

 | Congenital Heart Disease |  |
DOI: 10.32604/chd.2022.019126
ARTICLE
Effectiveness of Bilateral Pulmonary Artery Banding in Patients with Hypoplastic Left Heart Syndrome and Congenital Heart Defects with a Functional Single Ventricle: A Single-Center Retrospective Study
1Saint Petersburg State Pediatric Medical University, Saint-Petersburg, Russia
2Academisch Medisch Centrum Universiteit van Amsterdam, Amsterdam, Netherlands
*Corresponding Author: Vitaliy Suvorov. Email: vitalikkrak@gmail.com
Received: 04 September 2021; Accepted: 04 January 2022
Abstract: Background: Bilateral banding of the branches of the pulmonary artery in patients with hypoplastic left heart syndrome (HLHS) and other duct dependent critical neonatal heart malformations can significantly reduce the incidence of severe complications in the postoperative period, especially in severely unstable patients. In our study we compared different surgical techniques of bilateral pulmonary artery banding (PAB) in respect to their success in balancing systemic and pulmonary blood flow. Methods: We included 44 neonates with a HLHS and congenital heart diseases (CHD) with a functional single ventricle underwent a hybrid operation: bilateral PAB and patent ductus arteriosus stenting. The hybrid surgery for method No. 1 is performed as a one-stage procedure, together with patent ductus arteriosus (PDA) stenting. After median sternotomy, two Gore-Tex 1–2 mm wide bands with a diameter of 3–3.5 mm are put. When we apply method No. 2 then the thread is used to create bands. Method No. 3 is distinguished by intraoperative assessment of blood flow at the site of narrowing of the branches of the pulmonary artery and optional stenting of the PDA. The cuff for banding is made of Gore-Tex tubing. Effectiveness when applying method Nos. 1 and 2 is assessed by the change in invasive blood pressure and oxygen saturation after narrowing of the branches of the pulmonary artery. Also, with these techniques PDA stenting by inserting the introducer via pulmonary artery trunk is performed. Results: HLHS with mitral or aortic valve atresia or both was present in 19 patients (43.1%), with severe left heart obstruction resulting in PDA dependent systemic circulation in 16 babies (36.4%). CHD with single ventricle physiology occurred in 9 patients (20.5%). 14 babies (31.8%) undergo the procedure following the method No. 1, 8 patients (18.2%) method No. 2 and 22 patients (50%) method No. 3. Qp/Qs = 1/1 was achieved in 30 patients (30/44, 68.1%): as a result of the method No. 1 was achieved in 5 patients (5/14, 35.7%), method No. 2 in 4 patients (4/8, 50%), method No. 3 in 21 patients (21/22, 95.5%). Multivariate regression analysis revealed that method No. 3 significantly increases the chances of hemodynamic efficacy operations (OR = 35.0; p = 0.005; CI (95%) 3–411.5). Conclusion: Application of the operation technique No. 3 in combination with the intraoperative assessment of blood flow parameters at the site of banding of the branches of the pulmonary artery are the most optimal criteria for achieving Qp/Qs = 1/1. If there are signs of restriction at the level of the foramen ovale, atrioseptostomy should be done in the second stage after bilateral pulmonary banding.
Keywords: Bilateral banding; hybrid Norwood procedure; pulmonary banding; hybrid approach HLHS; surgery single ventricle
One of the congenital heart and great vessels defects, which has recently undergone significant changes in diagnosis, methods of treatment and their results, are pathologies associated with hypoplasia of the left heart, as well as ductus-dependent congenital heart diseases (CHD). The hypoplastic left heart syndrome (HLHS) is an extreme variant of this pathology. Survival and outcome of HLHS or HLHS complex patients depends on the maintenance of both the interartial and patent arterial duct (PDA) shunts established in the fetus. 30 years ago, the only available option for such patients was palliative care. Today, there are some therapeutic solutions and surgical methods for correcting these pathologies, but there is no consensus on the choice of the optimal approach. Nonetheless, most cardiac surgeons follow a 3-step procedure to treat patients with a functionally single ventricle. However, there are some differences in surgical technique, treatment management and results between many facilities around the world [1,2]. During the neonatal period the goal is to provide non-obstructive systemic and coronary blood flow, achieve a balanced pulmonary and systemic circulation and create a non-restrictive interatrial communication. The second stage of hemodynamic correction is performed to reduce the volume overload of the single ventricle supplying both systemic and pulmonary blood flow with with the progression of hypoxemia and hypovolemia in the pulmonary circulation. The formation of a bidirectional cavapulmonary anastomosis helps to reduce volume overload in the single ventricle. In the third stage remaining deoxygenated blood from the lower half of the body is redirected into the pulmonary circulation. Difficulties in choosing the best way of surgical correction appear already at the first stage of treatment, since all methods have their own risks and characteristics. Norwood’s procedure certainly fulfills the assumptions of the first stage of hemodynamic correction. Nevertheless, it requires a complex surgical reconstruction in conditions of extracorporeal circulation with a need to create an optimal balance of systemic and pulmonary blood flow in increased demand of oxygen by the myocardium after crossclamp [3,4]. In addition, the consequences of extracorporeal circulation in the neonatal period—deep hypothermia, cardiac arrest or isolated regional cerebral perfusion during the reconstruction of the aortic arch contribute to an increased risk of hemodynamic instability in the early postoperative period, cerebral stroke and death [5–7]. Due to the associated risk of developing severe complications, which significantly worsen the prognosis, a hybrid modification of Norwood’s procedure emerged as an alternative to the classic operation of the first stage of hemodynamic correction [8,9]. Unlike in the classic procedure, in the hybrid approach the extracorporeal circulation and induced hypothermia are not necessary. Thus, a less invasive hybrid intervention avoids the unintended risks associated with the classic variant of Norwood’s operation, which significantly improves the results of treatment [8–10]. Such differences make possible to consider hybrid approach as the treatment of choice for a group of patients with a high risk of developing fatal complications.
The classic indications for bilateral pulmonary banding are: the low weight and severe/critical condition of the patient. In many centers around the world, preference is given to Norwood’s surgery, and only patients at high risk undergo bilateral banding of the pulmonary artery. Also, one of the issues with this method is the need to maintain the PDA. Some prefer continuous infusion of prostaglandin, while others perform PDA stenting. Stenting of the PDA can be performed as a first step, before banding of the pulmonary artery branches or after (within the same procedure or after a few days). Despite the difficulties in achieving the hemodynamic target values while performing the hybrid surgery, in many clinics this method of correction remains preferred in the treatment of children with complex CHD [11,12]. One of the unsolved problems so far is to determine the most optimal perimeter of the band to achieve targeted hemodynamic values, which differ in variable forms of CHD. In the literature, several techniques for performing this operation were presented, all having their own advantages and disadvantages [8,10,12,13]. The main differences between these techniques were the criteria for assessing the effectiveness of performed surgery. The difficulty in achieving the optimal outcome is caused by the fact that the hemodynamics of the pulmonary and systemic circulation is influenced by many perioperative factors: the partial pressure of oxygen and carbon dioxide, mechanical ventilation, the usage of prostaglandins, vasopressors and inotropic agents, the infusion load and fluid balance, etc. It is impossible to avoid the influence of most of them, which requires the determination of universal criteria for assessing Qp/Qs in this group of patients.
The aim of this study was to compare the effectiveness of three methods proposed by Galantowicz [10], Kitahori et al. [12] and Berishvili [13] and to determination of intraoperative criteria for the effectiveness of banding of the branches of the pulmonary artery on patients with critical congenital heart defects.
The research was carried out based on the results of a retrospective single center analysis treatment of 44 patients with critical CHD who underwent a hybrid operation: bilateral pulmonary artery banding and PDA stenting. Our study was approved by the ethics committee (the local ethics committee at the Saint Petersburg State Pediatric Medical University, protocol No. 04/06, 26 April 2021). The patients were divided into two groups depending on the period of application of the operation technique: first—method No. 1 + method No. 2 and second—method No. 3. The performed surgical method was chosen based on the criteria for bilateral banding in order to balance pulmonary and systemic circulation for different patients. Techniques were described by Galantowicz [10], Berishvili [13], and Kitahori et al. [12].
2.1 Methods of Bilateral Pulmonary Artery Banding
(1) Method No. 1 [10]
The hybrid surgery is performed as a one-stage procedure, together with PDA stenting. After median sternotomy, two Gore-Tex 1–2 mm wide bands with a diameter of 3.5 mm (3 mm for patients with body weight < 2 kg) are prepared. Each band is cut and overlaid with horizontal mattress stitch using 5–0 thread. The right pulmonary artery (RPA) is mobilized within a small distance between ascending aorta and the superior vena cava. There should be no inclusion of any local tissue between the cuff and the pulmonary artery. The band must be inserted and tightened with a formed seam. To prevent displacement, the cuff is attached to the adventitia of the artery with additional seam. Degree of narrowing the pulmonary arteries is determined during the procedure and depends on the artery size, systemic blood pressure response, oxygen saturation and the child’s weight. The band is usually tightened to achieve an increase of blood pressure by 10 points mm Hg and decrease oxygen saturation (SpO2). Tightening the band to about 3.3 mm (slightly smaller than the diameter of the band), let to balance the systemic and pulmonary blood flow and do not lead to excessive narrowing of the arteries. Mobilization of the left pulmonary artery (LPA) is greatly facilitated by moving to the left side of the patient. This allows the surgeon for more accurate placement of the cuff in the proximal side of the LPA. On the left side, the upper lobe branches of the LPA start earlier than branches of RPA, which creates a bigger risk of compromising them if the cuff is located distally. The procedure of LPA mobilization is similar, after the cuff is properly positioned, the seam is tightened. An additional suture is used to fix the band to the adventitia of the artery. After narrowing of LPA, typical changes in hemodynamics occur: an increase in blood pressure by 10 points and decrease in saturation by 10 points. It is possible to perform an angiography to control if the narrowing of the arteries is sufficient. After bilateral banding, PDA stenting by inserting the introducer via pulmonary artery trunk is performed.
(2) Method No. 2 [13]
The first stage takes place in a standard cardiac surgery operating room. For the endovascular stage of the procedure, a mobile angiographic installation is prepared. The surgery is performed through the left lateral thoracotomy or median sternotomy. Pulmonary banding begins with the left pulmonary artery, then the right.
To create bands, 1 mm thick thread is used. Diameter of the cuff is 3 mm with a body weight >2.5 kg, and 2 mm for a child weighing <2.5 kg. PDA stenting was performed through an introducer installed in the pulmonary trunk (with median sternotomy). Length and diameter of PDA is measured from lateral projection during angiography. The size of the stent is selected to exceed the minimum size of the duct area by 2 mm.
(3) Method No. 3 [12]
The surgery is performed through median sternotomy. During the surgery, target values for blood oxygen saturation (SaO2) needs to be maintained at the level of 80%. The cuff for banding is made of Gore-Tex tubing and it is cut longitudinally to obtain a 2 mm wide stripe.
The length of the cuff required for the LPA is determined by the following formula: 7 mm + patient weight (1 mm for every 1 kg). For RPA: 7.5 mm + patient weight (1 mm for every 1 kg). Then, determined points are marked at each end of the cuff. If the child’s weight is not a round number, 0.5 mm is added for every 0.5 kg, and for every 0.25 kg and 0.75 kg rounded to lower or higher value, respectively. Thus, the length may vary by a multiple of 0.5 mm. During pulmonary banding, the lobe inhaled oxygen is recommended to be maintained at 21%.
Intraoperative echocardiography (ECHOKG) should be performed to detect reduced blood flow in the pulmonary vein and increased blood flow in pulmonary arteries. If after narrowing pulmonary arteries, SaO2 is more than 85%, and the flow velocity is less than 2 m/s, the additional tightening is required, starting with the RPA, then the LPA. On the contrary, if SaO2 is less than 75%, the cuff must be loosened in the reverse order. The optimal goal is to increase the flow velocity to >3 m/s in both pulmonary arteries, intraoperatively. However, flow velocity in the range from 2 m/s to 3 m/s is acceptable.
PDA stenting is performed, if necessary. The preference is given to constant infusion of prostaglandin to prevent closure of the PDA before performing the Norwood operation.
The first group consists of 22 patients (50%) who were operated on according to methods 1 [10] and 2 [13], the second group represents 22 patients (50%) who underwent surgery according to method 3 [12]. Also, the patients were divided into groups based on the diagnosis of congenital heart disease: HLHS, hypoplastic left heart complex (HLHC, cardiac malformation at the mildest end of the spectrum of hypoplastic left heart syndrome), other forms of a functionally single ventricle heart and critical CHD.
Patients with HLHS and HLHC were divided into groups depending on the pathology of the atrioventricular valves: with atresia or stenosis of mitral valve (MV) in combination with atresia or stenosis of aortic valve (AV). HLHS with mitral or aortic valve atresia or both was present in 19 patients (43.1%). HLHS with severe stenosis of these valves was diagnosed in 16 babies (36.4%). HLHC or CHD with single ventricle physiology occurred in 9 patients (20.5%).
2.3 Сriteria for the Effectiveness of Balancing Systemic and Pulmonary Blood Flow
The success of the surgery in our study was achieved when the following criteria were met:
(1) Target values of various indicators of balanced pulmonary and systemic circulation (Qp/Qs = 1). Qp/Qs was assessed in accordance with recommendations of Klauwer et al. [14]:
– PaO2 ~ 40 (±5) mm Hg,
– SpO2 75–85%—with normal lung function (i.e., with SO2 in the pulmonary veins = 100%), normal ventilation (FiO 21–30%), normal blood pressure and hemoglobin level.
(2) Intraoperative assessment of pulmonary arteries flow velocity in echocardiography: Vmax > 3 m/s, stenotic nature of the diastolic flow (Fig. 1).

Figure 1: Intraoperative assessment of blood flow at the site of narrowing of the right branch of the pulmonary artery using ECHOKG study in Doppler mode (CW)
The differences between the 2 groups were compared using t-tests for the continuous variables with normal distributions, Mann–Whitney U-test if abnormal distributions, and chi-square test for the qualitative data. To identify factors influencing the hemodynamic efficiency of bilateral pulmonary banding, determining the degree of their influence on the result (optimal Qp/Qs = 1/1), we performed univariate and multivariate logistic regression analysis. The analysis includes 32 risk factors. Correlation of each factor was determined using the Pearson χ2 test, before performing multiple analysis. Subsequently, to analyse the relationship of risk factors and the result of the surgery, we performed multiple logistic regression analysis. Statistical processing was carried out using SPSS for Windows. The level of statistical significance is accepted for p < 0.05.
In total, during the observation period in our clinic, 47 procedures of pulmonary banding were performed in patients with critical CHD. The statistics was carried out based on the treatment results of 44 patients. 3 patients were excluded from the study due to a lethal outcome intraoperatively: in two cases the operation was performed according to method No. 1, in one—according to method No. 2. All patients were HLHS. In all three cases, patients were admitted in severe conditions (metabolic acidosis, hyperlactatemia, hypotension, moderate doses of inotropic drugs, in one case there was an episode of circulatory arrest and required resuscitation). All babies were with Echo signs of restriction at the level of foramen ovale. The first stage of surgical treatment was atrioseptostomy. After atrioseptostomy severe hemodynamic disturbances (hypotension, bradycardia) developed with the progression of hypoxemia. Medical therapy and emergency surgery (bilateral pulmonary banding) were ineffective.
Among the total number of patients (n = 44), 14 (31.8%) undergo the procedure following the method No. 1 [10], 8 patients (18.2%) method No. 2 [13] and 22 patients (50%) method No. 3 [12].
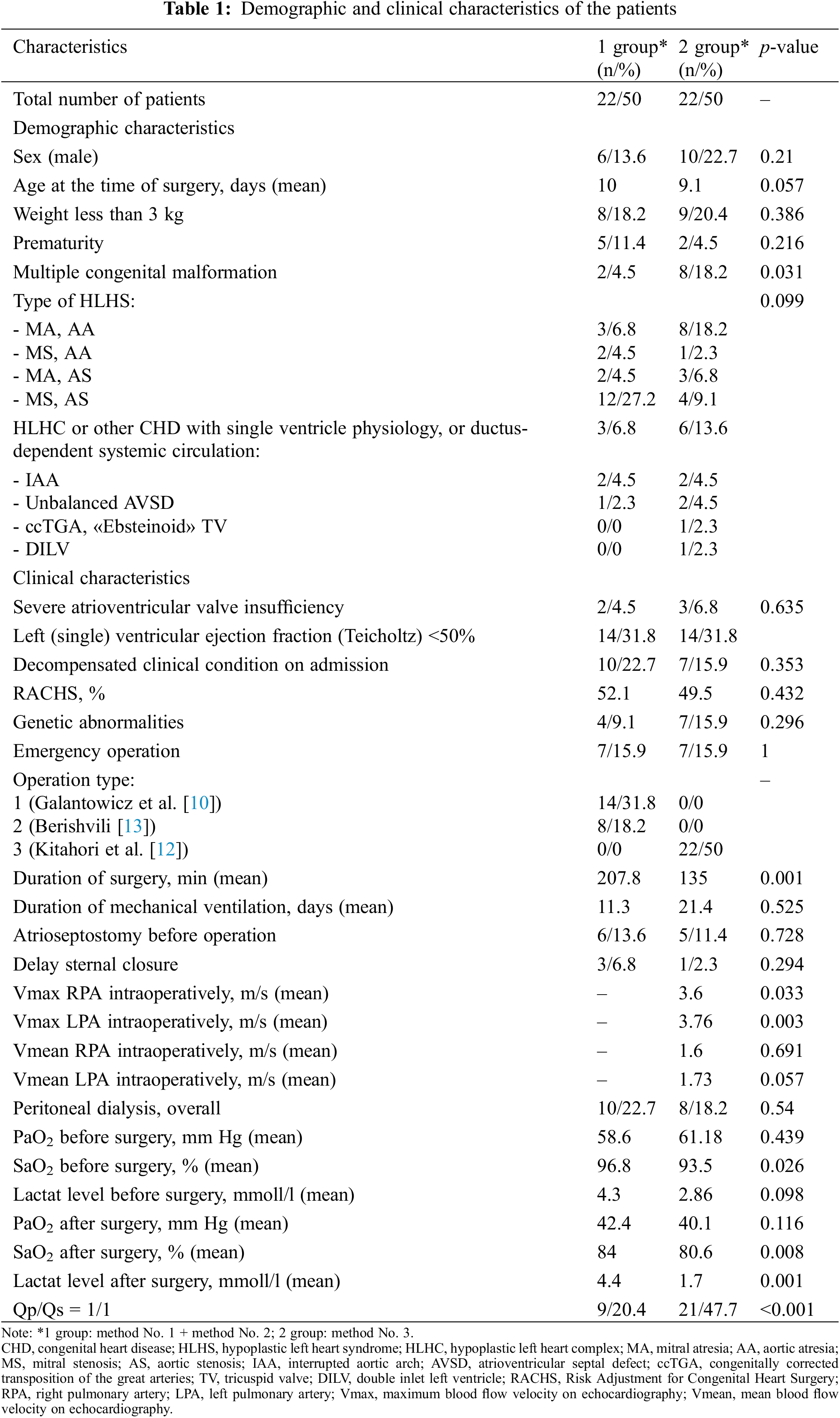
MV atresia with AV atresia occurred in 11 patients (25%), MV atresia in combination with AV stenosis were in 5 patients (11.4%), stenosis of MV and AV atresia occurred in 3 children (6.8%). Critical AV stenosis was present in 9 patients, while 3 children (6.8%) in the first hours after birth developed acute pulmonary edema, which led to cardiopulmonary resuscitation, followed by performing an emergency operation. 5 patients (11.4%) were diagnosed with interrupted aortic arch. Complete unbalanced atrioventricular septal defect (AVSD) in combination with critical coarctation of the aorta (CoA) was diagnosed in 3 children. 1 patient (2.3%) had a congenitally corrected transposition of the great arteries (TGA) with «Ebsteinoid» displacement of the tricuspid valves in combination with severe aortic valve insufficiency and stenosis. Mean age of patients at the time of the operation was 9.5 days (0–74 days). Mean weight was 3178 g (1800–4240 g). A more detailed description of patients is presented in Table 1.
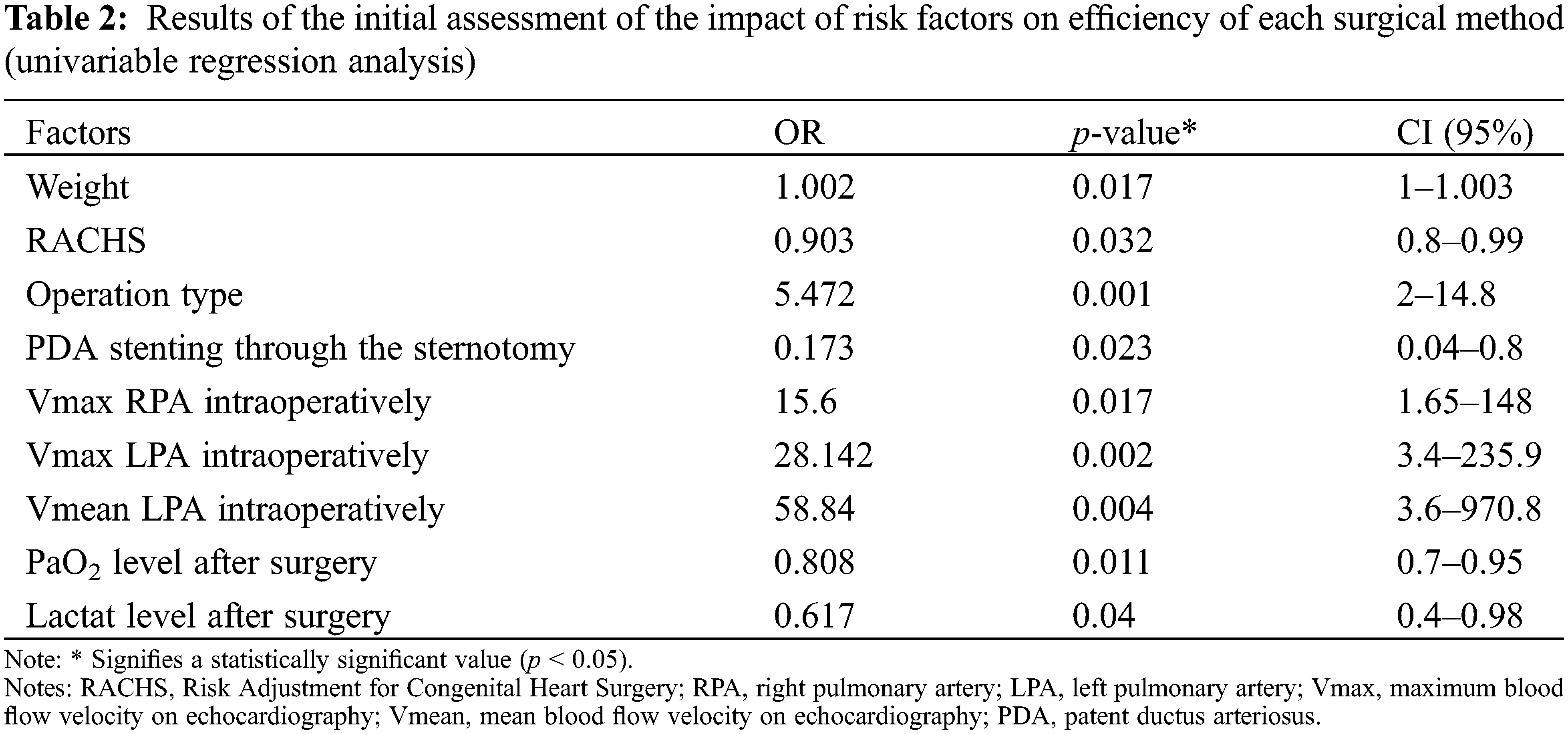
The success of the surgery (Qp/Qs = 1) was achieved in 30 patients (30/44, 68.1%): as a result of the method No. 1 was achieved in 5 patients (5/14, 35.7%), method No. 2 in 4 patients (4/8, 50%), method No. 3 in 21 patients (21/22, 95.5%). All factors that, as a result of univariable regression analysis, statistically significantly influencing the outcome of the surgery are presented in Table 2.
In order to assess the complex influence of risk factors on achieving the goal of the operation, the multivariable regression analysis was performed. The results of multiple regression analysis demonstrate that only «Method No. 3» is a confirmed predictor, the use of which increases the chances of achieving optimal balance between pulmonary and systemic circulation (Qp/Qs = 1/1) when performing bilateral in children with critical congenital heart disease (OR = 35.0; CI: 3–411.5; p = 0.005).
The main objective of this study was to identify intraoperative criteria for the hemodynamic efficiency of bilateral pulmonary banding, resulting in optimal balance between the pulmonary and systemic blood flow.
We analyzed the results using three methods of bilateral banding and PDA stenting in children with critical CHD. One of the effective criterion indicating a balance between pulmonary and systemic circulation is PaO2 and SpO2. However, for some critical defects, in which the antegrade flow into the aorta from the left ventricle is present, the levels of these indicators often do not correspond to the target values and do not reflect the true effect of a banding procedure. Among patients with a critical CHD, for instance, with acute pulmonary edema or of impaired systemic perfusion resulting in blood flow centralization, it becomes a challenge to assess the efficacy of the procedure performed according to these criteria. In our study, there were three such patients and it was impossible to evaluate the effectiveness of balancing systemic and pulmonary blood flow according to the data of invasive blood pressure measurements, as well as blood gas parameters. Therefore, symptoms of insufficient or excessive banding of pulmonary arteries are often present in the postoperative period and in consequence, a second intervention.
According to various authors, if the atrioseptostomy procedure is necessary, the safest solution is to perform the pulmonary banding in one stage [10,13]. The pathophysiological features of restriction at the level of the open PFO, especially in children older than 2 weeks, must certainly be taken into consideration. Due to a decrease in resistance in pulmonary vessels in newborns, especially in patients with CHD with hypervolemia in pulmonary circulation, the presence of restrictive PFO leads to an increase in pressure in the pulmonary circulation, which contributes to the progression of hypoxemia. Atrioseptostomy leads to a rapid decrease in pressure in the left atrium, which in turn aggravates hypervolemia in the pulmonary circulation. Children with critical CHD with ductus-dependent systemic and coronary circulation will therefore develop an acute failure of systemic perfusion, which manifests in the progression of hypotension and bradycardia. In this situation use of inotropic agents and vasopressors is ineffective, since the reason for the development of such condition is redistribution of blood volume towards the pulmonary circulation. Therefore, due to the high risk of such complications after the Rashkind procedure in patients with restrictive PFO, it is advised to perform the first stage of pulmonary banding. It should be mentioned that in three cases, patients were admitted to the clinic at the age of over 14 days with signs of restriction at the level of foramen ovale. The atrioseptostomy was performed at the first stage, after which severe hemodynamic disturbances (hypotension, bradycardia) developed with the progression of hypoxemia. Symptomatic therapy and emergency bilateral banding were ineffective. Subsequently, in similar situations, atrioseptostomy was performed in all patients at the second stage.
In our study, the efficiency of the pulmonary banding procedure was 68.1% (30 out of 44 cases). Three patients (6.8%) presented signs of pulmonary hypervolemia in the early postoperative period, requiring a second intervention. Kitahori et al. (2010) [12] note the high efficiency of intraoperative assessment of flow characteristics at the site of narrowing branches of the pulmonary artery using ECHOKG studies [12]. This technique was applied in 22 patients (50%), among whom only one case showed signs of insufficient restriction of blood flow in the right pulmonary artery, and subsequently, development of pulmonary hypertension. At the same time, the duration of mechanical ventilation in this patient was three days, which indicates an insignificant negative impact on hemodynamics. Among this group, the proportion of patients who managed to achieve the target values (Qp/Qs = 1/1) was 95.5%. According to the results of our study, this technique statistically significantly increases the chance of desirable hemodynamic effect of the pulmonary banding procedure.
A single-center retrospective study was performed, but an increase in the sample size is required to increase the statistical power of the results. Due to the retrospective version of the study, some parameters were not available for group No. 1 of patients.
The main task was to identify the most optimal method of banding of the pulmonary arteries on the basis of the available intraoperative criteria for balancing systemic and pulmonary blood flow. The results were obtained on the basis of intraoperative observations and in the early postoperative period. In addition, we consider it important to evaluate the long-term results of these methods of surgical correction. It is also advisable to conduct a study to compare groups of patients who underwent the operation of bilateral banding of the branches of the pulmonary artery and the classic Norwood operation.
Bilateral banding of the branches of the pulmonary artery allows you to effectively balance the volumetric load of the pulmonary and systemic circulation in patients with critical congenital heart defects. Application of the operation technique No. 3 in combination with the intraoperative assessment of blood flow parameters at the site of banding of the branches of the pulmonary artery are the most optimal criteria for achieving Qp/Qs = 1/1. If there are signs of restriction at the level of the foramen ovale, atrioseptostomy should be done in the second stage after bilateral pulmonary banding.
Acknowledgement: We thank Dietrich Klauwer, M.D for his help and support in the treatment of patients.
Authors Contribution: Vitaliy Suvorov has made substantial contributions to the intellectual content of an article in terms of the conception, drafting, and revising of the work, analysis, and interpretation of the data. Vladimir Zaitcev has made substantial contributions to the intellectual content of an article in terms of the conception, drafting of the article and the acquisition of the data. Karolina Andrzejczyk has made substantial contributions to the content of an article in terms of the drafting, and revising of the work and the acquisition of the data.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Schidlow, D. N., Anderson, J. B., Klitzner, T. S., Beekman, R. H.III, Jenkins, K. J. et al. (2011). Variation in interstage outpatient care after the Norwood procedure: A report from the joint council on congenital heart disease national quality improvement collaborative. Congenital Heart Disease, 6(2), 98–107. DOI 10.1111/j.1747-0803.2011.00509.x. [Google Scholar] [CrossRef]
2. Davies, R. R., Pizarro, C. (2015). Decision-making for surgery in the management of patients with univentricular heart. Frontiers in Pediatric, 3, 61–80. DOI 10.3389/fped.2015.00061. [Google Scholar] [CrossRef]
3. Norwood, W. I., Kirklin, J. K., Sanders, S. P. (1980). Hypoplastic left heart syndrome: Experience with palliative surgery. The American Journal of Cardiology, 45(1), 87–91. DOI 10.1016/0002-9149(80)90224-6. [Google Scholar] [CrossRef]
4. Ohye, R. G., Sleeper, L. A., Mahony, L., Newburger, J. W., Pearson, G. D. et al. (2010). Comparison of shunt types in the Norwood procedure for single-ventricle lesions. The New England Journal of Medicine, 362(21), 1980–1992. DOI 10.1056/NEJMoa0912461. [Google Scholar] [CrossRef]
5. Honjo, O., Benson, L. N., Mewhort, H. E., Predescu, D., Holtby, H. et al. (2009). Clinical outcomes, program evolution, and pulmonary artery growth in single ventricle palliation using hybrid and Norwood palliative strategies. The Annals of Thoracic Surgery, 87(6), 1885–1893. DOI 10.1016/j.athoracsur.2009.03.061. [Google Scholar] [CrossRef]
6. Kussman, B. D., Gauvreau, K., DiNardo, J. A., Newburger, J. W., Mackie, A. S. et al. (2007). Cerebral perfusion and oxygenation after the Norwood procedure: Comparison of right ventricle-pulmonary artery conduit with modified Blalock–Taussig shunt. The Journal of Thoracic and Cardiovascular Surgery, 133(3), 648–655. DOI 10.1016/j.jtcvs.2006.09.034. [Google Scholar] [CrossRef]
7. Khubulava, G. G., Marchenko, S. P., Dubova, E. V., Suvorov, V. V. (2016). Role of modified ultrafiltration in reduce of the systemic inflammatory response syndrome in cardiac surgery. Pediatrician, 7(1), 106–110. DOI 10.17816/PED71106-110. [Google Scholar] [CrossRef]
8. Brescia, A. A., Jureidini, S., Danon, S., Armbrecht, E., Fiore, A. C. et al. (2014). Hybrid versus Norwood procedure for hypoplastic left heart syndrome: Contemporary series from a single center. The Journal of Thoracic and Cardiovascular Surgery, 147(6), 1777–1782. DOI 10.1016/j.jtcvs.2014.02.066. [Google Scholar] [CrossRef]
9. Dave, H., Rosser, B., Knirsch, W., Hubler, M., Pretre, R. et al. (2014). Hybrid approach for hypoplastic left heart syndrome and its variants: The fate of the pulmonary arteries. European Journal of Cardio-Thoracic Surgery, 46(1), 14–19. DOI 10.1093/ejcts/ezt604. [Google Scholar] [CrossRef]
10. Galantowicz, M. (2009). The hybrid approach to hypoplastic left heart syndrome. Operative Techniques in Thoracic and Cardiovascular Surgery, 14(2), 74–85. DOI 10.1053/j.optechstcvs.2009.06.005. [Google Scholar] [CrossRef]
11. Sano, S., Ishino, K., Kawada, M., Arai, S., Kasahara, S. et al. (2003). Right ventricle-pulmonary artery shunt in first-stage palliation of hypoplastic left heart syndrome. The Journal of Thoracic and Cardiovascular Surgery, 126(2), 504–510. DOI 10.1016/S0022-5223(02)73575-7. [Google Scholar] [CrossRef]
12. Kitahori, K., Murakami, A., Takaoka, T., Takamoto, S., Ono, M. (2010). Precise evaluation of bilateral pulmonary artery banding for initial palliation in high-risk hypoplastic left heart syndrome. The Journal of Thoracic and Cardiovascular Surgery, 140(5), 1084–1091. DOI 10.1016/j.jtcvs.2010.07.084. [Google Scholar] [CrossRef]
13. Berishvili, D. O. (2010). Palliative operations without cardiopulmonary bypass as a means of emergency care for newborns with congenital heart defects (Dissertation, Doctor of Medical Sciences), pp. 218. Moscow. [Google Scholar]
14. Klauwer, D., Neuhaeuser, C., Thul, J., Zimmermann, R. (2019). A practical handbook on pediatric cardiac intensive care therapy, vol. 560, pp. 421–424. Cham, Switzerland, Springer. [Google Scholar]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |