

 | Congenital Heart Disease |  |
DOI: 10.32604/CHD.2021.015371
ARTICLE
Management of “Wall to Wall Heart” in a Transient Neonatal Tricuspid Regurgitation
Mediterranean Pediatric Cardiology Center “Bambino Gesù”, San Vincenzo Hospital, Taormina, Italy
*Corresponding Author: Elio Caruso. Email: carusoelio@gmail.com
Received: 14 December 2020; Accepted: 06 January 2021
Abstract: We present a case of a one-day-old newborn, without prenatal diagnosis, referred to our cardiologic intensive care unit in critical condition presenting sub-cyanosis and peripheral oxygen saturation of 80%. Echocardiography diagnosis was tricuspid valve dysplasia with severe regurgitation, functional pulmonary valve atresia with intact ventricular septum and reversal flow in the large patent ductus arteriosus (PDA). Chest X-ray showed severe cardiomegaly and wall to wall heart. Prostaglandin E1 infusion was started once after birth. After few days, clinical conditions progressively worsened because of right heart failure; a first pharmacological approach to close PDA failed and surgery ligation of PDA was necessary to restore anterograde pulmonary flow and heart size.
Keywords: Wall to wall heart; tricuspid regurgitation; pulmonary atresia; arterial duct
The wall-to-wall heart is a typically consequence either of Ebstein’s malformation or dysplasia of the normally tricuspid valve. Congenital isolated tricuspid regurgitation (TR) is a rare cardiac malformation, usually associated to functional pulmonary atresia (PA). In the setting of PA and intact ventricular septum with the “wall-to-wall” heart, the pulmonary valve is typically imperforate and, it is difficult to differentiate if the atresia is functional rather than anatomic [1,2]. We report a very rare case of severe TR, functional PA and wall-to-wall heart.
A full-term female neonate was born from eutocic delivery with 3,240 kg body weight, APGAR 1’ 7 and 5’ 8, without specific antenatal diagnosis. Patient was admitted to cardiologic intensive care unit (ICU) in critical condition, moderately cyanotic with peripheral oxygen saturation of 80%. Written informed consent was obtained from parents.
Echocardiography evaluation revealed tricuspid valve dysplasia with severe regurgitation and functional pulmonary valve atresia with intact ventricular septum. The massive tricuspid regurgitation was associated with enlarged right atrium (RA) (Fig. 1), without anterograde flow from right ventricle (RV) to pulmonary valve, resulting in a functional PA with reversal flow in the large patent ductus arteriosus (PDA) (Fig. 2) and pericardial effusion.
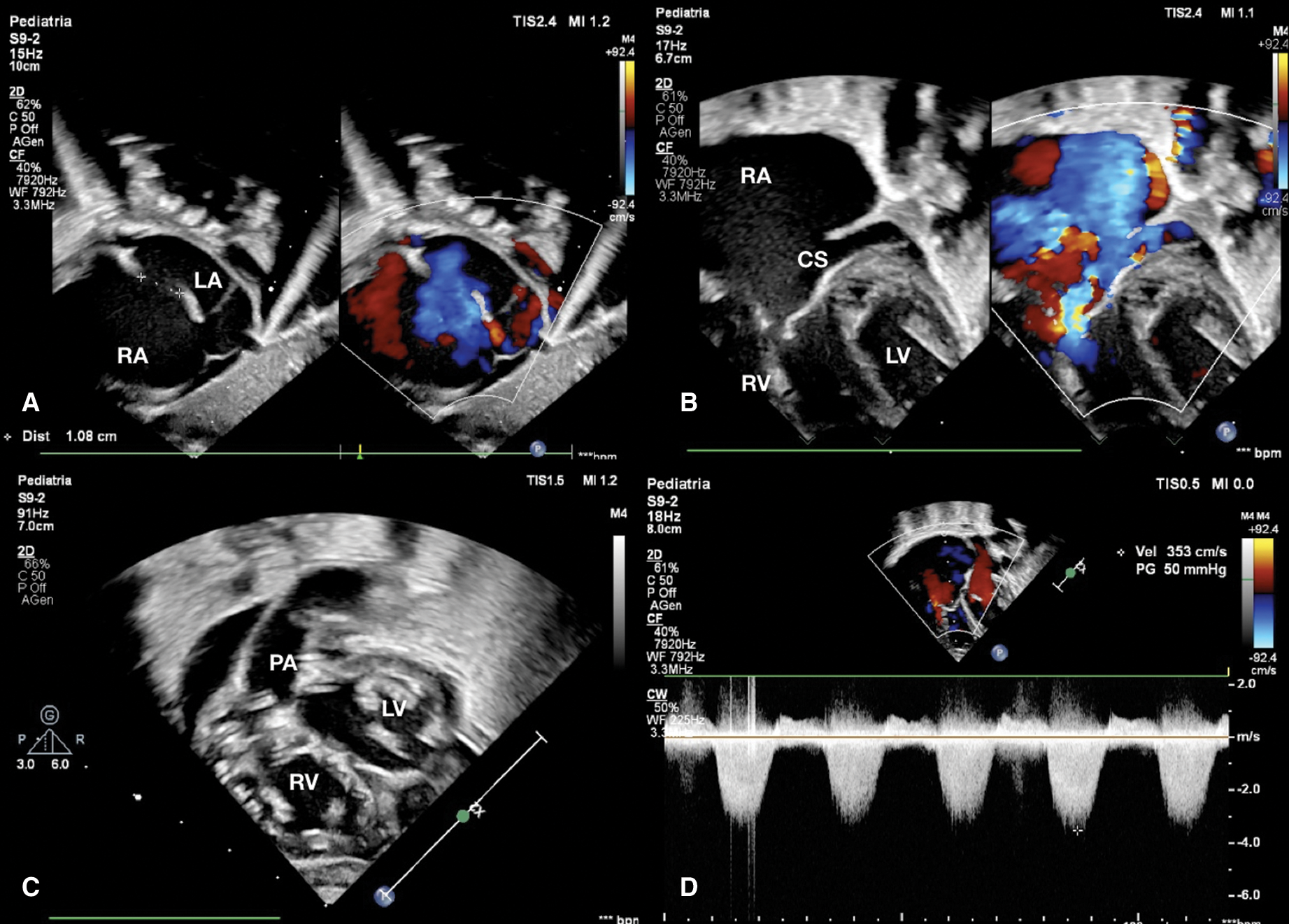
Figure 1: (A) Subcostal view shows expanded right atrium and atrial septal defect of 1.08 cm with right to left shunt. (B) Apical view shows severe tricuspid regurgitation and dilated coronary sinus for persistent left superior caval vein. (C) Ventricle short axis subcostal view shows atrioventricular valves and right outflow tract. (D) CW Doppler through tricuspid valve estimates a right ventricle pressure of 50 mmHg. CS coronary sinus, LA left atrium, LV left ventricle, PA pulmonary artery, RA right atrium, RV right ventricle
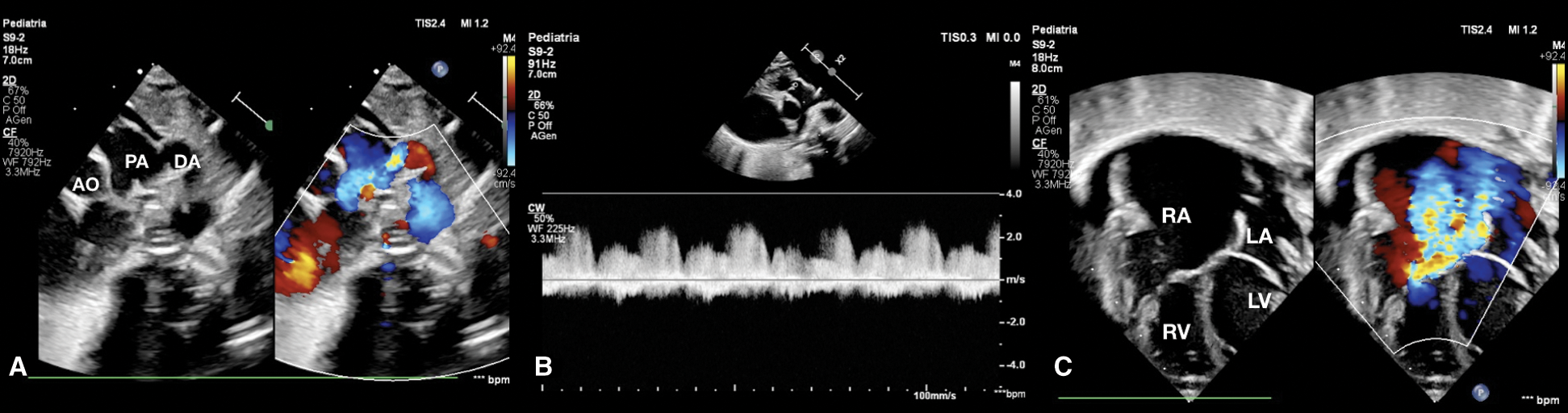
Figure 2: (A) Short axis parasternal view shows patent ductus arteriosus with reversal flow. (B) CW Doppler in pulmonary artery shows absence of anterograde flow. (C) Four-chamber view at birth shows severe tricuspid regurgitation, normal AV valves implantation, huge right atrium, pericardial effusion and normal size of right ventricle and left ventricle. AO aorta, DA ductus arteriosus, LA left atrium, LV left ventricle, PA pulmonary artery, RA right atrium, RV right ventricle
High intravenous dosage of Prostaglandin E1 (PGE1) was administered immediately. Chest X-ray showed severe cardiomegaly and wall to wall heart (Fig. 3A). After diuretic and dopamine therapy, TR improved from severe to trivial, but persisted functional PA with a thickened non-opening pulmonary valve without anterograde flow. Multidisciplinary team decision was to progressively reduce Prostaglandin’s dose and clinical observation. Clinical conditions progressively worsened with an imposing right heart failure characterized by ascites, hepatomegaly and anuria (creatinine: 2 mg/dl). Despite diuretic therapy and paracentesis drainage, we faced with further deterioration of clinical conditions such as increase lactates levels (6.5 mmol/l) and lower peripheral oxygen saturation of 75%. Serial echocardiograms documented persistence of a large PDA and limited opening of the pulmonic valve cusps, with initial mild anterograde flow. After 5 days of ineffective Ibuprofen administration, surgical PDA ligation was indicated and performed (Fig. 4). By sternotomy approach the first surgical findings were the evidence of cardiomegaly (Fig. 5) and a very large PDA that was ligated. Immediately after the procedure, progressive reduction of the right chambers’ enlargement and oxygen saturation improvement (up to 95%) was noted. Intraoperatory transesophageal echocardiography showed normal opening of the pulmonary valve and persistence of trivial tricuspid valve regurgitation.
Since right heart failure completely regressed three days after the procedure and heart size at chest X-ray markedly improved (Fig. 3B), we speculate that surgical PDA closure relieved “competitive” anterograde pulmonary blood flow with consequent decompression of the RV.
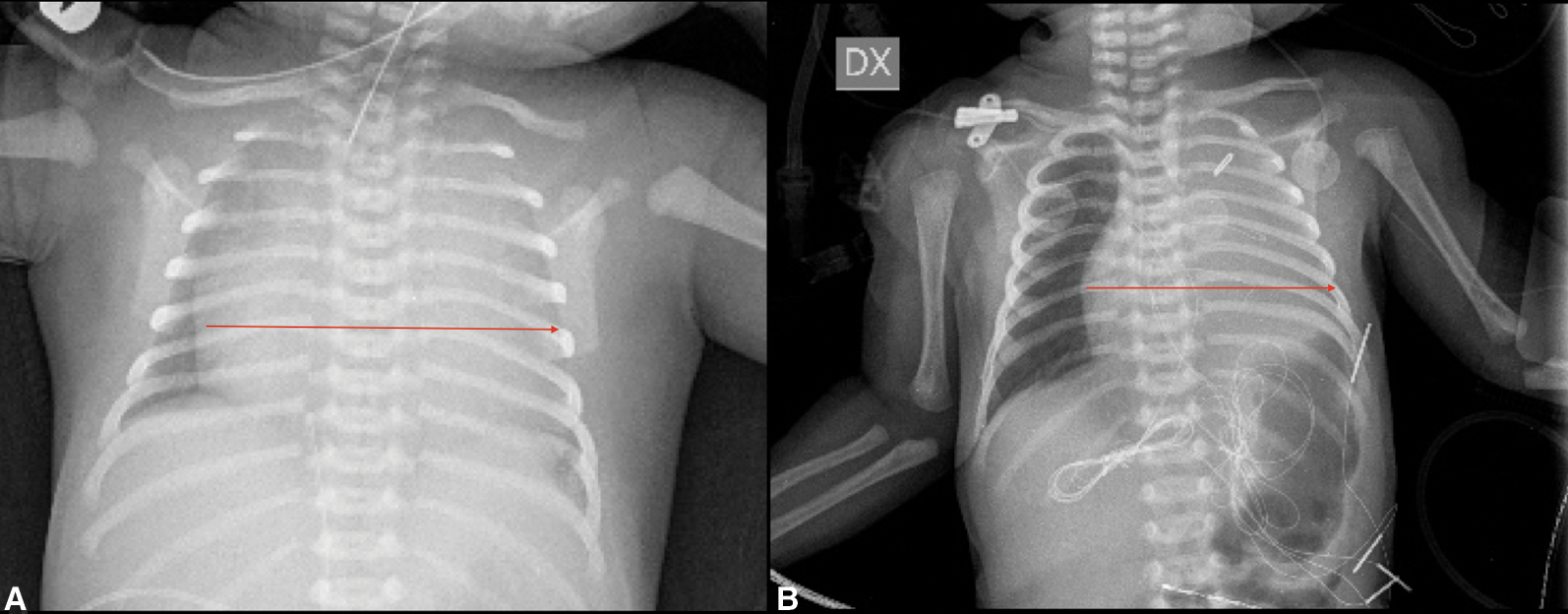
Figure 3: Chest X-ray (A) Red arrow: Wall to wall heart pre surgery (B) Red arrow: Heart dimension post-surgery
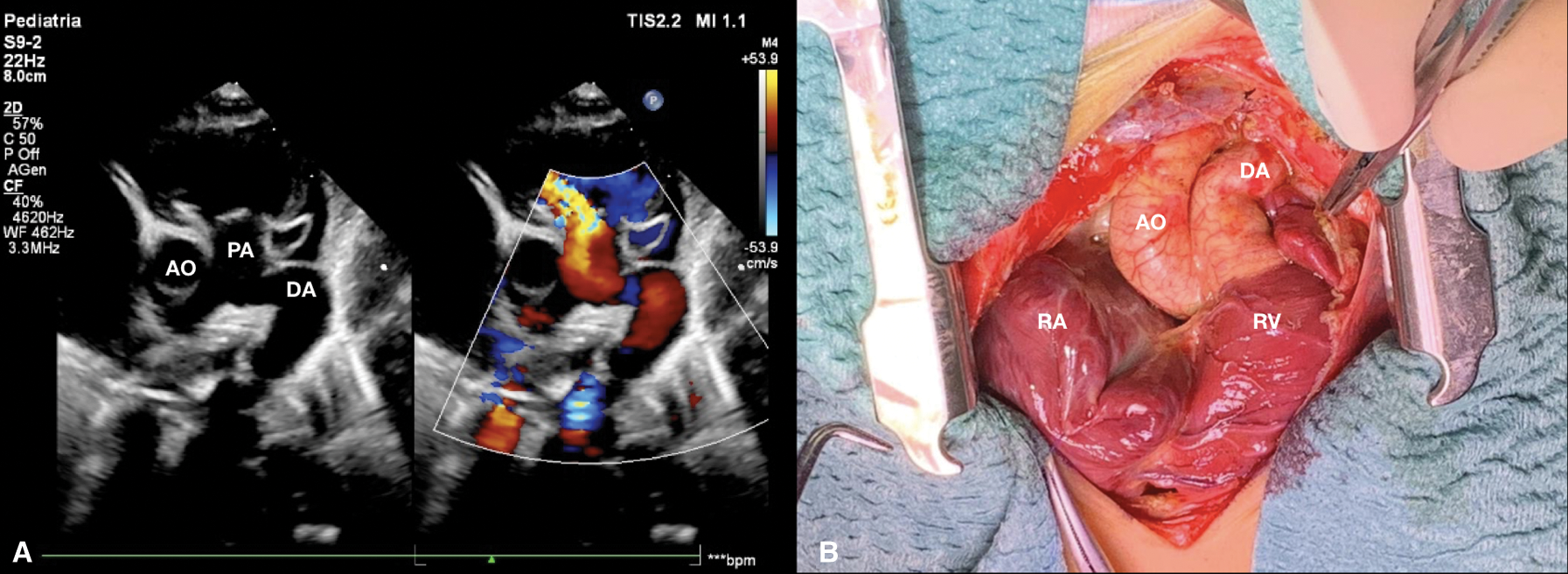
Figure 4: (A) Short axis parasternal view after few days from birth shows patent ductus arteriosus with reversal flow and pulmonary regurgitation. (B) Surgical picture of ductus arteriosus before ligation. AO aorta, DA ductus arteriosus, PA pulmonary artery, RA right atrium, RV right ventricle
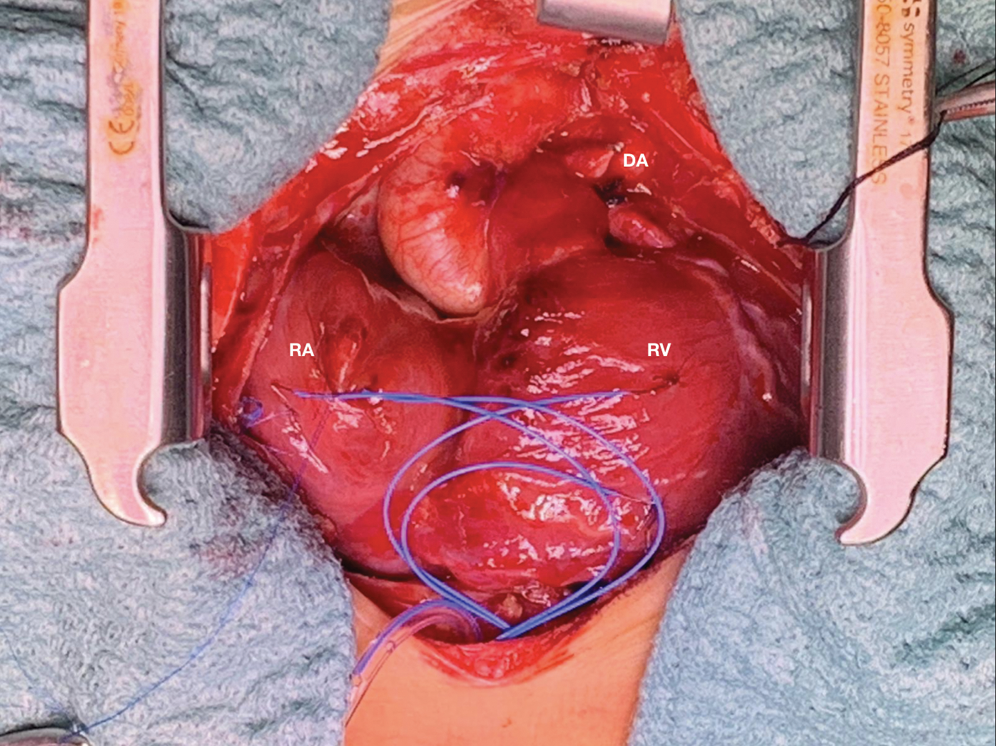
Figure 5: Surgical picture of wall to wall heart after ligation of ductus arteriosus. DA ductus arteriosus, RA right atrium, RV right ventricle
The essence of the wall-to-wall heart is its sheer size characterized by a grossly enlarged heart, in particular a huge right atrium and enlarged right ventricle. In fetal period, it can be caused by Ebstein’s malformation of tricuspid valve (TV) or PA with intact ventricle septum and ecochardiographic imaging shows the “big” heart occupying much of the volume within the bony thorax that should be available for the lungs [1].
In the case reported, the characteristic wall-to-wall radiological image is due to the association of TV dysplasia with a functional PA secondary to a huge PDA. The association of TV dysplasia with functional PA can manifest itself with the clinical characteristics of a severe Ebstein’s malformation.
The distinguishing pathognomonic feature between Ebstein’s malformation and tricuspid dysplasia is the displacement and rotation of the hinge points of the tricuspid valvar leaflets, which is well differentiated with two-dimensional echocardiography providing some basic anatomical insights, and even more with three-dimensional imaging which is rapidly emerging as an important new clinical tool in congenital heart disease [3]. In our case two-dimensional echocardiography allowed to exclude Ebstein’s malformation showing clearly the right closing in trifoliate fashion of the leaflets of the tricuspid valve, hinged at the right atrioventricular junction (Figs. 1B, 1C and 2C).
Congenital TR is unusual and may be due to morphological or functional anomaly derived from a transitional myocardial ischemia or rarely, from a primary disorder of the RV myocardium, as absence of RV myocardium (Uhl’s anomaly). In the neonatal period isolated anatomical abnormalities of TV are uncommon, usually associated to severe right ventricular outflow tract (RVOT) obstruction, as critical pulmonary stenosis or atresia with an intact ventricular septum, Ebstein’s anomaly or atrioventricular septal defect [2]. Transient neonatal severe TR with a structurally normal heart may be related to premature closure of the ductus arteriosus [4].
Many authors documented severe TR in isolation and, in some cases associated to functional PA [2,5–9]. Echocardiography is crucial to readily differentiate functional from organic PA. In the patient with functional PA despite an anatomically normal pulmonary outflow tract, pulmonary regurgitation is seen in diastole. After RV afterload reduction and pulmonary vascular bed development, organic TR can improve, and surgery for isolated congenital TR (escluding Ebstein’s anomaly) is extremely rare [2]. Whereas among organic PA, morphologically, PA type II (with tricuspid insufficiency) has a greater resemblance to Ebstein’s disease than PA type I (with tricuspid stenosis) [10]. In patients with PA and intact ventricular septum who exhibit the “wall-to-wall” malformation, the pulmonary valve is typically imperforate and, although in some instances the atresia is functional rather than anatomic, it is frequently difficult to make this distinction. Administration of prostaglandin to maintain ductal patency may be helpful, at least in the short-term, to differentiate the outflow obstruction due to either anatomic or functional PA in severely affected neonates with either Ebstein’s malformation or tricuspid valvar dysplasia [1].
Insufficiency of the TV will be addressed later and not in the neonatal period. A reduction in pulmonary artery pressure with surgical PDA closure would be resolutive to allow anterograde flow [11]. The inversion of the septal protrusion from the left ventricle to the right ventricle proves a clear sign of decompression of the RV. The presence of a valid anterograde flow resolves the critical condition allowing the child to grow.
As previously described, patients with transient neonatal TR, without an obvious anatomical and haemodynamic basis, should be followed up during growth for the possible development of late tricuspid regurgitation [12].
We report a challenging case of marked cardiomegaly, absence of anterograde flow in the pulmonary valve and reversal flow in the PDA, indicating a ductus-dependent congenital heart disease with poor prognosis. The early neonatal period could be complicated by congestive heart failure and PGE1 infusion was initially required for the maintenance of pulmonary perfusion. Successful surgical ligation of the large PDA demonstrated the functional PA resulting in normal RV anterograde flow. The differentiation between functional and anatomical PA is crucial to provide the right therapeutic approach and restore anterograde pulmonary perfusion. Patients with transient neonatal TR, without an obvious anatomical and haemodynamic basis, need a multidisciplinary approach and management in the first period of life and for long-term follow up [12].
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Freedom, R. M., Jaeggi, E., Perrin, D., Yoo, S. J., Anderson, R. H. (2006). The wall-to-wall heart in the patient with pulmonary atresia and intact ventricular septum. Cardiology in the Young, 16(1), 18–29. DOI 10.1017/S1047951105002040. [Google Scholar] [CrossRef]
2. Freedom, R. M., Benson, L. N., Smallhorn, J. F., Van Praagh, R. (1992). Neonatal heart disease. Berlin, Heidelberg, New York: Springer-Verlag. [Google Scholar]
3. Holst, K. A., Connolly, H. M., Dearani, J. A. (2019). Ebstein’s anomaly. Methodist DeBakey Cardiovascular Journal, 15(2), 138–144. [Google Scholar]
4. Berry, T. E., Muster, A. J., Paul, M. H. (1983). Transient neonatal tricuspid regurgitation: Possible relation with premature closure of the ductus arteriosus. Journal of the American College of Cardiology, 2(6), 1178–1182. DOI 10.1016/S0735-1097(83)80348-9. [Google Scholar] [CrossRef]
5. Berman, W., Jr., Whitman, V., Stanger, P., Rudolph, A. M. (1978). Congenital tricuspid incompetence simulating pulmonary atresia with intact ventricular septum: A report of two cases. American Heart Journal, 96(5), 655–661. DOI 10.1016/0002-8703(78)90203-X. [Google Scholar] [CrossRef]
6. Boucek, R. J., Jr., Graham, T. P., Jr., Morgan, J. P., Atwood, G. F., Boerth, R. C. (1976). Spontaneous resolution of massive congenital tricuspid insufficiency. Circulation, 54(5), 795–800. DOI 10.1161/01.CIR.54.5.795. [Google Scholar] [CrossRef]
7. Freedon, R. M., Culham, G., Moes, F., Olley, P. M., Rowe, R. D. (1978). Differentiation of functional and structural pulmonary atresia: Role of aortography. American Journal of Cardiology, 41(5), 914–920. DOI 10.1016/0002-9149(78)90733-6. [Google Scholar] [CrossRef]
8. Freymann, R., Kallfelz, H. C. (1975). Transient tricuspid incompetence in a newborn. European Journal of Cardiology, 2(4), 467–471. [Google Scholar]
9. Yeager, S. B., Parness, I. A., Sanders, S. P. (1988). Severe tricuspid regurgitation simulating pulmonary atresia in the fetus. American Heart Journal, 115(4), 906–908. DOI 10.1016/0002-8703(88)90898-8. [Google Scholar] [CrossRef]
10. Bharati, S., McAllister, H. A.,Jr., Chiemmongkoltip, P., Lev, M. (1977). Congenital pulmonary atresia with tricuspid insufficiency: Morphologic study. American Journal of Cardiology, 40(1), 70–75. DOI 10.1016/0002-9149(77)90103-5. [Google Scholar] [CrossRef]
11. Rato J, Sousa A., Teixeira A., Anjos R. (2019). Ebstein’s anomaly with ‘reversible’ functional pulmonary atresia. BMJ Case Reports, 12(12), e229809. DOI 10.1136/bcr-2019-229809. [Google Scholar] [CrossRef]
12. Boshoff, D., Mertens, L., Gewillig, M. (2001). Severe tricuspid regurgitation 14 years after diagnosis of transient neonatal tricuspid regurgitation. Heart (British Cardiac Society), 86(1), 88–90. DOI 10.1136/heart.86.1.88. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |