2012.12.055. [Google Scholar] [CrossRef]
13. Gurvitz, M. Z., Inkelas, M., Lee, M., Stout, K., Escarce, J. et al. (2007). Changes in hospitalization patterns among patients with Congenital Heart Disease during the transition from adolescence to adulthood. Journal of the American College of Cardiology, 49, 875–882. [Google Scholar]
14. Lu, Y., Agrawal, G., Lin, C. W., Williams, R. G. (2014). Inpatient admissions and costs of congenital heart disease from adolescence to young adulthood. American Heart Journal, 168(6), 948–955. DOI 10.1016/j.ahj.2014.08.006. [Google Scholar] [CrossRef]
15. Kim, Y. Y., Gauvreau, K., Bacha, E. A., Landzberg, M. J., Benavidez, O. J. (2011). Resource use among adult congenital heart surgery admissions in pediatric hospitals: Risk factors for high resource utilization and association with inpatient death. Circulation: Cardiovascular Quality and Outcomes, 4(6), 634–639. DOI 10.1161/CIRCOUTCOMES.111.963223. [Google Scholar] [CrossRef]
16. Wang, Y., Liu, G., Druschel, C. M., Kirby, R. S. (2013). Maternal race/ethnicity and survival experience of children with Congenital Heart Disease. Journal of Pediatrics, 163(5), 1437–1442.e2. DOI 10.1016/j.jpeds.2013.06.084. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |