

 | Congenital Heart Disease |  |
DOI: 10.32604/chd.2022.018666
ARTICLE
Long-Term Outcome and Risk Factor Analysis of Surgical Pulmonary Valve Replacement in Congenital Heart Disease
1Department of Pediatrics, Seoul National University Children’s Hospital, Seoul National University College of Medicine, Seoul, Korea
2Department of Thoracic and Cardiovascular Surgery, Seoul National University Children’s Hospital, Seoul National University College of Medicine, Seoul, Korea
*Corresponding Author: Gi Beom Kim. Email: ped9526@snu.ac.kr
Received: 09 September 2021; Accepted: 14 February 2022
Abstract: Objectives: To establish long-term outcome of surgical pulmonary valve replacement (PVR) in congenital heart disease (CHD) and to identify risk factors for overall mortality, operative mortality, and repetitive PVR. Methods: This is a retrospective study of 375 surgical PVR in 293 patients who underwent surgical PVR for CHD between January 2000 and May 2020. We only included patients with index PVR with previous open-heart surgery regardless of the number of PVRs. The previous surgical history of patients who underwent PVR during the study period was also included. Patients who underwent the Rastelli operation, and those who underwent single PVR without previous open-heart surgery were excluded. Results: The median age of the patients at the time of surgical PVR was 14.9 years (Interquartile range, IQR, 11.0–22.0). The median follow-up duration was 10.5 years (IQR, 5.5–14.8 years). There were 3 patients with operative mortality (1.0%) and 15 patients with overall mortality (5.1%). The survival rate was 95.1% over 20 years follow-up period. Multivariate analysis demonstrated that more than 3 times of previous open-heart surgeries before surgical PVR, older age at the first operation, longer cardiopulmonary bypass (CPB) time and longer intensive care unit (ICU) stay were predictors for overall mortality. Patients who underwent surgical PVR after more than 3 times of previous open-heart surgeries had significantly higher mortality than those who underwent open-heart surgeries less than 3 times (P < 0.001). Age younger than 10 years, male, multiple valve problems and longer ICU stay were significant predictors for repetitive PVR by multivariate analysis. Conclusions: Though surgical PVR has excellent long-term outcome, it should be performed with caution for those who previously underwent multiple open-heart surgeries, especially if patient received more than 3 times of open-heart surgeries.
Keywords: Pulmonary valve replacement; mortality; operative mortality; repetitive pulmonary valve replacement; risk factor analysis; childhood
As the adult congenital heart disease (ACHD) population grows, re-operation and long-term complications of previously corrected cardiac lesions have become evident [1]. Surgical palliation of many forms of complex congenital heart disease (CHD), including tetralogy of Fallot (TOF), double-outlet right ventricle (DORV), and transposition of the great arteries, commonly involves reconstruction of the right ventricular outflow tract (RVOT) [2]. Pulmonary valve replacement (PVR) is the most common reoperation in ACHD and is frequently required when pulmonary regurgitation (PR) gradually progresses even after the original lesions being corrected [3]. Although the majority of patients with significant PR could be asymptomatic for many years, they remain at risk for progressive right ventricular (RV) dilatation and reduced ventricular function, development of arrhythmia, or sudden death [4]. PVR in these patients has previously been demonstrated to significantly improve RV function [5,6].
Among the various valve substitutes for PVR, bioprosthetic valves are the most widely used because they are readily available and do not need permanent anticoagulation therapy in the low-pressure RV [7]. Several types of bioprosthetic valves have been used for surgical PVR, but all are prone to be failed and will likely require reintervention [8]. Although surgical PVR is the most common reoperation in ACHD, long-term outcomes and prognostic factors for surgical PVR in CHD have not yet been clearly defined. In this study, we aimed to establish long-term survival data of surgical PVR for CHD and to identify risk factors for overall mortality including operative mortality, and repetitive PVR.
We conducted a retrospective study of 375 surgical PVR in 293 consecutive patients who underwent surgical PVR for CHD in the Seoul National University Children’s Hospital between January 2000 and May 2020. We included patients with index PVR with previous open-heart surgery regardless of the number of PVRs. The previous surgical history of patients who underwent PVR during the study period was also included. In addition, surgeries only performed by congenital heart surgeons were included. Patients who underwent the Rastelli operation, and those who underwent single PVR without previous open-heart surgery were excluded.
Demographic, clinical, peri-operative and imaging data were obtained from the patients’ medical records. We collected data including patients’ age, sex, diagnosis, prior procedures, comorbidities, type and size of valve, operation time, cardiopulmonary bypass (CPB) and aortic cross clamp (ACC) time, duration of intensive care unit (ICU) and hospital stay. The type of implanted valve and other specifics of the surgical procedures were obtained from the operative reports. This study used clinical data retrieved from Seoul National University Hospital Patients Research Environment (SUPREME) system and was approved by the Institutional Review Board of the Seoul National University Hospital (IRB number: 1908-081-1054, approved date: September 6th, 2019).
The individual data linkage to mortality data was made under agreement between the National Health Insurance Service and Statistics Korea. After internal linkage processes in Statistics Korea, aggregate data without any personal identifiers were transferred to the researchers of this study for final analyses.
Open heart surgery (OHS) was defined as a surgery in which the thoracic cavity is opened to expose the heart and the blood is recirculated and oxygenated by a heart-lung machine. Complex TOF was defined as TOF with other congenital structural malformation such as major aortopulmonary collateral artery, absent pulmonary valve or pulmonary atresia. Overall mortality was defined as all deaths occurring after the index PVR with any cause. Operative mortality was defined as (1) all deaths occurring during hospitalization in which the operation was performed, even if after 30 days, and (2) after 30 days during the same hospitalization subsequent to the operation [9,10]. Pre-operation left ventricle (LV) dysfuction was defined as ejection fraction (EF) <50% as measured by echocardiography.
The primary outcome of this study was to reveal patients’ survival data and risk factor for mortality or operative mortality. The second outcome of this study was to find long-term outcome for repetitive surgical PVR and risk factor for repetitive surgical PVR.
Data were expressed as median and interquartile range (IQR). Continuous variables were analyzed using Wilcoxon rank-sum test and discrete variables were analyzed using chi-square tests. To select candidate factors for independent variables to be included in the Cox model, the continuous variables were checked to determine if the proportional risk assumption was satisfied using restricted cubic splines. For categorical variables, the proportional risk assumption was confirmed by using a log-log (survival) plot or from the interaction with the time variable. Only the variables that satisfied the proportional risk assumption were analyzed.
Univariate and multivariate analysis, reported as hazard ratios (HRs) with 95% confidence intervals (CIs), were carried out for risk factors of mortality and repetitive PVR. The survival rates of each group were compared using the log-rank test. As appropriate, P < 0.05 was considered statistically significant. All data analyses were performed using SAS (9.4 Version; SAS Institute Inc., Cary, NC, USA). This study was supported by the Medical Research Collaborating Center at Seoul National University College of Medicine and Seoul National University Hospital.
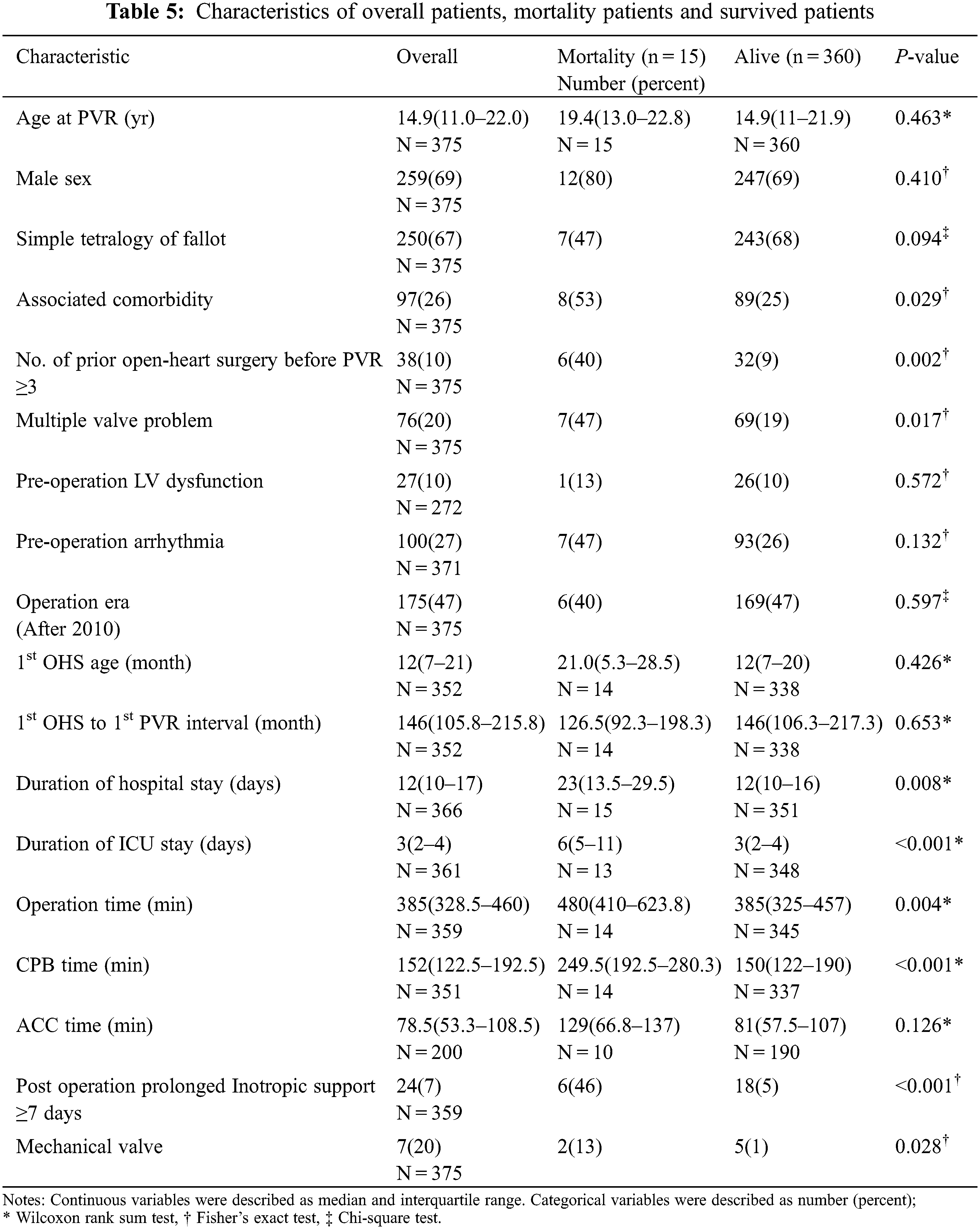
The demographic characteristics of the patients were described in Table 1. Overall, 293 patients were found to have undergone surgical PVR during the study period at a median age of 14.9 years (IQR, 11.0–22.0 years). The median age of the patients at initial open-heart surgery was 12 months (IQR, 7–21 months) and the median interval between initial open-heart surgery and first PVR was 146 months (range, 105.8–215.8 months). Of the total study participants, 99 were women (33.8%). The most common fundamental diagnosis was TOF (n = 244, 83.3%); among which, 215 cases were simple TOF and 29 cases were complex TOF. The median duration of ICU stay was 3 days (IQR, 2–4 days), the mean hospital stay was 12 days (IQR, 10–17 days), and the median follow-up duration was 10.5 years (IQR, 5.4–14.8 years).
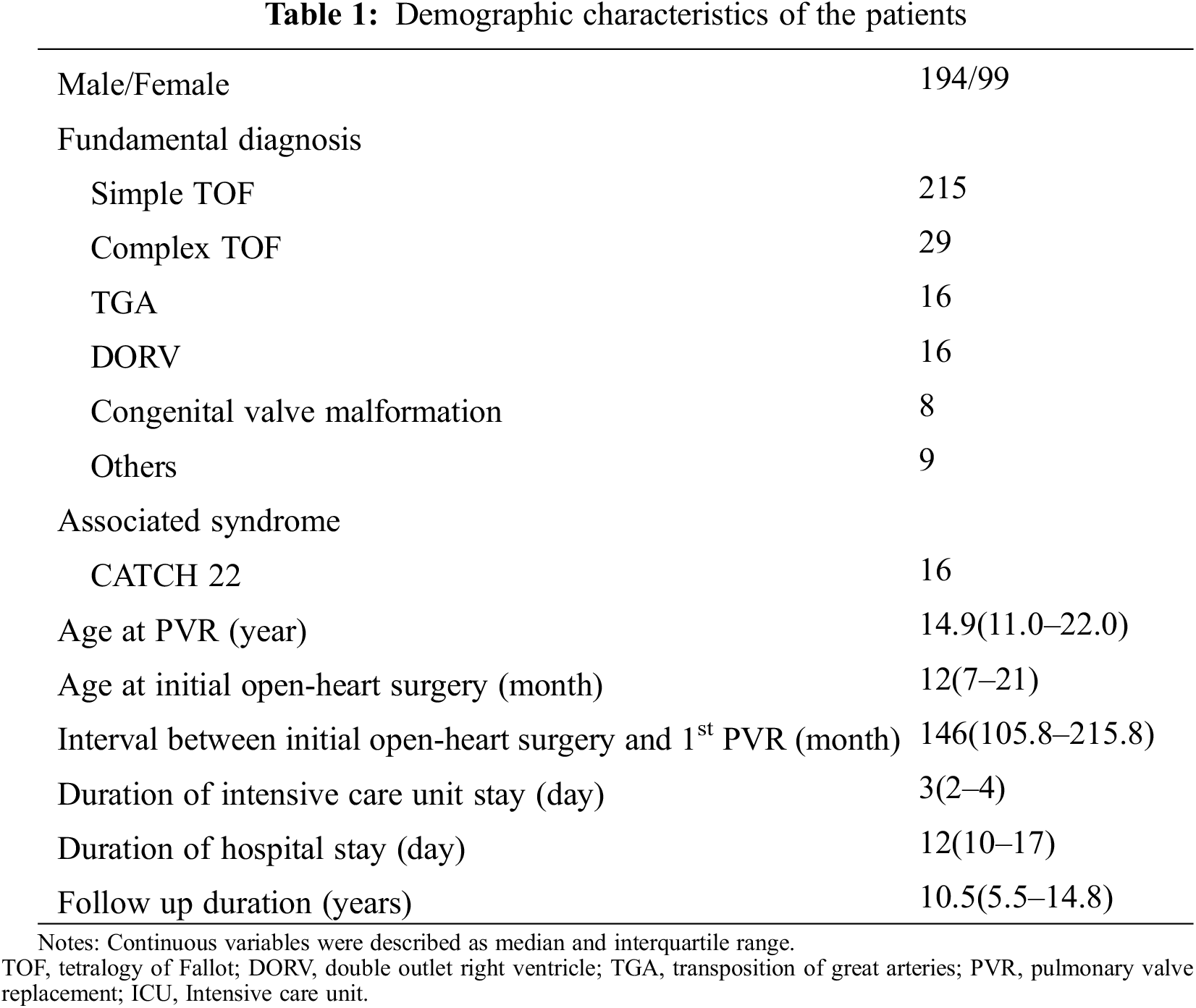
3.2 Surgical Pulmonary Valve Replacement
The surgical PVR data were summarized in Table 2. PVR was performed through a median sternotomy on cardiopulmonary bypass with mild hypothermia. Aortic cross-clamping was used or not dependent on each surgeon’s preference or concomitant procedures. The median operation time was 385 min (IQR, 328.5–460 min), the median CPB time was 152 min (IQR, 122.5–192.5 min), and the median ACC time was 78.5 min (IQR, 53.3–108.5 min). The most commonly used valve was the tissue valve (n = 368, 98.1%), and among them, the stented bioprosthetic porcine valve was the most commonly used valve (n = 270, 73.4%). The most commonly performed concomitant procedure was RVOT surgery including pulmonary artery angioplasty (n = 261, 81.6%).
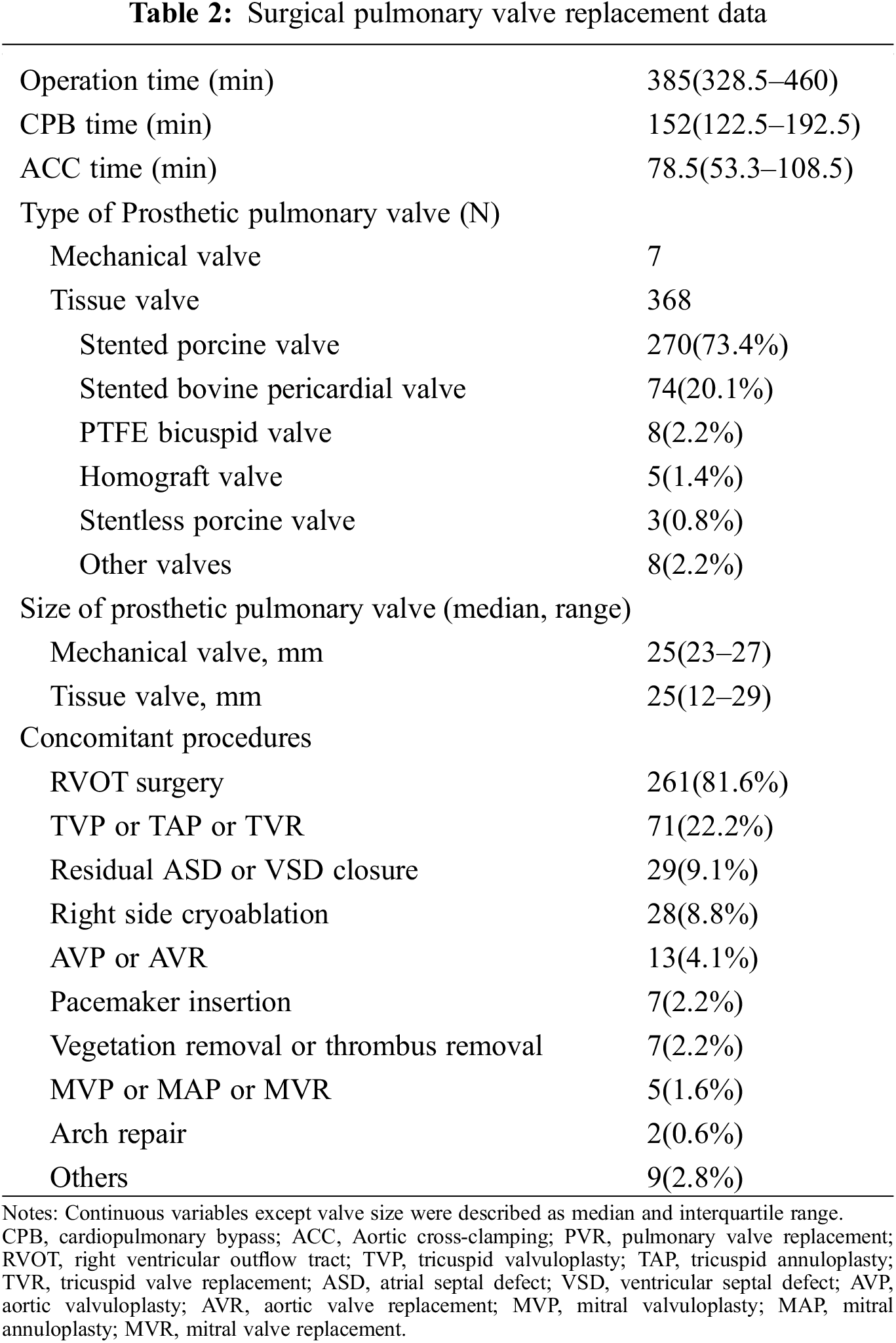
3.3 Follow-Up Outcomes and Comorbidity
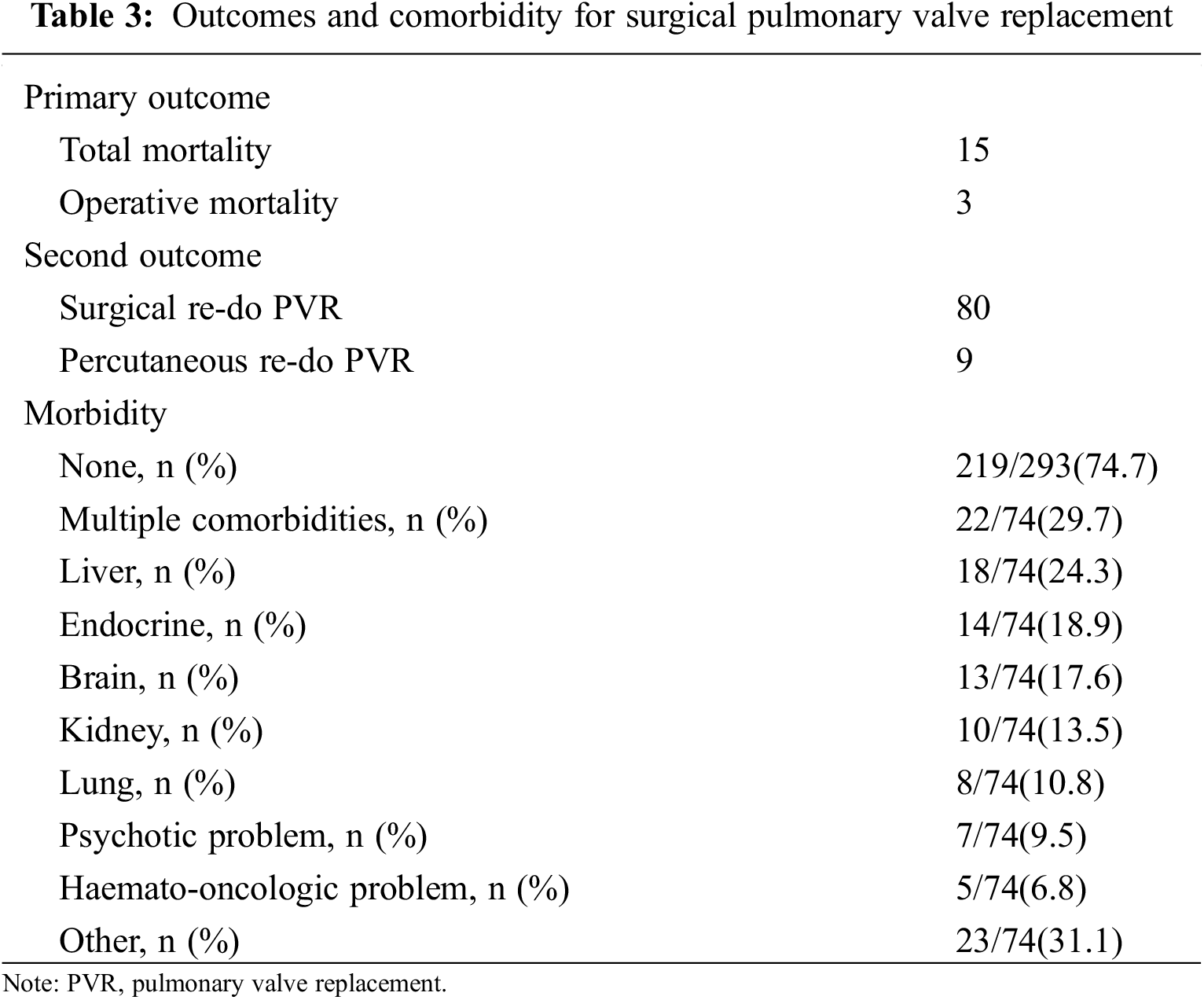
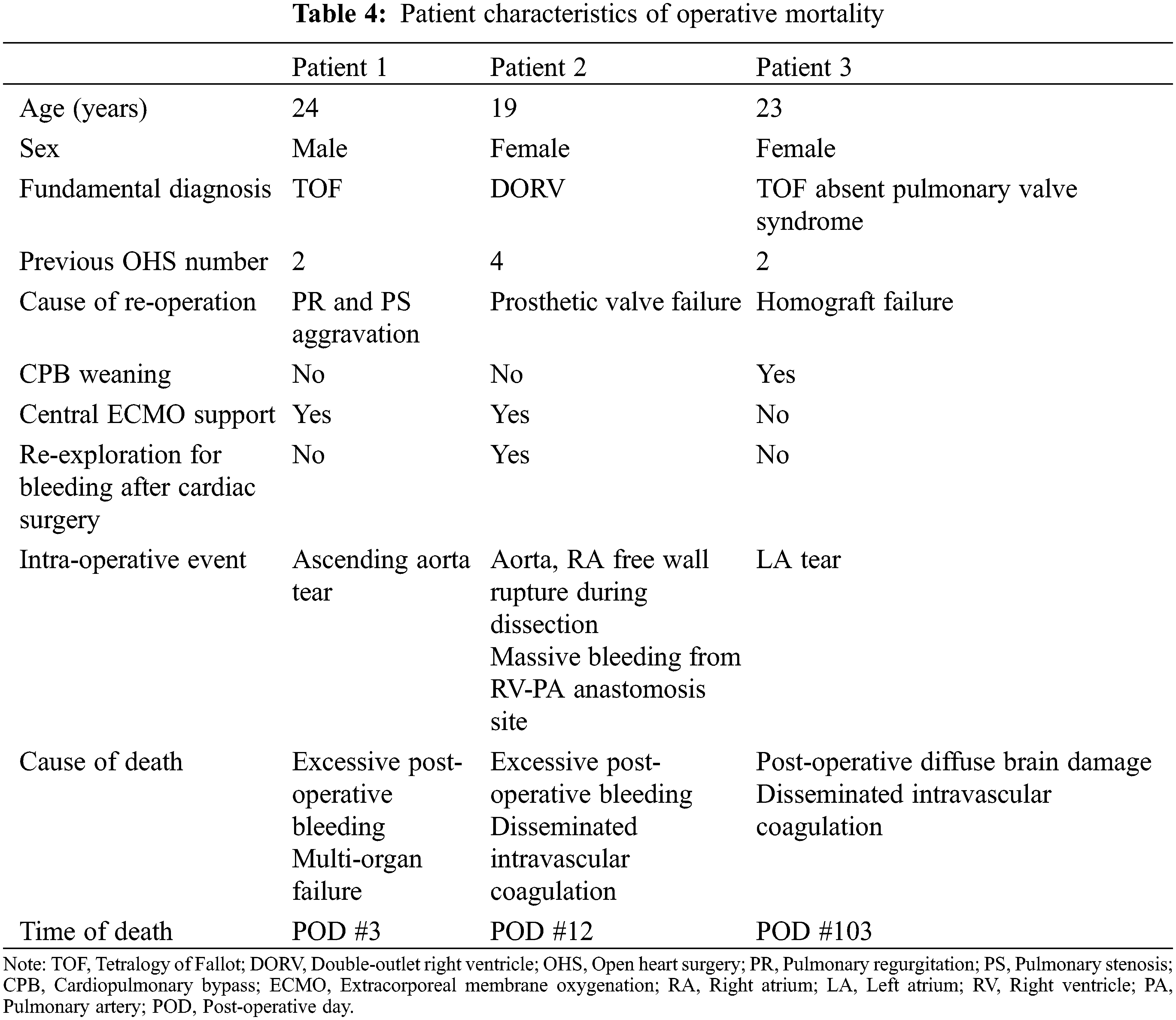
The outcomes and comorbidities of surgical PVR were summarized in Table 3. There were 3 patients with operative mortality and all three mortality cases were occurred during hospitalization in which the operation was performed, even if one of them was occurred after 30 days. The characteristics of the patients with operative mortality were summarized in Table 4. There was no additional death within 30 days after hospital discharge and the total number of mortalities during follow-up were 15. The characteristics of overall patients, overall mortality patients, and survived patients were summarized in Table 5. The survival rate was 95.1% over a 20-year follow-up period. (Fig. 1) There were 89 re-interventions, of which 80 were surgical PVR and 9 were percutaneous PVR. Seventy-four patients (74/293, 25.3%) had comorbidities from the initial open-heart surgery until the PVR and 22 patients (22/74, 29.7%) had multiple comorbidities. Most common comorbidity was liver disease (18/74, 24.3%).
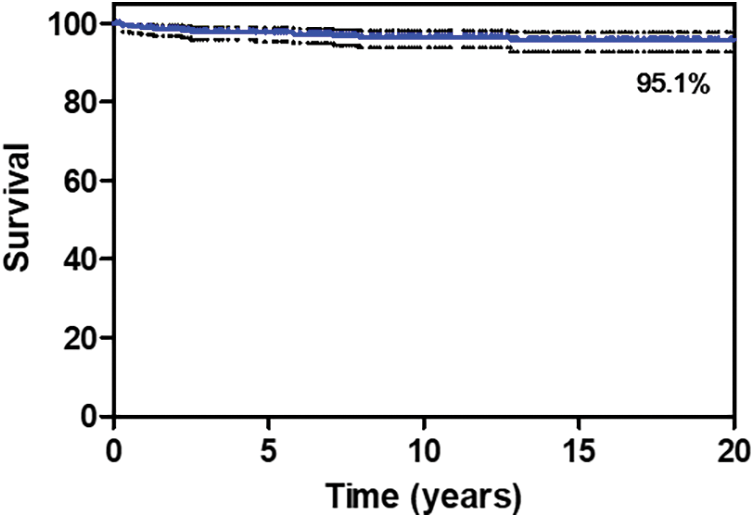
Figure 1: Overall survival from surgical pulmonary valve replacement. The survival rate after 20 years of follow-up was 95.1%
3.4 Prognostic Factors for Mortality of Pulmonary Valve Replacement
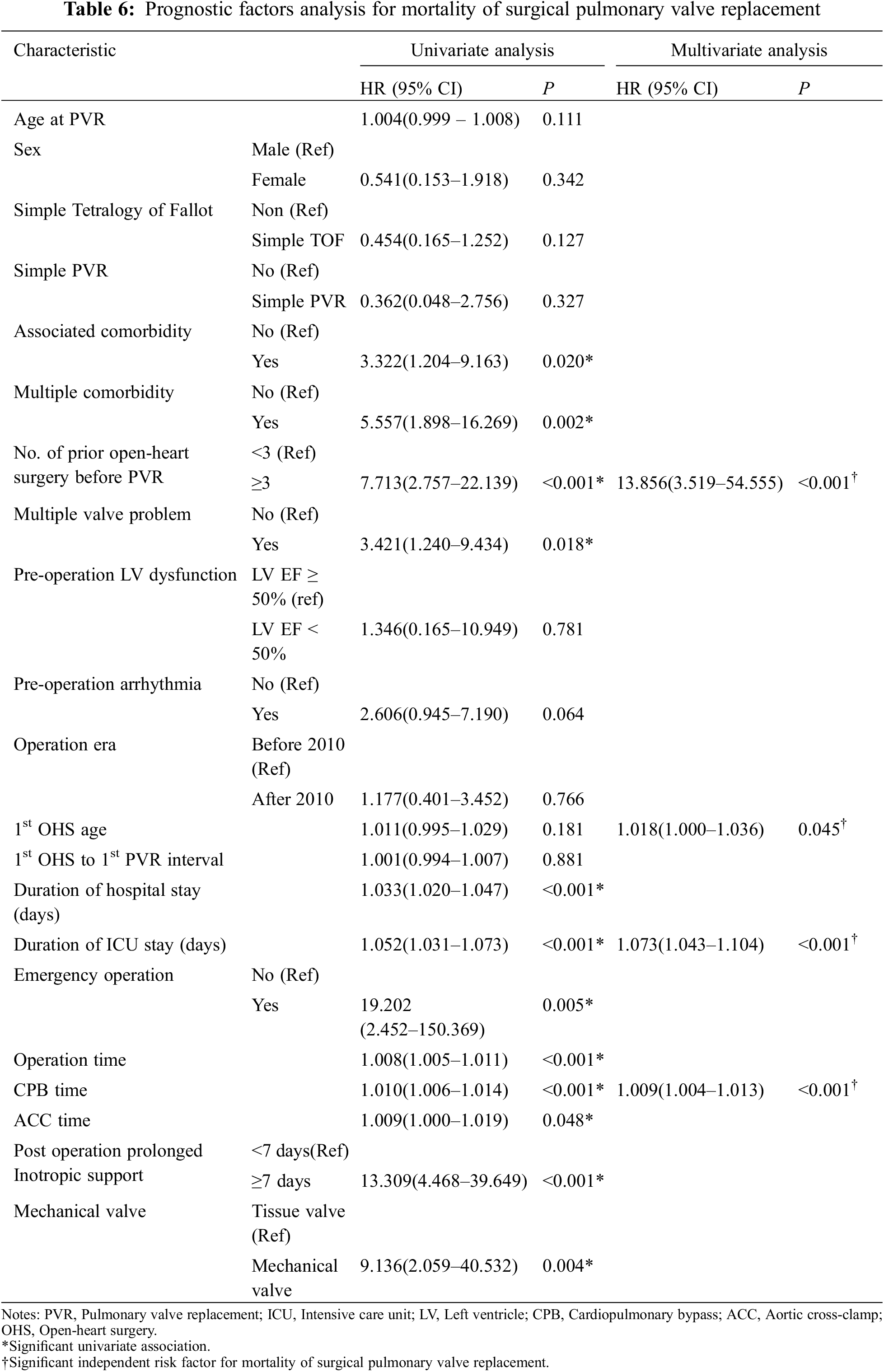
The characteristics of both patients and valves were assessed as potential prognostic factors for mortality of surgical PVR and were summarized in Table 6. Statistically significant variables at the 20% significance level in the univariate analysis were selected as independent variables to be included in the multivariate analysis. Univariate analysis showed that the presence of comorbidity or multiple comorbidities, number of prior open-heart surgeries before PVR, multiple valve problems, longer duration of hospital and ICU stay, emergency operation, longer operation time, longer CPB time, prolonged post-operative inotropic support, and use of mechanical valve were associated with mortality. Multivariate analysis demonstrated that prior open-heart surgery at least 3 times before PVR (HR, 13.856; 95% CI, 3.519–54.555; P < 0.001), age at the 1st operation (HR 1.018; 95% CI, 1.000–1.036; P = 0.045), longer CPB time (HR, 1.009; 95% CI, 1.004–1.013; P < 0.001) and longer ICU stay (HR, 1.073; 95% CI, 1.043–1.104; P < 0.001) were predictors of mortality from surgical PVR.
Patients who previously underwent multiple surgical PVR and open-heart surgeries had significantly higher mortality rates. The mortality rate increased with the number of PVR surgeries (P < 0.001, Fig. 2A). Patients who underwent surgical PVR after at least three previous open-heart surgeries had significantly higher mortality than those who underwent PVR with less than three (P < 0.001, Fig. 2B).
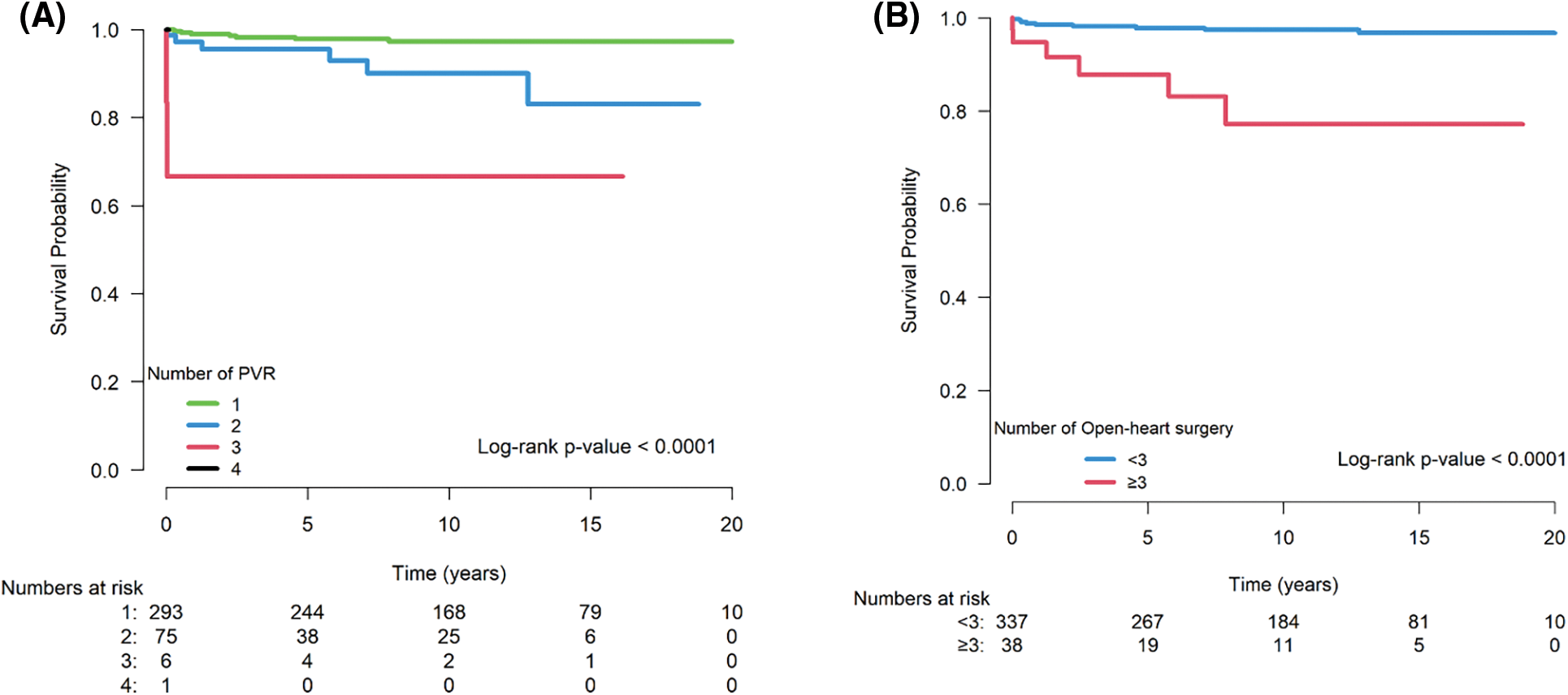
Figure 2: Log rank analysis of mortality after surgical PVR stratified by number of PVR (2A) and number of open-heart surgeries (2B). The mortality rate increased with the number of PVR surgeries (P < 0.001). In addition, patients who underwent PVR after 3 or more previous open-heart surgeries had significantly higher mortality than those underwent open-heart surgeries less than 3 times (P < 0.001).
3.5 Prognostic Factors for Operative Mortality of Surgical Pulmonary Valve Replacement
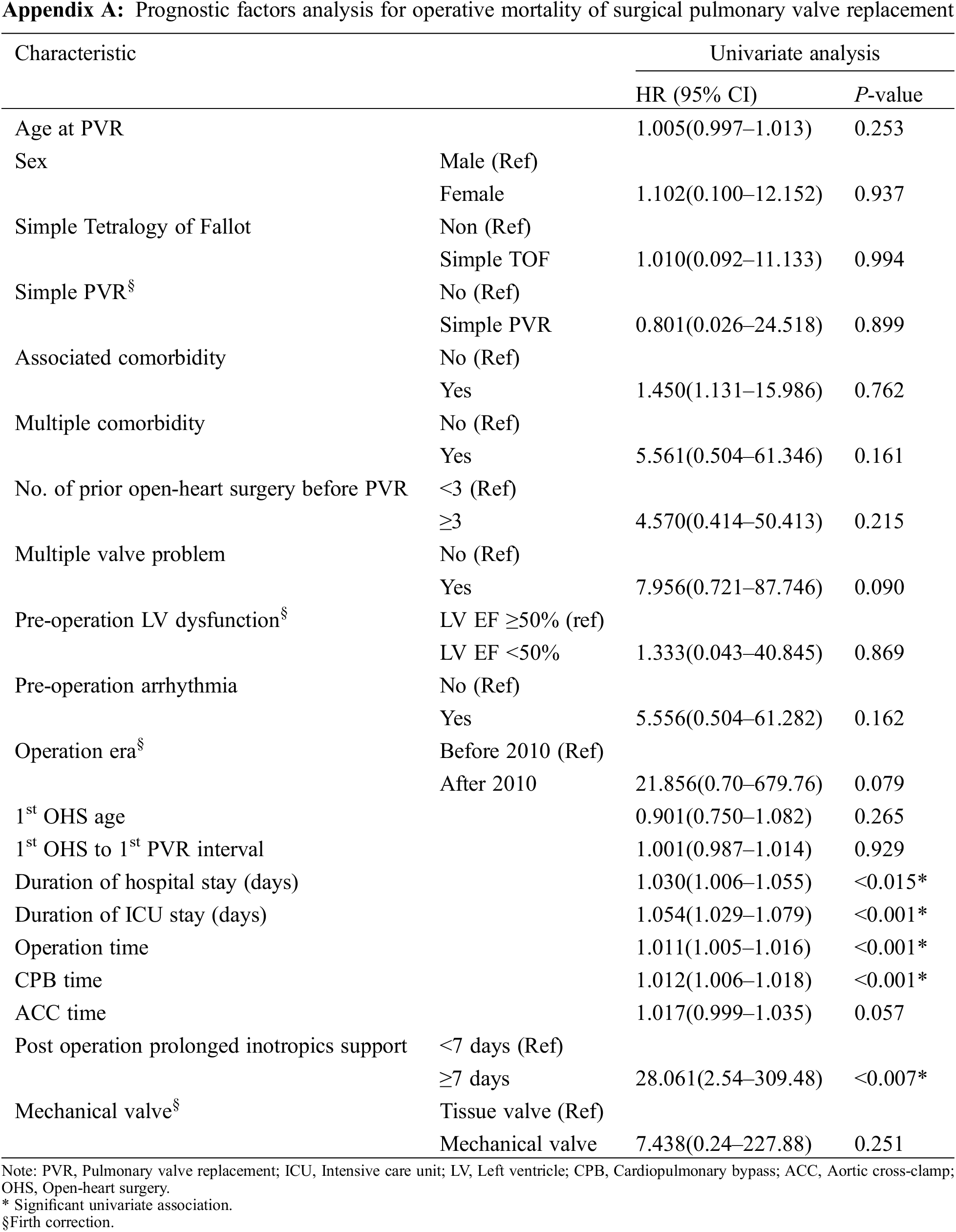
We additionally performed univariate analyses to evaluate the prognostic factors for operative mortality. The same factors as those used to evaluate hospital mortality were assessed as potential prognostic factors for operative mortality of surgical PVR and were summarized in Appendix A. Univariate analysis showed that longer hospital and ICU stay, longer operation time, longer CPB, and prolonged post-operative inotropic support were associated with mortality.
3.6 Prognostic Factors for Repetitive Pulmonary Valve Replacement
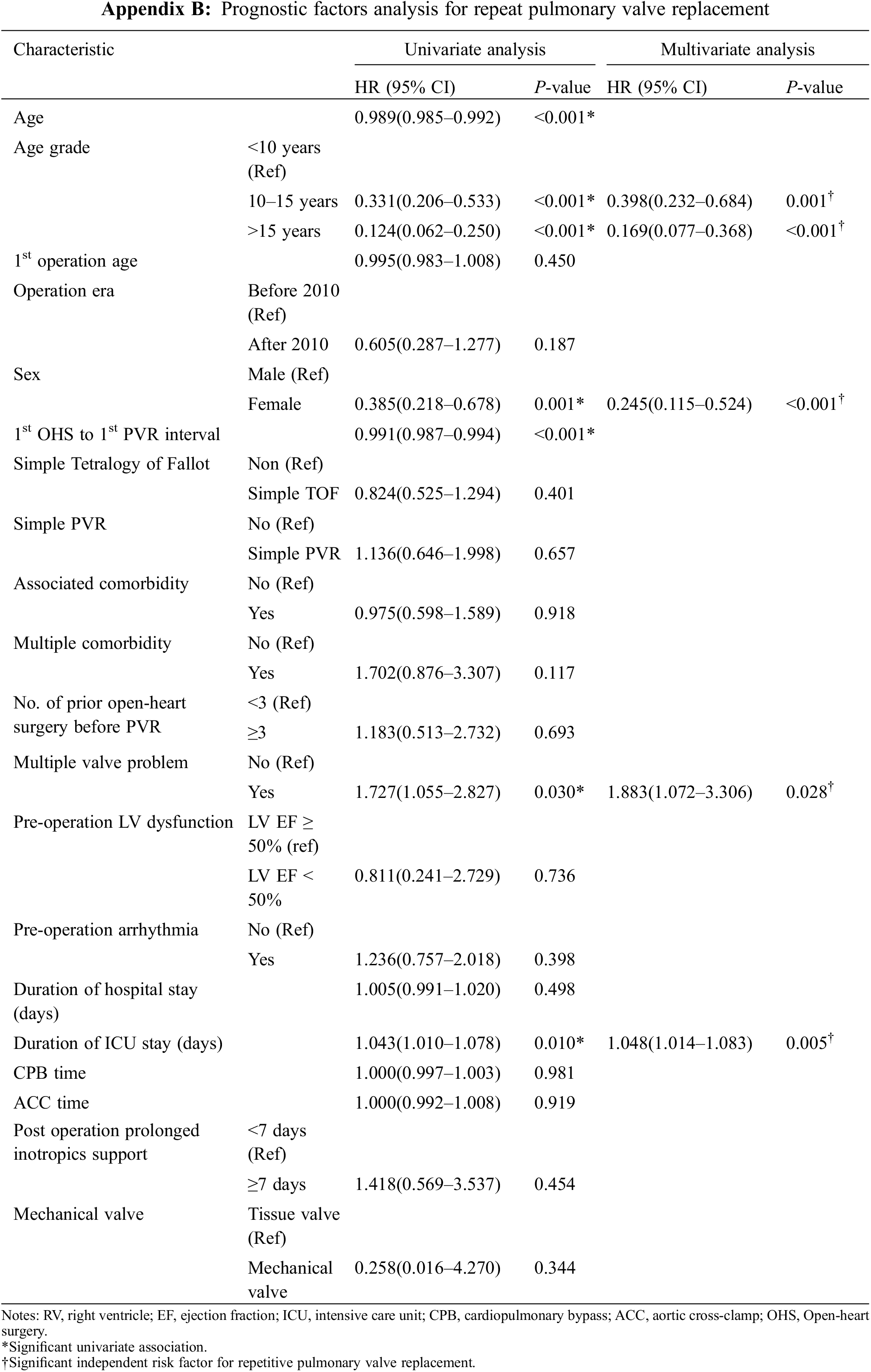
The potential prognostic factors for repetitive PVR were summarized in Appendix B. Univariate analysis showed that younger age, male sex, 1st OHS to 1st PVR interval, multiple valve problems, duration of ICU stay were associated with repetitive PVR. Age younger than 10 years at first PVR, male, multiple valve problems (HR, 1.883; 95% CI, 1.072–3.306; P = 0.028) and duration of ICU stay (HR 1.048; 95% CI, 1.014–1.083; P = 0.005) were predictors for repetitive PVR by multivariate analysis.
There were 80 surgical redo PVRs and 9 percutaneous re-do PVRs. Repetitive PVR was examined by dividing the patients into three groups by age <10 years, 10–14 years, and ≥15 years. The group aged <10 years had a higher incidence of repetitive PVR (P < 0.001, Appendix C).
As the ACHD population grows, it is becoming increasingly important to identify patients at the greatest risk for mortality and repetitive operations. PVR is a frequently performed procedure in the field of congenital heart surgery and is the most common cardiac operation and reoperation performed in adults with CHD [3,11]. Our study primarily focused on determining the long-term outcome and potential risk factors contributing to mortality and repetitive PVR after surgical PVR. This study showed good operative long-term outcomes of surgical PVR (95.1% survival rate over a follow-up period of 20 years) although there were 3 operative mortalities. The increasing number of prior open-heart surgeries, age at the OHS, longer ICU stay, and longer CPB time were risk factors for mortality after surgical PVR. In addition, the presence of multiple valve problems, longer ICU stay, younger age, and male sex were risk factors for redo PVR.
In our study, an increase in the number of prior open-heart surgeries was one of the main risk factors for mortality. This might be related to repetitive sternotomies and open-heart surgeries which increase the difficulty of surgery and CPB time. Although the Texas Children’s Hospital reported excellent results on repeat sternotomy in congenital heart surgery and concluded that repeat sternotomy was no longer a risk factor for morbidity or mortality [12], other groups have reported that an increase in the number of sternotomies was associated with an increase in operative mortality [11,13,14]. In addition, Giamberti et al. [15], in their study of 164 adults with CHD who underwent cardiac reoperations, reported that post-operative morbidity was associated with the number of previous operations. The median number of previous open-heart operations for mortality cases in our study (n = 15) was 2 (range, 1–5).
Patients with adverse events had a significantly longer duration of ICU stay. It is common finding in the literature and self-explanatory that the occurrence of post-operative complications after congenital heart surgery is associated with prolonged ICU stay [16]. We also found that overall mortality after PVR was associated with longer CPB time, although most cases of mortality were noted more than 30 days after PVR in our study. Longer CPB time is a well-known independent risk factor for post-operative complications after congenital heart surgery [15,17,18]. In the case of performing concomitant operation as well as simple PVR, the CPB time was longer, which means that the difficulty of the surgery increases CPB time and may be related with an increase in mortality. Simple PVR was not a statistically significant risk factor for operative mortality. However, among patients who received simple PVR, none showed operative mortality. This is possibly due to the small number of operative mortality cases (n = 3). We hope that further studies will reveal the relationship between simple PVR and operative mortality.
It turns out from univariate and multivariate analysis of prognostic factor analysis for repetitive PVR that repetitive PVR is closely related with multiple valve problems, and that the tricuspid valve is the most commonly involved in this problem. This result implies that the hemodynamic interaction between the valve lesions increases the hemodynamic burden to the replaced valve, which leads to shortening the durability of the replaced valve. It is worthy to note that, as compared with the patients with single valve problem, the patients with multiple valve problems are younger and have more multiple comorbidities, heart dysfunction, but the less simple-TOF and single PVR. This indicates that patients in the multiple valve problem group had more severe disease than those in the single valve problem group.
To improve long-term outcomes of PVR, percutaneous pulmonary valve implantation (PPVI) has recently emerged as an alternative treatment option for failed bioprosthetic valves [19]. Indications for PPVI are the same as for surgical interventions on pulmonary valve, with limits related to the maximum diameter of the available percutaneous prosthesis. Seven-year outcomes of the United States investigational device exemption trial of the Melody transcatheter pulmonary valve showed a high rate of procedural success and good clinical outcome [20]. This technique is expected to reduce the number of operations during the lifetime of patients who have undergone bioprosthetic PVR. New valves and developing technology are expected to extend indications to patients with very large RVOT also. However, a careful anatomical and hemodynamic assessment is mandatory in order to select good candidates for PPVI [21].
The present study was limited by its retrospective nature. In addition, statistical significance is likely to be reduced as a result of the insufficient number of patients with mortality, especially operative mortality.
As the ACHD population grows, repetitive PVR is inevitable and helpful for the patient with problems in pulmonary valve. Though surgical PVR has excellent long-term outcome, it should be performed with caution for those who previously underwent multiple open-heart surgeries, especially if the patient received more than 3 times of open-heart surgeries. PPVI is expected to reduce the total number of operations during lifetime of patients who have undergone bioprosthetic PVR.
Author Contributions: WYP and GBK contributed to the research conception and design, data analysis and interpretation, manuscript drafting, and critical revision of the manuscript. SYL, MKS, HWK, HSA, EJB, SC, JGK and WHK contributed to analyze and interpret data and revised the manuscript. All authors had full access to all of the graphs and tables in the study and finally approved for submission.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Jain, A., Oster, M., Kilgo, P., Grudziak, J., Jokhadar, M. et al. (2012). Risk factors associated with morbidity and mortality after pulmonary valve replacement in adult patients with previously corrected tetralogy of fallot. Pediatric Cardiology, 33(4), 601–606. DOI 10.1007/s00246-012-0185-z. [Google Scholar] [CrossRef]
2. Batlivala, S. P., Emani, S., Mayer, J. E., McElhinney, D. B. (2012). Pulmonary valve replacement function in adolescents: A comparison of bioprosthetic valves and homograft conduits. The Annals of Thoracic Surgery, 93(6), 2007–2016. DOI 10.1016/j.athoracsur.2012.02.039. [Google Scholar] [CrossRef]
3. Mascio, C. E., Pasquali, S. K., Jacobs, J. P., Jacobs, M. L., Austin, E. H., 3rd (2011). Outcomes in adult congenital heart surgery: Analysis of the society of thoracic surgeons database. The Journal of Thoracic and Cardiovascular Surgery, 142(5), 1090–1097. DOI 10.1016/j.jtcvs.2011.07.028. [Google Scholar] [CrossRef]
4. Gatzoulis, M. A., Balaji, S., Webber, S. A., Siu, S. C., Hokanson, J. S. et al. (2000). Risk factors for arrhythmia and sudden cardiac death late after repair of tetralogy of fallot: A multicentre study. Lancet, 356(9234), 975–981. DOI 10.1016/S0140-6736(00)02714-8. [Google Scholar] [CrossRef]
5. Bove, E. L., Kavey, R. E., Byrum, C. J., Sondheimer, H. M., Blackman, M. S. et al. (1985). Improved right ventricular function following late pulmonary valve replacement for residual pulmonary insufficiency or stenosis. The Journal of Thoracic and Cardiovascular Surgery, 90(1), 50–55. DOI 10.1016/S0022-5223(19)38661-1. [Google Scholar] [CrossRef]
6. Eyskens, B., Reybrouck, T., Bogaert, J., Dymarkowsky, S., Daenen, W. et al. (2000). Homograft insertion for pulmonary regurgitation after repair of tetralogy of fallot improves cardiorespiratory exercise performance. The American Journal of Cardiology, 85(2), 221–225. DOI 10.1016/S0002-9149(99)00640-2. [Google Scholar] [CrossRef]
7. Lee, C., Lee, C. H., Kwak, J. G. (2016). Outcomes of redo pulmonary valve replacement for bioprosthetic pulmonary valve failure in 61 patients with congenital heart disease. European Journal of Cardio-Thoracic Surgery, 50(3), 470–475. DOI 10.1093/ejcts/ezw037. [Google Scholar] [CrossRef]
8. Zubairi, R., Malik, S., Jaquiss, R. D., Imamura, M., Gossett, J. et al. (2011). Risk factors for prosthesis failure in pulmonary valve replacement. The Annals of Thoracic Surgery, 91(2), 561–565. DOI 10.1016/j.athoracsur.2010.07.111. [Google Scholar] [CrossRef]
9. Jacobs, J. P., Mavroudis, C., Jacobs, M. L., Maruszewski, B., Tchervenkov, C. I. et al. (2006). What is operative mortality? Defining death in a surgical registry database: A report of the STS congenital database taskforce and the joint EACTS-STS congenital database committee. The Annals of Thoracic Surgery, 81(5), 1937–1941. DOI 10.1016/j.athoracsur.2005.11.063. [Google Scholar] [CrossRef]
10. Overman, D. M., Jacobs, J. P., Prager, R. L., Wright, C. D., Clarke, D. R. et al. (2013). Report from the society of thoracic surgeons national database workforce: Clarifying the definition of operative mortality. World Journal for Pediatric & Congenital Heart Surgery, 4(1), 10–12. DOI 10.1177/2150135112461924. [Google Scholar] [CrossRef]
11. Jacobs, J. P., Mavroudis, C., Quintessenza, J. A., Chai, P. J., Pasquali, S. K. et al. (2014). Reoperations for pediatric and congenital heart disease: An analysis of the society of thoracic surgeons (STS) congenital heart surgery database. Seminars in Thoracic and Cardiovascular Surgery: Pediatric Cardiac Surgery Annual, 17(1), 2–8. DOI 10.1053/j.pcsu.2014.01.006. [Google Scholar] [CrossRef]
12. Morales, D. L., Zafar, F., Arrington, K. A., Gonzalez, S. M., McKenzie, E. D. et al. (2008). Repeat sternotomy in congenital heart surgery: No longer a risk factor. The Annals of Thoracic Surgery, 86(3), 897–902. DOI 10.1016/j.athoracsur.2008.04.044. [Google Scholar] [CrossRef]
13. Kirshbom, P. M., Myung, R. J., Simsic, J. M., Kramer, Z. B., Leong, T. et al. (2009). One thousand repeat sternotomies for congenital cardiac surgery: Risk factors for reentry injury. The Annals of Thoracic Surgery, 88(1), 158–161. DOI 10.1016/j.athoracsur.2009.03.082. [Google Scholar] [CrossRef]
14. Dearani, J. A., Connolly, H. M., Martinez, R., Fontanet, H., Webb, G. D. (2007). Caring for adults with congenital cardiac disease: Successes and challenges for 2007 and beyond. Cardiology in the Young, 17(Suppl 2), 87–96. DOI 10.1017/S1047951107001199. [Google Scholar] [CrossRef]
15. Giamberti, A., Chessa, M., Abella, R., Butera, G., Carlucci, C. et al. (2009). Morbidity and mortality risk factors in adults with congenital heart disease undergoing cardiac reoperations. The Annals of Thoracic Surgery, 88(4), 1284–1289. DOI 10.1016/j.athoracsur.2009.05.060. [Google Scholar] [CrossRef]
16. Brown, K. L., Ridout, D. A., Goldman, A. P., Hoskote, A., Penny, D. J. (2003). Risk factors for long intensive care unit stay after cardiopulmonary bypass in children. Critical Care Medicine, 31(1), 28–33. DOI 10.1097/00003246-200301000-00004. [Google Scholar] [CrossRef]
17. Kogon, B., Grudziak, J., Sahu, A., Jokhadar, M., McConnell, M. et al. (2013). Surgery in adults with congenital heart disease: Risk factors for morbidity and mortality. The Annals of Thoracic Surgery, 95(4), 1377–1382. DOI 10.1016/j.athoracsur.2012.11.076. [Google Scholar] [CrossRef]
18. Agarwal, H. S., Wolfram, K. B., Saville, B. R., Donahue, B. S., Bichell, D. P. (2014). Postoperative complications and association with outcomes in pediatric cardiac surgery. The Journal of Thoracic and Cardiovascular Surgery, 148(2), 609–616.e1. DOI 10.1016/j.jtcvs.2013.10.031. [Google Scholar] [CrossRef]
19. Gillespie, M. J., Rome, J. J., Levi, D. S., Williams, R. J., Rhodes, J. F. et al. (2012). Melody valve implant within failed bioprosthetic valves in the pulmonary position: A multicenter experience. Circulation: Cardiovascular Interventions, 5(6), 862–870. DOI 10.1161/CIRCINTERVENTIONS.112.972216. [Google Scholar] [CrossRef]
20. Cheatham, J. P., Hellenbrand, W. E., Zahn, E. M., Jones, T. K., Berman, D. P. et al. (2015). Clinical and hemodynamic outcomes up to 7 years after transcatheter pulmonary valve replacement in the US melody valve investigational device exemption trial. Circulation, 131(22), 1960–1970. DOI 10.1161/CIRCULATIONAHA.114.013588. [Google Scholar] [CrossRef]
21. Giugno, L., Faccini, A., Carminati, M. (2020). Percutaneous pulmonary valve implantation. Korean Circulation Journal, 50(4), 302–316. DOI 10.4070/kcj.2019.0291. [Google Scholar] [CrossRef]
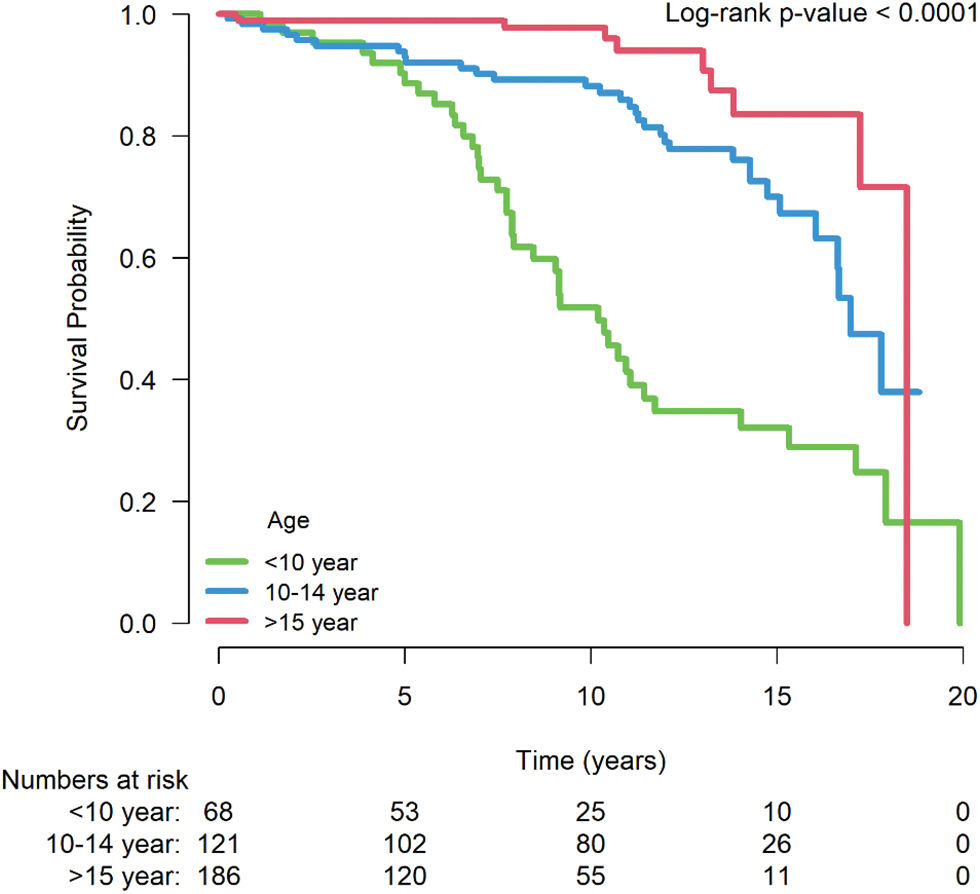
Appendix C: Log rank test of redo PVR after repetitive surgical PVR. The repetitive PVR was examined by dividing the patients into three groups by age: <10 years, 10–14 years, and ≥15 years. The <10 years group had a higher incidence of repetitive PVR (P < 0.001)
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |