

 | Congenital Heart Disease |  |
DOI: 10.32604/CHD.2021.015237
ARTICLE
Six-Year Outcome after Valve Replacement and Resynchronization Therapy in TGA Patient
1Department of Cardiovascular Diseases, Clinical Hospital Centre Zagreb, Zagreb, 10000, Croatia
2Department of Cardiac Surgery, Clinical Hospital Centre Zagreb, Zagreb, 10000, Croatia
*Corresponding Author: Marija Brestovac. Email: marija.brestovac@gmail.com
Received: 03 December 2020; Accepted: 01 March 2021
Abstract: Patients with complete transposition of the great arteries (TGA) treated by the Senning procedure have a higher risk of developing heart failure due to: a) additional work load of the systemic (morphologic right) ventricle (sRV), b) arrhythmias, mainly caused by surgical implications at the atria as well as c) worsening of systemic tricuspid regurgitation. We present a unique case of a female patient who developed all these complications, who was successfully treated and was able to carry out a twin pregnancy. This breakthrough approach was based on: 1. detecting reversibility potential of myocardial systolic dysfunction in a severe valvular lesion combined with continuous systemic afterload settings and permanent tachyarrhythmia, and 2. prevention of subsequently iatrogenic worsening of systemic ventricular function due to permanent pacing. Surgical replacement of systemic tricuspid valve (sTV) and cardiac resynchronization device (CRT) implantation after nodal ablation resulted in recovering of the systolic function and a positive remodeling of the sRV. The reversal of a further decline in systolic function was achieved by permanent arrhythmia control, synchronous pacing with epicardial leads of CRT, sTV replacement as well as echocardiographic monitoring during pregnancy to determine the right time for delivery. Two years after delivery, the patient remains in NYHA Class I.
Keywords: Transposition of the great arteries; Cardiac resynchronization therapy; Pregnancy in TGA patients
Congenitally corrected TGA (CCTGA) is characterized by atrioventricular (AV) and ventriculoarterial (VA) discordance, and is compatible with life. In the past, the Mustard and Senning procedures were mostly used, while nowadays arterial switch and the Rastelli procedures are used [1–3]. Increased loading conditions may cause systemic right ventricle dysfunction (up to 40%) as well as systemic tricuspid regurgitation (sTR) (10%–40%) [4]. Due to scarring after surgical procedures, consequent arrhythmias are often resistant to electrophysiological focus ablation methods [5,6]. AV nodal ablation and permanent pacing may induce iatrogenic intraventricular dyssynchronouos contractions and heart failure in such patients with a usually pre-existing right bundle branch block (RBBB) [7,8]. Postponing surgical tricuspid valve repair/replacement may lead to irreversible failure of the systemic ventricle due to chronic volume overload on top of systemic pressure load [9]. The potential of reversibility is difficult to evaluate, adding the fact that significant sTR may mask the decrease of ejection fraction (EF) [10]. Pregnancy in women with TGA leads to worsening of sTR and a higher rate of cardiac complications as well as obstetric complications and low birth weight [11–13]. We present a very successful outcome of a complicated twin pregnancy in a TGA patient, who initially presented with several complications.
We present a case of a 33-year-old female patient (written informed consent was signed by the patient) with TGA, treated with septostomy by Rashkind a few days after her birth and by the Senning procedure at the age of nine months. She was presented in our clinic with palpitations and dyspnea in 2011, at the age of 26. At that moment she was hemodynamically stabile, tachycardic 106/min with a systolic heart murmur (3/6) and no clinical signs of congestion. Auscultation of the lung revealed no signs of congestion and no peripheral edema was present. Auscultation of the lung revealed no signs of congestion and no peripheral edema was present. Monitoring electrocardiogram (ECG) showed a continuous atrial undulation (UA) and wide QRS, so antiarrhythmic therapy with bisoprolol and amiodarone was started. Beside the arrhythmia, echocardiographic examination (ECHO) revealed a dilated sRV with end-diastolic volume (EDV RV) of 220 ml (126 ml/m2), moderate sTR due to combined functional regurgitation with primary valve lesion-prolapse of posterior leaflet, and reduced EF of sRV 42%. Due to drug- and electrocardioversion resistant arrhythmia a successful radiofrequency ablation of the re-entry tachycardia was performed in December 2012. During the next four months of follow up the patient was clinically compensated, in New York Heart Association (NYHA) I functional status, with no change in effort tolerance. Control ECG monitoring in March 2013 revealed recurrence of UA while control ECHO verified a worsening of sTR (regurgitation volume > 50% of the stroke volume) to severe regurgitation and moderate reduction of sRV systolic function. Pulmonary artery pressure was not elevated. Due to a resistant and long-lasting UA, systolic function of sRV progressively declined implicating a component of tachycardia induced cardiomyopathy, followed by an increased regurgitation volume of sTR (Figs. 1a–1c). Gadolinium scan (magnetic resonance) of sRV showed a dilated systemic right atrium, dilated and hypertrophied sRV (EDV 161 ml, 92 ml/m2, end-systolic volume (ESV) 83 ml, stroke volume (SV) 78 ml, EF 48%), thin left ventricle (LV) walls (EDV 76 ml, ESV 30 ml, SV 46 ml, EF 60%), no regional contractility abnormalities and no signs of replacement fibrosis. Coronary stenosis was ruled out by coronary angiography. Native ECG showed RBBB with QRS width 140 ms. The patient was scheduled for sTV replacement and AV node ablation to be followed by implantation of pacemaker. During the surgery, a biological TV prothesis (pTV) (Edwards Lifesciences C-E Perimount Magma Mitral 33 mm) was implanted and AV node ablation was performed. In order to prevent mechanical iatrogenic dysynchrony of permanent pacing, a CRT with biventricular pacing was implanted during surgery, placing epicardial bipolar leads (Medtronic Model 4968, atrial lead on sRV and ventricular lead on LV in DVIR pacing mode, with AV delay of 30 ms). The optimization of lead placement was guided using transesophageal echocardiography in order to find the most favorable stimulation sites on the right and left ventricular walls that would achieve the most synchronous sRV contraction. An echocardiographic control (April 2013) revealed a positive remodelling of sRV based on reduction of EDV RV to 60 ml, an increase in EF RV 60% and synchronous myocardial contractions (Figs. 2a–2c) and QRS 130 ms. The CRT memory follow up data showed no arrhythmias, so betablockers could be discontinued and the risks during pregnancy decreased to an acceptable level. Nineteen months later an ovarium dermoid cyst removal and correction of septate uterus was performed. Three years after the surgery, the patient was in NYHA Class I, with normal sRV size and function and synchronous myocardial contractions (Fig. 2). The underlying atrial rhythm was UA. The patient was diagnosed with hereditary thrombophilia. Despite increased risk of cardiovascular events, our patient had a strong motivation and an increasing wish to become a mother that we approved. After several attempts of unsuccessful in vitro fertilization procedures, a twin pregnancy was achieved. In the 30th week of gestation, in July 2017, no clinical signs of heart failure (HF) were present but ECHO revealed a slight dilatation and worsening od EF of sRV and sTR (EDV 140 ml, ESV 70 ml, EF 45%). Despite the suggested restriction of physical exertion and bed rest in the following gestational weeks, an excessive volume overload and further dilatation of sRV were noticed.
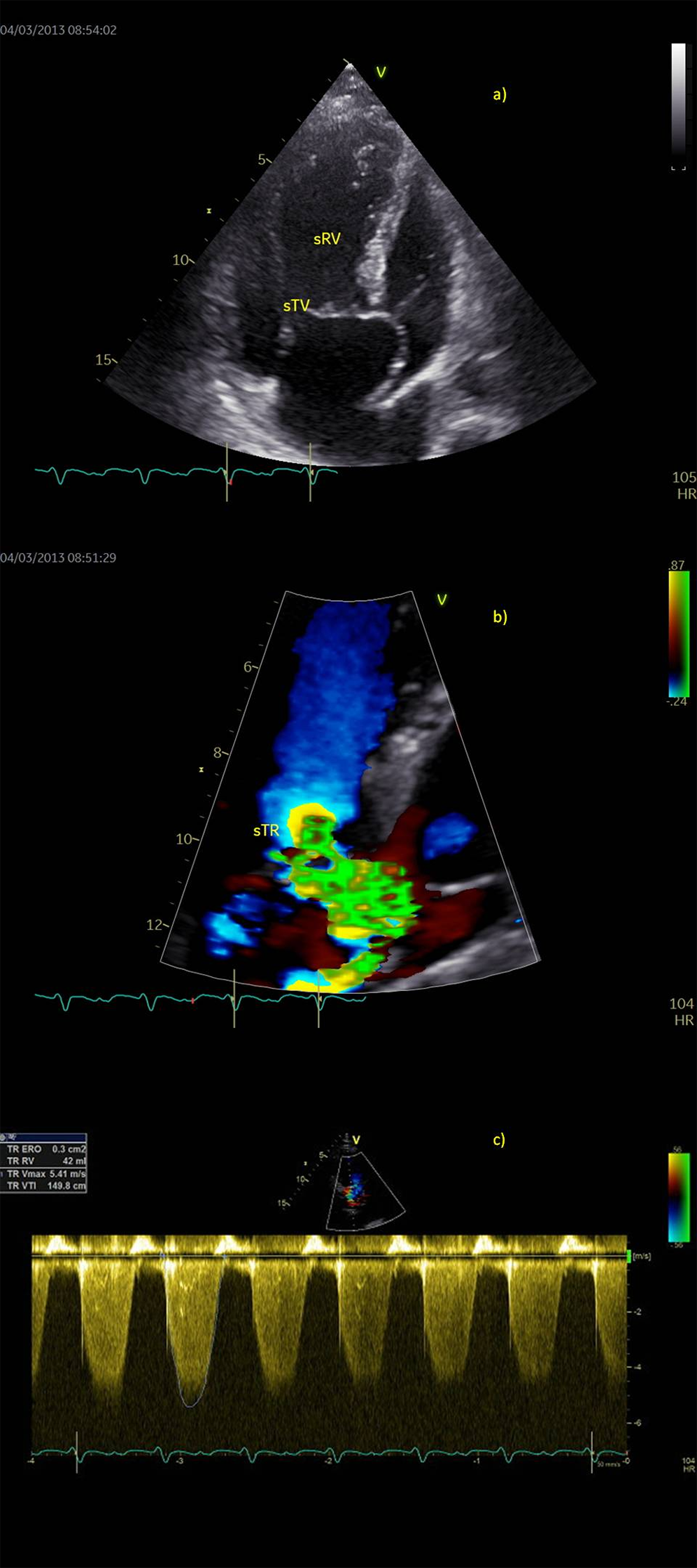
Figure 1: Preoperative echocardiography in a patient with failing systemic right ventricle (sRV), a) Apical four-chamber view, b) Color flow of severe tricuspid regurgitation (sTR), PISA 10 mm, regurgitant volume 42 ml. c) Holosystolic and dense continuous Doppler trace of tricuspid regurgitation flow (sTR VTI 150 cm)
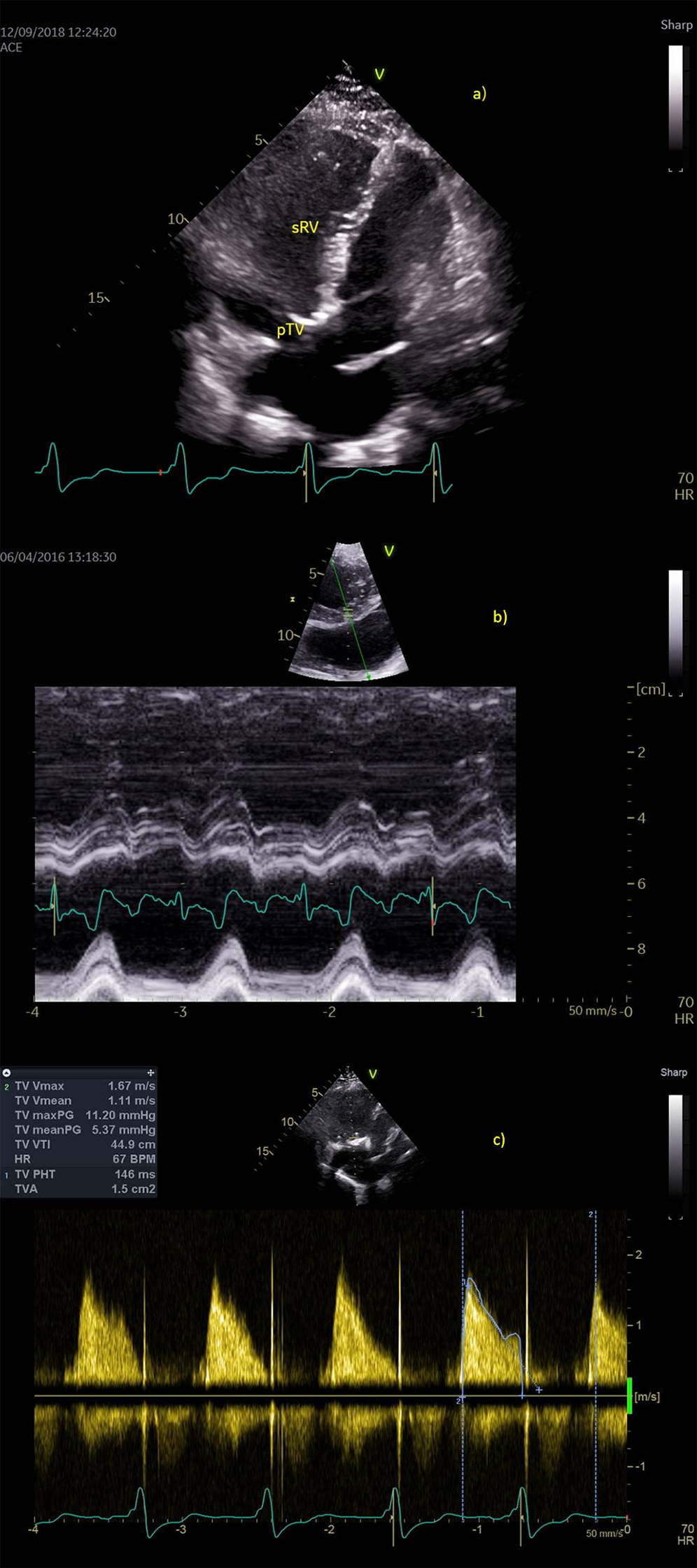
Figure 2: Postoperative echocardiography. a) Apical four-chamber view shows complete positive remodeling of systemic right ventricle (sRV), b) Synchronous contraction of the sRV walls (anatomic M mode of the short axis view, arrow points to systolic contraction of sRV) c) normal continuous Doppler trace across biological prosthesis at tricuspid place (pTV)
In order to prevent a possible irreversible stretching of the biological valve ring and myocardial dysfunction progression, we suggested that the pregnancy should be finished. In the 35th gestational week, a caesarean section was performed, and our patient gave birth to two healthy babies (04.09.2017, birth weights 2,370 and 2,320 g). Postpartum ECHO controls (in the 2nd and 4th post-partum weeks) showed a reversal in the sRV function and dimension of sRV (EDV 100 ml, ESV 70 ml, EF 50%). Due to UA and hereditary thrombophilia, we recommended to continue anticoagulant therapy with warfarin. The patient underwent regular CRT and ECHO checkups. Two years after delivery the patient is clinically stabile, in NYHA Class I.
Patients with TGA often develop complications like systemic RV failure, resistant arrhythmias and sTR. Despite all these complications combined in our young female TGA patient, we established a successful individual treatment approach based on the understanding of the pathophysiological process of the sRV function. The crucial first step was the diagnosis of the clinically relevant valve lesion, particularly to unmask severe sTR and its adverse effect followed by tachyarrhythmia. Both unfavorable effects, i.e., volume load as well as energy demanding long lasting rapid tachyarrhythmia induce reversible heart failure [14] in our patient, whose clinical course was previously uneventful. A continuous increased afterload of sRV in TGA patients induces remodeling and hypertrophy of the morphological right myocardium to compensate for the high wall stress. Unfortunately, such remodeled ventricles in tachyarrhythmia settings are not capable to compensate for the increased volume state without a rise in the pulmonary pressure and occurrence of heart failure signs. The increase in ventricular dilatation induces functional component on top of the primary regurgitation lesion as it was revealed in our case [15]. Previous surgical procedure followed by atrium scarring as well as volume-induced stretching of the right atrium induced persistent atrial undulation in our patient as well as a relapse of arrhythmia after radiofrequency atrial undulation ablation that caused arrhythmia induced cardiomyopathy. Once the whole pathophysiological process was understood, the best treatment and interventions option were planned. Consistent with that, once we revealed severe sTR as the major reversible unfavorable cause, a surgical treatment–sTV replacement was recommended. Recent data showed that in order to achieve positive remodeling and sRV function recovery, regurgitant valvular lesions in TGA patient should be diagnosed and treated on time [16]. However, our concern was directed towards unphysiological standard permanent pacing after AV nodal ablation, which was necessary to treat arrhythmia [8]. Namely, the reversibility potential of sRV systolic dysfunction after sTV replacement might have been compromised with iatrogenic dyssynchrony induced by one lead permanent epicardial pacing that was shown during lead testing in the operating room. The only option to overcome this unfavorable cause was to resynchronize sRV contraction by two epicardial leads, whose implantation was echocardiographycally guided, to achieve synchronous pacing [17,18].
As our patient wanted to become a mother, despite the increased risk of cardiovascular events in such patients and hereditary thrombophilia, a twin pregnancy was achieved. In a close cardiologist and ECHO monitoring during the pregnancy, sRV size and function were in our focus. Systemic right ventricle was under double physiological volume load, which increased during the second trimester when we revealed a slight progression of the sRV size, the final exaggerated dilatation and a decrease in the systolic sRV function, which was detected at the 35th gestational week. This was the marker of unmatched load and the potential hazard point, so we recommended delivery. Regular postpartum examinations revealed continuous clinical and ECHO improvement.
In this case report we described a very successful outcome of a complicated twin pregnancy in a TGA patient, who initially presented with several complications. Clinical decisions for treatment and prevention of further clinical decline were made, related to the entire individual pathway of heart failure. Decision for surgical replacement of systemic valve and cardiac resynchronization pacing resulted in a positive remodeling of the right ventricle and systolic function recovery. Reversal of a further decline in systole function was achieved by permanent arrhythmia control, ECHO guided implantation of epicardial CRT, tricuspid valve replacement and decision about the timing of the patient’s delivery.
This case could improve decision making in medical practice in such patient population.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Brawn, W. J., Barron, D. J. (2003). Technical aspects of the Rastelli and atrial switch procedure for congenitally corrected transposition of the great arteries with ventricular septal defect and pulmonary stenosis or atresia: Results of therapy. Seminars in Thoracic and Cardiovascular Surgery. Pediatric Cardiac Surgery Annual, 6(1), 4–8. DOI 10.1016/S1092-9126(03)70003-9. [Google Scholar] [CrossRef]
2. Devaney, E. J., Ohye, R. G., Bove, E. L. (2003). Technical aspects of the combined arterial switch and senning operation for congenitally corrected transposition of the great arteries. Seminars in Thoracic and Cardiovascular Surgery. Pediatric Cardiac Surgery Annual, 6(1), 9–15. DOI 10.1016/S1092-9126(03)70004-0. [Google Scholar] [CrossRef]
3. Konstantinov, I., Williams, W. (2003). Atrial switch and rastelli operation for congenitally corrected transposition with ventricular septal defect and pulmonary stenosis. Journal of Thoracic and Cardiovascular Surgery, 8(3), 160–166. [Google Scholar]
4. Chow, P. C., Liang, X. C., Lam, W. W., Cheung, E. W., Wong, K. T. et al. (2008). Mechanical right ventricular dyssynchrony in patients after atrial switch operation for transposition of the great arteries. American Journal of Cardiology, 101(6), 874–881. DOI 10.1016/j.amjcard.2007.11.033. [Google Scholar] [CrossRef]
5. Kanter, R. J., Papagiannis, J., Carboni, M. P., Ungerleider, R. M., Sanders, W. E. et al. (2000). Radiofrequency catheter ablation of supraventricular tachycardia substrates after mustard and senning operations for d-transposition of the great arteries. Journal of the American College of Cardiology, 35(2), 428–441. DOI 10.1016/S0735-1097(99)00557-4. [Google Scholar] [CrossRef]
6. Lobo, R. G., Griffith, M., de Bono, J. (2014). Ablation of arrhythmias in patients with adult congenital heart disease. Arrhythmia & Electrophysiology Review, 3(1), 36–39. DOI 10.15420/aer.2011.3.1.1. [Google Scholar] [CrossRef]
7. Mah, D. Y., Alexander, M. E., Banka, P., Abrams, D. J., Triedman, J. K. et al. (2013). The role of cardiac resynchronization therapy for arterial switch operations complicated by complete heart block. Annals of Thoracic Surgery, 96(3), 904–909. DOI 10.1016/j.athoracsur.2013.05.082. [Google Scholar] [CrossRef]
8. Nothroff, J., Norozi, K., Alpers, V., Arnhold, J. O., Wessel, A. et al. (2006). Pacemaker implantation as a risk factor for heart failure in young adults with congenital heart disease. Pacing and Clinical Electrophysiology: PACE, 29(4), 386–392. DOI 10.1111/j.1540-8159.2006.00358.x. [Google Scholar] [CrossRef]
9. Koolbergen, D. R., Ahmed, Y., Bouma, B. J., Scherptong, R. W., Bruggemans, E. F. et al. (2016). Follow-up after tricuspid valve surgery in adult patients with systemic right ventricles. European Journal of Cardio-Thoracic Surgery: Official Journal of the European Association for Cardio-thoracic Surgery, 50(3), 456–463. DOI 10.1093/ejcts/ezw059. [Google Scholar] [CrossRef]
10. Talwar, S., Ahmed, T., Saxena, A., Kothari, S. S., Juneja, R. et al. (2013). Morphology, surgical techniques, and outcomes in patients above 15 years undergoing surgery for congenitally corrected transposition of great arteries. World Journal for Pediatric and Congenital Heart Surgery, 4(3), 271–277. DOI 10.1177/2150135113476717. [Google Scholar] [CrossRef]
11. Cataldo, S., Doohan, M., Rice, K., Trinder, J., Stuart, A. G. et al. (2016). Pregnancy following Mustard or Senning correction of transposition of the great arteries: a retrospective study. BJOG: An International Journal of Obstetrics & Gynaecology, 123(5), 807–813. DOI 10.1111/1471-0528.13508. [Google Scholar] [CrossRef]
12. Chow, P. C., Liang, X. C., Lam, W. W., Cheung, E. W., Wong, K. T. et al. (2008). Mechanical right ventricular dyssynchrony in patients after atrial switch operation for transposition of the great arteries. American Journal of Cardiology, 101(6), 874–881. DOI 10.1016/j.amjcard.2007.11.033. [Google Scholar] [CrossRef]
13. Metz, T. D., Jackson, G. M., Yetman, A. T. (2011). Pregnancy outcomes in women who have undergone an atrial switch repair for congenital d-transposition of the great arteries. American Journal of Obstetrics and Gynecology, 205(3), 273.e1–273.e5. DOI 10.1016/j.ajog.2011.06.042. [Google Scholar] [CrossRef]
14. Gopinathannair, R., Etheridge, S. P., Marchlinski, F. E., Spinale, F. G., Lakkireddy, D. et al. (2015). Arrhythmia-Induced Cardiomyopathies: Mechanisms, Recognition, and Management. Journal of the American College of Cardiology, 66(15), 1714–1728. DOI 10.1016/j.jacc.2015.08.038. [Google Scholar] [CrossRef]
15. Grossman, W., Paulus, W. J. (2013). Myocardial stress and hypertrophy: A complex interface between biophysics and cardiac remodeling. Journal of Clinical Investigation, 123(9), 3701–3703. DOI 10.1172/JCI69830. [Google Scholar] [CrossRef]
16. Vlachos, K., Letsas, K. P., Korantzopoulos, P., Liu, T., Efremidis, M. et al. (2015). A review on atrioventricular junction ablation and pacing for heart rate control of atrial fibrillation. Journal of Geriatric Cardiology, 12(5), 547–554. [Google Scholar]
17. Dubin, A. M., Janousek, J., Rhee, E., Strieper, M. J., Cecchin, F. et al. (2005). Resynchronization therapy in pediatric and congenital heart disease patients: An international multicenter study. Journal of the American College of Cardiology, 46(12), 2277–2283. DOI 10.1016/j.jacc.2005.05.096. [Google Scholar] [CrossRef]
18. Mongeon, F. P., Connolly, H. M., Dearani, J. A., Li, Z., Warnes, C. A. (2011). Congenitally corrected transposition of the great arteries ventricular function at the time of systemic atrioventricular valve replacement predicts long-term ventricular function. Journal of the American College of Cardiology, 57(20), 2008–2017. DOI 10.1016/j.jacc.2010.11.021. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |