

 | Congenital Heart Disease |  |
DOI: 10.32604/chd.2022.018300
META-ANALYSIS
Prevalence of Bicuspid Aortic Valve in Turner Syndrome Patients Receiving Cardiac MRI and CT: A Meta-Analysis
Department of Pediatric Cardiology and Intensive Care, Medical Hospital of the University of Munich, Ludwig Maximilians University Munich, Munich, Germany
*Corresponding Author: Felix Sebastian Oberhoffer. Email: Felix.Oberhoffer@med.uni-muenchen.de
Received: 14 July 2021; Accepted: 29 October 2021
Abstract: Turner syndrome (TS) is a rare disorder affecting 25–50 in 100000 female newborns. Bicuspid aortic valve (BAV) is assumed to be the most common congenital heart defect (CHD) in TS. In literature, reported BAV prevalence in TS ranges between 14% and 34%. The specific BAV prevalence in TS is still unknown. The aim of this study was to give a more precise estimation of BAV prevalence in TS by conducting a meta-analysis of TS-studies, which detected BAV by either cardiac magnetic resonance imaging (MRI) or cardiac computed tomography (CT). We searched PubMed, Cochrane Library, and Web of Science databases to collect observational studies including the prevalence of BAV identified by cardiac MRI or cardiac CT in TS patients up to June 4th, 2021. After screening for inclusion, data extraction, and quality assessment by two independent reviewers, the meta-analysis was performed with R 4.1.1 software. Results are shown as proportion and weighted mean difference with 95% confidence intervals (95% CI). In total, 11 studies involving 1177 patients were included. Pooled data showed that the prevalence of BAV in TS patients was 23.7% (95% CI: 21.3% to 26.1%). No high heterogeneity was found between the included studies. The current meta-analysis reveals that BAV can be detected in 23.7% of TS patients receiving cardiac MRI or cardiac CT. Therefore, BAV can be considered as the most common CHD in TS. Compared to TTE, cardiac MRI and cardiac CT might represent superior imaging modalities in BAV assessment of adult TS patients.
Keywords: Turner syndrome; bicuspid aortic valve; magnetic resonance imaging (MRI); tomography; X-ray computed (CT)
| Abbreviations | |
| BAV: | Bicuspid aortic valve |
| CHD: | Congenital heart defect |
| CI: | Confidence interval |
| CME-SAM: | Continuing medical education-self-assessment module |
| CT: | Computed tomography |
| I2: | Inconsistency |
| IQR: | Interquartile range |
| MAStARI: | Meta-analysis of statistics assessment and review instrument |
| MRI: | Magnetic resonance imaging |
| PRISMA: | Preferred reporting items for systematic reviews and meta-analyses |
| RoB: | Risk of bias |
| SD: | Standard deviation |
| SE: | Standard error |
| TAV: | Tricuspid aortic valve |
| TEE: | Transesophageal echocardiography |
| TS: | Turner syndrome |
| TTE: | Transthoracic echocardiography |
Turner syndrome (TS) is a rare chromosomal disorder caused by a complete or partial absence of an X chromosome with an incidence of 25–50 in 100,000 live-born females [1,2]. Mortality in TS patients is reported to be up to threefold higher than in the general population [3]. The elevated cardiovascular morbidity is considered to be the main contributor leading to increased mortality [3]. Approximately half of TS patients display congenital heart defects (CHD) [4]. According to the latest clinical practice guidelines for the care of girls and women with TS, bicuspid aortic valve (BAV) is the most common CHD in TS affecting between 14–34% of individuals [1]. Aortic dilatation and aortic dissection, which are both associated with the presence of BAV, are common in TS [5]. The management of these acquired aortic conditions is crucial for the cardiovascular outcome of TS patients [6]. Regarding the cardiovascular care of TS girls and women, a more precise specification of BAV prevalence would be desirable.
Transthoracic echocardiography (TTE) is conventionally used to screen for BAV [7]. However, TS is often accompanied by excess weight and chest wall anomalies, particularly in adult subjects, hampering optimal echocardiographic image quality and thus the diagnosis of BAV [8]. Cardiac magnetic resonance imaging (MRI) has been shown to be potentially superior in the diagnosis of BAV compared to conventional TTE in an adult cohort [9]. Besides cardiac MRI, Alkadhi et al. [10] were able to demonstrate that cardiac computer tomography (CT) is highly accurate for the differentiation between BAV and tricuspid aortic valve (TAV).
The aim of this study was to give a more precise estimation of BAV prevalence in TS by conducting a meta-analysis of TS studies, which detected BAV by either cardiac MRI or cardiac CT.
Our study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. The protocol was registered at PROSPERO (Registration No. CRD42021242223).
The inclusion criteria were as follows: (1) patients diagnosed with TS; (2) presence of BAV in TS patients was evaluated by cardiac MRI or cardiac CT; (3) the prevalence of BAV in TS patients was reported, possible to calculate or possible to extrapolate. Exclusively English publications were searched, and no publication date restriction was defined. Any research type included relevant cross-sectional data was included.
The exclusion criteria were the following: (1) the inclusion criteria were not met; (2) the prevalence of BAV in TS patients was neither reported nor possible to calculate nor possible to extrapolate; (3) studies excluding TS patients who underwent cardiac surgery; (4) studies on specific TS karyotypes; (5) studies only containing pregnant TS patients; (6) reviews, meta-analyses, case reports, letters, conference abstracts, or protocols; (7) repeated publication of the same study population.
We searched the databases of PubMed, Cochrane Library, and Web of Science databases as of June 4th, 2021.
We searched for the following medical subject heading terms and free-text terms individually or in combination: “Turner Syndrome” or “Turner’s Syndrome” and “aortic valve” or “aortic valves” and “Magnetic Resonance Imaging” or “MRI” or “Tomography, X-Ray Computed” or “CT”. The search strategies for each database are available in Table 1.
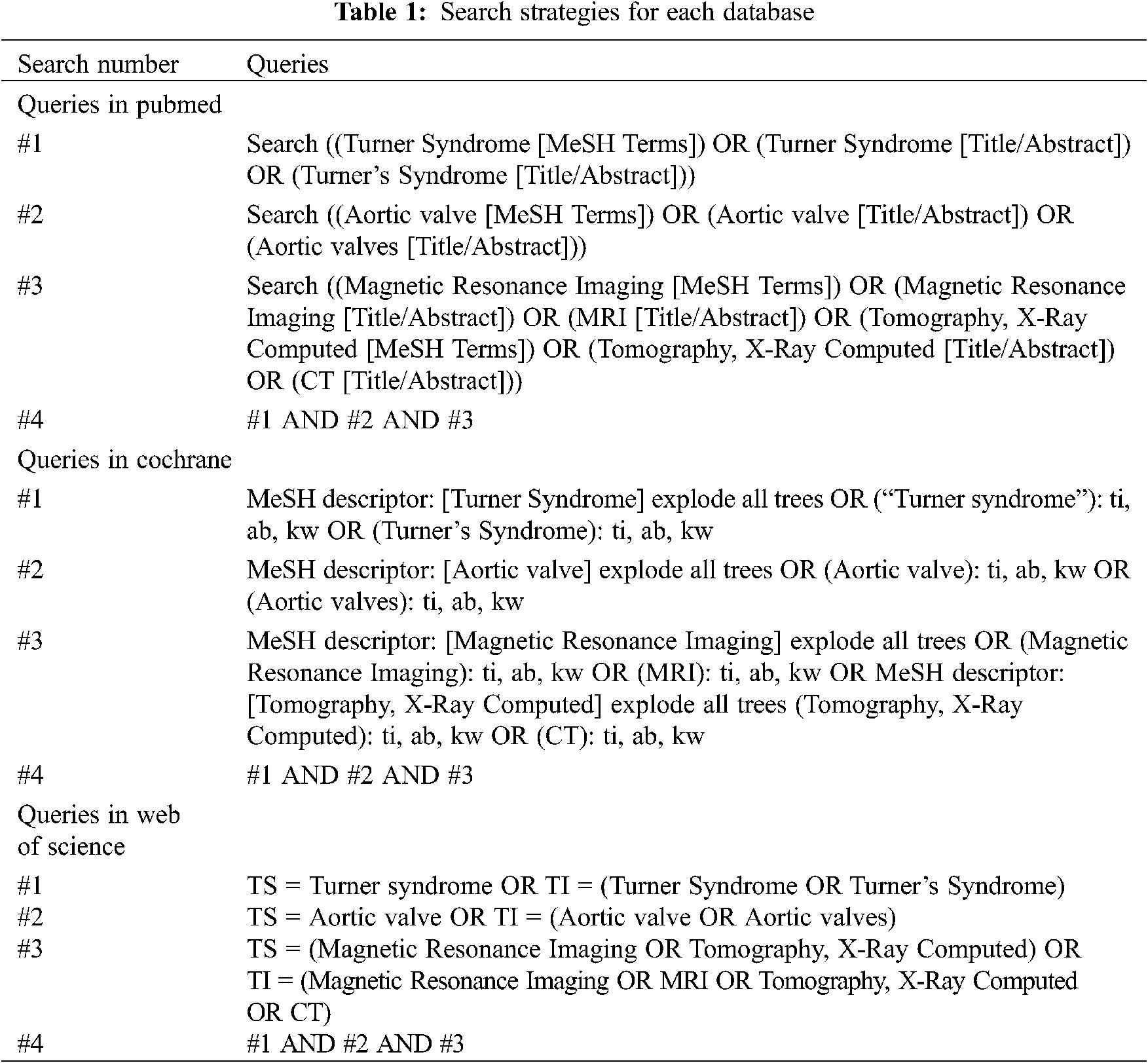
Two researchers independently screened titles and abstracts identified from the search strategy. Records that met all inclusion criteria were fully read by the same two researchers. Full texts that did not meet the inclusion criteria were excluded. Any disagreements between the two researchers were mediated through discussions with a third researcher to reach a final decision.
2.5 Data Collection Process and Items
Similarly, data was independently extracted from all the included articles by two researchers. Any disagreements concerning the availability of data were settled through discussion with a third researcher. From each study, first author, years of publication, country, case number, age distribution, and BAV prevalence were extracted. For studies that did not give BAV prevalence but only the number of TS patients with BAV, prevalence was manually calculated.
2.6 Study Risk of Bias Assessment
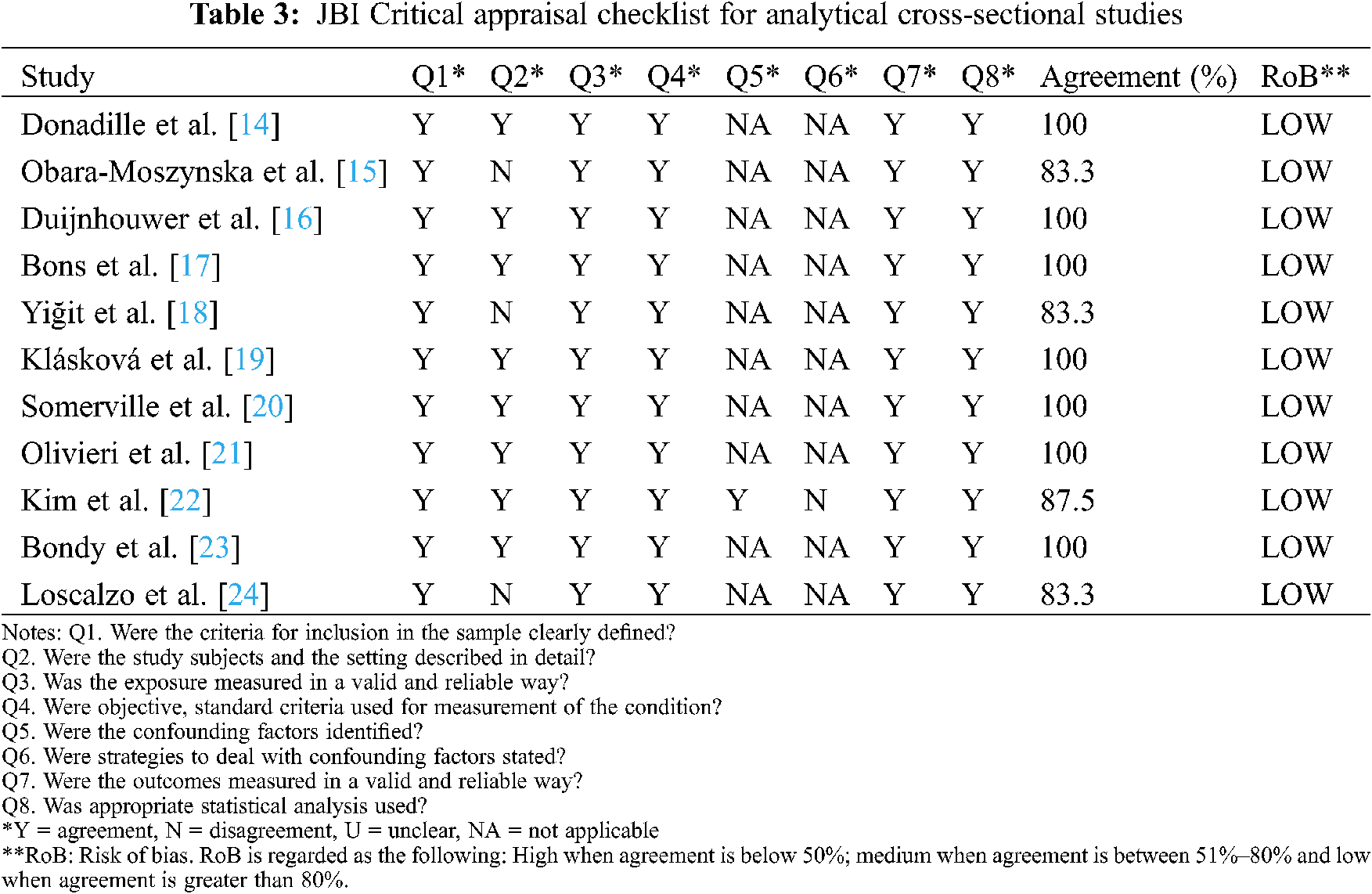
We used JBI Critical Appraisal checklist for Analytical Cross-Sectional Studies to evaluate studies’ risk of bias (RoB). Two researchers did the assessment independently. For non-cross-sectional-studies, RoB was determined by considering the cross-sectional study portion. RoB was regarded as the following: High when agreement is below 50%; medium when agreement is between 51%–80% and low when agreement is greater than 80%. If the two researchers disagreed on any aspect of the quality assessment, a third researcher provided his assessment to resolve the disagreement.
Estimated BAV prevalence was measured. R 4.1.1 software was used to conduct quantitative analyses of the included studies and to conduct the final meta-analysis. The result was reported with 95% confidence intervals (95% CI). The heterogeneity was calculated using inconsistency indexes (I2). In consistence with criteria used by previous meta-analyses, an I2 > 50% was determined as an indicator of substantial heterogeneity between studies [11,12]. If the heterogeneity was significant, a random-effects model was used. For homogeneity, a fixed-effects model was applied [13]. The significance level was set at 0.05. Funnel plot, Egger’s and Macaskill’s tests were used to indicate potential publication bias. A p-value < 0.05 was considered statistically significant.
The initial search consisted of 161 records. After excluding 55 duplicates, two researchers reviewed the titles and abstracts and excluded 57 records based on the type of article and related biases. The remaining 49 records were fully reviewed by the same two researchers and were selected according to the relevant inclusion and exclusion criteria. Ultimately, 11 articles were considered relevant for further analysis (Fig. 1).
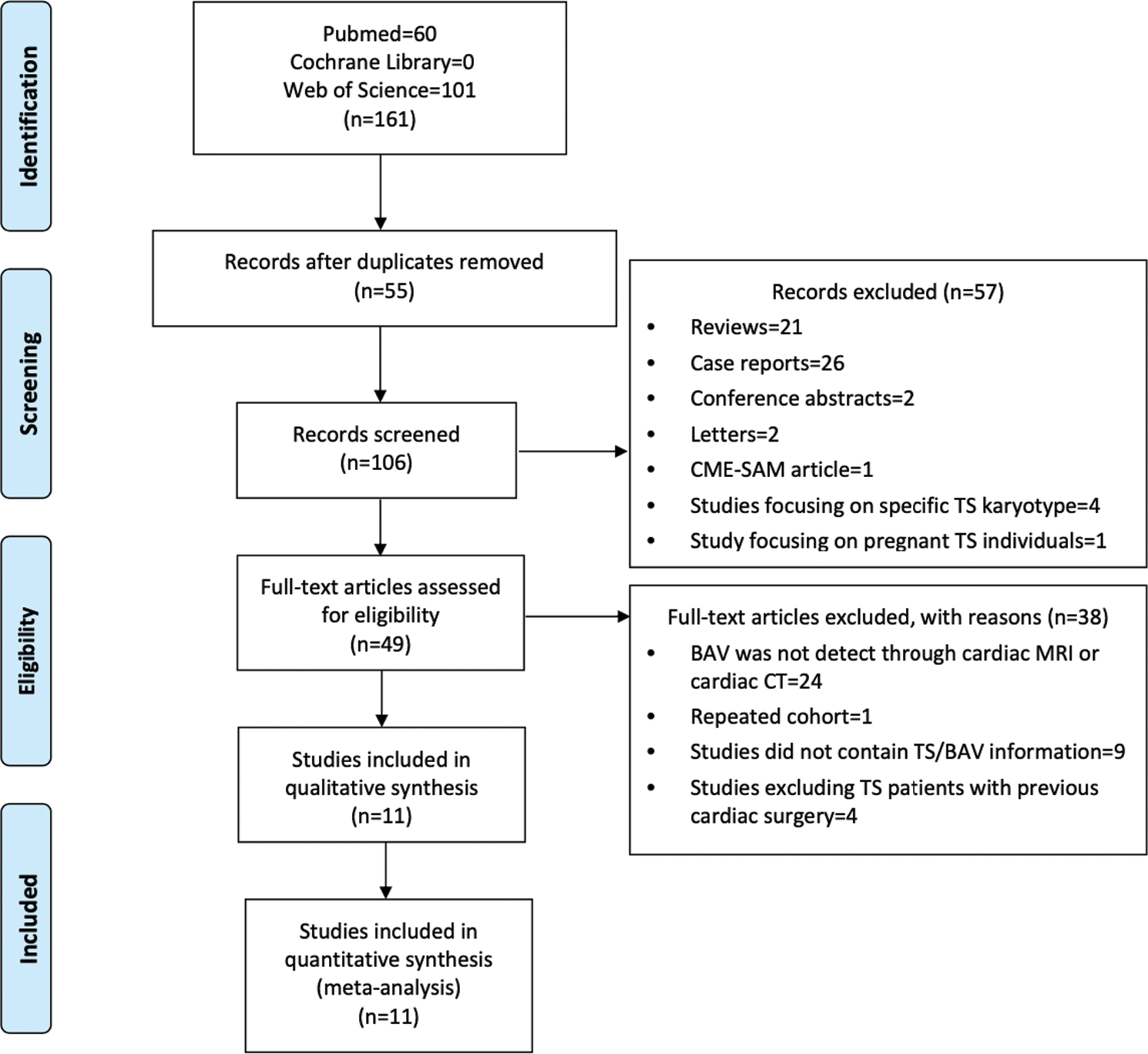
Figure 1: Preferred reporting items for meta-analysis diagram. BAV; bicuspid aortic valve. CME-SAM; continuing medical education-self-assessment module. CT; computed tomography, MRI; magnetic resonance imaging, TS; Turner syndrome
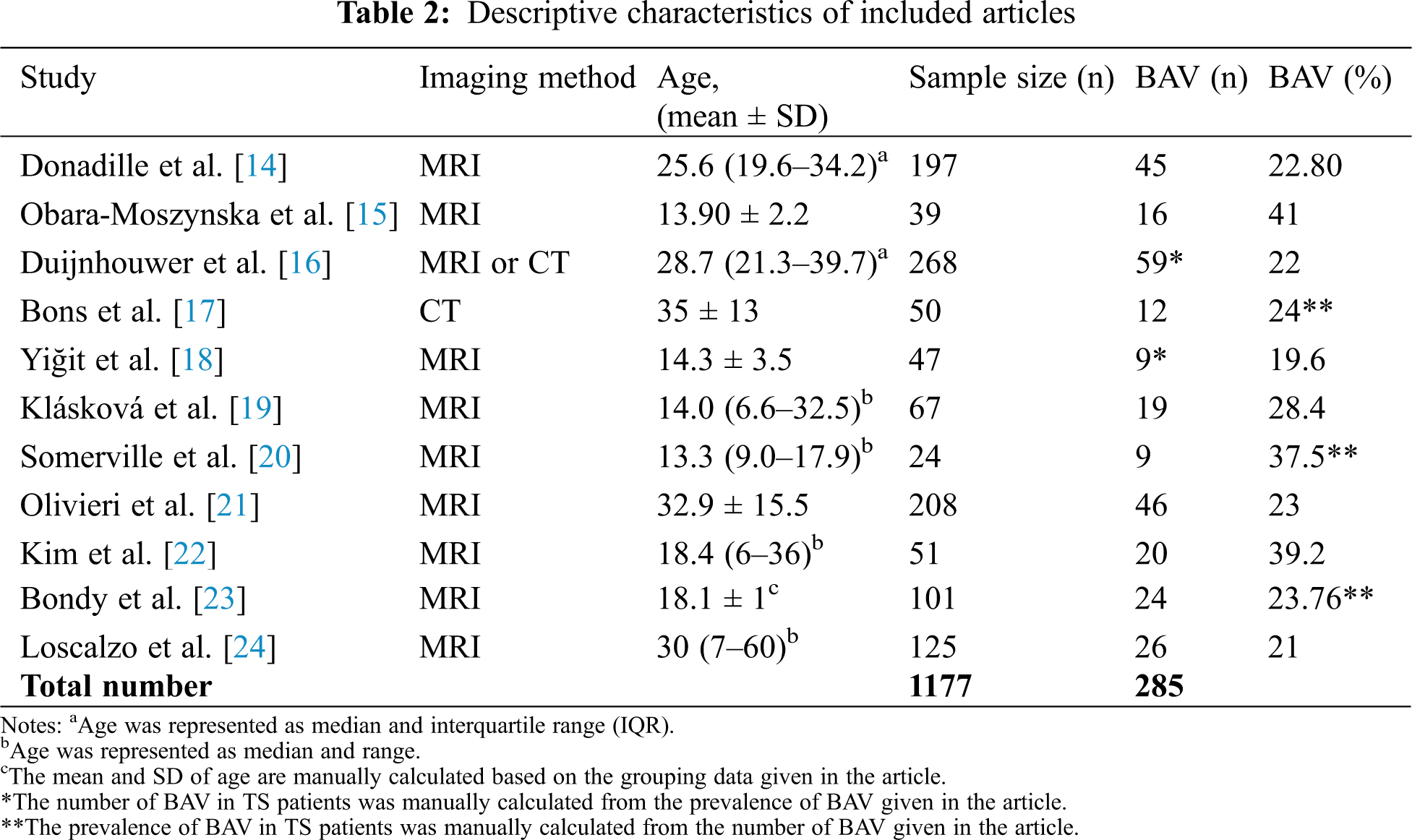
The included studies examined 1177 patients ranging from the age of 6 to 67 years. The sample size of the selected studies ranged from 24 to 268 participants. The study population consisted of TS patients from multiple countries including France, Poland, Czech Republic, the Netherlands, Turkey, Canada, and the USA. One study used cardiac CT to evaluate TS patients; one study used cardiac MRI and cardiac CT to evaluate patients, while nine studies solely used cardiac MRI. Table 2 provides detailed information on TS sample size, TS age distribution, the applied imaging method, number of TS patients with BAV, and BAV prevalence.
3.3 Quality of Eligible Studies
All 11 studies were shown to present low RoB according to the JBI Critical Appraisal checklist. Consequently, no article was excluded from the meta-analysis for quality reasons (Table 3).
Eleven studies evaluated the prevalence of BAV in TS and contained sufficient data to be included in our quantitative analysis (meta-analysis). These studies comprised 1177 TS patients. The BAV prevalence was analyzed for all studies. An I2 of 32.2% demonstrated a lack of high heterogeneity between the studies. Results from the meta-analysis indicated a BAV prevalence of 23.7% (CI: 21.3% to 26.1%) in the studied TS patients (Fig. 2). The funnel chart visualized no significant publication bias of the included studies (Fig. 3). Macaskill’s test result showed a p-value of 0.1228 implying no significant publication bias. However, Egger’s test result showed a p-value of 0.0088 indicating a potential publication bias.
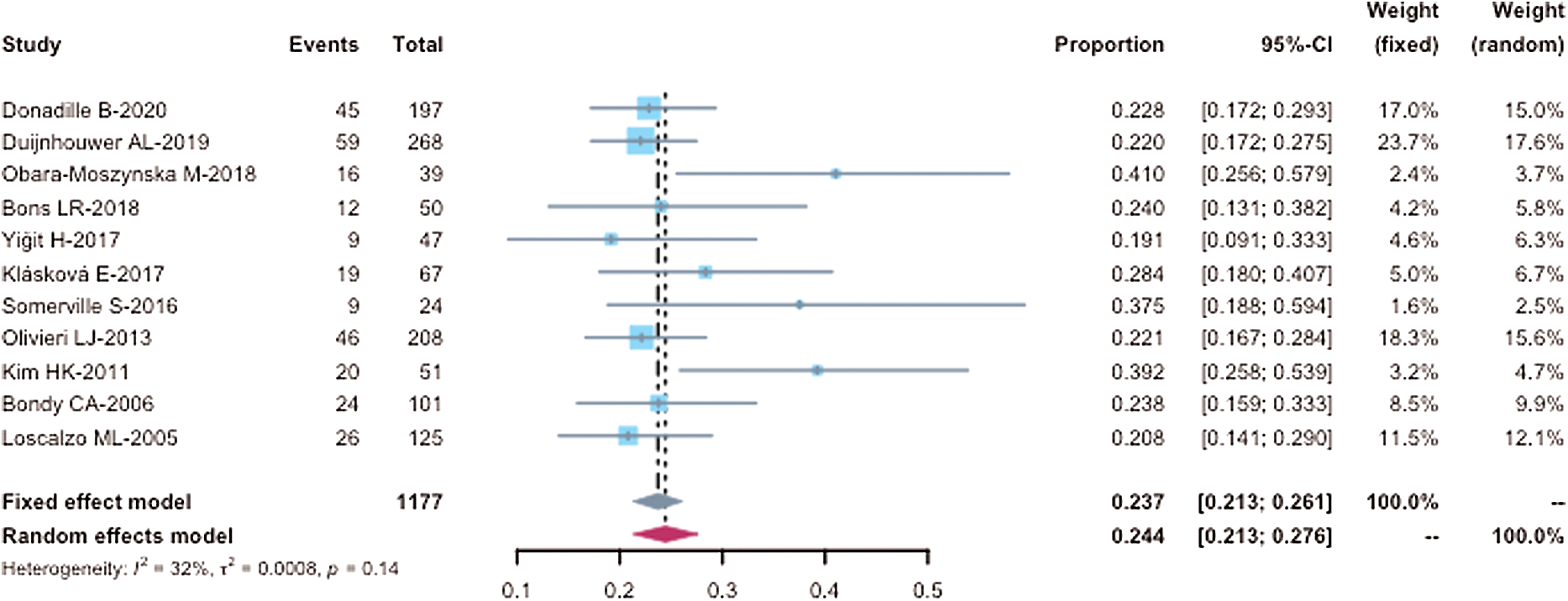
Figure 2: Forest plot of BAV prevalence in TS patients (n = 1177) receiving cardiac MRI or cardiac CT. CI, confidence intervals. Events, numbers of TS patents with BAV in each study; Total, total number of TS patients in each study; Proportion, BAV prevalence of TS patients in each study. Within the meta-analysis, the size of squares corresponds to the study weight. The diamond plot represents the overall results of the included studies. The horizontal lines represent the 95% CI
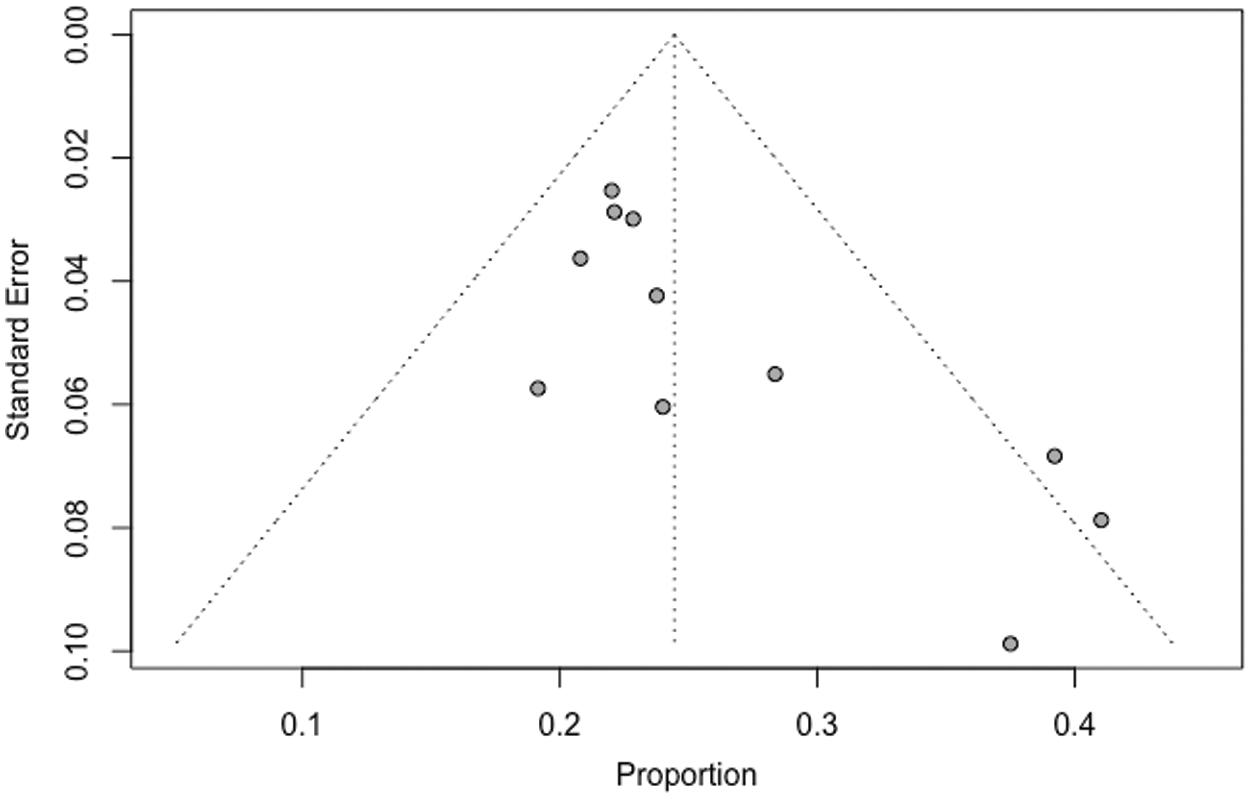
Figure 3: Funnel plot of BAV prevalence in TS patients receiving cardiac MRI and cardiac CT. SE; standard error = (P(1–P)/n)1/2. The symmetric shape of the funnel plot suggests no publication bias
To the best of our knowledge, this is the first meta-analysis investigating BAV prevalence in TS. Our study reveals that BAV can be detected in 23.7% of TS patients receiving cardiac MRI or cardiac CT. Therefore, BAV can be considered as the most common CHD in TS.
4.1 Cardiovascular Morbidity and Bicuspid Aortic Valve in Turner Syndrome
Mortality in TS patients is reported to be up to Threefold higher than in the general population [3]. The elevated cardiovascular morbidity is considered the main contributor leading to increased mortality [1,3]. TS is assumed to be accompanied by a general vasculopathy [25]. In addition, cardiovascular and cardiometabolic risk factors like excess weight or lipid anomalies are commonly seen in TS subjects, potentially leading to early atherosclerosis and further arterial stiffening [26]. A variety of CHD (e.g., BAV, coarctation of the aorta, partial anomalous pulmonary venous connection) can be found in TS [1]. This meta-analysis suggests that BAV is the most common CHD affecting approximately one out of four TS females. Changes in ascending aortic blood flow characteristics due to valve malformations (e.g., BAV) are widely considered to be one of the causes of aortic dilation and aortic dissection [27]. Compared to healthy controls, Hope et al. [28] were able to demonstrate that helical flow patterns which lead to increased wall stress can be observed during ascending aortic systole in patients with BAV. Additionally, coherent intrinsic factors engaged in the pathophysiology of both BAV and aortic wall deformations can be assumed [29]. During embryonic development, the aortic root and the aortic valve originate from the second heart field cells. The NOTCH1 gene has been shown to be substantially involved in TAV development [30]. Mutations in the NOTCH1 gene are associated with the presence of BAV and the occurrence of aortic wall deformations [31]. Within TS patients, Abu-Halima et al. [32] found that the abundance level of miR-126-3p in subjects with congenital aortic valve disease (including monocuspid aortic valve and BAV) was significantly lower compared to patients with TAV. Moreover, the abundance level of miR-126-3p significantly correlated with ascending aortic dimensions [32]. BAV is associated with acquired cardiovascular alterations which are most commonly seen in adult TS patients but can also be found in young TS subjects: Recent studies confirmed that TS patients with BAV display an increased prevalence of aortic dilatation [33]. Aortic dilatation itself can lead to chronic aortic regurgitation as well as spontaneous rupture and dissection of the aorta [34]. Compared to the general population, risk for acute aortic dissection is elevated by more than 100-fold in TS [35]. Within the TS cohort, Thunström et al. [36] were able to demonstrate that the existence of BAV or aortic coarctation is linked with a more than fourfold increased risk of aortic dissection. Hence, the precise assessment of BAV is crucial to discover TS patients at highest risk for acquired aortic complications.
4.2 Assessment of Bicuspid Aortic Valve in Turner Syndrome
4.2.1 Transthoracic and Transesophageal Echocardiography
The crucial role of TTE in the screening for CHD and in the assessment of cardiovascular function in TS patients should be pointed out. According to the clinical practice guidelines for the care of girls and women with TS, TTE should be performed at the onset of TS diagnosis to screen for CHD [1]. Besides BAV, TS is associated with multiple other CHD (e.g., coarctation of the aorta, partial anomalous pulmonary venous connection). Particularly in neonates and young children, TTE profits from a great echocardiographic window and therefore represents an excellent imaging modality to assess CHD and monitor cardiovascular function. TTE’s sensitivity in detecting CHD highly depends on the operator’s experience, underlining the essential role of cardiovascular care in eligible TS centers. In addition, TTE is cost-effective and does not require sedation, making it easily applicable in the pediatric and adult outpatient care. However, TS is often accompanied by excess weight and chest wall anomalies which lead to suboptimal echocardiographic image quality, particularly in adults subjects, and thus complicates BAV diagnosis at advanced age [37]. Hillebrand et al. [38] were able to reveal in a group of adult study participants that sensitivity of routine TTE for BAV is only 46.3% when performed by a primary investigator and only 59.7% when TTE recordings were additionally reevaluated by an expert. The low sensitivity of TTE might be one reason for the relatively low prevalence of BAV reported in previous TS studies that used TTE to assess cardiovascular malformations [39–42]. In these TS studies, BAV prevalence ranged between 12% and 18% and is hence distinctly lower than the BAV prevalence shown in the current meta-analysis [39–42]. In addition, the time of study conduction and the technological advances substantially influence the echocardiographic sensitivity of BAV in TS. Recent echocardiographic TS studies demonstrated a much higher BAV prevalence between 22% and 39% [43,44]. To overcome the limitation of suboptimal image quality, transesophageal echocardiography (TEE) might be applicable. With a sensitivity of 78%, TEE was shown to be superior in detecting BAV compared to cardiac MRI, with a sensitivity of 75%, and cardiac CT with a sensitivity of 67% in an adult study cohort [45]. However, sedation of the patient is required to carry out TEE and thus its practicality as a potential screening tool for the assessment of BAV is questionable. To the best of our knowledge, there are no studies that have investigated the presence of cardiovascular malformations in TS through TEE yet.
4.2.2 Cardiac MRI and Cardiac CT
The “Clinical practice guidelines for the care of girls and women with Turner syndrome” recommend that cardiac MRI is conducted in TS patients as soon as general anesthesia is not required [1]. If cardiac MRI is not tolerated or in case of emergency, the guidelines name cardiac CT as a further imaging option [1]. In the diagnosis of BAV, cardiac MRI and cardiac CT have been shown to be potentially superior compared to conventional TTE in adult patients [10,45,46]. In addition to the increased BAV sensitivity, cardiac MRI and cardiac CT are able to accurately visualize the entire aorta and give additional information on vascular function and flow dynamics [47]. Considering the particular aortic morbidity and the increased prevalence of CHD, cardiac MRI and cardiac CT represent excellent imaging modalities for the acute and chronic cardiovascular care of TS girls who do not require general anesthesia as well as adult women with TS [1].
The total number of included TS subjects in this meta-analysis can be considered as relatively small. Nevertheless, regarding the low incidence of TS, we believe the total number of included subjects to be adequate. As the sensitivity of detecting BAV with cardiac MRI and cardiac CT are 75% and 67% respectively, the BAV prevalence of 23.7% in TS shown in this meta-analysis might underestimate the “true” BAV prevalence [45]. In contrast, the potential publication bias of TS patients with a history of CHD who underwent advanced imaging might lead to a possible overestimation of “true” BAV prevalence. Due to the fact that primarily retrospective and cross-sectional studies were selected for this meta-analysis, some bias may be present. While the funnel chart and the Macaskill’s test result did not demonstrate significant publication bias, the Egger’s test result indicated potential publication bias. Our results show that the heterogeneity of BAV prevalence between the included studies is fairly small, suggesting that cardiac MRI and cardiac CT offer reproducible results in the detection of BAV in TS.
This meta-analysis reveals that BAV can be detected in 23.7% of TS patients receiving cardiac MRI or cardiac CT. BAV can be considered as the most common CHD in TS. Compared to TTE, cardiac MRI and cardiac CT might represent superior imaging modalities in BAV assessment of adults TS patients. In the future large multicenter studies are required which evaluate BAV prevalence in TS patients simultaneously through different imaging modalities (cardiac MRI, cardiac CT, TEE, and TTE).
Availability of Data and Materials: All articles and data can be obtained by searching relevant terms in the databases of Pubmed, Cochrane, and Web of Science.
Acknowledgement: We would like to thank Sabine Hoffmann for statistical assistance. We would like to thank Megan Crouse for editorial assistance.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Gravholt, C. H., Andersen, N. H., Conway, G. S., Dekkers, O. M., Geffner, M. E. et al. (2017). Clinical practice guidelines for the care of girls and women with Turner syndrome: Proceedings from the 2016 Cincinnati International Turner Syndrome Meeting. European Journal of Endocrinology of the European Federation of Endocrine Societies, 177(3), G1–G70. DOI 10.1530/EJE-17-0430. [Google Scholar] [CrossRef]
2. Sybert, V. P., McCauley, E. (2004). Turner’s syndrome. New England Journal of Medicine, 351(12), 1227–1238. DOI 10.1056/NEJMra030360. [Google Scholar] [CrossRef]
3. Schoemaker, M. J., Swerdlow, A. J., Higgins, C. D., Wright, A. F., Jacobs, P. A. (2008). Mortality in women with turner syndrome in great Britain: A national cohort study. The Journal of Clinical Endocrinology and Metabolism, 93(12), 4735–4742. DOI 10.1210/jc.2008-1049. [Google Scholar] [CrossRef]
4. Korkmaz, Ö., Savas, R., Levent, E., Ozen, S., Mecidov, İ. et al. (2019). The role of cardiac magnetic resonance İmaging in determination of cardiovascular anomalies in children and young adults with Turner syndrome. The Journal of Pediatric Research, 6(3), 203–207. DOI 10.4274/jpr.galenos.2018.63439. [Google Scholar] [CrossRef]
5. Mortensen, K. H., Wen, J., Erlandsen, M., Trolle, C., Ringgaard, S. et al. (2019). Aortic growth rates are not increased in Turner syndrome-a prospective CMR study. European Heart Journal Cardiovascular Imaging, 20(10), 1164–1170. DOI 10.1093/ehjci/jez065. [Google Scholar] [CrossRef]
6. Silberbach, M., Roos-Hesselink, J. W., Andersen, N. H., Braverman, A. C., Brown, N. et al. (2018). Cardiovascular health in Turner syndrome: A scientific statement from the American heart association. Circulation: Genomic and Precision Medicine, 11(10), e000048. DOI 10.1161/HCG.0000000000000048. [Google Scholar] [CrossRef]
7. Jain, R., Ammar, K. A., Kalvin, L., Ignatowski, D., Olet, S. et al. (2018). Diagnostic accuracy of bicuspid aortic valve by echocardiography. Echocardiography, 35(12), 1932–1938. DOI 10.1111/echo.14167. [Google Scholar] [CrossRef]
8. Donadille, B., Rousseau, A., Zenaty, D., Cabrol, S., Courtillot, C. et al. (2012). Cardiovascular findings and management in Turner syndrome: Insights from a French cohort. European Journal of Endocrinology of the European Federation of Endocrine Societies, 167(4), 517–522. DOI 10.1530/EJE-12-0434. [Google Scholar] [CrossRef]
9. Malaisrie, S. C., Carr, J., Mikati, I., Rigolin, V., Yip, B. K. et al. (2012). Cardiac magnetic resonance imaging is more diagnostic than 2-dimensional echocardiography in determining the presence of bicuspid aortic valve. The Journal of Thoracic and Cardiovascular Surgery, 144(2), 370–376. DOI 10.1016/j.jtcvs.2011.09.068. [Google Scholar] [CrossRef]
10. Alkadhi, H., Leschka, S., Trindade, P. T., Feuchtner, G., Stolzmann, P. et al. (2010). Cardiac CT for the differentiation of bicuspid and tricuspid aortic valves: Comparison with echocardiography and surgery. AJR American Journal of Roentgenology, 195(4), 900–908. DOI 10.2214/AJR.09.3813. [Google Scholar] [CrossRef]
11. Higgins, J. P., Thompson, S. G. (2002). Quantifying heterogeneity in a meta-analysis. Statistics in Medicine, 21(11), 1539–1558. DOI 10.1002/(ISSN)1097-0258. [Google Scholar] [CrossRef]
12. Mlika, M., Zorgati, M., Mezni, F. E. (2020). Classifying multiple lung cancers using morphological features: A meta-analysis. Journal of Immunoassay & Immunochemistry, 41(5), 817–832. DOI 10.1080/15321819.2020.1779740. [Google Scholar] [CrossRef]
13. Bae, J. M. (2020). Coffee consumption and colon cancer risk: A meta-epidemiological study of asian cohort studies. Asian Pacific Journal of Cancer Prevention, 21(5), 1177–1179. DOI 10.31557/APJCP.2020.21.5.1177. [Google Scholar] [CrossRef]
14. Donadille, B., Tuffet, S., Cholet, C., Nedelcu, M., Bourcigaux, N. et al. (2020). Prevalence and progression of aortic dilatation in adult patients with Turner syndrome: A cohort study. European Journal of Endocrinology of the European Federation of Endocrine Societies, 183(4), 463–470. DOI 10.1530/EJE-20-0284. [Google Scholar] [CrossRef]
15. Obara-Moszynska, M., Rajewska-Tabor, J., Rozmiarek, S., Karmelita-Katulska, K., Kociemba, A. et al. (2018). The usefulness of magnetic resonance imaging of the cardiovascular system in the diagnostic work-up of patients with Turner syndrome. Front Endocrinol (Lausanne), 9(609), 1694. DOI 10.3389/fendo.2018.00609. [Google Scholar] [CrossRef]
16. Duijnhouwer, A. L., Bons, L. R., Timmers, H., van Kimmenade, R. R. L., Snoeren, M. et al. (2019). Aortic dilatation and outcome in women with Turner syndrome. Heart, 105(9), 693–700. DOI 10.1136/heartjnl-2018-313716. [Google Scholar] [CrossRef]
17. Bons, L. R., Duijnhouwer, A. L., Boccalini, S., van den Hoven, A. T., van der Vlugt, M. J. et al. (2019). Intermodality variation of aortic dimensions: How, where and when to measure the ascending aorta. International Journal of Cardiology, 276, 230–235. DOI 10.1016/j.ijcard.2018.08.067. [Google Scholar] [CrossRef]
18. Yiğit, H., Önder, A., Özgür, S., Aycan, Z., Karademir, S. et al. (2017). Cardiac MRI and 3D contrast-enhanced MR angiography in pediatric and young adult patients with Turner syndrome. Turkish Journal of Medical Sciences, 47(1), 127–133. DOI 10.3906/sag-1511-3. [Google Scholar] [CrossRef]
19. Klásková, E., Zapletalová, J., Kaprálová, S., Šnajderová, M., Lebl, J. et al. (2017). Increased prevalence of bicuspid aortic valve in Turner syndrome links with karyotype: The crucial importance of detailed cardiovascular screening. Journal of Pediatric Endocrinology & Metabolism, 30(3), 319–325. DOI 10.1515/jpem-2016-0301. [Google Scholar] [CrossRef]
20. Somerville, S., Rosolowsky, E., Suntratonpipat, S., Girgis, R., Goot, B. H. et al. (2016). Cardiac magnetic resonance imaging in pediatric Turner syndrome. The Journal of Pediatrics, 175, 111–115.e111. DOI 10.1016/j.jpeds.2016.04.080. [Google Scholar] [CrossRef]
21. Olivieri, L. J., Baba, R. Y., Arai, A. E., Bandettini, W. P., Rosing, D. R. et al. (2013). Spectrum of aortic valve abnormalities associated with aortic dilation across age groups in Turner syndrome. Circulation: Cardiovascular Imaging, 6(6), 1018–1023. DOI 10.1161/CIRCIMAGING.113.000526. [Google Scholar] [CrossRef]
22. Kim, H. K., Gottliebson, W., Hor, K., Backeljauw, P., Gutmark-Little, I. et al. (2011). Cardiovascular anomalies in Turner syndrome: Spectrum, prevalence, and cardiac MRI findings in a pediatric and young adult population. American Journal of Roentgenology, 196(2), 454–460. DOI 10.2214/AJR.10.4973. [Google Scholar] [CrossRef]
23. Bondy, C. A., van, P. L., Bakalov, V. K., Ho, V. B. (2006). Growth hormone treatment and aortic dimensions in Turner syndrome. The Journal of Clinical Endocrinology and Metabolism, 91(5), 1785–1788. DOI 10.1210/jc.2005-2625. [Google Scholar] [CrossRef]
24. Loscalzo, M. L., van, P. L., Ho, V. B., Bakalov, V. K., Rosing, D. R. et al. (2005). Association between fetal lymphedema and congenital cardiovascular defects in Turner syndrome. Pediatrics, 115(3), 732–735. DOI 10.1542/peds.2004-1369. [Google Scholar] [CrossRef]
25. Ostberg, J. E., Donald, A. E., Halcox, J. P., Storry, C., McCarthy, C. et al. (2005). Vasculopathy in Turner syndrome: Arterial dilatation and intimal thickening without endothelial dysfunction. The Journal of Clinical Endocrinology and Metabolism, 90(9), 5161–5166. DOI 10.1210/jc.2005-0677. [Google Scholar] [CrossRef]
26. Oberhoffer, F. S., Abdul-Khaliq, H., Jung, A. M., Rohrer, T. R., Abd, E. et al. (2019). Two-dimensional speckle tracking of the abdominal aorta: A novel approach to evaluate arterial stiffness in patients with Turner syndrome. Cardiovascular Diagnosis and Therapy, 9(Suppl 2), S228–S237. DOI 10.21037/cdt.2019.03.01. [Google Scholar] [CrossRef]
27. Antequera-González, B., Martínez-Micaelo, N., Alegret, J. M. (2020). Bicuspid aortic valve and endothelial dysfunction: Current evidence and potential therapeutic targets. Frontiers in Physiology, 11, 1015. DOI 10.3389/fphys.2020.01015. [Google Scholar] [CrossRef]
28. Hope, M. D., Hope, T. A., Meadows, A. K., Ordovas, K. G., Urbania, T. H. et al. (2010). Bicuspid aortic valve: Four-dimensional MR evaluation of ascending aortic systolic flow patterns. Radiology, 255(1), 53–61. DOI 10.1148/radiol.09091437. [Google Scholar] [CrossRef]
29. Goudot, G., Mirault, T., Bruneval, P., Soulat, G., Pernot, M. et al. (2019). Aortic wall elastic properties in case of bicuspid aortic valve. Frontiers in Physiology, 10(299), 81. DOI 10.3389/fphys.2019.00299. [Google Scholar] [CrossRef]
30. Godby, R. C., Munjal, C., Opoka, A. M., Smith, J. M., Yutzey, K. E. et al. (2014). Cross talk between NOTCH signaling and biomechanics in human aortic valve disease pathogenesis. Journal of Cardiovascular Development and Disease, 1(3), 237–256. DOI 10.3390/jcdd1030237. [Google Scholar] [CrossRef]
31. McKellar, S. H., Tester, D. J., Yagubyan, M., Majumdar, R., Ackerman, M. J. et al. (2007). Novel NOTCH1 mutations in patients with bicuspid aortic valve disease and thoracic aortic aneurysms. The Journal of Thoracic and Cardiovascular Surgery, 134(2), 290–296. DOI 10.1016/j.jtcvs.2007.02.041. [Google Scholar] [CrossRef]
32. Abu-Halima, M., Oberhoffer, F. S., El Rahman, M. A., Jung, A. M., Zemlin, M. et al. (2020). Insights from circulating microRNAs in cardiovascular entities in Turner syndrome patients. PLoS One, 15(4), e0231402. DOI 10.1371/journal.pone.0231402. [Google Scholar] [CrossRef]
33. Mortensen, K. H., Hjerrild, B. E., Stochholm, K., Andersen, N. H., Sørensen, K. E. et al. (2011). Dilation of the ascending aorta in Turner syndrome-a prospective cardiovascular magnetic resonance study. Journal of Cardiovascular Magnetic Resonance: Official Journal of the Society for Cardiovascular Magnetic Resonance, 13(1), 24. DOI 10.1186/1532-429X-13-24. [Google Scholar] [CrossRef]
34. Kim, J. B., Spotnitz, M., Lindsay, M. E., MacGillivray, T. E., Isselbacher, E. M. et al. (2016). Risk of aortic dissection in the moderately dilated ascending aorta. Journal of the American College of Cardiology, 68(11), 1209–1219. DOI 10.1016/j.jacc.2016.06.025. [Google Scholar] [CrossRef]
35. Bondy, C. A. (2008). Aortic dissection in Turner syndrome. Current Opinion in Cardiology, 23(6), 519–526. DOI 10.1097/HCO.0b013e3283129b89. [Google Scholar] [CrossRef]
36. Thunström, S., Krantz, E., Thunström, E., Hanson, C., Bryman, I. et al. (2019). Incidence of aortic dissection in Turner syndrome. Circulation, 139(24), 2802–2804. DOI 10.1161/CIRCULATIONAHA.119.040552. [Google Scholar] [CrossRef]
37. Marin, A., Weir-McCall, J. R., Webb, D. J., van Beek, E. J., Mirsadraee, S. (2015). Imaging of cardiovascular risk in patients with Turner’s syndrome. Clinical Radiology, 70(8), 803–814. DOI 10.1016/j.crad.2015.03.009. [Google Scholar] [CrossRef]
38. Hillebrand, M., Koschyk, D., Ter Hark, P., Schüler, H., Rybczynski, M. et al. (2017). Diagnostic accuracy study of routine echocardiography for bicuspid aortic valve: A retrospective study and meta-analysis. Cardiovascular Diagnosis and Therapy, 7(4), 367–379. DOI 10.21037/cdt.2017.05.03. [Google Scholar] [CrossRef]
39. Gøtzsche, C. O., Krag-Olsen, B., Nielsen, J., Sørensen, K. E., Kristensen, B. O. (1994). Prevalence of cardiovascular malformations and association with karyotypes in Turner’s syndrome. Archives of Disease in Childhood, 71(5), 433–436. DOI 10.1136/adc.71.5.433. [Google Scholar] [CrossRef]
40. Mazzanti, L., Cacciari, E. (1998). Congenital heart disease in patients with Turner’s syndrome. The Journal of Pediatrics, 133(5), 688–692. DOI 10.1016/S0022-3476(98)70119-2. [Google Scholar] [CrossRef]
41. Sybert, V. P. (1998). Cardiovascular malformations and complications in Turner syndrome. Pediatrics, 101(1), E11. DOI 10.1542/peds.101.1.e11. [Google Scholar] [CrossRef]
42. Völkl, T. M., Degenhardt, K., Koch, A., Simm, D., Dörr, H. G. et al. (2005). Cardiovascular anomalies in children and young adults with Ullrich-Turner syndrome the erlangen experience. Clinical Cardiology, 28(2), 88–92. DOI 10.1002/clc.4960280209. [Google Scholar] [CrossRef]
43. Noordman, I. D., Fejzic, Z., Bos, M., Duijnhouwer, A. L., Weijers, G. et al. (2021). Cardiac abnormalities in girls with Turner syndrome: ECG abnormalities, myocardial strain imaging, and karyotype-phenotype associations. American Journal of Medical Genetics, 185(8), 2399–2408. DOI 10.1002/ajmg.a.62259. [Google Scholar] [CrossRef]
44. Yetman, A. T., Starr, L., Sanmann, J., Wilde, M., Murray, M. et al. (2018). Clinical and echocardiographic prevalence and detection of congenital and acquired cardiac abnormalities in girls and women with the Turner syndrome. The American Journal of Cardiology, 122(2), 327–330. DOI 10.1016/j.amjcard.2018.03.357. [Google Scholar] [CrossRef]
45. Cramer, P. M., Prakash, S. K. (2019). Misclassification of bicuspid aortic valves is common and varies by imaging modality and patient characteristics. Echocardiography, 36(4), 761–765. DOI 10.1111/echo.14295. [Google Scholar] [CrossRef]
46. Malaisrie, S. C., Carr, J., Mikati, I., Rigolin, V., Yip, B. K. et al. (2012). Cardiac magnetic resonance imaging is more diagnostic than 2-dimensional echocardiography in determining the presence of bicuspid aortic valve. The Journal of Thoracic and Cardiovascular Surgery, 144(2), 370–376. DOI 10.1016/j.jtcvs.2011.09.068. [Google Scholar] [CrossRef]
47. van Hout, M. J., Scholte, A. J., Juffermans, J. F., Westenberg, J. J., Zhong, L. et al. (2020). How to measure the aorta using MRI: A practical guide. Journal of Magnetic Resonance Imaging, 52(4), 971–977. DOI 10.1002/jmri.27183. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |