

 | Congenital Heart Disease |  |
DOI: 10.32604/CHD.2021.016271
ARTICLE
Epicardial Versus Endocardial Pacemakers in the Pediatric Population: A Comparative Inquiry
1Cardiovascular Medical and Research Center, Iran University of Medical Sciences, Tehran, Iran
2Cardiovascular Surgery Department, Rajaie Cardiovascular Medical and Research Center, Iran University of Medical Sciences, Tehran, Iran
3Department of Pediatric Cardiology, Mediterranean Pediatric Cardiology Center “Bambin Gesù”, San Vincenzo Hospital, Taormina, Italy
4Cardiac Primary Prevention Research Center, Tehran Heart Center and Department of Cardiology, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran
5Pediatric Cardiology, Bahrami Children’s Hospital, Tehran University of Medical Science, Tehran, Iran
6Adult Congenital Heart Disease Department, Rajaie Cardiovascular Medical and Research Center, Iran University of Medical Sciences, Tehran, Iran
*Corresponding Authors: Ali Sadeghpour Tabaei. Email: sadegpour@rhc.ac.ir; Elio Caruso. Email: carusoelio@gmail.com
Received: 22 February 2021; Accepted: 17 April 2021
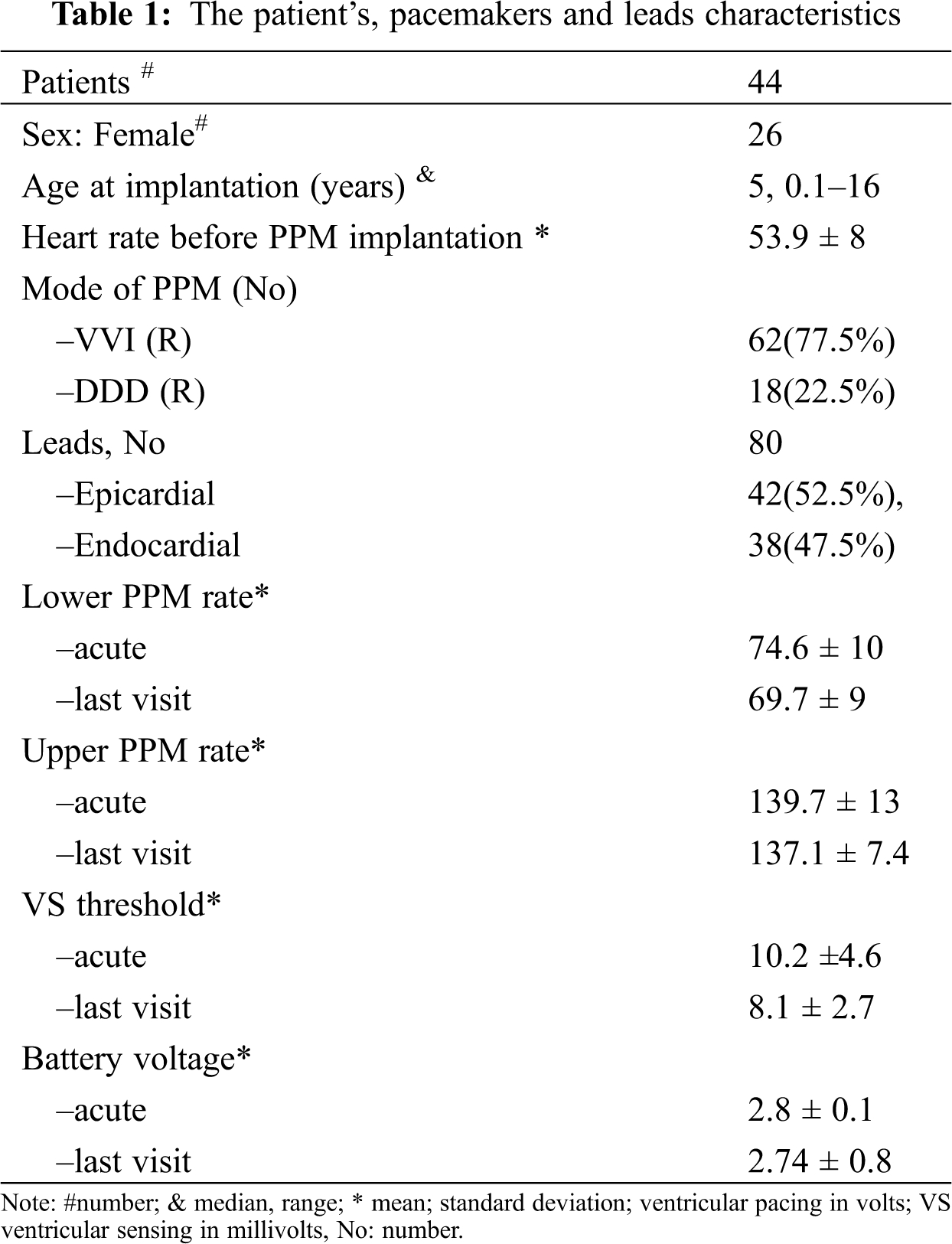
Abstract: Background: Most children in need of cardiac pacemakers remain dependent on the function of the permanent from childhood to adulthood. We sought to evaluate and compare the function between epicardial and endocardial pacemakers in pediatric groups with different conditions. Methods: Between 2012 and 2018, this single-canter study evaluated 44 pediatric patients with indications for epicardial or endocardial pacemakers. Results: The 2 groups, at a median age of 5 (0.1–16) years, were compared concerning the characteristics of the leads used (n = 80: bipolar, unipolar, steroid-eluting, and non–steroid-eluting), survival data, and complications. The reason for pacemaker implantation was congenital complete heart block in 11 (25%) cases and postoperative heart block in 33 (75%) cases. The commonest congenital heart disease accompanied by postoperative block was the ventricular septal defect. In the endocardial lead group, the mean ventricular pacing threshold immediately after the implantation and during the follow-up was less than that in the epicardial lead group (0.75 vs. 0.81 V; P = 0.01 and 0.8 vs. 2.4 V; P = 0.001). During the follow-up, the mean battery longevity was better in the endocardial group (last visit: 6.7 endocardial vs. 3.3 years epicardial). Lead failure was commoner in the epicardial pacemaker, and chronic high-pacing threshold pattern was seen in 14 patients in this group. After 3 years, freedom from lead failure was 94% and 63% in the endocardial and epicardial leads. Conclusions: Pacemakers with endocardial bipolar steroid-eluting leads showed better lead characteristics regarding survival and battery longevity than epicardial pacemakers without these lead characteristics. An appropriate pacemaker type should be selected based on the patient’s condition.
Keywords: Pacemaker; congenital heart defects; cardiac pacing; pediatrics
Although the implantation of the permanent pacemaker (PPM) in the pediatric began decades ago, what exactly constitutes an optimal cardiac pacemaker therapy is still deemed a challenging topic. Children require their own management and treatment protocols because the etiologies of the diseases that prompt PPM implantation in them are different from those in adults. Pediatric pacing requires unique challenges because of patient size, growth and development and, moreover, the frequent coexistence of congenital heart disease (CHD). A cardiac pacemaker is considered optimal when it is capable of providing pace with a substantial longevity, the most favourable physiological state, and the fewest complications. What is widely regarded as crucial vis-à-vis PPM implantation is how long the device can preserve its functional properties. The advantages of the endocardial PPMs include desirable pacemaker analysis results, low atrial and ventricular pacing threshold, low sensing thresholds, efficient energy saving, and acceptable battery and lead longevity; nonetheless. The disadvantages include limited venous accessibility (especially in children with a small body size), the risk of thrombi and residual intracardiac shunts and left ventricle (LV) remodelling due to right ventricular (RV) pacing [1–3]. The epicardial PPMs returned to competition with the endocardial PPM with the advent of steroid-eluting epicardial leads [4]. The epicardial PPMs can be inserted in smaller children and offers the possibility of implantation in the LV site as the systemic ventricle. The aim of the present study was to conduct a comprehensive evaluation and comparison of epicardial and endocardial PPMs during a long follow-up in pediatric groups with different etiologies and conditions.
All patients from infancy to age 18 years who were candidates for PPM implantation following structural cardiac surgery or primary cardiac conduction disorders in a single tertiary medical center between March 2012 and December 2018 were recruited. Those Patients that could not complete the follow-up period were excluded from the study. The study protocol was approved by the Ethics Committee and the Review Board of University of Medical Sciences, and informed consent was obtained from the children’s parents. During the study period, data on 44 consecutive patients and 80 leads—comprised of 42 epicardial and 38 endocardial PPMs—were collected. The epicardial approach was considered in the following conditions: a small body size, postoperative heart block immediately after surgical repair, venoocclusive problems or venous thromboses in the access pathway, the presence of left-to-right intracardiac shunting, or complex CHD. We considered the indication for PPM implantation for each patient based on the current guidelines of the European Heart Rhythm Association (EHRA) [5]. Contrast echocardiography was done with agitated saline before PPM implantation in the patients with heart block after cardiac surgical repair to rule out residual intracardiac shunts. The follow-up was carried out 1 month after PPM implantation and then every 6 months. In each visit, pacemaker analysis, surface electrocardiography (ECG), and echocardiography were conducted. Chest X-ray was done every 1 year or when the pacemaker analysis indicated pace malfunction. The follow-up period was shorter (every 3 months) if the pacemaker analysis or clinical manifestations showed evidence of pacemaker dysfunction or battery depletion.
The lifetime of a lead was considered to reflect the time duration from the implantation date to the date of documented lead failure, pacing problems, cardiac complications, removal, or the latest follow-up date with in the study period. Lead failure was defined as lead replacement or abandonment because of any cause—including lead fracture, insulation break, lead displacement, trauma, and infection, or pacing problems leading to high pacing and/or sensing threshold events and loss of capture.
For the endocardial PPM, a 7-F introducer removable sheath was inserted via the left and less commonly right, subclavian vein puncture. Under fluoroscopy guidance, bipolar active leads (Medtronic, Abbott/St Jude, Boston, Guidant)—48, 52, and 58 cm in length—were selected according to the child’s body surface area. For the single-chamber PPM, the RV leads were implanted by maneuvering the stylet in the apex or the interventricular septum in the RV site. For the dual-chamber PPM, the leads were fixed in the right atrium (mainly the appendage) and the RV. Given that there is no difference in the rate of lead failure in terms of the pocket location [6], all 3 surgical techniques of rectus, subxiphoid, and retrocostal were employed for the location of the epicardial PPM. The technique of “screw-in” technique with nonabsorbable suture was used in group of the patients for fixing, and the generator was inserted in the rectus sheath pocket. An infraclavicular pocket was created when thoracotomy was done.
2.3 Pacing and Lead Characteristics
In most of the patients who needed the epicardial PPM or had a previously implanted PPM, the single chamber ventricular PPM (ventricular demand [VVI] pacing or ventricular demand rate responsive [VVIR] pacing) was implanted. In the patients considered for the endocardial PPM, the single-chamber ventricular PPM was usually preferred if the patient was younger than 5 years or weighed below 15 kg, whereas in the patients who were older than 5 years or weighed over 15 to 20 kg, the first choice was the dual-chamber PPM (DDD or DDDR). For both epicardial and endocardial ventricular leads, lead function was considered acceptable if the R wave amplitude was greater than 5 mV, the ventricular pacing threshold was below 2 V (pulse width = 0.4–1 ms), and the lead impedance was between 200 and 1200 Ω. Normal values for the atrial leads consisted of a P-wave amplitude of greater than 2 mV, an atrial pacing threshold of below 1 V (pulse width = 0.4–1 ms). The upper and lower pacing rates were set based on the normal value of the heart rate according to the appropriate age of the children.
The patients’ data were collected and analysed with the SPSS software, version 21 (SPSS, Chicago, IL, USA). The variables evaluated in this study were comprised of demographic characteristics, types of CHD, lead characteristics (both epicardial and endocardial PPMs), and pacemaker analysis data. The quantitative data are expressed as means ± standard deviations (SDs) or as medians and ranges. The categorical variables are reported as percentages. AP value of less than 0.05 was considered statistically significant. The differences between the 2 groups were compared using t-tests for the continuous variables with normal distributions. Freedom from lead failure was evaluated using the log-rank test.
Forty-four patients, comprising 18 male and 26 female children, with a total of 80 procedures and 80 leads were evaluated. The median of the patients’ follow-up was 6 years. The patients’ and pacemakers’ characteristics are presented in Tab. 1. The youngest patient to receive an epicardial PPM was 1 month old. There was a significant difference between the age groups selected for epicardial and endocardial PPM implantation (P = 0.001). There was no death due to pacemaker procedures or complications during the follow-up. The reason for PPM implantation was congenital complete heart block (CCHB) in 11 (25%) patients and postoperative heart block after in CHD repair in 33 (75%). The types of CHD resulting in PPM implantation after surgery are presented in Tab. 2. The most frequent CHD accompanied by postoperative heart block were as the ventricular septal defect (VSD) (n = 10), followed by the tetralogy of Fallot (n = 6). Two cases of CCHB were accompanied by CHD. The conduction disorders prompting PPM implantation were complete heart block in 43 patients and sinus node dysfunction in only 1 patient. The mean heart rate was 50.6 ± 6.7 bpm in the patients with CCHB and 55.2 ± 7.9 bpm in the patients with non-CCHB. There was a significant difference in the mean heart rate between CCHB and postoperative heart block (P = 0.036).
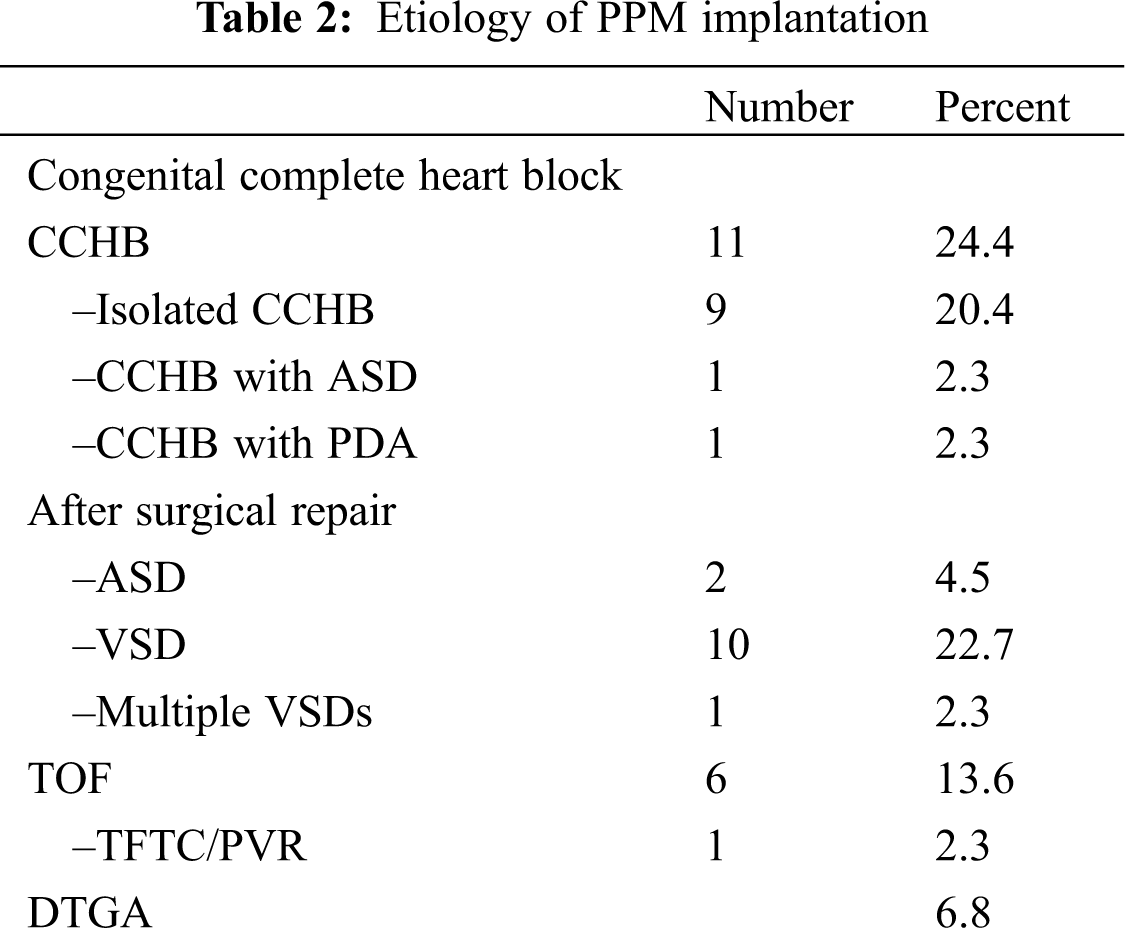
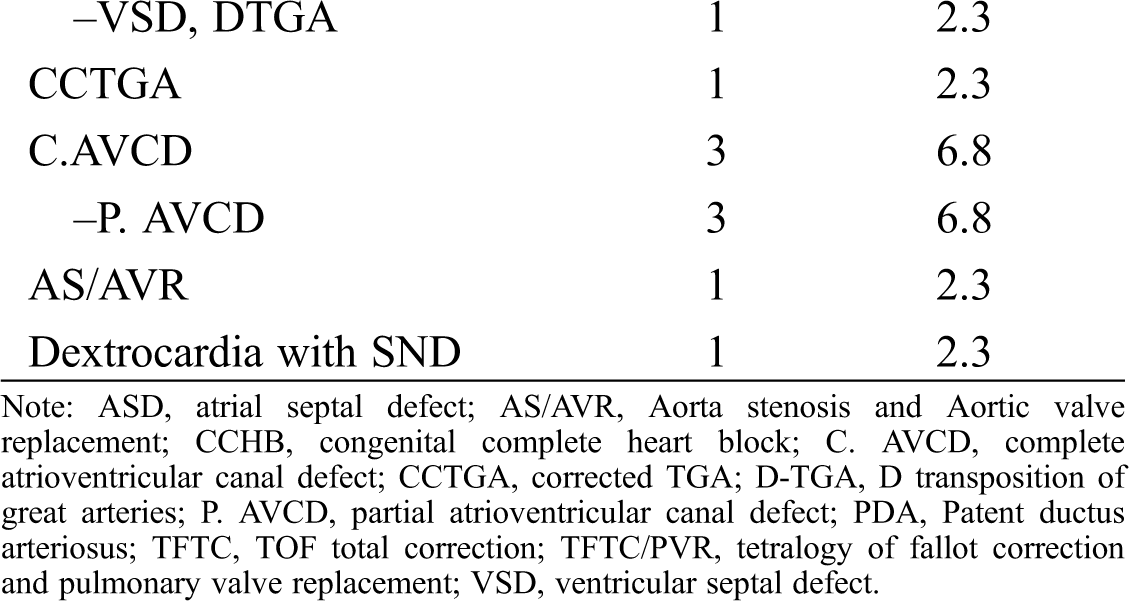
3.2 Characteristics of the PPMs and Leads
There were 80 cases of PPM leads were implanted: 38 endocardial and 42 epicardial. In 17 (42%) of the 42 epicardial PPMs, the pacemaker lead was steroid-eluting. In 14 of the 42 epicardial PPMs the lead was Bipolar. The dual-chamber PPM was implanted in 18 children: 16 endocardial and 2 epicardial procedures. In 26 cases, the epicardial PPM was replaced with the endocardial PPM. In 2 patients, the endocardial single-chamber PPM was upgraded to the dual chamber PPM.
The endocardial lead tip was inserted into the RV apex and the mid septum in 36 cases and in the RV outflow tract in 2 cases. Single-chamber ventricular leads were used in 62 PPMs. In all the patients, the rate modulation response was initially activated; however, it was switched automatically to non-rate modulatory mode in 4 cases due to end-of-life condition. The mean battery longevity was 7.7 ± 0.8 years in the first visit and 4.9 ± 2.5 years in the last visit. The mean lead impedance immediately after PPM implantation in the endocardial lead group was 563 ohm and in epicardial group was 539 ohm. There was no significant difference vis-à-vis the mean ventricular impedance between the epicardial and endocardial leads in the first visit and during the follow-up. The PPM programmable parameters during the follow-up are depicted in Tab. 3. The lead characteristics of the epicardial and endocardial lead groups were also compared in Figs. 1 and 2. The battery voltage was not significantly different between the groups early after PPM implantation (P = 0.2); nevertheless, in the last visit, the battery voltage was significantly lower in the epicardial lead group (P = 0.001).
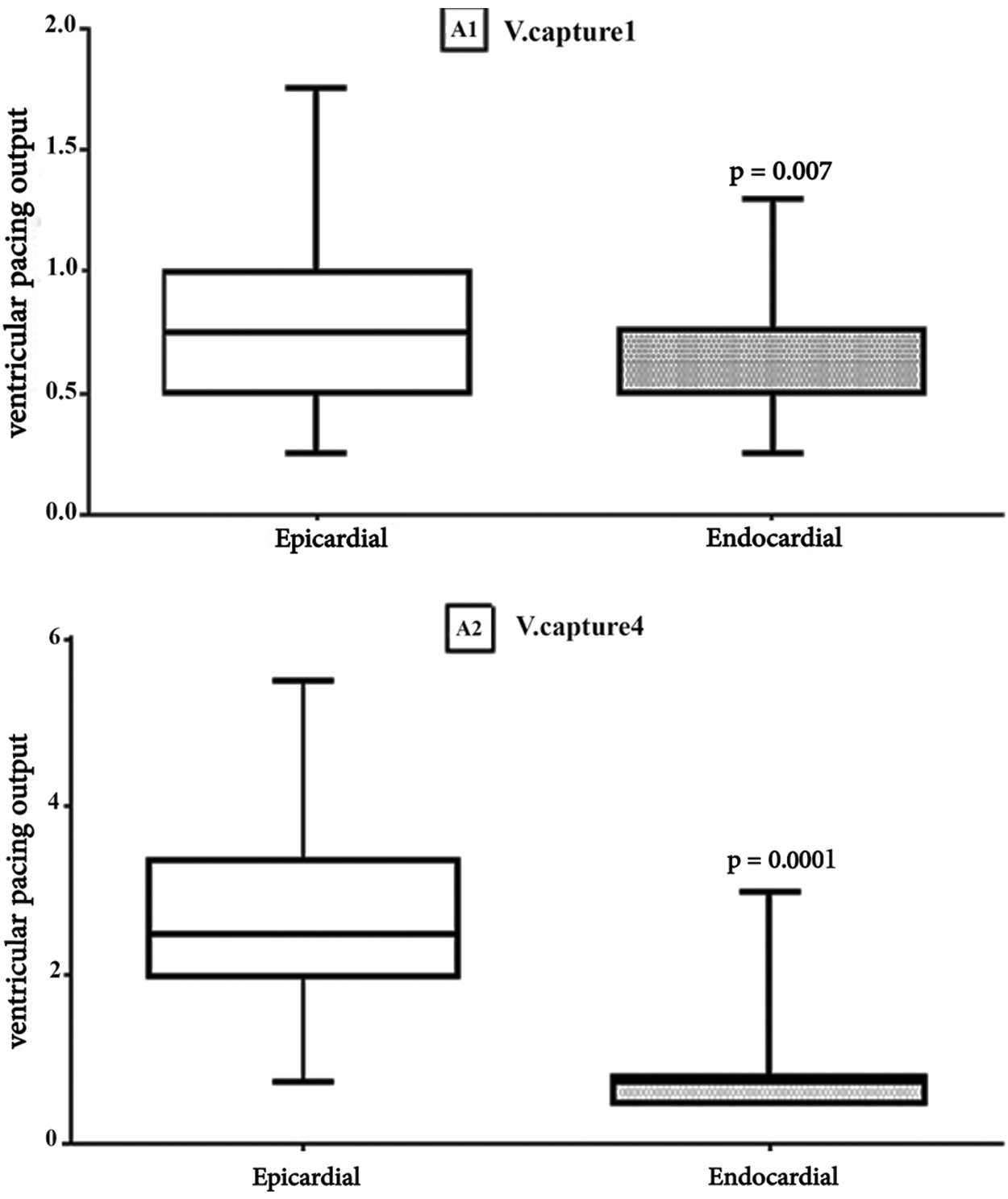
Figure 1: Comparison Results of Epicardial and Endocardial Ventricular pacing thresholds. Box plot for Ventricular capture. The horizontal in the middle each box is Median. A1: V. captur1, first visit (Median, Epi = 0.75 vs. Endo = 0. 5, p = 0.007), A2: V. captur4, last visit (Median, Epi = 2.5 vs. Endo = 0.75 p = 0.0001)
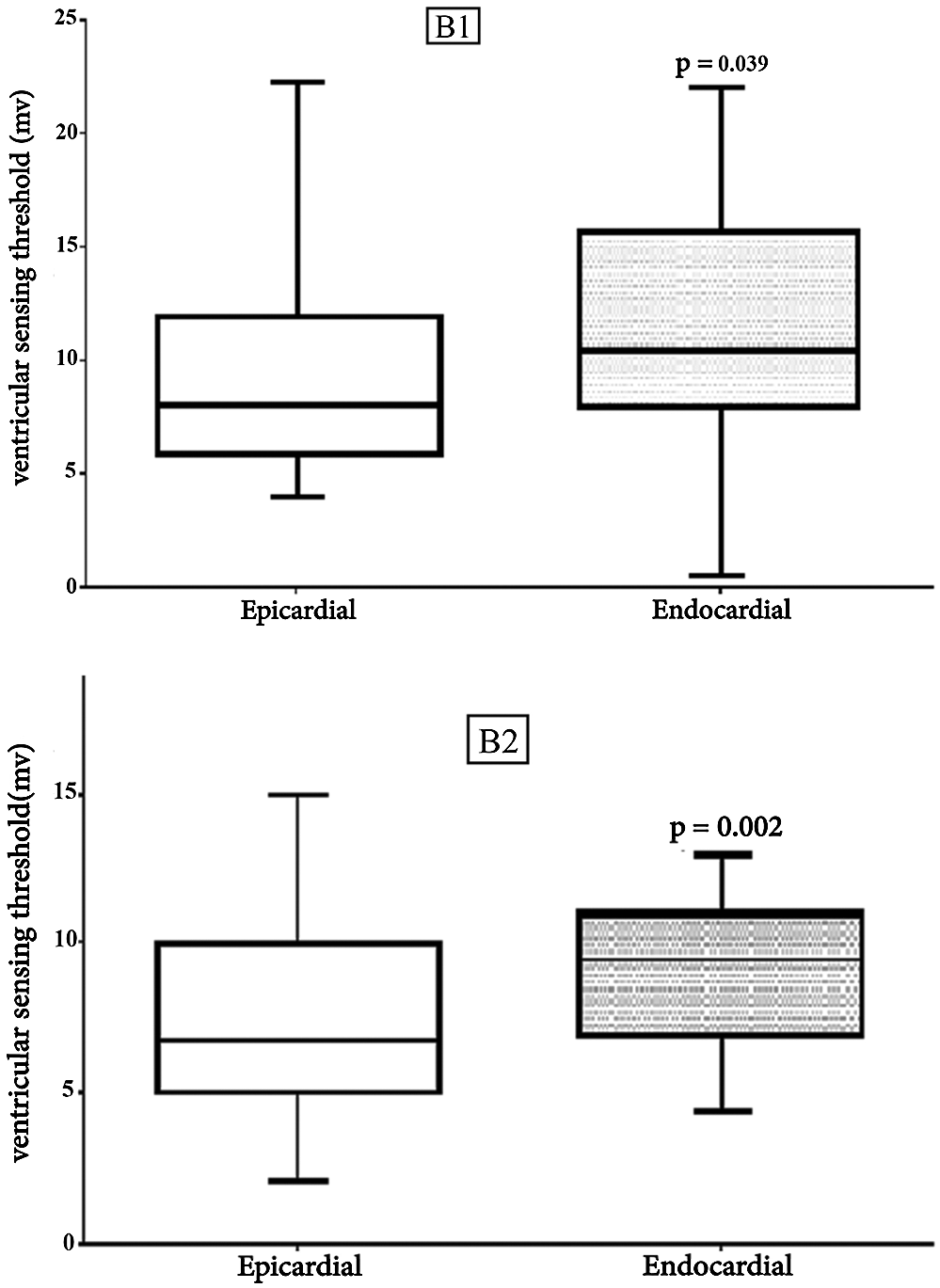
Figure 2: Comparison Results of Epicardial and Endocardial Ventricular sensing thresholds Box plot for Ventricular sensing. The horizontal in the middle each box is Median. B1: V. sens1, first visit (Median, Epi = 8 vs. Endo = 10. 5, p = 0.039), B2: V. sens4, last visit (Median, Epi = 6.75 vs. Endo = 9.5, p = 0.002)
Of the 38 endocardial leads, lead failure was seen in 4. Ventricular lead displacement was reported in 1 case, atrial lead displacement in 1, and twiddler’s syndrome in 1. Lead fracture was reported in 3 patients: 1 endocardial PPM (at proximal part of lead) and 2 epicardial PPMs. Lead failure was significantly different between the epicardial and endocardial leads (P = 0.001). In 2 patients, both of the implanted epicardial and endocardial leads developed problems. In one of these patients, the epicardial lead was replaced with an endocardial lead due to a chronic high-pacing threshold pattern; however, the endocardial lead was subsequently fractured and had to be re-inserted. In the other patient, the endocardial lead was re-looped. Of the 42 epicardial leads, an early high-pacing threshold pattern was seen in 4 leads. Acute lead fracture and lead-tip displacement were each responsible for 1 case. A chronic high-pacing threshold pattern was seen in 14 patients. The other causes of chronic lead failure included 3 cases of insulation break and 1 case of lead fracture. Twenty leads (18 cases) did not develop any problems and had end-of-life generators. For different reasons, 26 of the 42 patients had their epicardial PPMs replaced with endocardial PPMs. The rate of freedom from lead failure is shown in Fig. 3.
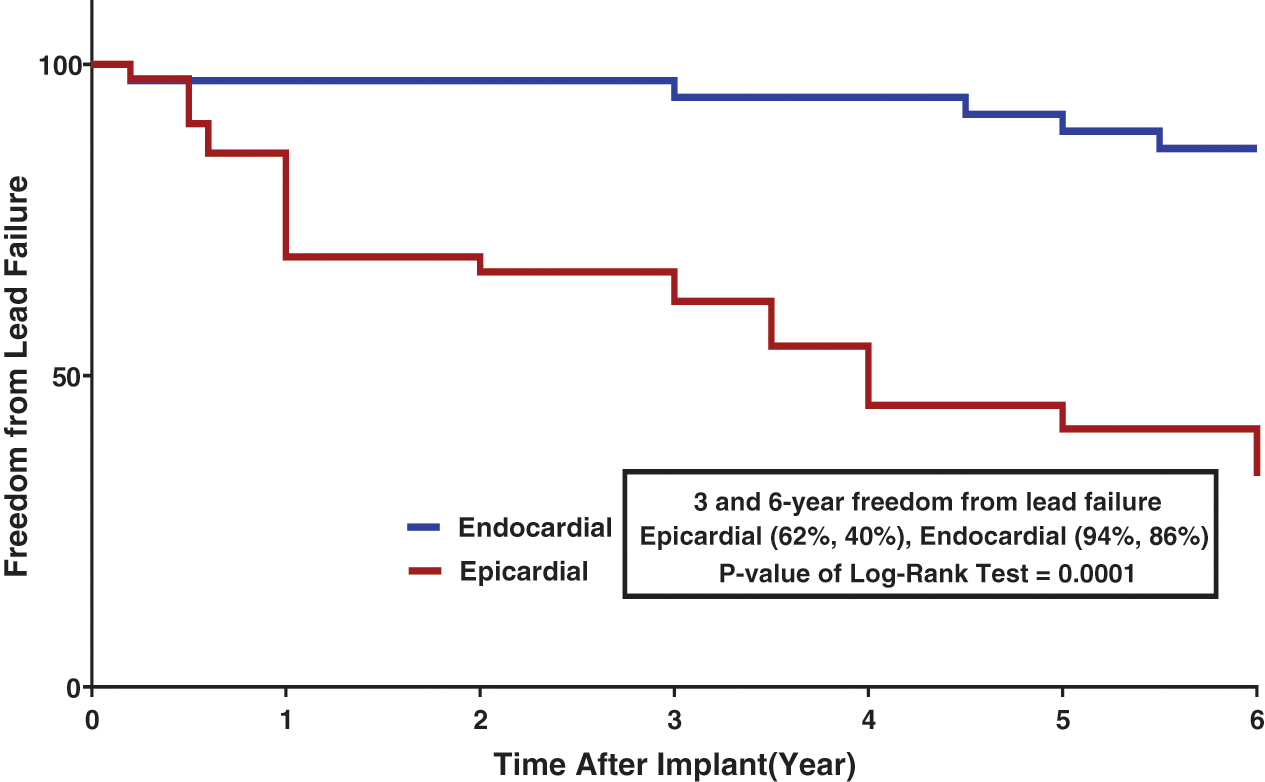
Figure 3: Freedom from Lead Failure stratified by Lead Type
Post-PPM implantation complications were reported in 3 cases. Whereas there was no incidence of pocket infection after endocardial PPM implantation, 1 patient with an epicardial PPM developed pocket infection after 1 month and had to receive a new device system. One Pneumothorax was seen in 1 case experienced pneumothorax during venous access, which was treated conservatively. There were no cases of cardiac tamponade or perforation, and nor any cases of acute venous thrombosis in the venous system. One patient developed pocket hematoma, which was subsequently evacuated. There were no cases of paradoxical embolism in the endocardial PPMs during the follow-up. Pulmonary thromboembolism and superior vena cava syndrome were not reported.
The advancements in the surgical correction of CHD with increased number of CHDs repair have been in tandem with a rise in the rate of surgical complications such as postoperative heart block, which is one of the reasons why the issue of pacemaker therapy in children has attracted so much attention. Our investigation is a single-center study, but what should be borne in mind is that our hospital is the sole center for cardiac device implantation in children in the country. Over a 6-year period, we evaluated 44 patients at a mean age of 6.47 years and 80 cases of lead implantation. The mean age at PPM implantation in the epicardial lead group was significantly lower than that in the endocardial lead group. The indication for PPM implantation in almost all of our patients (except 1 patient) was complete heart block. It is in contrast to a study by Paech et al. [7], who reported that 24% of their 82 pediatric patients with postoperative PPM had sick sinus syndrome. Apropos of the cause of heart block, in line with other studies, postoperative heart block was more common than complete heart block in our study. Most of the cases of CCHB were isolated; nevertheless, in a few cases, they were accompanied by other CHD. Surgical isolated VSD closure was the most common cause of postoperative heart block (25%), followed by the tetralogy of Fallot repair. Additionally, one-quarter of all the cases of PPM implantation were due to CCHB. In a study by Papadopoulos et al. [8] on 45 children, the most common cause of post operation block was the atrioventricular canal, followed by the VSD. The authors also reported that nearly half of their cases of PPM implantation were in consequence of CCHB. In a study by Kelvin et al. [9], the epicardial PPM was replaced with the endocardial PPM in a large number of the patients (62%) during the follow-up. The most common cause of lead failure was a chronic high ventricular threshold. The use of unipolar non–steroid-eluting leads predisposes patients to high threshold events, both early and chronic. In the current study, we observed an increase in the ventricular threshold usually after 6 months; however, in the endocardial leads, thresholds were in a steady state and did not change significantly during the follow-up. Lead fracture and lead displacement were reported in a few patients in both groups, with the difference between the endocardial and epicardial PPMs not constituting statistical significance. It appears that the most notable cause of acute lead failure is technical problems during the procedure. In a study by Silvetti et al. [10], the early complications of PPM implantation were seen more frequently in the epicardial PPMs than in the endocardial ones. Thomson et al. [11] evaluated 96 cases of epicardial lead implantation with a median age of 1.9 years (68% steroid-eluting and32% non–steroid-eluting) and found that lead fracture was the most common cause of lead failure (47%), followed by an increase in pacing thresholds. In a considerably large study, Fortescue et al. [12] concluded that the risks for lead failure were younger age, structural heart diseases, and epicardial PPMs. While pocket infection is one of the most frequent causes of PPM complications in adults, it is usually less common in pediatrics. In our study, only 1 patient developed late pocket infection after epicardial PPM implantation. In another study on 256 epicardial leads, Fortescue et al. [13] reported 4 cases of lead failure due to infection. In the current study, during the follow-up of the endocardial PPMs, we observed no significant lead related tricuspid regurgitation necessitating re-intervention. We also had no significant diaphragmatic stimulation prompting re-intervention; nevertheless, in some studies, it is the cause of PPM failure [14]. The LBBB pattern usually was observed in the ECG of patients with endocardial PPM, although the RBBB pattern may be observed in cases of high-septum lead implantation. The RV pacing with LBBB pattern results in interventricular asynchrony, which—with time—may lead to a decreased cardiac function—especially in small children with PPMs [15]. During our follow-up, 1 patient with an endocardial lead had a gradually decreased LV function. In our study, the differences in the pacemaker indices were significant between the endocardial and epicardial lead groups. Although immediately after PPM implantation, the ventricular pacing threshold was different between the 2 groups, the values were almost similar. In a study by Sachweh et al. [1], this difference was also significant and acute ventricular stimulation was higher in the cases with epicardial PPMs. During their follow-up, the authors reported a gradual increase in the difference until it reached statistical significance at 3 years. They reported that the main cause was a chronic high ventricular pacing threshold, probably due to the inflammation of the epicardial lead tip connection. This finding can be explained by various reasons, including fibrosis and scar in the location of epicardial lead insertion and fat and myocardial damage, especially after the repair of CHD [16]. In a study by Lofty et al. [17] on 101 PPM implantation procedures, the mean pacing output of the endocardial leads was lower than that of the epicardial leads (3.06 V in the epicardial leads vs. 2.3 V in the endocardial leads). The mean pacing output of the endocardial leads in our study is better than that in the study by Lofty and colleagues. In contrast, this index in the study by Udink et al. [18], during a 2-year follow-up, is more favorable than ours. However, Udink and colleagues used steroid-eluting leads in both of their epicardial and endocardial lead groups. Silvetti et al. [10] compared endocardial (mean weight = 4.9 kg) and epicardial (mean weight = 3.8 kg) PPMs in 56 infants less than 1 year old and reported that the epicardial leads had a 21-fold increase in the risk of failure in comparison with the endocardial leads. Additionally, there was no significant difference in the outcome between the non–steroid-eluting and steroid-eluting epicardial PPMs in their study. Likewise, in study by Wilhelm et al. [19] on 149 pacemaker procedures, increased pacing thresholds observed in 17.2% of the epicardial leads compared with 2.9% of the endocardial leads. In our study, the rate of freedom from lead failure at 3 years’ follow-up was significantly higher in the endocardial PPMs than in the epicardial PPMs (94% vs. 63%), and this difference continued with time. Kubus et al. [20] assessed 119 patients and reported that the rate of freedom from epicardial pacing dysfunction was 70% at 5 years’ follow-up. Additionally, 83% of the epicardial leads were steroid-eluting. In another study, a lead survival comparison between 2 groups at 1 and 3 years’ follow-ups yielded almost similar results, although the difference was significant at 5 years’ follow-up (endocardial 85% vs. endocardial 58%) [10]. Despite the fact that single-chamber ventricular PPMs may be adequate for the cardiac pump function [21], the need for physiological or atrioventricular pacing may be more palpable in complex CHD with single-ventricle physiologies even in children with a low weight and a small body size. The pacing site presents yet another challenge in PPM implantation among children. It may not be possible to achieve normal electrophysiological cardiac pacing with artificial pacemakers [22], but it is necessary that an optimal condition—not least in children needing long-life pacing—be provided. Recent years have witnessed a rise in the trend of the RV lead insertion in the mid to high septum because of narrow-paced QRS patterns and probably better hemodynamic conditions, but it appears that the LV apex pacing might provide a suitable cardiac hemodynamic state in comparison with the RV site, which is only possible with epicardial PPM implantation [23,24]. This is one of the main points on which the advocators of epicardial PPMs tend to focus. Gebauer et al. [3] concluded that the major independent risk factor for pathologic LV remodelling was epicardial RV free-wall pacing. One of our concerns regarding epicardial PPMs is their unpredictable longevity and the different behaviours of some of their types, which may be problematic in children living far from our hospital as a referral centre for a regular close follow-up. First and foremost, among the limitations of the present study are the different cardiac diseases prompting PPM implantation, rendering a correct comparison between endocardial and epicardial leads somewhat difficult. There is no doubt that a larger sample size and a longer follow-up would have altered our results. We compared function between epicardial and endocardial PPMs. It should, however, be borne in mind that there were differences in the characteristics of the leads (unipolar, bipolar, steroid and non–steroid-eluting), which affected the final results because of the limitations of facilities available to us.
To sum up, in the selection between endocardial and epicardial PPMs, any prejudgment about the superiority of one type is inadvisable inasmuch as each type has its own advantages and disadvantages. What determines the appropriate PPM type should be based on the patient’s condition.
Acknowledgement: We thank the staff and participants in Rajaie Cardiovascular, Medical, and Research Center in the pediatric field.
Funding Statement: We have not any financial support from person, industry and organs.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Sachweh, J. S., Vazquez-Jimenez, J. F., Schöndube, F. A., Daebritz, S. H., Dörge, H. et al. (2000). Twenty years’ experience with pediatric pacing: Epicardial and transvenous stimulation. European Journal of Cardio-Thoracic Surgery: Official Journal of the European Association for Cardio-Thoracic Surgery, 17(4), 455–461. [Google Scholar]
2. Silvetti, M. S., Drago, F., Grutter, G., De Santis, A., Di Ciommo, V. et al. (2006). Twenty years of paediatric cardiac pacing: 515 pacemakers and 480 leads implanted in 292 patients. Europace: European Pacing, Arrhythmias, and Cardiac Electrophysiology: Journal of the Working Groups on Cardiac Pacing, Arrhythmias, and Cardiac Cellular Electrophysiology of the European Society of Cardiology, 8(7), 530–536. [Google Scholar]
3. Gebauer, R. A., Tomek, V., Salameh, A., Marek, J., Chaloupecký, V. et al. (2009). Predictors of left ventricular remodelling and failure in right ventricular pacing in the young. European Heart Journal, 30(9), 1097–1104. [Google Scholar]
4. Tomaske, M., Gerritse, B., Kretzers, L., Pretre, R., Dodge-Khatami, A. et al. (2008). A 12-year experience of bipolar steroid-eluting epicardial pacing leads in children. Annals of Thoracic Surgery, 85(5), 1704–1711. [Google Scholar]
5. Brugada, J., Blom, N., Sarquella-Brugada, G., Blomstrom-Lundqvist, C., Deanfield, J. et al. (2013). European Heart Rhythm Association, & Association for European Paediatric and Congenital Cardiology, Pharmacological and non-pharmacological therapy for arrhythmias in the pediatric population: EHRA and AEPC-Arrhythmia Working Group joint consensus statement. Europace: European Pacing, Arrhythmias, and Cardiac Electrophysiology: Journal of the Working Groups on Cardiac Pacing, Arrhythmias, and Cardiac Cellular Electrophysiology of the European Society of Cardiology, 15(9), 1337–1382. [Google Scholar]
6. Lichtenstein, B. J., Bichell, D. P., Connolly, D. M., Lamberti, J. J., Shepard, S. M. et al. (2010). Surgical approaches to epicardial pacemaker placement: Does pocket location affect lead survival? Pediatric Cardiology, 31(7), 1016–1024. [Google Scholar]
7. Paech, C., Kostelka, M., Dähnert, I., Flosdorff, P., Riede, F. T. et al. (2014). Performance of steroid eluting bipolar epicardial leads in pediatric and congenital heart disease patients: 15 years of single center experience. Journal of Cardiothoracic Surgery, 9, 84. [Google Scholar]
8. Papadopoulos, N., Rouhollapour, A., Kleine, P., Moritz, A., Bakhtiary, F. (2010). Long-term follow-up after steroid-eluting epicardial pacemaker implantation in young children: A single centre experience. Europace: European Pacing, Arrhythmias, and Cardiac Electrophysiology: Journal of the Working Groups on Cardiac Pacing, Arrhythmias, and Cardiac Cellular Electrophysiology of the European Society of Cardiology, 12(4), 540–543. [Google Scholar]
9. Lau, K. C., William Gaynor, J., Fuller, S. M., Smoots, K. A., Shah, M. J. (2015). Long-term atrial and ventricular epicardial pacemaker lead survival after cardiac operations in pediatric patients with congenital heart disease. Heart Rhythm, 12(3), 566–573. [Google Scholar]
10. Silvetti, M. S., Drago, F., De Santis, A., Grutter, G., Ravà, L. et al. (2007). Single-centre experience on endocardial and epicardial pacemaker system function in neonates and infants. Europace: European Pacing, Arrhythmias, and Cardiac Electrophysiology: Journal of the Working Groups on Cardiac Pacing, Arrhythmias, and Cardiac Cellular Electrophysiology of the European Society of Cardiology, 9(6), 426–431. [Google Scholar]
11. Thomson, J. D., Blackburn, M. E., van Doorn, C., Nicholls, A., Watterson, K. G. (2004). Pacing activity, patient and lead survival over 20 years of permanent epicardial pacing in children. Annals of Thoracic Surgery, 77(4), 1366–1370. [Google Scholar]
12. Fortescue, E. B., Berul, C. I., Cecchin, F., Walsh, E. P., Triedman, J. K. et al. (2004). Patient, procedural, and hardware factors associated with pacemaker lead failures in pediatrics and congenital heart disease. Heart Rhythm, 1(2), 150–159. [Google Scholar]
13. Fortescue, E. B., Berul, C. I., Cecchin, F., Walsh, E. P., Triedman, J. K. et al. (2005). Comparison of modern steroid-eluting epicardial and thin transvenous pacemaker leads in pediatric and congenital heart disease patients. Journal of Interventional Cardiac Electrophysiology: An International Journal of Arrhythmias and Pacing, 14(1), 27–36. [Google Scholar]
14. Ector, B., Willems, R., Heidbüchel, H., Gewillig, M., Mertens, L. et al. (2006). Epicardial pacing: A single-centre study on 321 leads in 138 patients. Acta Cardiologica, 61(3), 343–351. [Google Scholar]
15. Tops, L. F., Schalij, M. J., Bax, J. J. (2009). The effects of right ventricular apical pacing on ventricular function and dyssynchrony implications for therapy. Journal of the American College of Cardiology, 54(9), 764–776. [Google Scholar]
16. Murayama, H., Maeda, M., Sakurai, H., Usui, A., Ueda, Y. (2008). Predictors affecting durability of epicardial pacemaker leads in pediatric patients. Journal of Thoracic and Cardiovascular Surgery, 135(2), 361–366. [Google Scholar]
17. Lotfy, W., Hegazy, R., AbdElAziz, O., Sobhy, R., Hasanein, H. et al. (2013). Permanent cardiac pacing in pediatric patients. Pediatric Cardiology, 34(2), 273–280. [Google Scholar]
18. Ten Cate, F. U., Breur, J., Boramanand, N., Crosson, J., Friedman, A. et al. (2002). Endocardial and epicardial steroid lead pacing in the neonatal and paediatric age group. Heart (British Cardiac Society), 88(4), 392–396. [Google Scholar]
19. Wilhelm, B. J., Thöne, M., El-Scheich, T., Livert, D., Angelico, R. et al. (2015). Complications and risk assessment of 25 years in pediatric pacing. Annals of Thoracic Surgery, 100(1), 147–153. [Google Scholar]
20. Kubus, P., Materna, O., Gebauer, R. A., Matejka, T., Gebauer, R. et al. (2012). Permanent epicardial pacing in children: Long-term results and factors modifying outcome. Europace: European Pacing, Arrhythmias, and Cardiac Electrophysiology: Journal of the Working Groups on Cardiac Pacing, Arrhythmias, and Cardiac Cellular Electrophysiology of the European Society of Cardiology, 14(4), 509–514. [Google Scholar]
21. Horenstein, M. S., Karpawich, P. P. (2004). Pacemaker syndrome in the young: Do children need dual chamber as the initial pacing mode? Pacing and Clinical Electrophysiology: PACE, 27(5), 600–605. [Google Scholar]
22. Motonaga, K. S., Punn, R., Axelrod, D. M., Ceresnak, S. R., Hanisch, D. et al. (2015). Diminished exercise capacity and chronotropic incompetence in pediatric patients with congenital complete heart block and chronic right ventricular pacing. Heart Rhythm, 12(3), 560–565. [Google Scholar]
23. Vanagt, W. Y., Verbeek, X. A., Delhaas, T., Mertens, L., Daenen, W. J. et al. (2004). The left ventricular apex is the optimal site for pediatric pacing: correlation with animal experience. Pacing and Clinical Electrophysiology: PACE, 27(6p2), 837–843. [Google Scholar]
24. Antretter, H., Colvin, J., Schweigmann, U., Hangler, H., Hofer, D. et al. (2003). Special problems of pacing in children. Indian Pacing and Electrophysiology Journal, 3(1), 23–33. [Google Scholar]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |