

 | Congenital Heart Disease |  |
DOI: 10.32604/CHD.2021.015470
REVIEW
Fetal Bradyarrhythmias: Etiopathogenesis, Diagnosis and Treatment: Between Literature Review and Experience of a Tertiary Center
1Mediterranean Pediatric Cardiology Center “Bambino Gesù” San Vincenzo Hospital, Contrada Sirina, Taormina, 98039, Italy
*Corresponding Author: Elio Caruso. Email: carusoelio@gmail.com
Received: 21 December 2020; Accepted: 08 February 2021
Abstract: Fetal arrhythmias reach up around 10% of the total third-level perinatal cardiology references. Sustained bradycardia is defined as a baseline fetal heart rate (FHR) of less than 110 bpm sustained for at least 10 min. The overall incidence of malignant fetal bradyarrhythmias, such as complete atrioventricular block (AVB) and channellopathies, is relatively rare, 1:5000 pregnancies, but represents a serious emergency for the gynecologist, neonatologists, and pediatric cardiologists. Fetal complete AVB is strongly associated with maternal connective tissue disease, but it can be also associated with congenital heart disease and usually with a poorer prognosis with high risk of fetal hydrops and abortion. Currently, the treatment of severe fetal bradyarrhythmias is principally pharmacological and aims to increase the FHR, besides an early resolution of underlying causes, when possible, and a promptly management of fetal heart failure. Intrauterine electrostimulation nowadays is an experimental pioneering method, reserved for limited selected cases.
Keywords: Fetal arrhythmias; hydrops; bradyarrhythmia; atrioventricular block
Life-threatening fetal arrhythmias warrant sophisticated specialty prenatal care, often provided by maternal-fetal medicine obstetricians and pediatric and fetal cardiologists.
Fetal arrhythmias are uncommon diseases which occurs in 1–3% of all fetus, and account for 10–20% of the referrals to fetal cardiology. The overall incidence of malignant fetal arrhythmias, such as complete AV block and SVT, are relatively rare, found in 1:5000 pregnancies [1]. According to gestational age and degree of fetal activity, normal fetal heart rate (FHR) is between the range of 110 and 180 beats per minute (bpm) and with a 1:1 atrio-ventricular (AV) electromechanical relationship. Fetal bradycardia according to the guidelines of the American College of Obstertricians and Gynecologists is defined as an FHR less than 110 bpm sustained for at least 10 min. This differs from a deceleration, which resolves in less than 10 minutes [2–4]. Short decelerations of FHR may be observed during the first and second trimester which may occur by vagal stimulation due to umbilical cord compression during ultrasound scans, but are usually short in duration and are clinically irrelevant.
The most common causes of bradyarrhythmia are due to blocked premature atrial contractions (PAC), sinus node dysfunction, advanced or complete atrio-ventricular block (AVB) and channelopathies mainly represented by long QT syndrome (LQTS) [5–9]. Sometimes fetal bradycardia is associated with intrauterine distress, maternal hypothermia, and medications (Tabs. 1, 2 [10]).
Table 1: Causes of fetal bradycardia. Courtesy of the book Yagel S. (ed.), Silverman N. H. (ed.), Gembruch U. (ed.) (2019). Fetal Cardiology. Boca Raton: CRC Press [10]

Table 2: Characteristics of atrioventricular conduction. Courtesy of the book Yagel S. (ed.), Silverman N.H. (ed.), Gembruch U. (ed.) (2019). Fetal Cardiology. Boca Raton: CRC Press [10]

1.1 Embriological Development of Conduction System
The cardiac conduction system has a unique embryological origin that is distinct from that of the working myocardium; this system involves more structures than originally thought, since it englobes the AV rings, a third retroaortic node and the pulmonary and aortic sleeves [11,12].
During embryological development the first morphological signs of sinoatrial node (SAN) are present by 5 weeks of gestation and complete its development at 16 weeks of gestation [4].
The SAN is characterized by specific gene expression profile, with minimal expression of KCNJ2 (inwardly rectifying K channel, Kir2.1) and SCN5A (cardiac Na channel, Nav1.5) and higher expression of HCN4 (the pacemaker channel) [5]. Mutations of KCNJ2, SCN5A, SCN1B, HCNJ2, GATA4 and GATA6, HF-1b, bHLH, TRPM4, Nkx2-5, Msx2, Hop and T-box family of transcription factors (especially Tbx5 because of haploinsufficiency cause Holt-Oram syndrome) genes may lead to congenital heart disease (CHD) and conduction disorders [1,13–15].
Specifically, the forced expression pattern of Tbx3 and deficiency of Pitx2 are related to the development of ectopic pacemaker tissues and to a dual SAN, respectively [4].
In fetal sinus bradycardia, atrial and ventricular events are absolutely regular, the AV ratio is consistently 1:1 but the heart rate is <110 bpm. It can occur if the SAN is normal, absent, displaced or dysfunction.
Immune-mediated diseases (Sjögren-Syndrome-Related antigen A and B), maternal infections (TORCH, Parvovirus), or viral myocarditis lead inflammation and fibrosis of the SAN and its dysfunction. Other causes are maternal treatment with beta-blockers, rare metabolic disorders (e.g., Pompes disease) or idiopathic origin. Some CHD are associated with conduction system anomalies such as left atrial isomerism resulting in low atrial rhythm due to absence of the SAN, right atrial isomerism due to dual SAN, or discordant AV connection also commonly associated to AVB [16].
No specific treatment is required for sinus or low-atrial bradycardia if hemodynamically well tolerated at birth [4].
Sinus bradycardia can be a marker of fetal LQTS (Figs. 1, 2). Indeed, many authors have confirmed that the fetus with repeated heart rate measures less than the third percentile for gestational age or <133 bpm in the third trimester may have LQTS [10]. In this channelopathy, SAN is normal and bradycardia is due to sympathetic imbalance or, more rarely, to AV conduction defects (see below). Identification of LQTS in fetal life is important because it accounts for approximately 8–10% each of sudden infant deaths and unexplained intrauterine fetal deaths [17]. Furthermore, the fetal diagnosis of LQTS has implications for limiting the maternal drugs that can prolong the QT interval and optimizing maternal magnesium, vitamin D and calcium levels, reducing the risk of fetal ventricular arrhythmias [18].
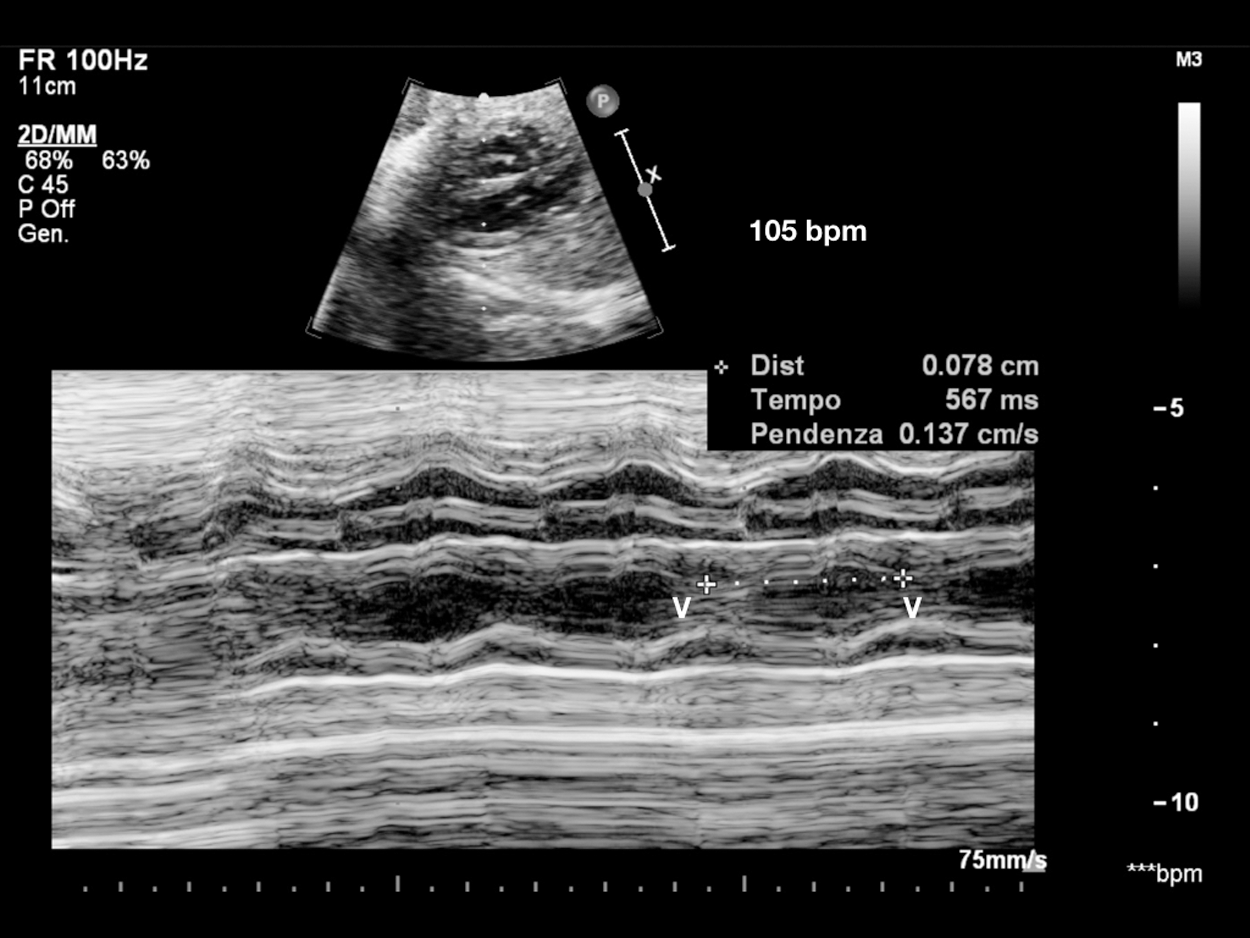
Figure 1: Sinus bradycardia. V-V interval 567 msec in patient with LQTS syndrome at birth. Consanguineous couple, both carriers of KCNQ1 and LAMC2 heterozygous mutations (epidermolysis bullosa). Two previous sons died, one at 14 months of S. Jervell-Langel-Nielsen (S. JLN), and one at 1 month of Epidermolysis bullosa. Third pregnancy amniocentesis: heterozygous KCNQ1 mutation (S. of JLN, LQT1). V = ventricle
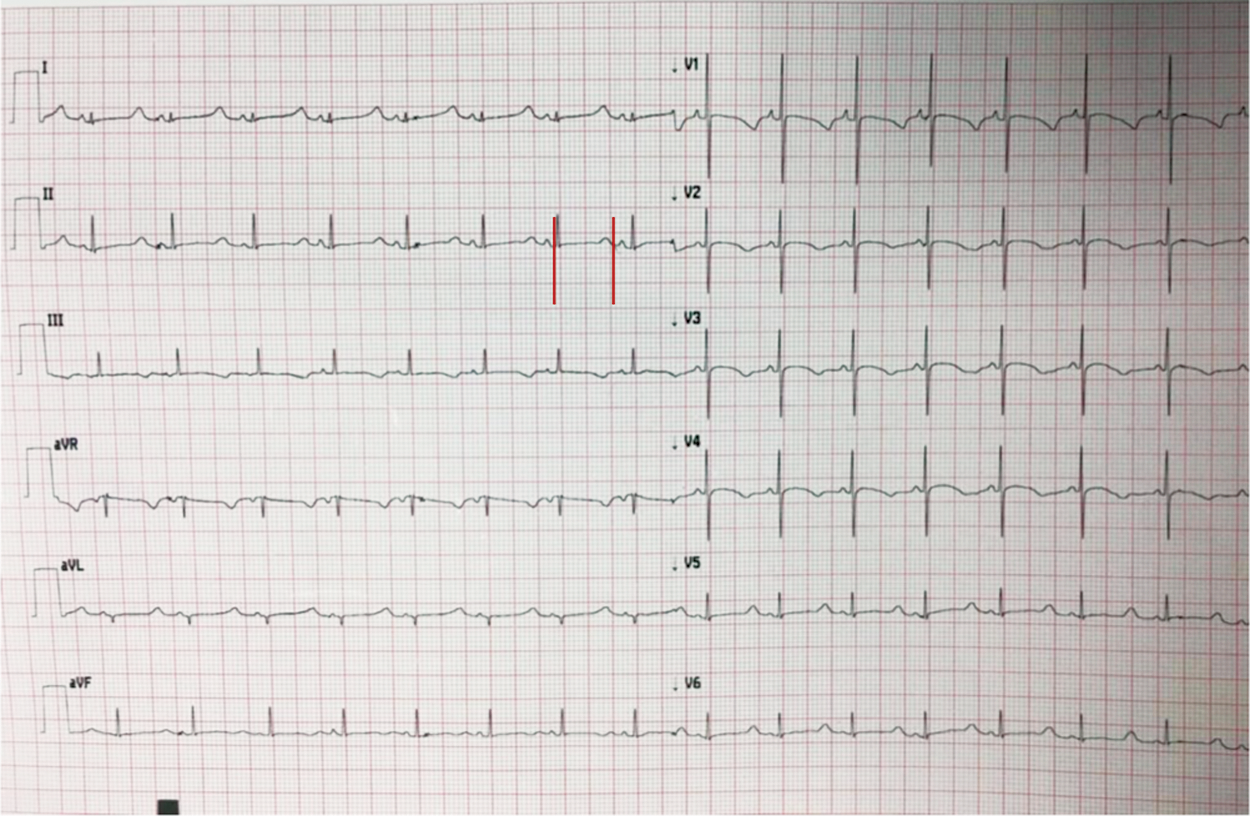
Figure 2: 12-lead electrocardiogram of a 1-day-old patient with LQTS born from a couple of consanguineous parents, both carriers of KCNQ1 and LAMC2 heterozygous mutations (epidermolysis bullosa). Red line: QTc interval. Using Bazett’s formula derives a QTc of 594 ms
Anecdotally, a further cause of reduced FHR could be Sick Sinus Syndrome (SSS), caused by recessive mutations in SCN5A gene or, more rarely, in HCN4 gene [19,20].
2.2 Blocked Premature Atrial Contractions
Irregular sinus rhythm at normal FHR occurs with atrial or, less frequently, with ventricular premature contractions (10:1), which are clinically benign if brief and isolated. If an extrasystole follows every sinus beat it is termed bigeminy (Figs. 3, 4), if it follows every third beat it is termed trigeminy, and if it follows every fourth beat the rhythm is quadrigeminy. Fetal ectopy is associated with CHD or myocardial diseases or intracardiac tumors in approximately 1% of cases [16].
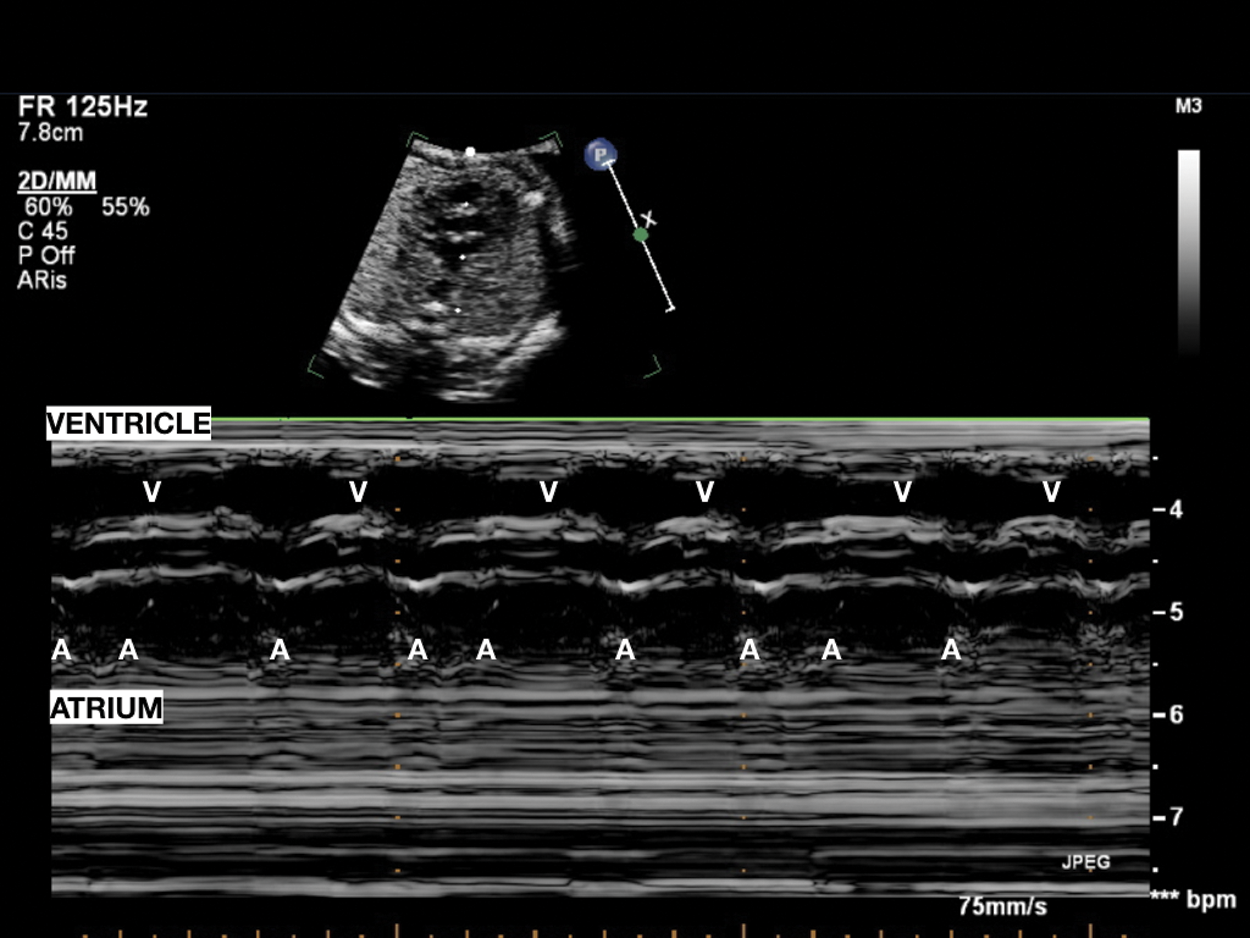
Figure 3: Pseudobradycardia: Atrial bigeminy with blocked premature beats; atrial rhythm with an alternating, shorter–longer AA time interval induces low ventricular heart rate

Figure 4: Pulsed-wave Doppler recording from the LV inflow and LV outflow in a fetus with atrial bigeminy. One atrial is conducted, but PAC occurred sooner, and the inflow Doppler waveform showed only an A wave since it occurred earlier and fused with the E wave. There is a ventricular contraction following this PAC, indicating the PAC is conducted. A, atrial contractions; LV, left ventricle, V, ventricular contractions; PAC, premature atrial contraction
Blocked PAC can occur at regular intervals and last over long period of time leading to persistent fetal bradycardia. The typical pattern of regular bradycardia due to ectopic beats corresponds to blocked atrial bigeminy and produces FHR between 75 and 90 bpm when conduction is in a 2:1 AV pattern. It is a common cause of regular bradycardia and management is the same as for isolated PAC. The risk of supraventricular tachycardia is about 10% of fetal atrial bigeminy with block [2]. Fetal echocardiography is recommended to assess cardiac structure and weekly FHR. Persistent atrial bigeminy can be confused with pathological 2:1 AVB.
Differential diagnosis is essential for therapeutic strategies, because long lasting 2:1 AV block has severe consequences. Echocardiography allows to evaluate FHR and regularity of atrial and ventricular systolic events and the AV relationship through M-mode (Fig. 3) and Pulsed-wave Doppler method (Fig. 4). However, it is often complex to distinguish 2:1 AVB from prolonged atrial bigeminy using only echocardiography: Lately, the more routine use of fetal magnetocardiography (see later) allows precise and rapid differential diagnosis [21].
AVB is a prolongation or blockage of AV conduction of the normal sinus beat through the AV node. A delayed conduction is present in the first-degree block and FHR reflects the normal pacemaker rate. In the second-degree block Mobitz I (Luciani-Wenckebach phenomenon) AV interval progressively lengthen until a blockage occurs, in Mobitz II some beats are conducted and some are blocked. A 2:1–3:1 AV conduction often causes regular bradycardia with an FHR of 60–80 bpm [22].
The high degree or complete AVB is one of the most dangerous fetal bradyarrhythmias due to increased fetal loss risk [23]. It occurs when the beat of the atria is completely dissociated from the ventricular beat and causes significant bradycardia with an FHR of up to 40–90 bpm [24]. Congenital AVB has two predominant presentations:
1. The usual case is that of a fetus with normal cardiac structure and exposure to specific maternal autoantibodies (immune-mediated AVB)
2. AVB can be present in fetuses with complex CHD
Other rare maternal risk factors for developing fetal AVB may be: Metabolic diseases (i.e., Type 2 diabetes mellitus), medication exposures, and viral infections [25]. In addition, inherited or (apparently) idiopathic forms of intrauterine atrioventricular conduction defects are been described.
Immune mediated AVB (Fig. 5A) is caused by passive placental transfer of maternal autoantibodies specific for Ro/SSA and/or La/SSB and can occur as between 18 and 24 weeks of gestation [7]; anecdotally a late onset of intrauterine conduction defect has been described, even up to gestational week 34 [26]. As demonstrated by Costedoat-Chalumeanu, specific maternal autoantibodies can be detected in over 95% of fetuses or newborns with congenital AVB without CHD [27], and maternal autoimmune disease accounts for 90–99% of cases of neonatal/infant complete AVB in absence of CHD [13]. Furthermore, in the literature it is widely reported as these autoantibodies have been detected in a minority of children diagnosed with AVB beyond the neonatal period [28,29]. The most accredited theory to explain the pathogenesis of the immune-mediated AVB is known as the “calcium channel hypothesis,” according to which maternal anti-Ro/SSA and/or anti-La/SSB autoantibodies interact directly with L-type calcium channels on fetal cardiomiyocytes. This phenomenon would determine the inflammatory damage process resulting in fibrosis and calcification of the cardiac conduction system [30,31]. In the last years, Alvarez et al. [32,33] and, even more recently Izmirly [34], have proposed an alternative theory for the genesis of the fibrosis responsible for the damage of the fetal excitoconduction system, according to which the complete AVB is due to the activation of the Toll-like receptor with consequent triggering of pro-inflammatory and pro-fibrotic cytokine secretion.

Figure 5: A. Complete immune fetal AV block. Atrial contractions occur regularly and independently of ventricular contractions. B. PW Doppler cava-aorta BAV II 2:1. A = atrium; V = ventricle
It has long been known in the literature that 2–5% of fetuses and infants (<1 month) whose mothers are autoantibody-positive develop AVB, and the risk increases to 12–15% in case of mothers with previous children with complete AVB [35]. The incidence of mortality in utero due to AVB is not well defined, and has certainly decreased over the years thanks to early diagnosis and appropriate therapeutic interventions [24,25]. Eliasson and colleagues, demonstrated in a multicentre study of 175 patients, how mortality is significantly burdened by concomitant risk factors such as: Fetal hydrops (Fig. 6), early onset of block, ventricular escape rate <55 bpm, and impaired left ventricular function [36].

Figure 6: Fetal hydrops due to congenital AVB at 25 gestational age
Abnormalities in the conduction system are highly prevalent in congenital heart defects. The nature of the conduction system in CHD is intricately related to the underlying lesion, and no description can occur without reference to the unique structural anatomy of this population. Although only subtle differences between simple congenital heart lesions and normal may exist, almost every patient with CHD harbours an important variation in the conduction system anatomy. In particular, fetal bradycardia can be associated with a CHD in 25–50% of cases [37], and it has a poorer prognosis than that associated with collagen vascular disease. Intrauterine hydrops and demise are seen in about 7% of fetuses, and another 10–15% of cases succumb in infancy to severe CHD with atrioventricular conduction disease [38]. Miyoshy described that fetal hydrops was associated with a 14-fold increased risk of perinatal death and myocardial dysfunction was a significant risk factor for poor prognosis.
Moreover, fetuses without CHD who develop hydrops, have a poor prognosis because of myocardial dysfunction, rather than the severity of bradyarrhythmia [39,40]. Same authors studied umbilical vein natriuretic peptide (UV NP) concentrations in fetuses with heart failure and found that UV NP levels are correlated with the severity of heart failure in fetuses with CHD and/or arrhythmia. Higher UV NP levels are found in fetuses with tachy- or brady-arrhythmia than in fetuses with CHD because wall stress induces an increased myocardial oxygen consumption and aggravates myocardial dysfunction. As a result, the end-diastolic filling pressure increases cardiac output and offset the reduction in ventricular compliance, resulting in more release of UV NP from the fetal heart. High UV NP levels may be associated with rapid progression to hydrops in fetuses with tachy- or brady-arrhythmia [41]. In a further recent study, Miyoshy demonstrated that UV NP levels reflect the severity of fetal arrhythmia and responses to fetal therapy, suggesting that it could be used as a biomarker to evaluate the effect of fetal therapy [42].
AVB associated with structural cardiac defects presents much earlier than that secondary to immunological causes, often before the fifteenth week of gestation. The most frequent cardiac malformations (Fig. 7) associated with severe bradyarrhythmia are: Left atrial isomerism (LAI) [43], congenital corrected transposition of the great arteries (CC-TGV) [44]; atrioventricular septal defect (AVSD), especially in the context of Trisomy 21 [45,46]; double outlet right ventricle (DORV) [47]; and, albeit more rarely, tetralogy of Fallot (TOF) [48]. Over the past decade, genetic findings have been shown to suggest that about 10% of sporadic CHD may have de novo mutations that significantly contribute to the disease process [49–51]. Mutations in genes encoding for transcription factors critical for cardiac chamber formation, endocardial cushion remodelling, conduction system development (i.e., Nkx2-5, Tbx5, and Id2), may lead to AVB associated with CHD [52,53].

Figure 7: A: CCTGA a normally positioned posterior AV node is present at the apex of the triangle of Koch, the posterior node in CCTGA is generally hypoplastic without a true connection to the AV bundle. The anterior node is located at the atrial aspect of the pulmonary-mitral continuity, connecting to a penetrating AV bundle. This bundle in its course around the pulmonary valve annulus may become extensively infiltrated by fibrous tissue and this is believed to explain the high rate of spontaneous AV block [47]. B: Right atrial isomerism with duplication of the AV node; an anterior and posterior AV node each give rise to a separate AV bundle that join together distally, forming a conduction sling [47]
It appears extremely rare, but already reported in the literature, as AVB may be secondary to the presence of rhabdomyomas [54].
Hereditary anomalies of the cardiac ion channel subunits or the proteins that regulate them can cause AVB and complex ventricular tachyarrhythmias during fetal life, with a very high risk of death in utero in the immediate neonatal period. The inherited arrhythmias may be suspected from a family or obstetrical history [55], supported by features of the current pregnancy including heart rhythm analysis of the fetus and confirmed by genetic testing. LQTS is the only channelopathy currently believed to be responsible for possible fetal AV conduction disturbances: This can result in AVB because the ventricular repolarization can be so prolonged that it activates the atrium before the ventricle fully repolarizes and not due to a disease of the system of conduction per se [6]. One speaks in this case of “functional AVB,” with atrio-ventricular ratio exclusively 2:1 or, rarely, 3:1 [56], and this tends to occur after 30 weeks’ gestation [57]. In about 70% of cases the “functional AVB,” if not associated with ventricular tachycardia and/or Torsade de Pointes, is usually well tolerated by the fetus without haemodynamic consequences [16,58–60].
However, albeit in limited case reports, the association between LQTS and complete AVB has been described as a likely expression of multiple genetic anomalies that impact the intraventricular and atrioventricular conduction system [61,62].
In Fig. 9, we report our “nightmare” case of a newborn affected by LQTS and complete AVB, with a fetal diagnosis of “bradycardia and non-sustained episodes of polymorphic ventricular tachycardia.”
Rarely, AVB of unknown origin appears during the fetal period, in absence of specific maternal autoantibodies, CHD, LQTS, or metabolic diseases, maternal medication exposures, or viral infections. Currently, the specialized literature is extremely poor in data regarding the incidence, etiology and clinical course of patients with “apparent” idiopathic AVB which, however, seems to have a better prognosis in the neonatal period, as recently described in a French multicentre study with long neonatal follow-up conducted on 141 children with isolated non-immune fetal AVB [63]. It appears that the cause of idiopathic AVB is genetic, and therefore involves the genes responsible for the conduction disorders previously described (see Paragraph 1.1).
Understand the underlying electrophysiological mechanism leading to fetal bradycardia is essential as management therapies depend on achieving a correct diagnosis (Tab. 3).
Table 3: Techniques to diagnose fetal bradycardia. Courtesy of the book Yagel S. (ed.), Silverman N. H. (ed.), Gembruch U. (ed.) (2019). Fetal Cardiology. Boca Raton: CRC Press [10]

Fetal heart rhythm can be evaluate by ultrasound using several techniques to the assessment, including 2D, M-mode, and pulsed Doppler imaging. Any of the techniques mentioned may be used to evaluate arrhythmia mechanism and should be included as part of the expanded fetal echocardiogram to assess the fetus with a suspected or documented arrhythmia. M-mode echocardiography was the first modality used to define arrhythmia mechanism by Wang in the 1964. M-mode imaging is one of the most frequently used ultrasound techniques in clinical practice; it records at the same time the atrial and ventricular contractions assessing AV relationship and rates [6].
Sinus bradycardia shows regular intervals between ventricular contractions (V-V interval) measured by M-mode or Doppler recordings through which accurate assessment of the pattern of atrial activity (regular or irregular A-A interval) and the temporal relationship between each atrial and ventricular activity (AV interval) can be performed. This analysis is defined as “the cornerstone” that allows correct diagnosis of any form of arrhythmia’s underlying electrophysiological mechanism. When the A-A intervals have little different lengths (Fig. 11), it needs to measure the A-A intervals over a period of time and the AV interval for each cardiac cycle. Five to 10 cardiac cycles are usually sufficient to evaluate the electrophysiological mechanism, but it is recommended to repeat various assessments to confirm diagnosis [2].
Pulsed Doppler (Figs. 4–12) recordings of simultaneous left ventricular inflow and outflow, superior vena cava and ascending aortic flow, or pulmonary artery and pulmonary venous flow permit documentation of the relationship between mechanical atrial and ventricular systole [6].

Figure 8: (A) Female newborn with antenatal study of fetal bradycardia without hemodynamic compromise, at fetal echocardiography an AVB 2:1 with prolongated phases was observable. After birth, LQTS type 8 diagnosis was established, also known as Timothy Syndrome Type 2 (p.Gly406Arg in exon 8 mutation associated with CACNA1C gen, which is associated with functional anomalies of calcium channels CAV 1.2); as well the presence of T-wave alternans were showed. Therefore, a partially functional AVB by marked prolongation of the QT interval was confirmed. (B) During neonatal period, secondary to a ventricular fibrillation episode; an epicardial ICD pace-maker was settled as shown in Chest X ray, the ICD was placed on the abdomen

Figure 9: (A) Representative electrocardiogram (ECG) from an infant with marked fetal bradycardia with finding of LQTS at birth associated to complete AVB with ventricular escape at FC 55–60 bpm (mutation “pR1135C” in the KCNH2 gene). Family history of AV conduction disorders at young age and father positive for the same genetic mutation. (B) On the second day of life, the newborn underwent to surgical implantation of a single chamber epicardial pacemaker placing the generator in the right pleural cavity and, subsequently, she began antiarrhythmic therapy with beta-blocker with optimal control of the anomalies of ventricular repolarization and ventricular arrhythmias. (C) ECG at birth showing subsequent episodes of non-sustained polymorphic ventricular tachycardia in the same patient

Figure 10: Progression of compensation for fetuses with complete AVB. HRV: Heart rate variability, TWA: T wave alternans

Figure 11: Non-immune congenital AVB
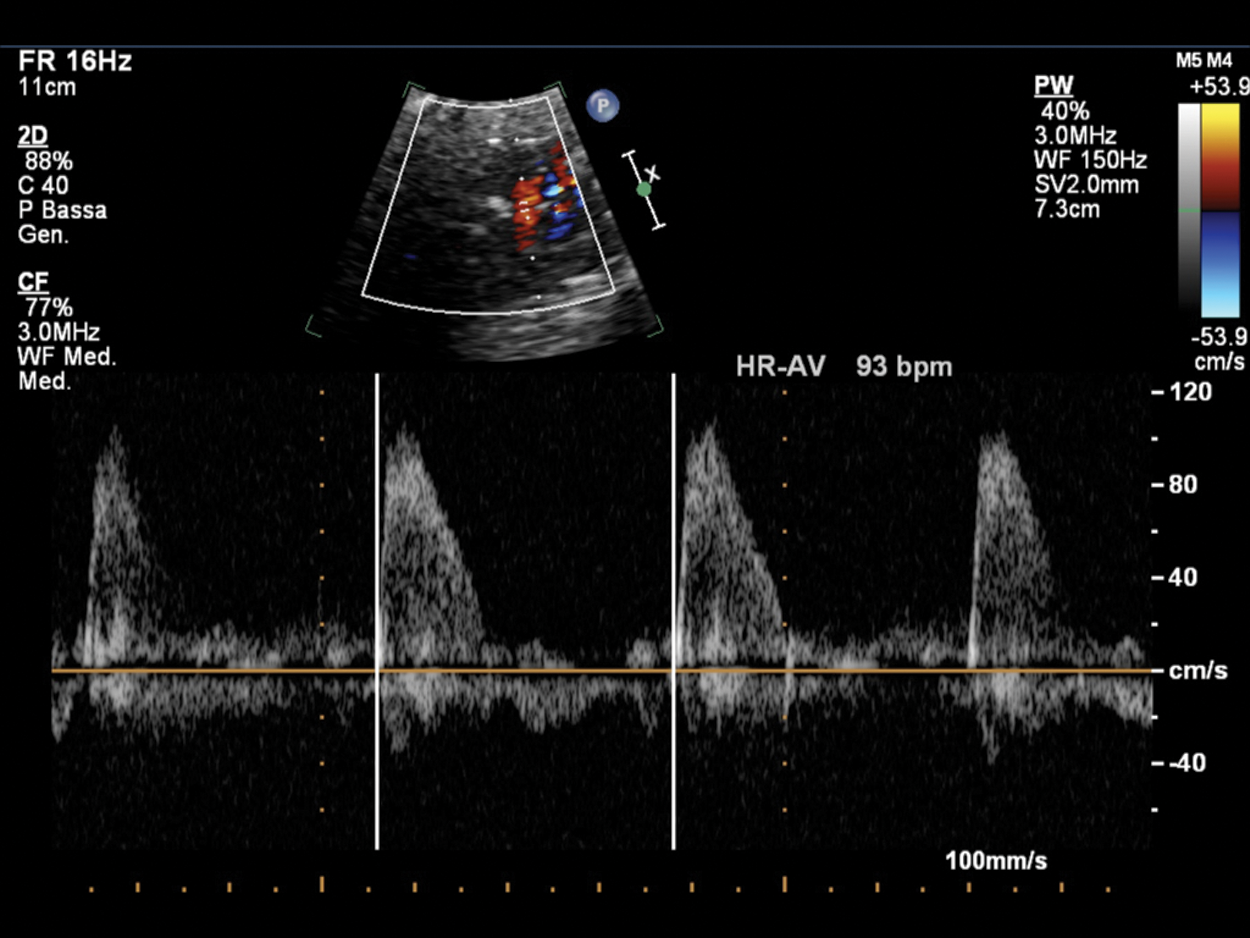
Figure 12: Fetal bradycardia (FHR 93 bpm) in a pregnant at 35 GA probably due to LQTS; the suspicion is related to the family history of a previous son who died at 2 months of life for LQTS and noncompaction myocardium. PW Doppler cursor placed through aortic valve. HR-AV: Heart rate—aortic valve
A simultaneous record of Doppler waveforms at the superior venous cava (SVC) and the ascending aorta (aAo) (Fig. 5B) was introduced as a useful method of assessing cardiac arrhythmias, beginning of reverse flow at the SVC created by atrial contraction and the beginning of forward flow at the aAo created by ventricular contraction corresponds to the P wave of the ECG. In contrast, during ventricular systole, the aortic or pulmonary flow Doppler ultrasound wave corresponds to the QRS complex of the ECG. At the start of the ventricular diastole, the valves are closed until the ventricle pressures drop below atrial pressure and consequently, the AV valves open and the ventricles fill passively, which corresponds to the E wave in the Doppler record of the AV valve flow. The pulmonary vein and the pulmonary artery, or the innominate vein and the aortic arch can also be used as alternative methods of assessing simultaneous venous and arterial waveforms [4,16].
Simple measurement of the time length of A wave may be another method to screen the prolongation of AV interval.
Fetal electrocardiography (fECG) can assess fetal cardiac waveform morphology, cardiac intervals, and repolarization characteristics, but doesn’t provide beat-to-beat analysis and the electric signals from the fetal heart are attenuated by amniotic fluid and fetal vernix [10]. Thus, it does not help diagnose fetal rhythm and conduction disorders with irregular heart rates [1].
Fetal cardiac magnetic resonance imaging (MRI) has been implemented over the last several years and its use is still not routinely widespread. Nevertheless, fetal cardiac MRI is reserved to assess complex fetal abnormalities since allow to detect congenital heart diseases and extracardiac malformations [64].
Fetal magnetocardiography (fMCG) is the magnetic analogous of fetal fECG, a non-invasive technique for recording the electrical activity of the fetal heart [65]. It is performed using external leads affixed to the maternal abdomen and detects magnetic fields caused by the external excitation of the fetal heart (see example in Fig. 13). These magnetic fields are plotted against time resulting in information equivalent to the surface ECG. Uses of fMCG have importance in the analysis of T waveforms and measurement of the QT interval. Although this technique is especially important for elucidating the mechanisms of tachyarrhythmias and identifying some high-risk conditions such as long-QT syndrome, it is still a very expensive method and not routinely available for clinical use [4,16].
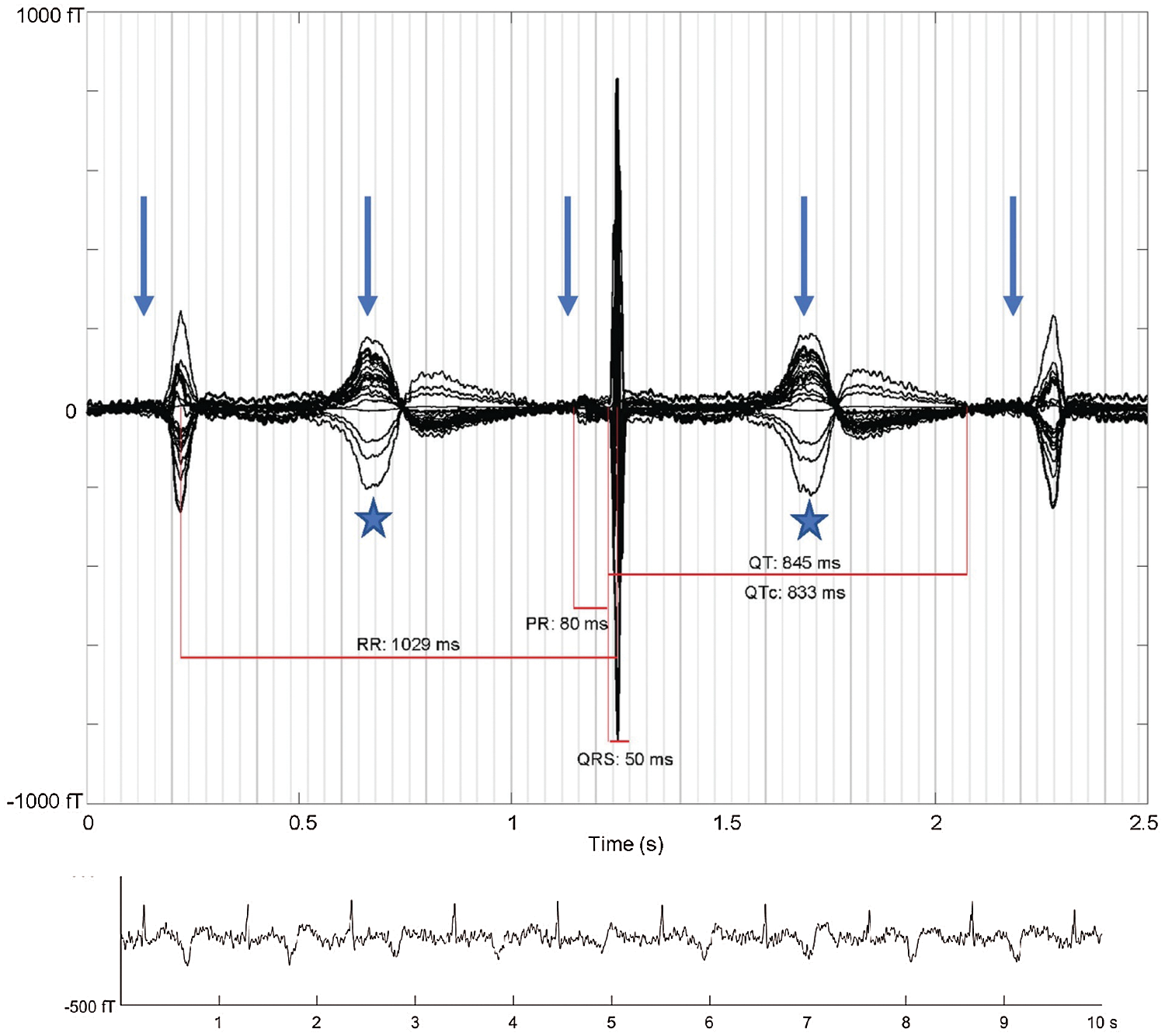
Figure 13: “Averaged fMCG waveform showing 2:1 atrioventricular block with three QRS complexes (arrows: P-waves, stars: T-waves). The averaging procedure time aligns the middle QRS complex, and due to heart rate variation, the other two QRS complexes are of reduced amplitude. The QTc is markedly prolonged with late-peaking T-wave morphology characteristic of fetal long QT syndrome. The rhythm tracing at the bottom of the figure shows variation in T-wave morphology during 2:1 atrioventricular block.” Courtesy of Desai, L., Wakai, R., Tsao, S., Strasburger, J., Gotteiner, N. & Patel, A. (2020). Fetal diagnosis of KCNQ1-variant long QT syndrome using fetal echocardiography and magnetocardiography. Pacing and Clinical Electrophysiology: PACE, 43(4), 430–433 [66]
It is demonstrated [67] that a LQTS prenatal diagnosis improves postnatal outcome because is the most common cause of arrhythmic death in fetuses and infants, and may identify family members at risk for SCD before symptoms. After LQTS diagnosis, the pregnant have to avoid QT prolonging drugs and to check maternal electrolytes levels (magnesium, vitamin D, calcium) to reduce risk of Torsade de Pointes/polymorphic ventricular arrhytmia to the LQTS fetus [68].
The fMCG uses a multichannel superconducting quantum interference device (SQUID) system, placed in a magnetically shielded room. By two-dimensional ultrasonography is defined fetal heart position, then fMCG is performed through a probe positioned on the maternal abdomen and is obtained simultaneously at nine points by directing the sensor array as close as possible to the centre of the fetal heart. Signal averaging is applied to more than 50 sinus beats to improve the signal-to-noise. Using the QRS complexes as triggers, averaged waveforms are computed during periods when the fetal heart rate was at baseline, during fetal quiescence (absence of fetal movement at the fMCG actography) and the heart rate is within 5 bpm of a stable minimum seen over the duration of the recording [69].
Fetal actograms, tracings of fetal activity derived from movement-related changes in signal amplitude, are derived from the instantaneous QRS amplitudes and examined to identify movement-related changes in fetal rhythm and FHR. Fetal QRS morphology is defined as narrow (<95th % for gestational age) or wide (>95th %) and Torsades de Pointes as paroxysms of a wide complex rhythm with a variable QRS morphology that exceeded the baseline ventricular rate. Since P-waves are not always visible on the tracings, a rhythm with a regular R-R interval, a narrow QRS, and a rate of 90–160 bpm was defined as sinus rhythm [70].
Cuneo et al. demonstrated that fMCG can successfully identify LQTS in utero using a threshold of QTc = 490 ms with high accuracy, sensitivity (89%) and specificity (89%) [69]. Moreover, fMCG was able to predict electrophysiological findings of fetal and neonatal risk of life-threatening ventricular arrhythmias; a markedly prolonged QTc (≥620 ms) was associated to high risk of perinatal torsades de pointes/polymorphic ventricular tachycardia. QTc assessment by fMCG has a diagnostic and prognostic value for identification of fetuses with LQTS, for prediction of a severe phenotype and planning of postnatal cares.
2.5 Treatment of Congenital Atrio-Ventricular Block
Treatment strategy of AVB depends on the cause (immune mediated, CHD, channelopathy) (Fig. 14), the presence of heart failure, the degree of heart block and the ventricular rate.

Figure 14: Flow-chart of fetal bradycardia diagnosis. Courtesy of the book Yagel S. (ed.), Silverman N.H. (ed.), Gembruch U. (ed.). (2019). Fetal Cardiology. Boca Raton: CRC Press [10]
About immune-mediated AVB, fetal AV block phenotype and natural history are variable, and there are no evidence-based guidelines to direct treatment strategies. The question is how prevent AV block progression avoiding the dilated cardiomyophathy and fetal hydrops [6,18]. AVB is common in congenital heart disease like CC-TGV, left atrial isomerism, AVSD and DORV, and the cause is an abnormal development of the AV node; treatment is the use of sympathomimetics for rate low 55 bpm. Channelopathies (including predominantly SSS and LQTS), can be associated to structural cardiac defects, dilated cardiomyopathy and conduction system diseases and led AV block, then it is necessary avoiding QT-prolonging drugs and observation for ventricular arrhythmias.
Miyoshi et al. discovered that maternal serum proinflammatory cytokines and apoptotic and angiogenic factors are potential candidates for predicting fetal heart failure in fetuses with CHDs or arrhythmias; in particular, maternal serum concentrations of tumor necrosis factor-a (TNF-a), vascular endothelial growth factor-D (VEGF-D), and heparin-binding epidermal growth factor-like growth factor (HB-EGF) were associated with fetal heart failure. Only fetuses with arrhythmias maternal serum soluble CD40 ligand concentrations were increased. Future studies are needed to verify if these maternal biomarkers can assess the severity of heart failure and the efficacy of fetal therapy [71].
Prophylactic therapies used are administration of transplacental fluorinates steroids, plasmapheresis, and intravenous immune globulin, but these therapies have not been successful in preventing AV block. Furthermore, prolonged steroid treatment has severe side effects on the fetus and on the mother. The only prophylactic treatment able to reduce recurrence of anti-Ro/SSA-mediated AV block is hydroxychloroquine (HCQ), by inhibiting the cellular immune response through the block of toll-like receptors. The results of PATCH-trial showed that HCQ used at dose of 200 mg twice a day, initiated in the first gestational age of pregnancy (before 10 weeks of gestation) in mothers anti-Ro/SSA-positive with a previously fetal AV block, reduced significantly the recurrence rate of AV block by more than one-half and should be considered for secondary prevention [72].
Nowadays the use of HCQ is widespread as prophylaxis and treatment of COVID-19 pandemia. In a PATCH sub-study was evaluated the potential fetal and neonatal cardiotoxity, evaluating neonatal ECGs and HCQ blood levels in the cord blood. Results demonstrated that the maternal use of HCQ is associated with low incidence of infant QTc prolongation [73].
β-sympathomimetics (terbutaline, salbutamol, isoprenaline) are used to augment fetal ventricular rates when <55 bpm or in fetuses with higher heart rates if there is underlying severe CHD or symptoms of fetal heart failure or hydrops. Terbutaline appears to be well tolerated; although may increase fetal heart rate and prolong pregnancy, no studies have shown survival benefit. The response to terbutaline differs on the basis of the origin of AVB; the atrial beat rate increases more than the ventricular beat rate in case of immune-mediated AVB rather than left atrial isomerism because in the latter the sinus node is absent and the subsidiary atrial pacemakers may not be capable of an increased beat rate [10].
Immune-mediated block, in spite of AVB due to congenital malformation of the conduction system, may be treated in utero with fluorinated steroids. Dexamethasone, a glucocorticoid agonist which crosses membranes, is only partially metabolized by placental enzyme (11β-hydroxysteroid dehydrogenase) with the reminder available to the fetus in an active form. It can be used for I–II block with findings of cardiac inflammation (echogenicity, valve regurgitation, cardiac dysfunction, effusion, etc) and may be considered to prevent progression to AVB, although its usefulness is not well established. In fetal AVB without signs of heart failure, dexamethasone may be used to improve survival and avoid dilated cardiomyopathy. Recent observations suggest a beneficial effect if dexamethasone and/or IVIG is started within 24 h from birth [74].
There is little agreement of improvement of AVB with treatment because data are retrospective and not randomized. Dosage of 4–8 mg/d may induce the reduction of inflammation, reversal or stabilization of incomplete block, and improvement or resolution of hydrops or endocardial fibroelastosis [6]. Complications of dexamethasone reported are growth restriction, oligohydramnios, hypoadrenalism, ductal constriction (conveyed also by the collagen vascular disease itself), maternal DM and hypertension, and central nervous system side effect [18]. Because of fetal and maternal side effects occur, steroid treatment may be considered only in recently developed AVB or compromised fetuses [22].
In our experience, we obtained good results using dexamethasone at a dosage of 4–8 mg/d with regression of AVB and without fetal or maternal complications.
Another option is intravenous infusion of γ-globulin (IVIG) that has been of benefit in immune-mediated and inflammatory diseases in pregnant woman and pediatric population. IVIG inhibits phagocytosis of IgG-opsonized blood cells (in the case of AV block predicted to be cardiocytes opsonized by anti-Ro independent of Ig-Fc sialylation). Usually are administered with dexamethasone to improve survival in presence of cardiac dysfunction. There is not a consensus about optimal timing of administration and intervals of repeat dosing, furthermore IVIG prophylaxis in early pregnancy is not recommended because of exposure to blood products and allergic reactions [6,18].
If corticosteroid treatment fail, hydrops does not regress and fetus is in a life-threatening condition, it could be considered the possibility of in utero pacing or ex utero intrapartum treatment to ventricular pacing, although are experimental and not recommended as part of usual care because of significant technical limitations [75,76].
Further randomized trials are indicated to establish treatment recommendations for the fetus with AVB. It is necessary a good maternal counseling before to start corticosteroid treatment because of significant maternal and fetal side effects.
In the last years several attempts have described both via open uterus procedures as well as by minimally invasive approaches. The first fetal pacing was implanted in 1986 by Carpentier who used a bipolar pigtail pacing catheter placed directly into RV via 19G needle. Another attempt to place pacing leads directly into the ventricle was tried in 2003 by Assad. Minimally invasive techniques were performed by Walkinshaw in 1994 placing a Teflon coated pacing lead through fetal umbilical vein and guided through IVC and RA into RV. All these procedures were successfully completed but fetuses demised after some hours for surgical complications [25].
Open fetal surgery for fetuses with complete AVB who were refractory to medical therapy is an extreme option. The first attempt of direct epicardial pacemaker implantation was performed in 2011 [77] using a unipolar pacemaker in a case of fetal hydrops and multi-organ failure. Pacing was successful for 5 days, but fetus demised from chronic multi-organ failure.
Recently a novel therapeutic strategy was lead on a 36-week fetus exposed via uterine incision and referred to ex utero intrapartum treatment (EXIT). Temporary epicardial pacing wires were sutured directly into the RV by a subxiphoid approach, then umbilical cord was clamped, and the patient was delivered [75,76].
These studies encourage to an early intervention strategy before a severe end-organ damage occurs, decreasing the risk of in utero demise. Percutaneous in utero pacing has been investigated as a method for providing heart rate support, however it appears to be a very high-risk procedure, with high fetal mortality.
New implantable micropacemaker are being developed and many studies are focus on a mininvasive approach to pace fetal patients with congenital AVB and reverse fetal hydrops. This new device could change outcome of congenital AVB and allow a successfully management in utero.
A potential implantable micropacemaker was developed by the group of Pruetz [25]. The size of micropacemaker is 3.48 mm in diameter and 2.4 cm in length and it could be recharged in utero until delivery via a transmitting coil placed over the mother’s abdomen. Other miniaturized pacemakers, known as “leadless” pacemakers, are generally used in adults and are delivered percutaneously through the femoral vein to the apex of the right ventricle.
Further studies are required to improve our understanding of the natural history of congenital AV block in order to identify more accurately the fetuses at the highest risk from not being paced. Moreover, development in the techniques and technologies available to deliver in utero pacing are required before this treatment can be adopted into routine clinical practice.
Although very rare, fetal bradyarrhythmias can represent a dramatic urgency even at risk of intrauterine or neonatal death. Fetal echocardiography is useful to the early detection of AVB and to the fetal heart rhythm monitoring. Maternal biomarkers can assess the severity of heart failure and the efficacy of fetal therapy, but further studies need to determine whether maternal serum biomarkers can predict fetal heart failure. Management of AVB may be challenging as few therapeutic options are available now. In the immune-mediated block steroid treatment may reduce inflammation and improve hydrops or endocardial fibroelastosis. IVIG are usually administered in association with corticosteroids to improve survival in presence of cardiac dysfunction. Hydroxychloroquine is effective to prevent the recurrence of AVB in anti-SSA/Ro exposed pregnancies. In fetus who are in a life-threatening condition, in utero pacing or ex utero intrapartum treatment could be considered rescue treatment although are not recommended as part of usual care because of significant technical limitations. Future perspective will consider new implantable micropacemaker in order to focus on a mininvasive approach to pace fetuses with congenital AVB and to treat fetal hydrops.
Funding Statement: The author(s) received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Yuan, S. M., Xu, Z. Y. (2020). Fetal arrhythmias: Prenatal evaluation and intrauterine therapeutics. Italian Journal of Pediatrics, 46(1), 21. DOI 10.1186/s13052-020-0785-9. [Google Scholar] [CrossRef]
2. Carvalho, J. S. (2014). Primary bradycardia: Keys and pitfalls in diagnosis. Ultrasound in Obstetrics & Gynecology, 44(2), 125–130. DOI 10.1002/uog.13451. [Google Scholar] [CrossRef]
3. ACOG Practice Bulletin No. 106 (2009). Intrapartum fetal heart rate monitoring: Nomenclature, interpretation, and general management principles. Obstetrics & Gynecology, 114(1), 192–202. DOI 10.1097/AOG.0b013e3181aef106. [Google Scholar] [CrossRef]
4. Bravo-Valenzuela, N. J., Rocha, L. A., Machado Nardozza, L. M., Araujo Júnior, E. (2018). Fetal cardiac arrhythmias: Current evidence. Annals of Pediatric Cardiology, 11(2), 148–163. DOI 10.4103/apc.APC_134_17. [Google Scholar] [CrossRef]
5. Ishikawa, T., Tsuji, Y., Makita, N. (2016). Inherited bradyarrhythmia: A diverse genetic background. Journal of Arrhythmia, 32(5), 352–358. DOI 10.1016/j.joa.2015.09.009. [Google Scholar] [CrossRef]
6. Donofrio, M. T., Moon-Grady, A. J., Hornberger, L. K., Copel, J. A., Sklansky, M. S. et al. (2014). Diagnosis and treatment of fetal cardiac disease: A scientific statement from the American Heart Association. Circulation, 129(21), 2183–2242. DOI 10.1161/01.cir.0000437597.44550.5d. [Google Scholar] [CrossRef]
7. Hornberger, L. K., Sahn, D. J. (2007). Rhythm abnormalities of the fetus. Heart, 93(10), 1294–1300. DOI 10.1136/hrt.2005.069369. [Google Scholar] [CrossRef]
8. Mitchell, J. L., Cuneo, B. F., Etheridge, S. P., Horigome, H., Weng, H. Y. et al. (2012). Fetal heart rate predictors of long QT syndrome. Circulation, 126(23), 2688–2695. DOI 10.1161/CIRCULATIONAHA.112.114132. [Google Scholar] [CrossRef]
9. Beinder, E., Grancay, T., Menéndez, T., Singer, H., Hofbeck, M. (2001). Fetal sinus bradycardia and the long QT syndrome. American Journal of Obstetrics and Gynecology, 185(3), 743–747. DOI 10.1067/mob.2001.117973. [Google Scholar] [CrossRef]
10. Yagel, S., Silverman, N. H., Gembruch, U. (2019). Fetal cardiology: embryology, genetics, physiology, echocardiographic evaluation, diagnosis, and perinatal management of cardiac diseases. Third edition. CRC Press. DOI 10.1201/9780429461118. [Google Scholar] [CrossRef]
11. Yanni, J., Boyett, M. R., Anderson, R. H., Dobrzynski, H. (2009). The extent of the specialized atrioventricular ring tissues. Heart Rhythm, 6(5), 672–680. DOI 10.1016/j.hrthm.2009.01.021. [Google Scholar] [CrossRef]
12. Gourdie, R. G., Harris, B. S., Bond, J., Justus, C., Hewett, K. W. et al. (2003). Development of the cardiac pacemaking and conduction system. Birth Defects Research. Part C, Embryo Today: Reviews, 69(1), 46–57. DOI 10.1002/bdrc.10008. [Google Scholar] [CrossRef]
13. Baruteau, A. E., Probst, V., Abriel, H. (2015). Inherited progressive cardiac conduction disorders. Current Opinion in Cardiology, 30(1), 33–39. DOI 10.1097/HCO.0000000000000134. [Google Scholar] [CrossRef]
14. Harris, B. S., Jay, P. Y., Rackley, M. S., Izumo, S., O’brien, T. X. et al. (2004). Transcriptional regulation of cardiac conduction system development: 2004 FASEB cardiac conduction system minimeeting. Washington, DC. Anatomical Record, 280A(2), 1036–1045. DOI 10.1002/ar.a.20101. [Google Scholar] [CrossRef]
15. Maitra, M., Schluterman, M. K., Nichols, H. A., Richardson, J. A., Cecilia, L. W. et al. (2009). Interaction of Gata4 and Gata6 with Tbx5 is critical for normal cardiac development. Developmental Biology, 326(2), 368–377. DOI 10.1016/j.ydbio.2008.11.004. [Google Scholar] [CrossRef]
16. Batra, A. S., Balaji, S. (2019). Fetal arrhythmias: Diagnosis and management. Indian Pacing and Electrophysiology Journal, 19(3), 104–109. DOI 10.1016/j.ipej.2019.02.007. [Google Scholar] [CrossRef]
17. Crotti, L., Tester, D. J., White, W. M., Bartos, D. C., Insolia, R. et al. (2013). Long QT syndrome-associated mutations in intrauterine fetal death. JAMA, 309(14), 1473–1482. DOI 10.1001/jama.2013.3219. [Google Scholar] [CrossRef]
18. Cuneo, B. F., Drose, J., Benson, D. W. (2021). Diagnosis and management of fetal arrhythmias. China: Wolters Kluwer. [Google Scholar]
19. Benson, D. W., Wang, D. W., Dyment, M., Knilans, T. K., Fish, F. A. et al. (2003). Congenital sick sinus syndrome caused by recessive mutations in the cardiac sodium channel gene (SCN5A). Journal of Clinical Investigation, 112(7), 1019–1028. DOI 10.1172/JCI200318062. [Google Scholar] [CrossRef]
20. Ishikawa, T., Ohno, S., Murakami, T., Yoshida, K., Mishima, H. et al. (2017). Sick sinus syndrome with HCN4 mutations shows early onset and frequent association with atrial fibrillation and left ventricular noncompaction. Heart Rhythm, 14(5), 717–724. DOI 10.1016/j.hrthm.2017.01.020. [Google Scholar] [CrossRef]
21. Wiggins, D. L., Strasburger, J. F., Gotteiner, N. L., Cuneo, B., Wakai, R. T. (2013). Magnetophysiologic and echocardiographic comparison of blocked atrial bigeminy and 2:1 atrioventricular block in the fetus. Heart Rhythm, 10(8), 1192–1198. DOI 10.1016/j.hrthm.2013.04.020. [Google Scholar] [CrossRef]
22. Carvalho, J. S. (2019). Fetal dysrhythmias. Best Practice & Research. Clinical Obstetrics & Gynaecology, 58(2), 28–41. DOI 10.1016/j.bpobgyn.2019.01.002. [Google Scholar] [CrossRef]
23. Brucato, A., Cimaz, R., Caporali, R., Ramoni, V., Buyon, J. (2011). Pregnancy outcomes in patients with autoimmune diseases and anti-Ro/SSA antibodies. Clinical Reviews in Allergy & Immunology, 40(1), 27–41. DOI 10.1007/s12016-009-8190-6. [Google Scholar] [CrossRef]
24. Brito-Zerón, P., Izmirly, P. M., Ramos-Casals, M., Buyon, J. P., Khamashta, M. A. (2015). The clinical spectrum of autoimmune congenital heart block. Nature Reviews Rheumatology, 11(5), 301–312. DOI 10.1038/nrrheum.2015.29. [Google Scholar] [CrossRef]
25. Pruetz, J. D., Miller, J. C., Loeb, G. E., Silka, M. J., Bar-Cohen, Y. et al. (2019). Prenatal diagnosis and management of congenital complete heart block. Birth Defects Research, 111(8), 380–388. DOI 10.1002/bdr2.1459. [Google Scholar] [CrossRef]
26. Rein, A. J., Mevorach, D., Perles, Z., Gavri, S., Nadjari, M. et al. (2009). Early diagnosis and treatment of atrioventricular block in the fetus exposed to maternal anti-SSA/Ro-SSB/La antibodies: A prospective, observational, fetal kinetocardiogram-based study. Circulation, 119(14), 1867–1872. DOI 10.1161/CIRCULATIONAHA.108.773143. [Google Scholar] [CrossRef]
27. Costedoat-Chalumeau, N., Georgin-Lavialle, S., Amoura, Z., Piette, J. C. (2016). Anti-SSA/Ro and anti-SSB/La antibody-mediated congenital heart block. Lupus, 14(9), 660–664. DOI 10.1191/0961203305lu2195oa. [Google Scholar] [CrossRef]
28. Brucato, A., Jonzon, A., Friedman, D., Allan, L. D., Vignati, G. et al. (2016). Proposal for a new definition of congenital complete atrioventricular block. Lupus, 12(6), 427–435. DOI 10.1191/0961203303lu408oa. [Google Scholar] [CrossRef]
29. Villain, V., Coastedoat-Chalumeau, E., Marijon, N., Boudjemline, E., Piette, Y. et al. (2006). Presentation and prognosis of complete atrioventricular block in childhood, according to maternal antibody status. Journal of the American College of Cardiology, 48(8), 1682–1687. DOI 10.1016/j.jacc.2006.07.034. [Google Scholar] [CrossRef]
30. Ambrosi, A., Wahren-Herlenius, M. (2012). Congenital heart block: Evidence for a pathogenic role of maternal autoantibodies. Arthritis Research & Therapy, 14(2), 208. DOI 10.1186/ar3787. [Google Scholar] [CrossRef]
31. Taylor, P. V., Scott, J. S., Gerlis, L. M., Esscher, E., Scott, O. (1986). Maternal antibodies against fetal cardiac antigens in congenital complete heart block. The New England Journal of Medicine, 315(11), 667–672. DOI 10.1056/NEJM198609113151103. [Google Scholar] [CrossRef]
32. Alvarez, D., Briassouli, P., Clancy, R. M., Zavadil, J., Reed, J. H. et al. (2011). A novel role of endothelin-1 in linking Toll-like receptor 7-mediated inflammation to fibrosis in congenital heart block. Journal of Biological Chemistry, 286(35), 30444–30454. DOI 10.1074/jbc.M111.263657. [Google Scholar] [CrossRef]
33. Clancy, R. M., Alvarez, D., Komissarova, E., Barrat, F. J., Swartz, J. et al. (2010). Ro60-associated single-stranded RNA links inflammation with fetal cardiac fibrosis via ligation of TLRs: a novel pathway to autoimmune-associated heart block. Journal of Immunology, 184(4), 2148–2155. DOI 10.4049/jimmunol.0902248. [Google Scholar] [CrossRef]
34. Izmirly, P. M., Halushka, M. K., Rosenberg, A. Z., Whelton, S., Rais-Bahrami, K. et al. (2017). Clinical and pathologic implications of extending the spectrum of maternal autoantibodies reactive with ribonucleoproteins associated with cutaneous and now cardiac neonatal lupus from SSA/Ro and SSB/La to U1RNP. Autoimmunity Reviews, 16(9), 980–983. DOI 10.1016/j.autrev.2017.07.013. [Google Scholar] [CrossRef]
35. Buyon, J. P., Hiebert, R., Copel, J., Craft, J., Friedman, D. et al. (1998). Autoimmune-associated congenital heart block: Demographics, mortality, morbidity and recurrence rates obtained from a national neonatal lupus registry. Journal of the American College of Cardiology, 31(7), 1658–1666. DOI 10.1016/S0735-1097(98)00161-2. [Google Scholar] [CrossRef]
36. Eliasson, H., Sonesson, S. E., Sharland, G., Granath, F., Simpson, J. M. et al. (2011). Isolated atrioventricular block in the fetus. Circulation, 124(18), 1919–1926. DOI 10.1161/CIRCULATIONAHA.111.041970. [Google Scholar] [CrossRef]
37. Jaeggi, E. T., Hamilton, R. M., Silverman, E. D., Zamora, S. A., Hornberger, L. K. (2002). Outcome of children with fetal, neonatal or childhood diagnosis of isolated congenital atrioventricular block. A single institution’s experience of 30 years. Journal of the American College of Cardiology, 39(1), 130–137. DOI 10.1016/S0735-1097(01)01697-7. [Google Scholar] [CrossRef]
38. Eronen, M., Sirèn, M. K., Ekblad, H., Tikanoja, T., Julkunen, H. et al. (2000). Short- and long-term outcome of children with congenital complete heart block diagnosed in utero or as a newborn. Pediatrics, 106(1), 86–91. DOI 10.1542/peds.106.1.86. [Google Scholar] [CrossRef]
39. Miyoshi, T., Maeno, Y., Sago, H., Inamura, N., Yasukouchi, S. et al. (2015). Fetal bradyarrhythmia associated with congenital heart defects–nationwide survey in Japan. Circulation Journal, 79(4), 854–861. DOI 10.1253/circj.CJ-14-0978. [Google Scholar] [CrossRef]
40. Miyoshi, T., Maeno, Y., Sago, H., Inamura, N., Yasukouchi, S. et al. (2015). Fetal bradyarrhythmia associated with congenital heart defects—nationwide survey in Japan. Circulation Journal, 79(4), 854–861. DOI 10.1253/circj.CJ-14-0978. [Google Scholar] [CrossRef]
41. Miyoshi, T., Umekawa, T., Hosoda, H., Asada, T., Fujiwara, A. et al. (2018). Plasma natriuretic peptide levels in fetuses with congenital heart defect and/or arrhythmia. Ultrasound in Obstetrics & Gynecology, 52(5), 609–616. DOI 10.1002/uog.18925. [Google Scholar] [CrossRef]
42. Miyoshi, T., Hosoda, H., Kurosaki, K. I., Shiraishi, I., Nakai, M. et al. (2019). Plasma natriuretic peptide levels reflect the status of the heart failure in fetuses with arrhythmia. Journal of Maternal-Fetal & Neonatal Medicine, 32, 1–7. Advance Online Publication. DOI 10.1080/14767058.2019.1651271. [Google Scholar] [CrossRef]
43. Ozawa, Y., Asakai, H., Shiraga, K., Shindo, T., Hirata, Y. et al. (2019). Cardiac rhythm disturbances in heterotaxy syndrome. Pediatric Cardiology, 40(5), 909–913. DOI 10.1007/s00246-019-02087-2. [Google Scholar] [CrossRef]
44. Baruteau, A. E., Abrams, D. J., Ho, S. Y., Thambo, J. B., McLeod, C. J. et al. (2017). Cardiac conduction system in congenitally corrected transposition of the great arteries and its clinical relevance. Journal of the American Heart Association, 6(12), 57. DOI 10.1161/JAHA.117.007759. [Google Scholar] [CrossRef]
45. Blom, N. A., Ottenkamp, J., Deruiter, M. C., Wenink, A. C., Gittenberger-de Groot, A. C. (2005). Development of the cardiac conduction system in atrioventricular septal defect in human trisomy 21. Pediatric Research, 58(3), 516–520. DOI 10.1203/01.PDR.0000179388.10921.44. [Google Scholar] [CrossRef]
46. di Mambro, C., Calvieri, C., Silvetti, M. S., Tamburri, I., Giannico, S. et al. (2018). Bradyarrhythmias in repaired atrioventricular septal defects: Single-center experience based on 34 years of follow-up of 522 patients. Pediatric Cardiology, 39(8), 1590–1597. DOI 10.1007/s00246-018-1934-4. [Google Scholar] [CrossRef]
47. Moore, J. P., Aboulhosn, J. A. (2017). Introduction to the congenital heart defects. Cardiac Electrophysiology Clinics, 9(2), 167–175. DOI 10.1016/j.ccep.2017.02.001. [Google Scholar] [CrossRef]
48. McElhinney, D. B., Geiger, E., Blinder, J., Benson, D. W., Goldmuntz, E. (2003). NKX2.5 mutations in patients with congenital heart disease. Journal of the American College of Cardiology, 42(9), 1650–1655. DOI 10.1016/j.jacc.2003.05.004. [Google Scholar] [CrossRef]
49. Zaidi, S., Choi, M., Wakimoto, H., Ma, L., Jiang, J. et al. (2013). De novo mutations in histone-modifying genes in congenital heart disease. Nature, 498(7453), 220–223. DOI 10.1038/nature12141. [Google Scholar] [CrossRef]
50. Baban, A., Olivini, N., Cantarutti, N., Calì, F., Vitello, C. et al. (2020). Differences in morbidity and mortality in Down syndrome are related to the type of congenital heart defect. American Journal of Medical Genetics Part A, 182(6), 1342–1350. DOI 10.1002/ajmg.a.61586. [Google Scholar] [CrossRef]
51. Bruneau, B. G. (2008). The developmental genetics of congenital heart disease. Nature, 451(7181), 943–948. DOI 10.1038/nature06801. [Google Scholar] [CrossRef]
52. McCulley, D. J., Black, B. L. (2012). Transcription factor pathways and congenital heart disease. Current Topics in Developmental Biology, 100, 253–277. [Google Scholar]
53. Moskowitz, I. P. G., Kim, J. B., Moore, M. L., Wolf, C. M., Peterson, M. A. et al. (2007). A molecular pathway including Id2, Tbx5, and Nkx2-5 required for cardiac conduction system development. Cell, 129(7), 1365–1376. DOI 10.1016/j.cell.2007.04.036. [Google Scholar] [CrossRef]
54. Wacker-Gussmann, A., Strasburger, J. F., Cuneo, B. F., Wiggins, D. L., Gotteiner, N. L. et al. (2014). Fetal arrhythmias associated with cardiac rhabdomyomas. Heart Rhythm, 11(4), 677–683. DOI 10.1016/j.hrthm.2013.12.018. [Google Scholar] [CrossRef]
55. Cuneo, B. F., Kaizer, A. M., Clur, S. A., Swan, H., Herberg, U. et al. (2020). Mothers with long QT syndrome are at increased risk for fetal death: Findings from a multicenter international study. American Journal of Obstetrics and Gynecology, 222(3), 263.e1–263.e11. DOI 10.1016/j.ajog.2019.09.004. [Google Scholar] [CrossRef]
56. Aziz, P. F., Tanel, R. E., Zelster, I. J., Pass, R. H., Wieand, T. S. et al. (2010). Congenital long QT syndrome and 2:1 atrioventricular block: an optimistic outcome in the current era. Heart Rhythm, 7(6), 781–785. DOI 10.1016/j.hrthm.2010.02.035. [Google Scholar] [CrossRef]
57. Horigome, H., Nagashima, M., Sumitomo, N., Yoshinaga, M., Ushinohama, H. et al. (2010). Clinical characteristics and genetic background of congenital long-QT syndrome diagnosed in fetal, neonatal, and infantile life: A nationwide questionnaire survey in Japan. Circulation, 3(1), 10–17. [Google Scholar]
58. Drago, F., Vignati, G., Bloise, R., Bronzetti, G., Cantù, F. et al. (2012). Diagnosi, trattamento e follow-up delle aritmie in età neonatale e fetale. Area di Aritmologia Pediatrica dell’Associazione Italiana di Aritmologia e Cardiostimolazione (AIAC). Documento congiunto AIAC–SICP. GIAC, 15(3), 173–185. [Google Scholar]
59. Cimaz, R., Stramba-Badiale, M., Brucato, A., Catelli, L., Panzeri, P. et al. (2000). QT interval prolongation in asymptomatic anti-SSA/Ro positive infants without congenital heart block. Arthritis and Rheumatism, 43(5), 1049–1053. DOI 10.1002/1529-0131(200005)43:5<1049::AID-ANR13>3.0.CO;2-X. [Google Scholar] [CrossRef]
60. Lazzerini, P. E., Acampa, M., Guideri, F., Capecchi, P. L., Campanella, V. et al. (2004). Prolongation of the corrected QT interval in adult patients with anti-Ro/SSA-positive connective tissue diseases. Arthritis & Rheumatism, 50(4), 1248–1252. DOI 10.1002/art.20130. [Google Scholar] [CrossRef]
61. Koçak, G., Atalay, S., Tutar, E., Imamoğlu, A., Uysalel, A. et al. (1998). Congenital complete atrioventricular block in an infant with long QT syndrome. Acta Cardiologica, 53(3), 153–155. [Google Scholar]
62. Ellesøe, S. G., Reimers, J. I., Andersen, H. Ø. (2014). Normalisation of left ventricular systolic function after change from VVI pacing to biventricular pacing in a child with congenital complete atrioventricular block, long-QT syndrome, and congenital muscular dystrophy: A 10-year follow-up. Cardiology in the Young, 24(3), 520–523. DOI 10.1017/S1047951113000541. [Google Scholar] [CrossRef]
63. Baruteau, A. E., Fouchard, S., Behaghel, A., Mabo, P., Villain, E. et al. (2012). Characteristics and long-term outcome of non-immune isolated atrioventricular block diagnosed in utero or early childhood: A multicentre study. European Heart Journal, 33(5), 622–629. DOI 10.1093/eurheartj/ehr347. [Google Scholar] [CrossRef]
64. Wielandner, A., Mlczoch, E., Prayer, D., Berger-Kulemann, V. (2013). Potential of magnetic resonance for imaging the fetal heart. Seminars in Fetal and Neonatal Medicine, 18(5), 286–297. DOI 10.1016/j.siny.2013.05.006. [Google Scholar] [CrossRef]
65. Strand, S. A., Strasburger, J. F., Wakai, R. T. (2019). Fetal magnetocardiogram waveform characteristics. Physiological Measurement, 40(3), 035002. DOI 10.1088/1361-6579/ab0a2c. [Google Scholar] [CrossRef]
66. Desai, L., Wakai, R., Tsao, S., Strasburger, J., Gotteiner, N. et al. (2020). Fetal diagnosis of KCNQ1-variant long QT syndrome using fetal echocardiography and magnetocardiography. Pacing and Clinical Electrophysiology, 43(4), 430–433. DOI 10.1111/pace.13900. [Google Scholar] [CrossRef]
67. Greene, E. A., Berul, C. I., Donofrio, M. T. (2013). Prenatal diagnosis of long QT syndrome: Implications for delivery room and neonatal management. Cardiology in the Young, 23(1), 141–145. DOI 10.1017/S1047951112000583. [Google Scholar] [CrossRef]
68. Cuneo, B. F., Strasburger, J. F., Wakai, R. T. (2016). The natural history of fetal long QT syndrome. Journal of Electrocardiology, 49(6), 807–813. DOI 10.1016/j.jelectrocard.2016.07.023. [Google Scholar] [CrossRef]
69. Strand, S. A., Strasburger, J. F., Wakai, R. T. (2019). Fetal magnetocardiogram waveform characteristics. Physiological Measurement, 40(3), 035002. DOI 10.1088/1361-6579/ab0a2c. [Google Scholar] [CrossRef]
70. Cuneo, B. F., Strasburger, J. F., Yu, S., Horigome, H., Hosono, T. et al. (2013). In utero diagnosis of long QT syndrome by magnetocardiography. Circulation, 128(20), 2183–2191. DOI 10.1161/CIRCULATIONAHA.113.004840. [Google Scholar] [CrossRef]
71. Miyoshi, T., Hosoda, H., Nakai, M., Nishimura, K., Miyazato, M. (2019). Maternal biomarkers for fetal heart failure in fetuses with congenital heart defects or arrhythmias. American Journal of Obstetrics and Gynecology, 220(1), 104.e1–104.e15. DOI 10.1016/j.ajog.2018.09.024. [Google Scholar] [CrossRef]
72. Izmirly, P., Kim, M., Friedman, D. M., Costedoat-Chalumeau, N., Clancy, R. et al. (2020). Hydroxychloroquine to prevent recurrent congenital heart block in fetuses of anti-SSA/Ro-positive mothers. Journal of the American College of Cardiology, 76(3), 292–302. DOI 10.1016/j.jacc.2020.05.045. [Google Scholar] [CrossRef]
73. Friedman, D. M., Kim, M., Costedoat-Chalumeau, N., Clancy, R., Copel, J. et al. (2020). Electrocardiographic QT intervals in infants exposed to hydroxychloroquine throughout gestation. Circulation: Arrhythmia and Electrophysiology, 13(10), 215. DOI 10.1161/CIRCEP.120.008686. [Google Scholar] [CrossRef]
74. Cuneo, B. F., Ambrose, S. E., Tworetzky, W. (2016). Detection and successful treatment of emergent anti-SSA–mediated fetal atrioventricular block. American Journal of Obstetrics and Gynecology, 215(4), 527–528. DOI 10.1016/j.ajog.2016.07.002. [Google Scholar] [CrossRef]
75. Cuneo, B. F., Mitchell, M. B., Marwan, A. I., Green, M., von Alvensleben, J. C. et al. (2017). Ex utero intrapartum treatment to ventricular pacing: A novel delivery strategy for complete atrioventricular block with severe bradycardia. Fetal Diagnosis and Therapy, 42(4), 311–314. DOI 10.1159/000475815. [Google Scholar] [CrossRef]
76. Walkinshaw, S. A., Welch, C. R., McCormack, J., Walsh, K. (1994). In utero pacing for fetal congenital heart block. Fetal Diagnosis and Therapy, 9(3), 183–185. DOI 10.1159/000263929. [Google Scholar] [CrossRef]
77. Eghtesady, P., Michelfelder, E. C., Knilans, T. K., Witte, D. P., Manning, P. B. et al. (2011). Fetal surgical management of congenital heart block in a hydropic fetus: Lessons learned from a clinical experience. Journal of Thoracic and Cardiovascular Surgery, 141(3), 835–837. DOI 10.1016/j.jtcvs.2010.06.048. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |