

 | Congenital Heart Disease |  |
DOI: 10.32604/CHD.2021.014881
ARTICLE
A Novel Perspective on Histopathology Provides Novel Insights into Surgical Effects in Pulmonary Atresia, Ventricular Septal Defect, and Major Aortopulmonary Collateral Arteries: A Case-Series Study
1Center for Pediatric Cardiac Surgery, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
2National Clinical Research Center for Cardiovascular Diseases, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
3Department of Pathology, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
4Department of Cardiopulmonary Bypass, Fuwai Hospital, National Center of Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
5Department of Anesthesiology, Fuwai Hospital, National Center of Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
6Department of Nutriology, Fuwai Hospital, National Center of Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
*Corresponding Author: Qiang Wang. Email: wq.cory@163.com
Received: 05 November 2020; Accepted: 07 January 2021
Abstract: Background: Never had literatures characterized the relationship between the property of major aortopulmonary collateral arteries (MAPCAs) and outcomes of selective unifocalization of pulmonary atresia with ventricular septal defects and MAPCAs. Methods: This is a case-series study. Thirteen patients were included. Angiography-based assessment was conducted to determine whether collateral arteries should be unifocalized or treated with intraoperative ligature. Specimens were collected and stained by HE and ET+VG. Results: Twelve patients underwent one-stage unifocalization at a median age of 37 months (range: 6–228 months) and a median weight of 14.0 kg (range: 5.0–49.0 kg), which produced a favorable right ventricle to aortic systolic pressure ratio of no more than 0.5 except in one patient who died. Patients were divided into three groups: Group 1 (n = 6), had no native pulmonary arteries, and collateral arteries supplied all pulmonary blood; Group 2 (n = 6) presented dysplastic native pulmonary arteries on one or both sides, and in some lung lobes or segments, blood was supplied only by collateral arteries; Group 3 (n = 1) had well-developed left and right pulmonary arteries, and collateral arteries, and pulmonary arteries provided blood flow to the same segments. Pathological reports demonstrated two types of collateral arteries: Elastic arteries presented an arborization distribution similar to native pulmonary artery walls, while muscular arteries showed high resistance and distortion. We selectively unifocalized single-supply collateral arteries with morphologic features based on the arborization distribution. Conclusions: We found that there were two kinds of MAPCAs with different histology, and we performed selective UF for MAPCAs that might belong to the elastic artery. Selective unifocalization achieved a low right ventricle to aortic systolic pressure ratio and favorable surgical effects.
Keywords: Histopathology; selective unifocalization; pulmonary atresia; major aortopulmonary collateral arteries
There is great variability in the anatomy of the true pulmonary arteries in patients with pulmonary atresia, ventricular septal defect, and major aortopulmonary collateral arteries (PA/VSD/MAPCAs). In more complex cases, pulmonary arteries on one or both sides are poorly developed or absent, and major aortopulmonary collateral arteries are responsible for supplying blood to the pulmonary vascular bed. Treatment for these complex individuals has seen substantial progress in recent years. Compared with the high rate of attrition in untreated cases [1,2], 51% to 92.6% of patients can now, with reasonable management, live long lives [3–6]. The management of PA/VSD/MAPCAs remains controversial, with the Stanford and Melbourne groups representing the 2 antithetical extremes in this debate [4–6]. However, it is evident that the unifocalization (UF) approach has achieved favorable outcomes in the majority of cases [3,4,7] since it was proposed by Reddy and colleagues [8]. To determine the origin and nature of collateral arteries, Schulze-Neick et al. [9] examined autopsy specimens and found that there was an intimate relationship between some of collateral arteries and the tracheobronchial tree. One study even suggested that collateral arteries in pulmonary atresia and ventricular septal defect were dilated bronchial arteries [10], while others have disagreed and suggested that the path of development of collateral arteries or bronchial arteries is likely to be profoundly influenced by the local environment [11,12].
In our clinical practice, there is wide variability in the characteristics of MAPCAs, including in their anatomy, morphology and pathology, and to date, there are limited data regarding characteristics of MAPCAs. Therefore, the purpose of this article is to identify the characteristics of collateral arteries, report on the relevant clinicopathological findings, propose a novel concept for selective unifocalization in MAPCAs, and provide novel insight into disease prognosis in this population.
2.1 Review Methods and Definitions
This was a retrospective case-series study. The protocol for this study was reviewed and approved by the Medical Ethics Review Committee of Fuwai Hospital (2020-1318). Informed consent was waived. The study was supported by the Central Public-interest Scientific Institution Basal Research Fund (2019XK320050) and Central University Basic Research Fund (APL20100410010302004). Clinical data were collected from the hospital database and extracted from medical records and outpatient records for the available follow-up. This study was registered at www.chictr.org.cn (ChiCTR2000033401).
Echocardiography, cardiac catheterization and computed tomography angiography were performed in all patients. Every patient underwent major aortopulmonary collateral artery coding (Fig. 1) to determine the number and features of major aortopulmonary collateral arteries. The distribution and morphology of collateral arteries were identified by posterior-anterior and lateral angiography views. Functional collateral arteries were defined as those that on view of the arborization distribution were considered able to be incorporated definitively to maximize recruitment of the lung segments that each MAPCAs dominated. Nonfunctional collateral arteries were defined as those that showed high resistance and distortion and recruited few segments. Dual collateral arteries were defined as those with collaterals forming a “dual supply” if the relevant bronchopulmonary segments were also supplied by a branch of the true pulmonary artery or other collateral arteries. The patients were classified into three groups based primarily on cardiac catherization and partly on CTA. Early mortality was defined as death occurring during the hospital stay after the index operation. Postoperative complications were defined as complications that occurred within 30 days after discharge.
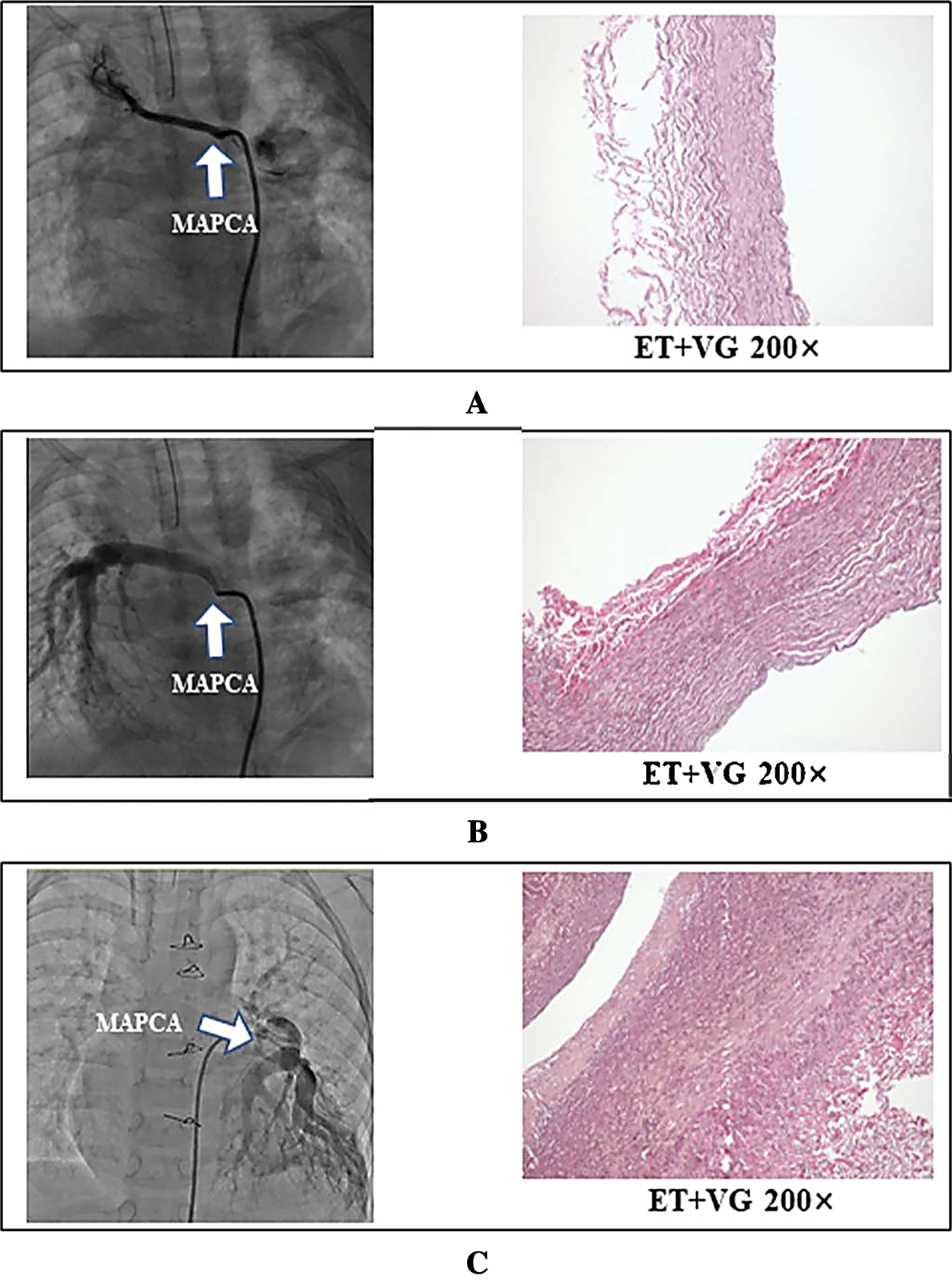
Figure 1: Comparison of selective angiogram and histology in proximal major aortopulmonary collateral arteries. Representative angiogram-based anatomic form and ET+VG staining of the corresponding sections in Clusters 1 (A), 2 (B), and 3 (C). A. MAPCA in cluster 1, which demonstrates high resistance changes and distortion without any arborization; these are classified as muscular arteries, in which the aortic media is composed mostly of smooth muscle fibers (ET+VG 200×). B. Major aortopulmonary collateral artery in Cluster 2, which presents an arborization distribution and provides a single blood supply into the lung lobes; these are classified as elastic arteries. C. Major aortopulmonary collateral artery in Cluster 3, which presents an arborization distribution and provides a blood supply together with the native branch pulmonary artery into the same lung lobes; these were classified as elastic arteries. B and C are both elastic arteries, in which the aortic media is composed of many layers of elastic membrane and a large number of elastic fibers (ET+VG 200×)
2.2 Collection and Preparation of Tissues
Intraoperative specimens (5 to 10 mm in length) were obtained from the proximal end of the collateral arteries. These tissues were paraffin-embedded, cut into sections with a thickness of 0.3 to 0.4 mm, and submitted to HE and ET+VG staining. The parameters, including myocyte and interstitial fibrosis (perivascular or interfiber type), were further determined by light microscopy.
As part of institutional standard procedures, all surgical patients who were discharged alive from the hospital were required to return for outpatient follow-up visits at 3, 6 and 12 months after the initial operation and then annually. During the follow-up period, echocardiography and electrocardiography were routinely performed.
We describe baseline data obtained at admission; angiogram, computed tomography angiography and clinicopathology findings; operative procedures for collateral arteries; and clinical outcomes.
The unifocalization procedure was performed with a median sternotomy. Following full heparinization, placement of the arterial cannula in the ascending aorta and bicaval cannulation bypass commenced while the heart was beating. Then, complete dissociation and release extending to the hilum of each collateral artery was performed before aortic crossclamping. We adopted intraoperative ligature or suture ligature to nonfunctional collateral arteries or dual collateral arteries and unifocalized functional single-supply major aortopulmonary collateral arteries. We ensured satisfactory intraoperative visualization using pump suckers. In some cases, a temporal reduction of bypass flow and deep hypothermic circulation arrest were adopted to facilitate exposure. Several common features of these techniques have been reported by other centers [13–15].
The results are reported as medians and ranges where appropriate. We used SPSS (version 24.0) for all analyses.
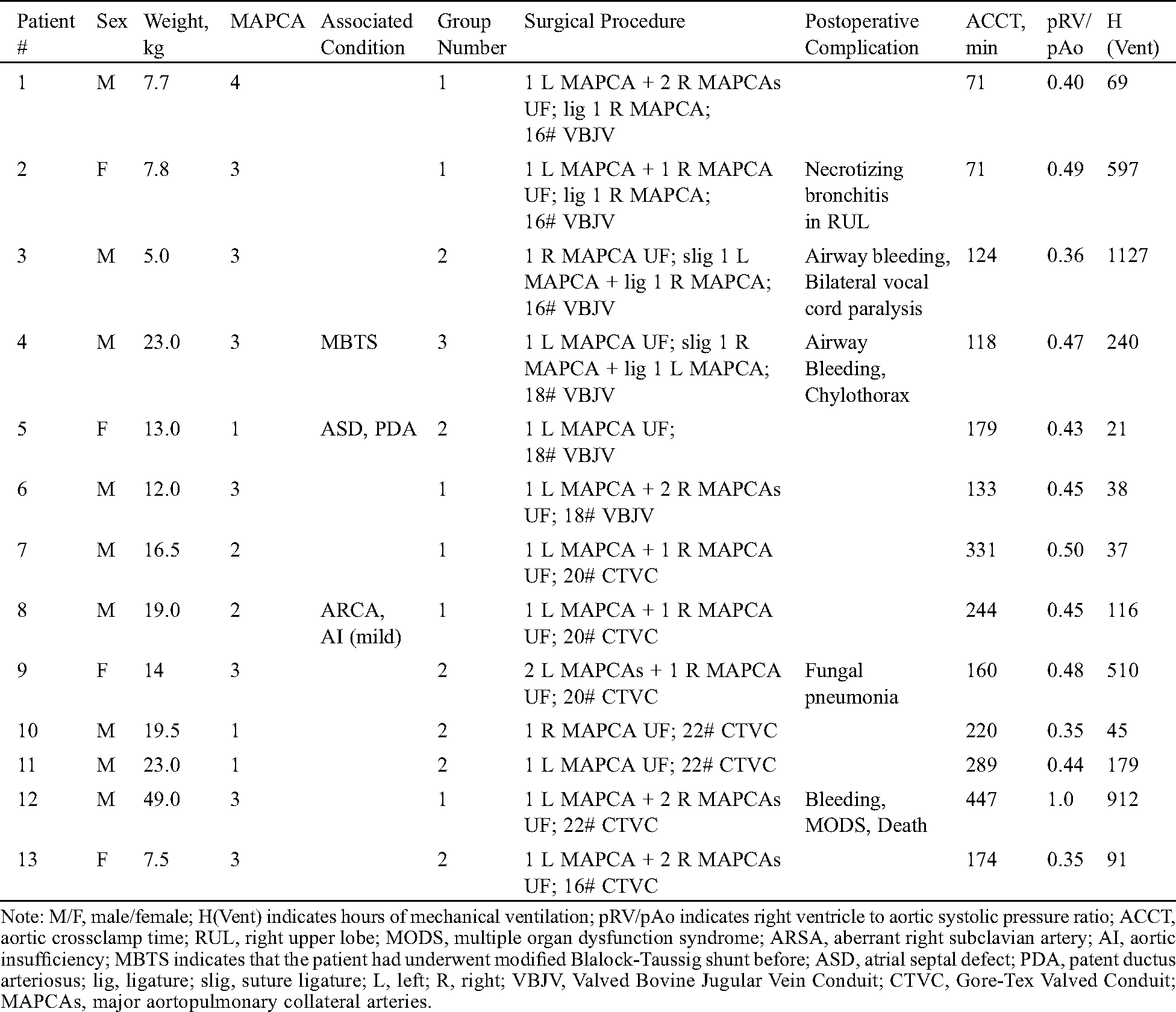
Tab. 1 shows the characteristics of the patients included in this study. Thirteen patients were directed to one-stage unifocalization and ventricular septal defect closure from June 2017 to June 2019. Twelve of these thirteen patients underwent one-stage unifocalization and ventricular septal defect closure at a median age of 37 months (range: 6–228 months) and a median weight of 14.0 kg (range: 5.0–49.0 kg). These patients had a favorable right ventricle to aortic systolic pressure ratio of no more than 0.5 except in one patient who died after 2 weeks of supportive treatment by extracorporeal membrane oxygenation (ECMO).
Table 1: Clinical History and preoperative angiograms or cardiac computed tomography angiography in pulmonary atresia, ventricular septal defect, and major aortopulmonary collateral arteries
3.2 Anatomy, Morphology, Grouping and Surgical Procedures
In our study, most MAPCAs arose from the descending aorta, and the remaining had origins in the subclavian arteries, aortic arches and ascending aorta (Fig. 2). According to cardiac angiography and computed tomography angiography, the patients were classified into three groups:
1. Group 1 (n = 6) had no native pulmonary arteries, and collateral arteries supplied all pulmonary blood. MAPCAs originated from the subclavian artery and ascending and descending aorta (Fig. 2A). The three angiograms demonstrated two types of distribution patterns in these collateral arteries: arborization distribution, distortion and high vascular resistance. We ligated collateral artery 1, which presented high vascular resistance, and collateral arteries 2 to 4 were unifocalized. Collateral artery 4 was not observed on angiogram but was found on a preoperative routine computed tomography scan.
2. Group 2 (n = 6) presented dysplastic native pulmonary arteries on one or both sides, and the blood supply to some lung lobes or segments was provided only by collateral arteries. MAPCAs originated from the subclavian artery and descending aorta (Fig. 2B). Collateral artery 1 demonstrated distortion and high vascular resistance and was submitted to intraoperative ligature. The left lower lung lobe received a “dual” blood supply by collateral artery 3 and well-developed left pulmonary arteries. Collateral artery 3 was ligated or closed with suturing during the operation. The blood flow to the right middle and lower lung lobe was provided only by collateral artery 2, which was unifocalized.
3. Group 3 (n = 1) showed well-developed left and right pulmonary arteries, and MAPCAs and pulmonary arteries provided the blood flow to the same lobes or segments. MAPCAs originated from the descending aorta (Fig. 2C). MAPCAs associated with branched pulmonary arteries or other major aortopulmonary collateral arteries dominated the same lobes or segments. Dual collateral arteries, such as collateral artery 2 and 3, were ligated or closed by sutures during the operation.
Angiogram-based grouping related anatomic atlas data and the surgical algorithm are shown in Fig. 2. Additional surgical procedures are shown in Tab. 1.
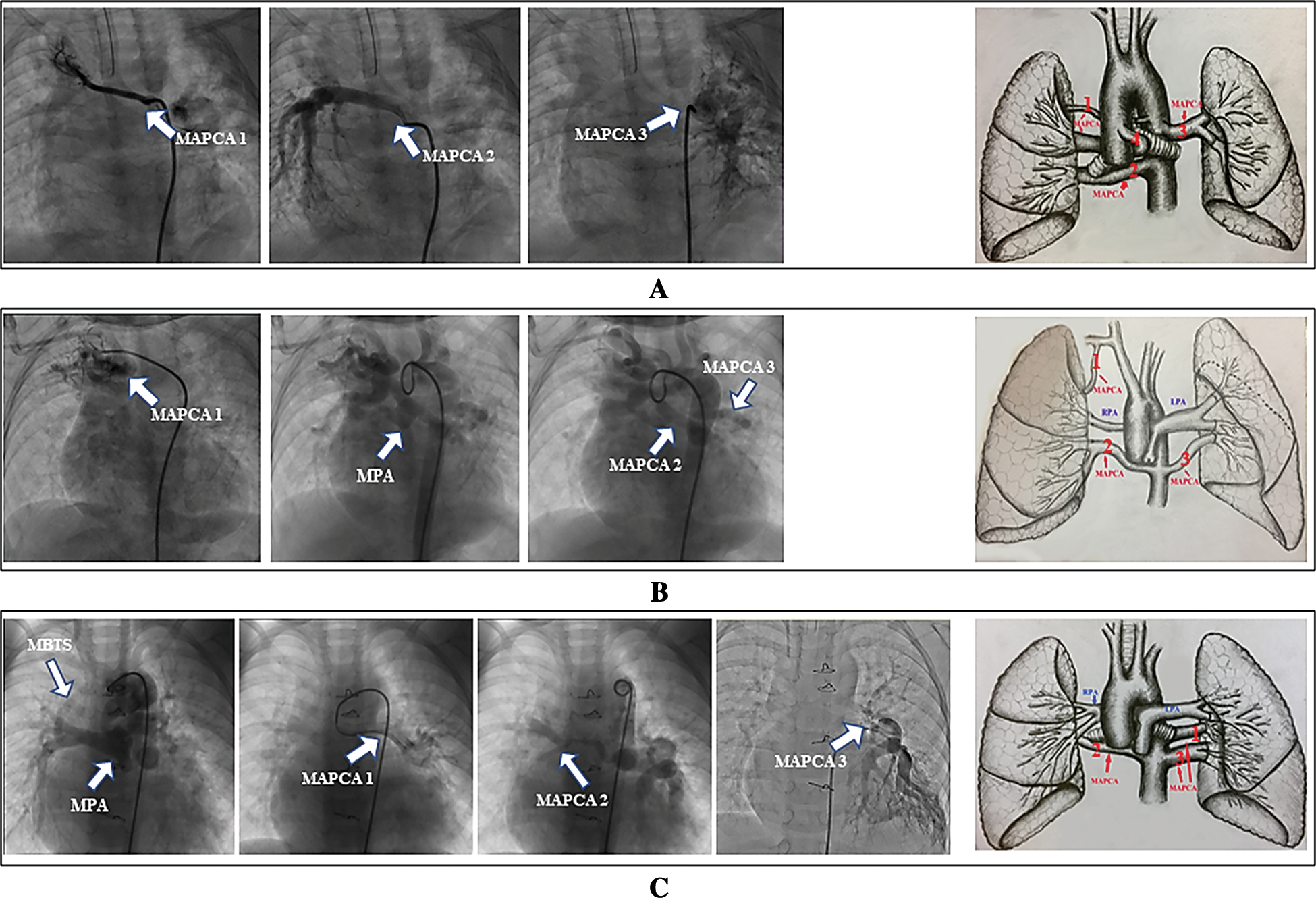
Figure 2: Angiogram-based grouping and anatomic atlas. Representative angiogram-based anatomic form and anatomic atlas of the corresponding patient in Groups 1 (A), 2 (B), and 3 (C), respectively. Group 1 shows no native pulmonary arteries, and major aortopulmonary collateral arteries supply all pulmonary blood. The collateral arteries can originate from the subclavian artery and the ascending and descending aorta (collateral artery 4 was demonstrated by preoperative computed tomography angiography); Group 2 presents native pulmonary arteries that cannot provide complete blood supply to the whole pulmonary vascular bed. This group of patients shows dysplastic native pulmonary arteries on one or both sides, and the blood supply in some lung lobes or segments is provided by only collateral arteries. Group 3 includes patients with well-developed left and right pulmonary arteries. Collateral arteries 1 to 3 and the pulmonary arteries provide blood flow to the same lobes or segments. The major aortopulmonary collateral arteries originate from the descending aorta
The pathological report of ET+VG data demonstrates that there are two histological types of collateral arteries, as shown in Fig. 1. The histopathological pattern of unifocalized and suture ligature collateral arteries (dual blood supply to same lung lobes) showed an arborization distribution and pulmonary artery walls similar to those of native structures, and these were classified as elastic arteries. Major aortopulmonary collateral arteries were ligated or sutured if they demonstrated high resistance and distortion according to a preoperative angiogram, and these were classified as muscular arteries. There was no difference in the HE staining patterns.
Overall survival was 92.3% for a median follow-up of 18 months (range, 6–30 months). Patient #2, as shown in Tab. 1, was a ten-month-old girl who underwent resection of the right upper lobe due to postoperative necrotizing bronchitis. She was in good physical condition with NYHA functional class I during the most recent follow-up. Patient #3, as shown in Tab. 1, was a seven-month-old boy who suffered from bilateral vocal cord paralysis 2 weeks after discharge and then underwent tracheotomy. He was treated by vocal cord reconstruction and recovered from dyspnea after a year of observation with NYHA functional class I.
This study was performed to investigate the surgical effects of patients treated with selective unifocalization. It presents brand-new data on the histopathology of MAPCAs and adds details about morphological features. The included patients were slightly older than those typically encountered in North America [7], with proportion of patients older than 1 year old as high as 69.2%, and this may provide some useful information about this complex congenital heart disease.
4.1 Classification and Operative Concept
We did not distinguish two types of pulmonary atresia, ventricular septal defect, and MAPCAs according to the Congenital Heart Surgery Nomenclature and Database Project [16]. We observed a greater number of angiogram-based morphologic features in collateral arteries and believed that these different features could play different roles and be attributed to different effects. Therefore, we defined functional and nonfunctional collateral arteries and performed patient grouping to obtain a more detailed and specific classification system in which outcomes could be correlated to management strategies among the various subsets of patients who present with this complex condition. Functional collateral arteries can present a favorable arborization distribution similar to that of a true pulmonary artery and have either a single or dual blood supply. The latter forms if the relevant bronchopulmonary segments are also supplied by a branch of the true pulmonary artery or other collateral arteries, as shown in Fig. 2C. Nonfunctional collateral arteries can also show a single vessel supplying more than 1 lung segment but present with high resistance and distortion. Whereas the philosophy of Stanford suggests that all single-supply collateral arteries that supply more than one lung segment should be unifocalized [7], we closed some of these nonfunctional single-supply collateral arteries even when the size of these vessels was large enough for unifocalization. The acceptable surgical effects achieved in these patients support our hypothesis, and postoperative pathologic findings demonstrated that the nonfunctional collateral arteries were muscular arteries. We believe that segments of collaterals that are muscular are particularly prone to the development of severe stenoses, which are often progressive. However, the other major part of our strategy applies to dual-supply functional collateral arteries, as shown in Fig. 2C. In these patients, this type of collateral artery shows excellent arborization and shows postoperative pathological findings similar to those of true pulmonary arteries; however, they should also be closed to prevent postoperative competitive blood flow and adverse outcomes, as might be reported by Stanford, who showed that mortality was 3-fold higher in patients with dual-supply collateral arteries with hypoplastic pulmonary arteries but confluent and normally arborizing pulmonary arteries who were treated with staged procedures than in patients with single-supply collateral arteries treated with single-stage complete repair [7].
Therefore, the surgical management of collateral arteries should be based on a comprehensive assessment. UF is not an all-or-nothing treatment. The critical goal in the management of this type of pulmonary atresia is the complete construction of the pulmonary vascular bed and the maximization of the increasing cross-sectional vascular bed area to achieve low right ventricle pressure. However, some indexes, such as the size and cross-sectional area of the neopulmonary artery, were rarely considered during our decision-making process.
4.2 Complications and Early Mortality
In our study cohort, selective unifocalization for functional collateral arteries was associated with an acceptable early survival rate comparable to that reported in Stanford [7]. Postoperative complications were also similar to those in previously reported studies [9,17,18]. Although we demonstrate there are associations between complications and prolonged ventilation duration (Tab. 1), these children were in good physical health after they were discharged. The patient who died was a 19-year-old male with a 30-mm ventricular septal defect. We do not recommend performing this operation in older children, but we had no choice because of the strong demands of his parents. Nevertheless, we can learn from this case. While in the early stage after complete repair, the patient achieved a good hemodynamic state, we later had to reperform bypass due to extensive bleeding in the wound. Inflammatory factors caused by a prolonged cardiopulmonary bypass time led to further deterioration of the fragile pathophysiological environment, and the pulmonary artery pressure began to increase. Increasing forward resistance caused increasing right ventricular pressure and the deterioration of right ventricular function. This vicious cycle increased the instability of the patient’s hemodynamics. To reduce right ventricular pressure, we performed ventricular septal defect patch fenestration for approximately 10 mm. Later, additional support by extracorporeal membrane oxygenation was used to remove the patient from bypass. During his period in the ICU, we administered large doses of vasoactive drugs to maintain hemodynamics and nitric oxide inhalation to reduce pulmonary artery resistance, but these approaches did not work. Extracorporeal membrane oxygenation support did not improve his condition, and the patient eventually died of severe infection and multiple organ dysfunction syndrome. In this case, if we had completely reopened the ventricular septal defect in time, which would have been equivalent to restoring his pathophysiology to the original state, the patient might have survived.
Although some studies have reported achieving successful repair in older cases [3,19,20], at what ages these repairs should be performed remains a controversial issue. When faced with older children, the question arises as to whether it is better to leave them untreated or to perform unifocalization. Our experience shows that the increased difficulty of achieving dissociation and hemostasis in older cases can prolong the operation time and increase the risk of a poor prognosis. However, many patients may, under similar circumstances, survive for many years with good quality of life.
This study has several limitations. First, only 13 patients were included. It would be better to include as many patients as possible who exhibit other morphologic features or even more collateral artery tissues from the proximal, middle and distal collateral artery trunks to obtain a more comprehensive understanding of the association between collateral artery quality and outcomes. Second, more detailed follow-up data, including reassessment by cardiac catherization and quantitative assessment of the distribution of pulmonary blood flow by lung perfusion scintigraphy, were unavailable at the time of analysis; however, the data in this study permit an early assessment of the clinical characteristics of collateral arteries and the surgical effect in and disease prognosis of these patients.
In summary, the concept that selective unifocalization can achieve a low right ventricle to aortic systolic pressure ratio and excellent surgical effects should be considered in a particular group of people. We found that there were two kinds of MAPCAs with different histology, and we performed selective UF for MAPCAs that might belong to the elastic artery. The long-term effect of the operation still needs to be further qualitatively and quantitatively evaluated by long-term follow-up cardiac catheterization and pulmonary perfusion scan.
Ethical Statement: This study was reviewed and approved by the Medical Ethics Review Committee of Fuwai Hospital (2020-1318). Informed consent was waived.
Funding Statement: The study was supported by the Central Public-interest Scientific Institution Basal Research Fund (2019XK320050) and Central University Basic Research Fund (APL20100410010302004).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Ugenti, V., Romano, A. C., Tibirica, E. (2018). Microvascular endothelial dysfunction during cardiopulmonary bypass in surgery for correction of cyanotic and acyanotic congenital heart disease. Microvascular Research, 120, 55–58. DOI 10.1016/j.mvr.2018.06.004. [Google Scholar] [CrossRef]
2. Leonard, H., Derrick, G., O’Sullivan, J., Wren, C. (2000). Natural and unnatural history of pulmonary atresia. Heart (British Cardiac Society), 84(5), 499–503. DOI 10.1136/heart.84.5.499. [Google Scholar] [CrossRef]
3. Babliak, O. D., Mykychak, Y. B., Motrechko, O. O., Yemets, I. M. (2017). Surgical treatment of pulmonary atresia with major aortopulmonary collateral arteries in 83 consecutive patients. European Journal of Cardio-Thoracic Surgery: Official Journal of the European Association for Cardio-Thoracic Surgery, 52(1), 96–104. DOI 10.1093/ejcts/ezx043. [Google Scholar] [CrossRef]
4. Bauser-Heaton, H., Borquez, A., Han, B., Ladd, M., Asija, R. et al. (2017). Programmatic approach to management of tetralogy of fallot with major aortopulmonary collateral arteries: A 15-year experience with 458 patients. Circulation. Cardiovascular Interventions, 10(4), e004952. DOI 10.1161/CIRCINTERVENTIONS.116.004952. [Google Scholar] [CrossRef]
5. d'Udekem, Y., Alphonso, N., Nørgaard, M. A., Cochrane, A. D., Grigg, L. E. et al. (2005). Pulmonary atresia with ventricular septal defects and major aortopulmonary collateral arteries: Unifocalization brings no long-term benefits. Journal of Thoracic and Cardiovascular Surgery, 130(6), 1496–1502. DOI 10.1016/j.jtcvs.2005.07.034. [Google Scholar] [CrossRef]
6. Soquet, J., Liava'a, M., Eastaugh, L., Konstantinov, I. E., Brink, J. et al. (2017). Achievements and limitations of a strategy of rehabilitation of native pulmonary vessels in pulmonary atresia, ventricular septal defect, and major aortopulmonary collateral arteries. Annals of Thoracic Surgery, 103(5), 1519–1526. DOI 10.1016/j.athoracsur.2016.08.113. [Google Scholar] [CrossRef]
7. Mainwaring, R. D., Patrick, W. L., Roth, S. J., Kamra, K., Wise-Faberowski, L. et al. (2018). Surgical algorithm and results for repair of pulmonary atresia with ventricular septal defect and major aortopulmonary collaterals. Journal of Thoracic and Cardiovascular Surgery, 156(3), 1194–1204. DOI 10.1016/j.jtcvs.2018.03.153. [Google Scholar] [CrossRef]
8. Reddy, V. M., Liddicoat, J. R., Hanley, F. L. (1995). Midline one-stage complete unifocalization and repair of pulmonary atresia with ventricular septal defect and major aortopulmonary collaterals. Journal of Thoracic and Cardiovascular Surgery, 109(5), 832–845. DOI 10.1016/S0022-5223(95)70305-5. [Google Scholar] [CrossRef]
9. Schulze-Neick, I., Ho, S. Y., Bush, A., Rosenthal, M., Franklin, R. C. et al. (2000). Severe airflow limitation after the unifocalization procedure: Clinical and morphological correlates. Circulation, 102(19 Suppl 3), III142–III147. DOI 10.1161/01.CIR.102.suppl_3.III-142. [Google Scholar] [CrossRef]
10. Nørgaard, M. A., Alphonso, N., Cochrane, A. D., Menahem, S., Brizard, C. P. et al. (2006). Major aorto-pulmonary collateral arteries of patients with pulmonary atresia and ventricular septal defect are dilated bronchial arteries. European Journal of Cardio-Thoracic Surgery: Official Journal of the European Association for Cardio-Thoracic Surgery, 29(5), 653–658. DOI 10.1016/j.ejcts.2005.12.054. [Google Scholar] [CrossRef]
11. Hanley, F. L. (2006). Major aortopulmonary collateral arteries, bronchials, monkeys, and men. European Journal of Cardio-Thoracic Surgery: Official Journal of the European Association for Cardio-Thoracic Surgery, 29, 643–644. [Google Scholar]
12. Watanabe, N., Mainwaring, R. D., Reddy, V. M., Palmon, M., Hanley, F. L. (2014). Early complete repair of pulmonary atresia with ventricular septal defect and major aortopulmonary collaterals. Annals of Thoracic Surgery, 97(3), 909–915. DOI 10.1016/j.athoracsur.2013.10.115. [Google Scholar] [CrossRef]
13. Cho, J. M., Puga, F. J., Danielson, G. K., Dearani, J. A., Mair, D. D. et al. (2002). Early and long-term results of the surgical treatment of tetralogy of Fallot with pulmonary atresia, with or without major aortopulmonary collateral arteries. Journal of Thoracic and Cardiovascular Surgery, 124(1), 70–81. DOI 10.1067/mtc.2002.120711. [Google Scholar] [CrossRef]
14. Reddy, V. M., McElhinney, D. B., Amin, Z., Moore, P., Parry, A. J. et al. (2000). Early and intermediate outcomes after repair of pulmonary atresia with ventricular septal defect and major aortopulmonary collateral arteries: Experience with 85 patients. Circulation, 101(15), 1826–1832. DOI 10.1161/01.CIR.101.15.1826. [Google Scholar] [CrossRef]
15. Tchervenkov, C. I., Salasidis, G., Cecere, R., Béland, M. J., Jutras, L. et al. (1997). One-stage midline unifocalization and complete repair in infancy versus multiple-stage unifocalization followed by repair for complex heart disease with major aortopulmonary collaterals. Journal of Thoracic and Cardiovascular Surgery, 114(5), 727–737. DOI 10.1016/S0022-5223(97)70076-X. [Google Scholar] [CrossRef]
16. Tchervenkov, C. I., Roy, N. (2000). Congenital Heart Surgery Nomenclature and Database Project: Pulmonary atresia--ventricular septal defect. Annals of Thoracic Surgery, 69(4 Suppl), S97–S105. DOI 10.1016/S0003-4975(99)01285-0. [Google Scholar] [CrossRef]
17. Asija, R., Roth, S. J., Hanley, F. L., Peng, L., Liu, K. et al. (2014). Reperfusion pulmonary edema in children with tetralogy of Fallot, pulmonary atresia, and major aortopulmonary collateral arteries undergoing unifocalization procedures: A pilot study examining potential pathophysiologic mechanisms and clinical significance. Journal of Thoracic and Cardiovascular Surgery, 148(4), 1560–1565. DOI 10.1016/j.jtcvs.2014.01.017. [Google Scholar] [CrossRef]
18. Maskatia, S. A., Feinstein, J. A., Newman, B., Hanley, F. L., Roth, S. J. (2012). Pulmonary reperfusion injury after the unifocalization procedure for tetralogy of Fallot, pulmonary atresia, and major aortopulmonary collateral arteries. Journal of Thoracic and Cardiovascular Surgery, 144(1), 184–189. DOI 10.1016/j.jtcvs.2011.12.030. [Google Scholar] [CrossRef]
19. Davies, B., Mussa, S., Davies, P., Stickley, J., Jones, T. J. et al. (2009). Unifocalization of major aortopulmonary collateral arteries in pulmonary atresia with ventricular septal defect is essential to achieve excellent outcomes irrespective of native pulmonary artery morphology. Journal of Thoracic and Cardiovascular Surgery, 138(6), 1269–1275.e1. DOI 10.1016/j.jtcvs.2009.08.011. [Google Scholar] [CrossRef]
20. Ishibashi, N., Shin'oka, T., Ishiyama, M., Sakamoto, T., Kurosawa, H. (2007). Clinical results of staged repair with complete unifocalization for pulmonary atresia with ventricular septal defect and major aortopulmonary collateral arteries. European Journal of Cardio-Thoracic Surgery: Official Journal of the European Association for Cardio-Thoracic Surgery, 32(2), 202–208. DOI 10.1016/j.ejcts.2007.04.022. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |