

 | Congenital Heart Disease |  |
DOI: 10.32604/CHD.2021.015222
ARTICLE
Use of the GORE® DrySeal Flex Introducer Sheath to Facilitate Implantation of the Transcatheter Venus P-valve
1Department of Congenital Cardiology, Evelina London Children’s Hospital, Guy’s & St Thomas’ NHS Foundation Trust, London, UK
2Department of Cardiology, Mater Misericordiae University Hospital, Dublin, Ireland
3Department of Pediatric Cardiology, Our Lady’s Children’s Hospital, Dublin, Ireland
4School of Biomedical Engineering and Imaging Sciences, Kings College, London, UK
*Corresponding Author: Gianfranco Butera. Email: gianfrancobutera@libero.it
Received: 02 December 2020; Accepted: 12 January 2021
Abstract: Objectives: We report our experience of using the 65 cm large diameter GORE® DrySeal Flex Introducer sheath to facilitate transcatheter implantation of the Venus P-valve in the pulmonary position. Background: Transcatheter implantation of pulmonary valves can be difficult due to rigidity of the valve delivery system or the anatomy of the RVOT and pulmonary artery bifurcation and the risk of iatrogenic damage to the tricuspid valve support apparatus. Using long sheaths to pass and protect the tricuspid valve may facilitate the procedure. Methods: Multi-centre registry of patients who underwent transcatheter pulmonary valve implantation of the Venus P-valve using the GORE® DrySeal Flex introducer sheath to facilitate passage of the valve to the right ventricular outflow tract. Procedural success, time to valve implantation and radiation safety parameters were analyzed. These data were compared to a control group of subjects treated between July 2014 and May 2016 with the same valve but without the use of GORE® DrySeal. Results: Between December 2016 and September 2018, the Venus P-valve was successfully deployed through the GORE® DrySeal in 12 patients. There were no procedure-related complications. As a control group, 10 subjects treated between July 2014 and May 2016 were included. Total procedure time was significantly shorter in the GORE® DrySeal group compared to the control group 96 ± 27 min vs. 164 ± 12 min (p < 0.001). Total screening time was significantly shorter in the GORE® DrySeal group (24 ± 11 min) when compared with the control group (32 ± 14 min, p < 0.001). Conclusions: We describe a modification to the previously described techniques of implanting the Venus P-valve in the pulmonary position after surgical repair of congenital heart disease. In our experience, the GORE® DrySeal sheath has considerably facilitated the procedure and reduced the potential risks.
Keywords: Congenital heart disease; percutaneous; pulmonary valve
Pulmonary valve regurgitation is a frequent consequence of surgery for Tetralogy of Fallot (ToF) [1]. During follow-up pulmonary regurgitation may lead to progressive dilatation and dysfunction of the right ventricle resulting in clinical outcomes such as arrhythmias, impairment of functional capacity and sudden arrhythmic death. Since the first-in-man case performed by Bonhoeffer et al. [2], percutaneous pulmonary valve implantation has evolved with the introduction of various valves that are limited by their maximum size. More recently percutaneous valves have been designed for use in large right ventricular outflow tracts (RVOTs). The most developed of these are the Venus P valve, the Harmony valve and the Pulsta valve. The Venus P-valve has the largest number of cases performed worldwide [3–7]. However, usually, the delivery systems available for valve implantation of all valves used, may have some significant limitations [3–7]. Here, we describe the use of GORE® DrySeal [8] sheath to facilitate percutaneous pulmonary valve implantation using the Venus P-valve in three centres across the United Kingdom and Ireland.
Multicentre, (international) retrospective study of patients who had undergone implantation of a Venus P-valve in the pulmonary position using the GORE® DrySeal sheath to facilitate manoeuvring of the valve assembly in the RVOT between December 2016 and September 2018. Three institutions submitted data to the study. We compared the data to subjects treated with Venus P-valve implantation prior to the adoption of the GORE® Dry-Seal between July 2014 and May 2016 as a control group. Investigators submitted and obtained the approval of the retrospective study to local IRB and ethical committee.
2.2 The Valve and the GORE DrySeal Sheath
The Venus P-valve (Venus MedTech, Hangzhou, China) is a self-expanding percutaneous valve comprising a nitinol stent and a tri-leaflet porcine pericardial tissue valve hand-sewn inside the nitinol frame. It is designed to be implanted into a native or reconstructed RVOT [3–7].
The GORE® DrySeal Flex introducer Sheath (WL GORE, Delaware, USA) (Fig. 1) is an introducer sheath with a hydrophilic coating, designed to offer enhanced flexibility and kink resistance and to provide support in accessing challenging anatomies during endovascular repair of abdominal aortic (AAA) and thoracic aortic (TAA) aneurysms. The GORE® DrySeal valve minimises blood loss and enables introduction of multiple devices with haemostatic control. It is available in two lengths (33 cm and 65 cm) with the longer sheath diameters ranging from 20–26F.

Figure 1: GORE® DrySeal long sheath
Pre-procedural process of approval, procedural steps of valve implantation, post-procedural care, early and mid-term results has been already reported in previous papers [3–7].
Between December 2016 and September 2018, in a further subset of patients not previously reported, implantation of a Venus P-valve was attempted and successful in all 12 patients (6 male, 6 female). Mean age was 21 ± 9 years (Range 13 to 39 years) and mean weight 53 ± 15 kg (Range 36 to 81 kg).
Nine patients had previously undergone surgical repair of ToF or pulmonary stenosis/atresia, with or without ventricular septal defect (VSD), with a transannular patch. One of these patients also had a previously implanted stent in the left pulmonary artery (LPA). Two patients had undergone transcatheter pulmonary valvoplasty and one patient had undergone surgical pulmonary valvotomy. All patients had severe pulmonary regurgitation by transthoracic echocardiography and/or magnetic resonance imaging (MRI) and met conventional criteria for implantation of a competent pulmonary valve. The control group included 10 subjects who underwent successful implantation of a Venus P-valve was (8 male, 2 female). Mean age was 26.5 ± 15.9 years (Range 13 to 62 years) and mean weight 77.5 ± 29 kg (Range 60 to 135 kgs). Nine patients had previously undergone surgical repair of ToF or pulmonary stenosis/atresia, with or without ventricular septal defect (VSD), with a transannular patch. One patient had undergone a previous Ross operation. All patients had severe pulmonary regurgitation by transthoracic echocardiography and/or magnetic resonance imaging (MRI) and met conventional criteria for implantation of a competent pulmonary valve. Data from the control group has been included in previous publications [4,7].
All the procedures were performed under general anaesthesia. Femoral vein access was obtained using ultrasound guidance in five patients and using anatomical landmarks in seven patients. After obtaining satisfactory wire position in the distal left pulmonary artery using a 0.035” Lunderquist guidewire (Cook Medical, Bloomington, IN, USA), the vein was serially dilated and the GORE® DrySeal sheath inserted. A 65 cm long 26F GORE DrySeal sheath was used in the GORE® DrySeal group. The distal end of the sheath was advanced to the proximal LPA in 7 patients and in the distal main pulmonary artery (MPA) in 5 patients. In the patient with the previously implanted LPA stent, the stent was predilated in order to facilitate passage of the sheath to the LPA. The Venus P-valve was crimped onto the delivery system using the previously described technique [3–7] and inserted over the distally placed wire through the GORE® DrySeal sheath to the distal position while opening the proximal “valve” system (Fig. 2). The GORE® Dryseal sheath was then withdrawn to uncover the valve and deployed in the usual fashion in the RV outflow [3–7] (Fig. 2).
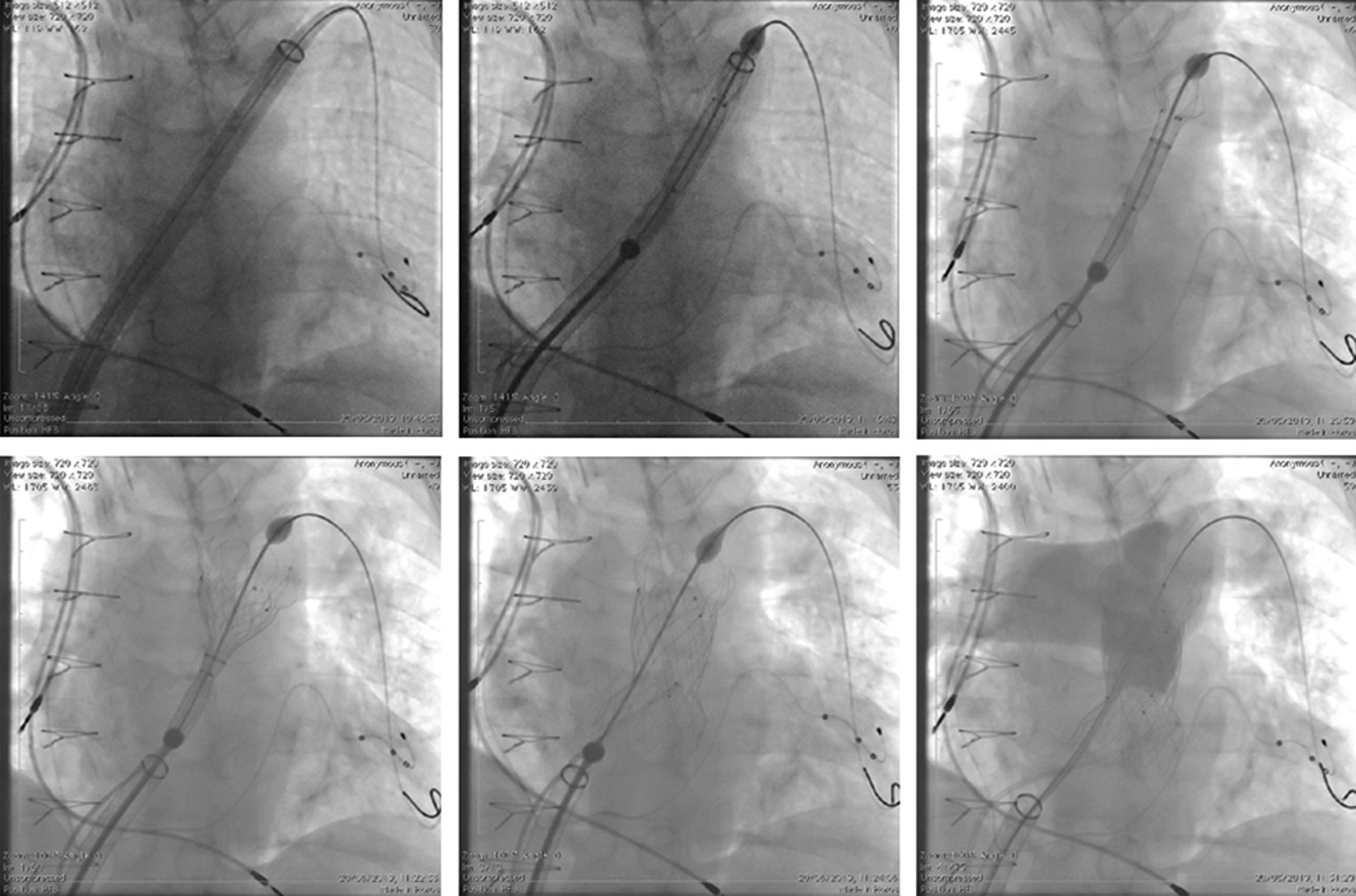
Figure 2: Fluoroscopic AP view showing the steps of the Venus-P valve implantation
The Venus P-valve was successfully deployed in all procedures. There were no procedure-related complications. Total procedure time, including balloon interrogation of the right ventricular outflow tract for valve sizing, was shorter in the GORE® DrySeal group (96 ± 27 min (Range 53 to 143 min) when compared to 164 ± 12 min in the control group (Range 113–280 min) p < 0.001). Total screening time was shorter for GORE® DrySeal group (24 ± 11 min (Range 7.5 to 45 min) vs. 32 ± 14 min (Range 21–70 min) p < 0.001). Several technical manoeuvres were needed to advance the valve through the right heart in the group where the GORE® DrySeal was not used while no major difficulties were reported by operators in the GORE® DrySeal group. Median follow-up was shorter in the GORE® DrySeal group (median16 months (range 6–28 months) vs. 58 months (range 48–72 months); p < 0,001). No complications occurred in both groups.
Surgical right ventricular outflow tract (RVOT) enlargement for congenital heart diseases such as tetralogy of Fallot (ToF), particularly including a transannular patch to abolish obstruction, results in pulmonary regurgitation. Severe pulmonary regurgitation may cause progressive dilatation and dysfunction of the right ventricle, a decrease in exercise tolerance, arrhythmias, and eventually right heart failure and increased risk of sudden death [1]. Historically, most children and young adults in whom there was an indication for implantation of a competent pulmonary valve were referred for surgery. However, many centres now consider a percutaneous approach to their management to be the first-line therapy. Use of the commercially available transcatheter valves-the Melody valve (Medtronic, Minneapolis, MN, USA) and the SAPIEN series of valves (Edwards Lifesciences, Irvine, CA, USA) - is limited by their maximum dimensions and the fact that a significant proportion of patients, who have undergone ToF repair using a transannular patch, have RVOT dimensions larger than those suitable for either of these valves. The Venus P-valve (Venus MedTech, Hangzhou, China) is a self-expanding percutaneous valve that has been used in several studies in humans in series from Asia (3), Europe (4) and South America (5) with good medium-term results both in Chinese (6) and international (7) studies. Transcatheter implantation of pulmonary valves can be difficult due to various reasons including rigidity of the valve delivery system, as for Venus P-Valve, softness of delivery system, as with the Melody or SAPIEN valves, or the anatomy of the RVOT and pulmonary artery bifurcation. Furthermore, in particular in patients in whom a SAPIEN valve is implanted, the fact that the valve is manoeuvred without being covered may result in tricuspid valve injury [9]. Experienced operators have reported modifications of techniques to overcome these difficulties [10]. The use of a long sheath rigid enough to provide safe delivery may ease the procedure and reduce the risks [11]. Use of the GORE® DrySeal sheath has been proven useful in the implantation of SAPIEN valves in a recently published multicentre study [8]. The implantation of Venus P valve has also its own difficulties due to stiffness of commercially available long-sheath. Therefore, we decided to modify the previously described techniques of implanting the Venus P-valve in the pulmonary position after surgical repair of congenital heart disease [3–7]. To date, all attempts to deploy the Venus P-valve using this technique have been successful. Furthermore, lower procedural and fluoroscopy times were found when compared with the group where the GORE® DrySeal was not used. These differences may be related to multiple factors, and not only to GORE® DrySeal use, including institutional learning curve. Furthermore, previous studies have described failure to deploy a Venus P-valve in a patient with a previously placed LPA stent due to difficulty in advancing the delivery sheath into the LPA, causing a break in the delivery sheath. Subsequently, the presence of an LPA stent has been considered a relative contraindication to Venus P-valve implantation [7]. In our patient with an in situ LPA stent, it was straightforward to advance the GORE® DrySeal sheath over a Lunderquist wire and through the LPA stent after pre-dilatation of the stent with a high-pressure balloon allowing uneventful implantation of a Venus-P-valve without complications (Fig. 3). Finally, even it is usually considered that the placement of the guidewire in the left pulmonary artery is advised and/or preferred, it is also possible to consider placing the guidewire in the right pulmonary artery in selected cases when a better orientation of the delivery system is anticipated.
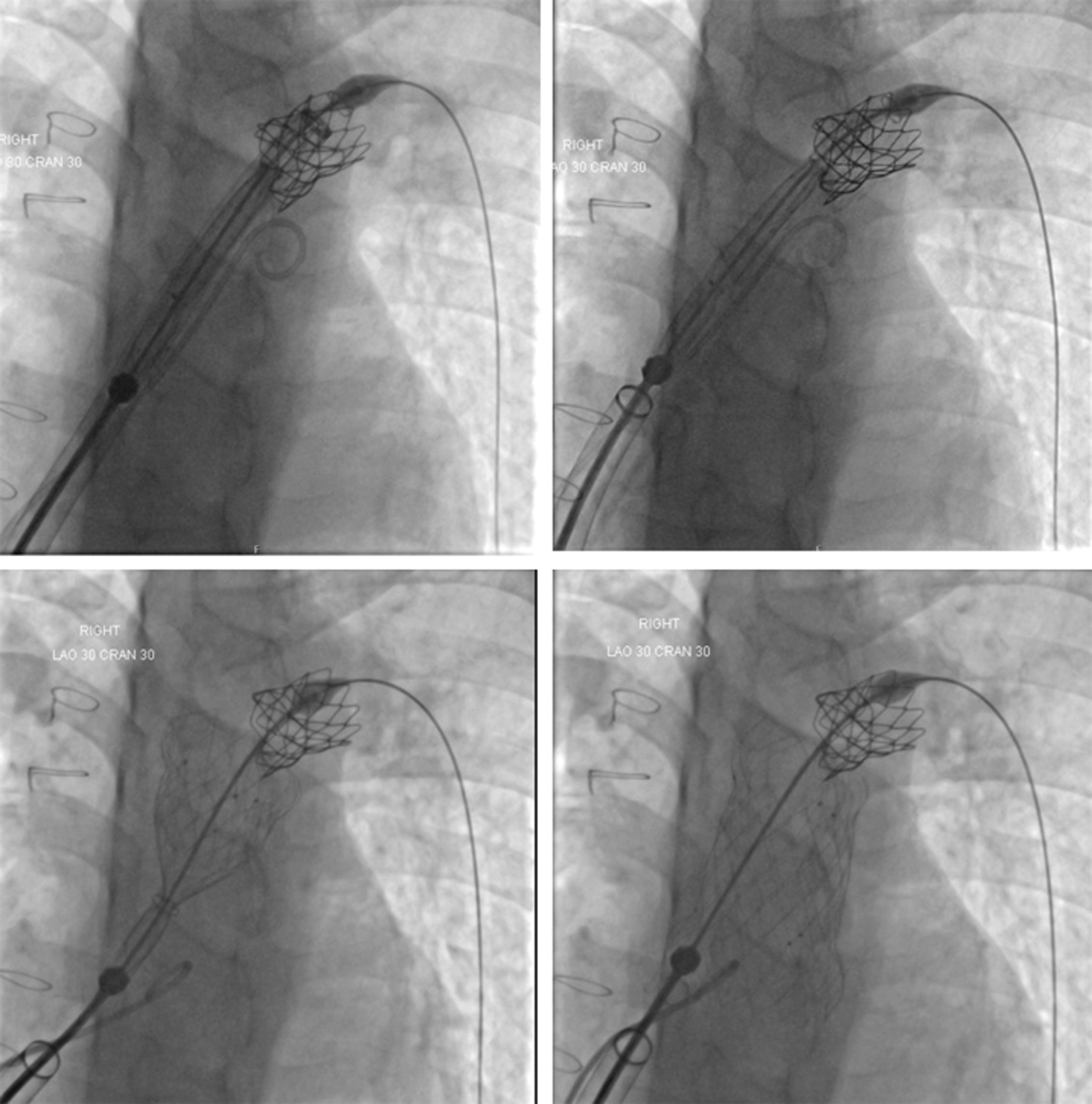
Figure 3: Fluoroscopic AP with cranial angulation views
In conclusion, in our experience, the GORE® DrySeal sheath has considerably facilitated the passage of the Venus P-valve through the RVOT, thus making the procedure safer and faster. Nowadays, in our practice, the use of the GORE® DrySeal sheath is used routinely in Venous P-Valve cases.
Authors’ contributions: Shakeel A Qureshi is principal investigator for the Venus P-valve CE study, Matthew Jones and Eric Rosenthal were sub-investigators for the Venus P-valve CE study, Kevin P Walsh, Damien Kenny were clinical investigators for the Venus p-valve CE study.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Babu-Narayan, S. V., Diller, G. P., Gheta, R. R., Bastin, A. J., Karonis, T. et al. (2014). Clinical outcomes of surgical pulmonary valve replacement after repair of tetralogy of Fallot and potential prognostic value of preoperative cardiopulmonary exercise testing. Circulation, 129(1), 18–27. DOI 10.1161/CIRCULATIONAHA.113.001485. [Google Scholar] [CrossRef]
2. Bonhoeffer, P., Boudjemline, Y., Saliba, Z., Merckx, J., Aggoun, Y. et al. (2000). Percutaneous replacement of pulmonary valve in a right-ventricle to pulmonary-artery prosthetic conduit with valve dysfunction. Lancet (London, England), 356(9239), 1403–1405. DOI 10.1016/S0140-6736(00)02844-0. [Google Scholar] [CrossRef]
3. Promphan, W., Prachasilchai, P., Siripornpitak, S., Qureshi, S. A., Layangool, T. (2016). Percutaneous pulmonary valve implantation with the Venus P-valve: clinical experience and early results. Cardiology in the Young, 26(4), 698–710. DOI 10.1017/S1047951115001067. [Google Scholar] [CrossRef]
4. Husain, J., Praichasilchai, P., Gilbert, Y., Qureshi, S. A., Morgan, G. J. (2016). Early European experience with the Venus P-valve®: Filling the gap in percutaneous pulmonary valve implantation. EuroIntervention: Journal of EuroPCR in Collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology, 12(5), e643–e651. DOI 10.4244/EIJV12I5A105. [Google Scholar] [CrossRef]
5. Garay, F., Pan, X., Zhang, Y. J., Wang, C., Springmuller, D. (2017). Early experience with the Venus P-valve for percutaneous pulmonary valve implantation in native outflow tract. Netherlands Heart Journal: Monthly Journal of the Netherlands Society of Cardiology and the Netherlands Heart Foundation, 25(2), 76–81. DOI 10.1007/s12471-016-0932-5. [Google Scholar] [CrossRef]
6. Zhou, D., Pan, W., Jilaihawi, H., Zhang, G., Feng, Y. et al. (2019). A self-expanding percutaneous valve for patients with pulmonary regurgitation and an enlarged native right ventricular outflow tract: One-year results. EuroIntervention: Journal of EuroPCR in Collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology, 14(13), 1371–1377. DOI 10.4244/EIJ-D-18-00715. [Google Scholar] [CrossRef]
7. Morgan, G., Prachasilchai, P., Promphan, W., Rosenthal, E., Sivakumar, K. et al. (2019). Medium-term results of percutaneous pulmonary valve implantation using the Venus P-valve: International experience. EuroIntervention: Journal of EuroPCR in Collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology, 14(13), 1363–1370. DOI 10.4244/EIJ-D-18-00299. [Google Scholar] [CrossRef]
8. Kenny, D., Morgan, G. J., Murphy, M., AlAlwi, K., Giugno, L. et al. (2019). Use of 65 cm large caliber Dryseal sheaths to facilitate delivery of the Edwards SAPIEN valve to dysfunctional right ventricular outflow tracts. Catheterization and Cardiovascular Interventions: Official Journal of the Society for Cardiac Angiography & Interventions, 94(3), 409–413. DOI 10.1002/ccd.28409. [Google Scholar] [CrossRef]
9. Faccini, A., Butera, G. (2018). Tricuspid regurgitation as a complication of Edwards Sapien XT valve implantation in pulmonary position a problem to deal with. Catheterization and Cardiovascular Interventions: Official Journal of the Society for Cardiac Angiography & Interventions, 91(5), 927–931. DOI 10.1002/ccd.27527. [Google Scholar] [CrossRef]
10. Shah, R. R., Poommipanit, P., Law, M. A., Amin, Z. (2018). Anchor balloon, buddy wire, and wire and sheath techniques to deploy percutaneous pulmonary valves in tetralogy of fallot patients. Catheterization and Cardiovascular Interventions: Official Journal of the Society for Cardiac Angiography & Interventions, 92(5), 915–920. DOI 10.1002/ccd.27022. [Google Scholar] [CrossRef]
11. Butera, G., Hansen, J. H., Jones, M. I. (2019). Tricuspid regurgitation complicating SAPIEN 3 valve implantation in pulmonary position. Catheterization and Cardiovascular Interventions: Official Journal of the Society for Cardiac Angiography & Interventions, 94(6), 894. DOI 10.1002/ccd.28083. [Google Scholar] [CrossRef]
Appendix
Video Legends
Video 1. Anteroposterior fluoroscopy showing the GORE® DrySeal advanced over the wire.
Video 2. The sheath is in place in the LPA and the Venous-P-Valve is easily advanced.
Video 5. The distal end of the valve is unsheathed.
Video 6. The delivery sheath is pulled back and the valve placed in the target area.
Video 7. Angiography in the main pulmonary artery showing a perfectly and continent valve.
 | This work is licensed under a Creative Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |