

 | Congenital Heart Disease |  |
DOI: 10.32604/CHD.2021.016923
ARTICLE
Combined Surgical Treatment of Atherosclerotic Coronary Artery Disease and Moderate Aortic Valve Stenosis in Patient with Concomitant Lipton’s R-III Type of Single Coronary Artery Anomaly
1Centre for Anesthesiology and Reanimatology, UC Clinical Centre of Serbia, Belgrade, 11000, Serbia
2Clinic for Cardiac Surgery, UC Clinical Centre of Serbia, Belgrade, 11000, Serbia
3Clinic for Vascular and Endovascular Surgery, UC Clinical Centre of Serbia, Belgrade, 11000, Serbia
*Corresponding Author: Mladen Kocica. Email: kocica@sbb.rs
Received: 09 April 2021; Accepted: 31 May 2021
Abstract: A single coronary artery is a very rare condition, commonly associated with other congenital anomalies. It could be generally considered as neither benign nor malignant form of congenital coronary artery anomalies since its pathophysiological and clinical implications grossly depend on different anatomical patterns defined by the site of origin and distribution of the branches. By presenting the patient who underwent successful coronary artery bypass grafting and aortic valve replacement surgery in a presence of isolated single coronary artery, we intend to emphasize natural and procedural risks and distinguish casual from causal in this extremely rare clinical and surgical scenario.
Keywords: Single coronary artery; aortic stenosis; angina pectoris; aortic valve surgery; coronary artery bypass grafting surgery
By definition, a single coronary artery (SCA) is a congenital coronary artery anomaly (CCAA) with anomalous coronary artery (CA) connection to the aorta/systemic circulation in which all of the coronary blood flow is provided by an artery that arises by one ostium from an arterial trunk [1,2].
Although this condition, accounts for less than 3% of all CCAA, affecting 0.0024%–0.066% of those undergoing coronary angiography and 0.019%–0.4% of the general population, SCA carries both natural and/or procedural risks that may affect normal life expectancy, causing sudden cardiac death in 23% of carriers. In more than 40% of the reported cases, SCA is associated with other complex congenital anomalies [2–5].
We present a patient with unstable angina pectoris who underwent successful combined coronary artery bypass grafting (CABG) and aortic valve replacement (AVR) surgery for moderate aortic stenosis, in a presence of Lipton’s R-III type of isolated SCA [6].
Since we could not find such preoperative and surgical characteristics in the available literature, we found it useful to emphasize some natural and procedural risks and to distinguish casual from causal in this extremely rare clinical and surgical scenario [7].
A 55 years-old (176 cm, 92 kg) male patient was admitted for scheduled combined CABG and AVR surgery. Except for mild hyperlipidemia, smoking, and heredity, no other risk factors for atherosclerosis were documented. No history of previous myocardial infarctions (MI), chronic diseases, and hospitalizations. Screening color Doppler of the extracranial brain blood vessels revealed the chronic asymptomatic occlusion of the right internal carotid artery and 30% stenosis at the opposite side. De novo, crescendo exertional angina appeared two months before the admission so that he underwent a complete cardiological examination. Prehospital therapy included: Trimetazidine 2 × 35 mg, Metoprolol 2 × 25 mg, Rosuvastatin 1 × 10 mg, Aspirine 1 × 100 mg, and Clopidogrel 1 × 75 mg, On repeated electrocardiographic (ECG) records, a sinus rhythm, with 65–70 beats/min, with rS and negative T in D3 and aVF was recorded. Cardiac exercise stress testing (Bruce protocol) was interrupted in the first minute of the second stage, with the onset of chest pain at a heart rate of 112 beats per minute and downward ST depression of 1.5 mm in leads II, III, aVF and V4–V6. Trans-thoracic echocardiography (TT-ECHO) indicated the presence of moderate trileaflet aortic valve (AoV) stenosis (Area 1.2 cm2, MPG 19 mmHg, Velocity 3.2 m/s) and mild regurgitation, with preserved left ventricular kinetics, morphology, and function (LV-EDD 5.3 cm, LV-ESD 3.3 cm, S and PV 0.9 cm, EF 65%, FS 35%). Subsequent trans-radial selective coronary angiography (Fig. 1) revealed an SCA anomaly arising from the right sinus of Valsalva. Triple vessel CA disease was present, with proximal occlusion of dominant right coronary artery (RCA), distal 70%–90% stenosis of retroaortic circumflex artery (RCx), and mid-to-distal < 50% stenosis of left anterior descending (LAD) artery. Distal RCA branches had good retrograde filling from the LAD.
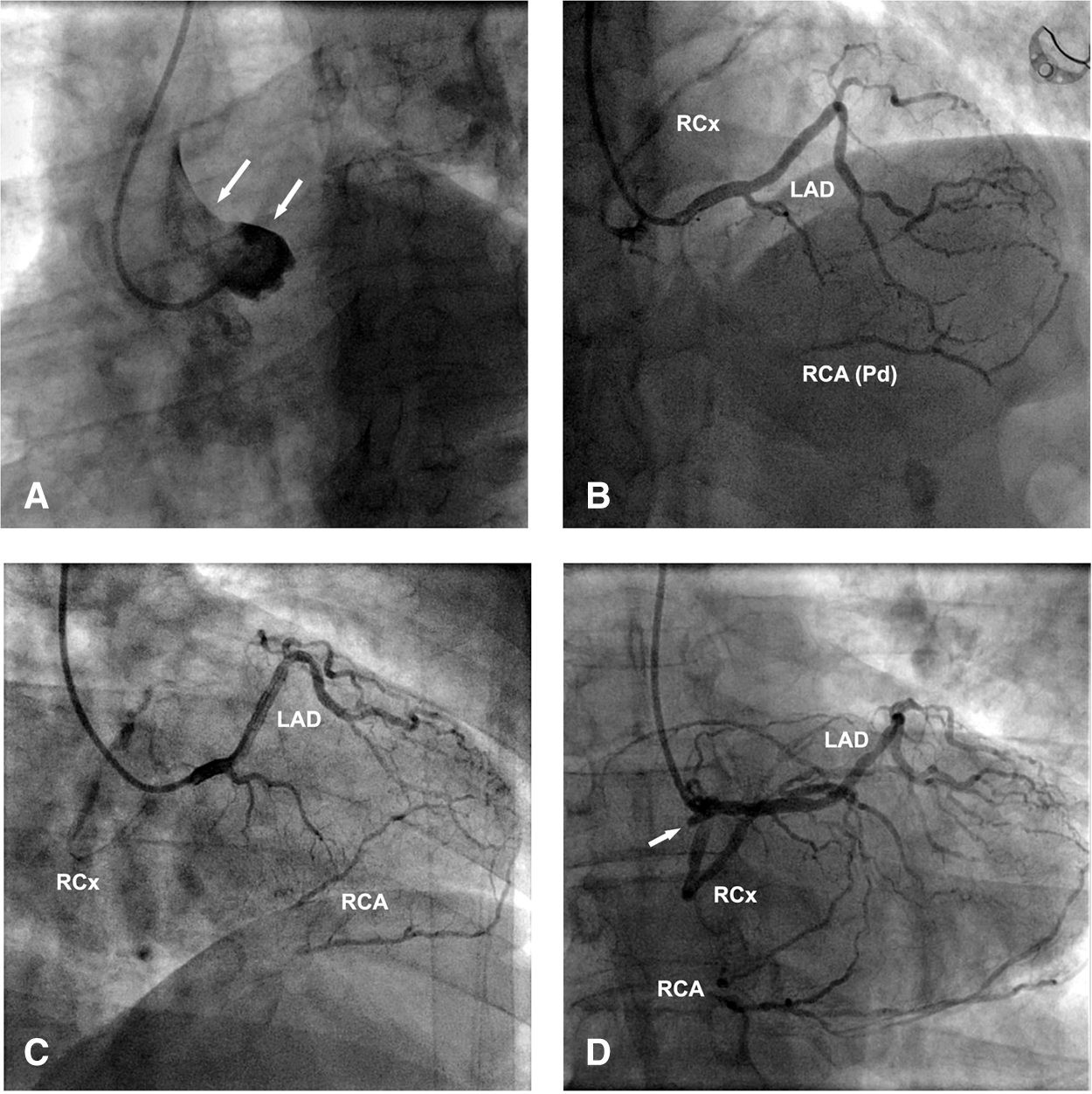
Figure 1: Selective coronary angiography: A–Absence of CAs (arrows) from the left sinus of Valsalva; B–SCA (Lipton’s R-III type) from the right sinus of Valsalva, with anterior LAD course, retroaortic RCx course (distal stenosis 70%–90%) and proximally occluded RCA (retrograde Pd filling from LAD); C–Same as B adjusted for better LAD and RCx tracing; D–Same as B adjusted for the proximal RCA stump (arrow) tracing. Legend: LAD–Left anterior descending; RCx–Circumflex; RCA–Right coronary artery
For better spatial orientation, multidetector computed tomography (MDCT) coronary angiography was performed and subsequent 3-D coronary network reconstruction was done (Fig. 2). This confirmed the retroaortic RCx course and its tight proximity to the aortic root, a fact of great importance for the forthcoming surgical procedure. Also, the anterior course of the LAD was evident, being a better option for future hemodynamics. Thus, it was possible to classify this SCA anatomy into Lipton’s R-III type. The patient was prepared and referred for combined CABG and AVR surgery.
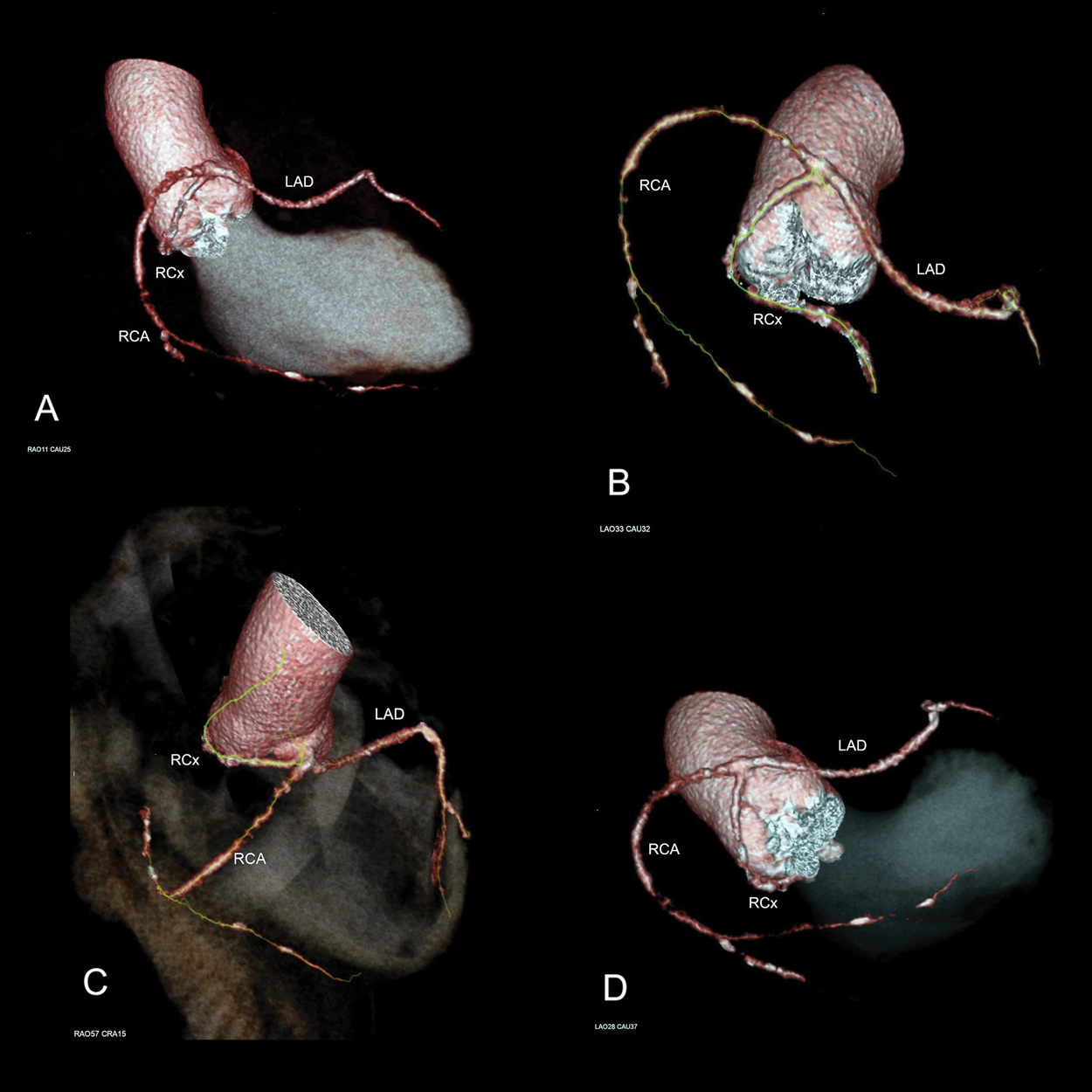
Figure 2: Multidetector computed tomography (MDCT) 3-D coronary network reconstruction: Different angles analysis to get a clear insight in distal branching patterns and particularly in retroaortic RCx course in respect to the aortic root. A–RAO: 11, Caudal: 25; B–LAO: 33, Caudal: 32; C–RAO: 57, Cranial: 15; D–LAO: 28, Caudal: 37. Legend: LAO–Left anterior oblique; RAO–Right anterior oblique
After the standard median sternotomy and heart exposure, no apparent macroscopical evidence of additional congenital heart malformation was present. Mid-to-distal portions of RCA, RCx, and LAD were easily identified at their usual locations. Partial cardiopulmonary bypass was established and the heart was arrested with cold St.Thomas cardioplegic solution. Transverse aortotomy exposed tricuspid AoV with degenerative nodular fibro-calcified changes predominantly at leaflets’ base. The free edge of the right leaflet was elongated with the cleft-like formation, probably caused by coalescing and subsequent rupture of the para-commissural fenestrations. There were no coronary ostia in the left sinus of Valsalva (Fig. 3).
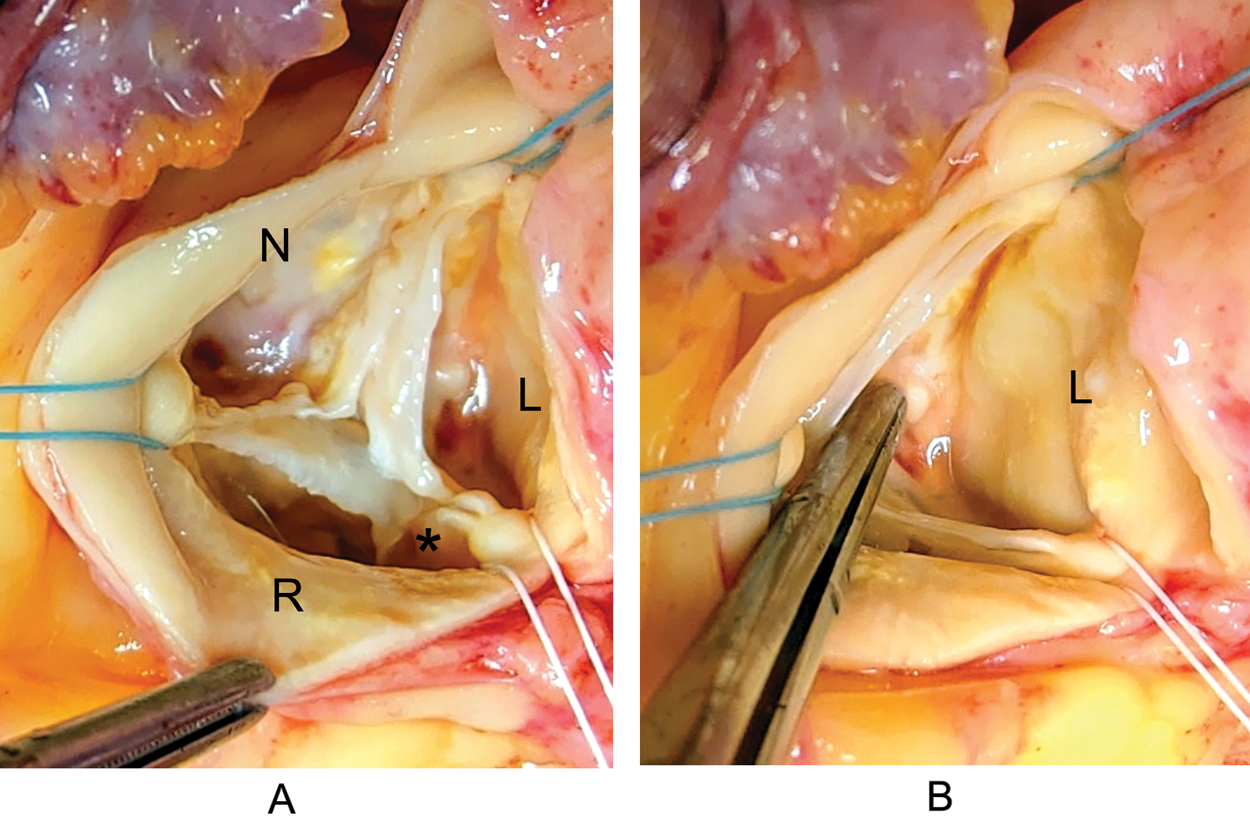
Figure 3: Aortic valve and Valsalva sinuses-intraoperatively: A–Trileaflet AoV, with degenerative nodular fibro-calcified changes predominantly at leaflets’ base. Elongation of the free edge of the R leaflet with para-commissural cleft-like formation (asterisk); B–The absence of coronary ostium in the left sinus of Valsalva. Legend: L–left; R–right; N–non-coronary
The single, wide para-commissural ostium of the common mixed trunk [1] with slightly dilated periostial aortic wall, forming a kind of secondary sinus, was identified within the right sinus of Valsalva (Fig. 4).
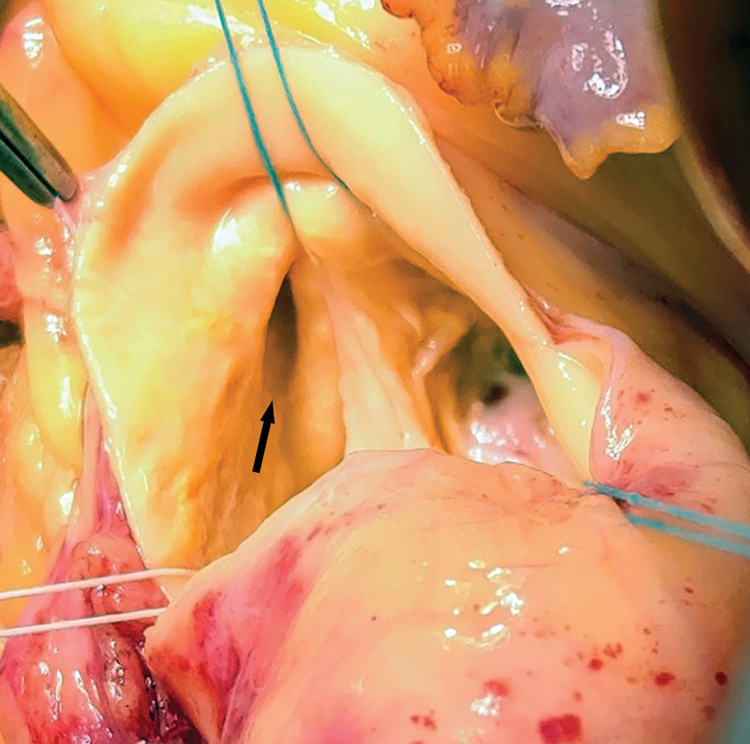
Figure 4: SCA ostium-intraoperatively: Wide para-commissural ostium of the common mixed trunk (arrow), arising from the R sinus of Valsalva. Periostial aortic wall is slightly dilated, forming a kind of secondary sinus within the R sinus of Valsalva (see also Fig. 2C)
Valvular excision and meticulous decalcification were done so that a 21 mm St. Jude Medical™ Regent™ (St. Jude Medical Inc., St. Paul, MN, USA) mechanical aortic prosthesis could be easily implanted. We decided to choose smaller (i.e., 21 mm) of two prosthetic sizes (i.e., 21 mm and 23 mm) with effective orifice area index greater than 1 cm2/m2 to avoid a “tight fit” scenario and subsequent retroaortic RCx compression. Having completed the distal anastomoses on RCx (OM1) and RCA (Pd) with saphenous vein grafts, the aortic valve prosthesis was sewn in place. Extreme care was taken not to go too deep with the stitches, to avoid the inadvertent injury of the retroaortic RCx. To ensure equal distribution during repeated cardioplegia delivery, we used the widest direct coronary cannula with a silicone cuff, to fit the single ostium. The next concern was to precisely identify the LAD course over the RV cone, to avoid its injury during the epicardial pacing wire implantation. The entire procedure was uneventful, and the patient was easily weaned from cardiopulmonary bypass. He was stable and discharged on the fifth postoperative day. Postoperative ECHO revealed the peak PG of 19 mmHg (a value which is normal for the size and type of implanted prosthesis). Four months after the surgery he is asymptomatic, and he went back to his routine activities.
The combination of underlying pathologies, clinical presentation, and surgical treatment in the case we presented here is extremely rare. Having performed an extensive literature review, we could not find a single case report of an adult patient with isolated SCA, CA disease, and AoV stenosis submitted to combined CABG and AVR surgery. Katsetos have reported a similar case in 2003, but in a patient with AoV insufficiency [8]. Kawano et al. also reported AVR for AoV insufficiency but without CABG [9,10]. In four other cases, AVR was done in patients with SCA and associated congenital anomalies [8,9].
Clinical or morphologic evidence of myocardial ischemia, as a natural risk, is present in about 15% of isolated SCA cases, without any angiographic evidence of atherosclerotic CA disease. On the other hand, 40%–45% of isolated SCA cases have significant atherosclerotic narrowing of one or more major epicardial CA [11]. Rigatelli found that benign forms of CCAA, in the absence of significant known risk factors, do not accelerate CA atherosclerosis [12]. Accordingly, the isolated SCA in our case is probably just a casual finding in a presence of CA atherosclerosis. On the other hand, Lo Rito has found that RCA with anomalous aortic origin fails to expand its lumen and increase flow at incremental loading conditions, suggesting that this may be the anatomic substrate responsible for the reduction of coronary blood flow reserve, regardless of inability to demonstrate inducible ischemia on the exercise stress testing [13]. Accordingly, if not related to atherogenesis, SCA may be a reason for different reactivity of the CA network, facilitating the clinical presentation of accompanying atherosclerotic CA disease.
Beyond SCA and CA disease, the third part of this complex underlying pathology was moderate AoV stenosis. In a series of 1099 consecutive patients submitted to AoV surgery, Naito found CCAA in 4% of cases, but none of them with SCA. They believe that younger patients, who were referred for AoV surgery, had a certain congenital component in their AoV disease, leading towards a higher prevalence of anomalous coronary ostium, thus giving a possible causal link for these pathologies. He could not find any significant correlation between CCAA and functional AoV lesions, but they did so for the root-type aortopathy [14]. As the risk factors for atherosclerosis and degenerative disease of the trileaflet AoV are similar [15], it is reasonable to consider that the presence of SCA in our case is only a casual finding. However, the complex embryological blueprint is linking the aortic root and proximal CAs, thus making different anomalous origins and courses of CAs commonly associated with aortic root malformations and patterning anomalies of great arteries [16,17]. Accordingly, a slightly dilated periostial aortic wall, forming a kind of secondary sinus within the right sinus of Valsalva, is probably congenital (see Figs. 4 and 2C). Its presence may subsequently induce adaptive modification and degenerative changes of the AoV, by changing the stress loading of the aortic valve leaflets [9,15,18]. Thus, again, the line between casual and causal becomes obscure.
Invasive coronary angiography remains the gold standard in patients with a high clinical likelihood of CA disease but has limited ability in demonstrating the anatomy of complex CCAA. Accordingly, whenever it accidentally reveals the presence of CCAA, an additional preoperative MDCT coronary angiography is an excellent complementary technique to precisely delineate the origin, branching patterns, and the course of the anomalous arteries [19]. In our case (Fig. 2), MDCT revealed three important SCA features, being specific procedural risks: 1) very short common mixed trunk-bearing possible difficulties during the direct cardioplegia delivery, 2) anterior LAD course-being prognostically better than interarterial course but in the risk of injury during the epicardial pacing, and 3) retroaortic RCx course-running very close to the aortic root and in the risk of injury during and after AoV implantation.
Isolated SCA is an extremely rare, neither benign nor malignant form of CCAA, carrying both the natural risk for sudden cardiac death of 23% and significant procedural risks if casually and/or causally associated with CA and/or AoV diseases. As for all other CCAA, both invasive and MDCT coronary angiography is mandatory for patients with isolated SCA. By presenting the patient who underwent successful combined CABG and AVR surgery in a presence of Lipton’s R-III type of isolated SCA, we emphasized the importance of meticulous, case-specific preoperative analysis, to ensure the optimal outcome.
Ethical Statement: The manuscript was prepared with consent from the patient.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Angelini, P., Villason, S., Chan, A. V., Diez, J. G. (1999). Normal and anomalous coronary arteries in humans. In: Angelini, P., Fairchild, V. D. (eds.pp. 27–79, Coronary artery anomalies: A comprehensive approach. Lippincott Williams & Wilkins. [Google Scholar]
2. Pérez-Pomares, J. M., de la Pompa, J. L., Franco, D., Henderson, D., Ho, S. Y. et al. (2016). Congenital coronary artery anomalies: A bridge from embryology to anatomy and pathophysiology--A position statement of the development, anatomy, and pathology ESC Working Group. Cardiovascular Research, 109(2), 204–216. [Google Scholar]
3. Kočica, M., Karadžić, M., Grujić, M., Cvetković, D., Šoškić, L. (2018). Anomalous aortic origin of right and circumflex coronary arteries–procedural risks during combined aortic valve replacement and coronary artery bypass grafting. Serbian Archives of Medicine, 146(7–8), 440–444. [Google Scholar]
4. Mavroudis, C., Dodge-Khatami, A., Stewart, R. D., Jacobs, M. L., Backer, C. L. et al. (2010). An overview of surgery options for congenital coronary artery anomalies. Future Cardiology, 6(5), 627–645. [Google Scholar]
5. Shah, J. R., Priya, C., Om, T. (2011). Single coronary artery: Classification and MDCTA diagnosis. European Journal of Radiology, 77(1), e1–e4. [Google Scholar]
6. Lipton, M. J., Barry, W. H., Obrez, I., Silverman, J. F., Wexler, L. et al. (1979). Isolated single coronary artery: Diagnosis, angiographic classification, and clinical significance. Radiology, 130(1), 39–47. [Google Scholar]
7. Angelini, P. (2004). A casual versus causal relationship in coronary artery anomalies: A question of method. Texas Heart Institute Journal, 31(3), 276–277. [Google Scholar]
8. Katsetos, M. C., Toce, D. T. (2003). Single coronary artery with aortic regurgitation. Cardiovascular and Interventional Radiology, 26(6), 567–568. [Google Scholar]
9. Kawano, H., Hayashi, T., Minami, T., Koide, Y., Eishi, K. et al. (2012). Cleft-like formation of the aortic valve in an adult patient with a single coronary artery. Internal Medicine, 51(17), 2341–2345. [Google Scholar]
10. Gallo, M., Lika, A., Gerosa, G., Colli, A. (2013). Aortic valve replacement in a single coronary artery arising from the right Valsalva sinus. European Journal of Cardio-Thoracic Surgery, 43(5), e141. [Google Scholar]
11. Shirani, J., Roberts, W. C. (1993). Solitary coronary ostium in the aorta in the absence of other major congenital cardiovascular anomalies. Journal of the American College of Cardiology, 21(1), 137–143. [Google Scholar]
12. Rigatelli, G., Gemelli, M., Zamboni, A., Docali, G., Rossi, P. et al. (2004). Are coronary artery anomalies an accelerating factor for coronary atherosclerosis development? Angiology, 55(1), 29–35. [Google Scholar]
13. Lo Rito, M., Romarowski, R. M., Rosato, A., Pica, S., Secchi, F. et al. (2021). Anomalous aortic origin of coronary artery biomechanical modeling: Toward clinical application. Journal of Thoracic and Cardiovascular Surgery, 161(1), 191–201.e1. [Google Scholar]
14. Naito, S., Petersen, J., Reichenspurner, H., Girdauskas, E. (2018). The impact of coronary anomalies on the outcome in aortic valve surgery: Comparison of bicuspid aortic valve versus tricuspid aortic valve morphotype. Interactive CardioVascular and Thoracic Surgery, 26(4), 617–622. [Google Scholar]
15. Robicsek, F., Thubrikar, M. J., Fokin, A. A. (2002). Cause of degenerative disease of the trileaflet aortic valve: Review of subject and presentation of a new theory. Annals of Thoracic Surgery, 73(4), 1346–1354. [Google Scholar]
16. Angelini, P., Velasco, J. A., Flamm, S. (2002). Coronary anomalies: Incidence, pathophysiology, and clinical relevance. Circulation, 105(20), 2449–2454. [Google Scholar]
17. Tomanek, R. J. (1996). Formation of the coronary vasculature: A brief review. Cardiovascular Research, 31(supp1), E46–51. [Google Scholar]
18. Robicsek, F., Thubrikar, M. J. (1999). Role of sinus wall compliance in aortic leaflet function. American Journal of Cardiology, 84(8), 944–946, a947. [Google Scholar]
19. Al Umairi, R., Al-Khouri, M. (2019). Prevalence, spectrum, and outcomes of single coronary artery detected on Coronary Computed Tomography Angiography (CCTA). Radiology Research and Practice, 2019, 2940148. [Google Scholar]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |