

 | Congenital Heart Disease |  |
DOI: 10.32604/chd.2022.019279
ARTICLE
Coronary Artery Anomalies in D-Transposition of the Great Artery Following Arterial Switch Operation
Division of Cardiology, Department of Pediatrics, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand
*Corresponding Author: Jarupim Soongswang. Email: jarupim.soo@mahidol.ac.th
Received: 18 September 2021; Accepted: 08 January 2022
Abstract: Background: The survival rate of patients following arterial switch operation (ASO) exceeds 95%, but coronary artery anomalies (CAA) contribute to a 2% incidence of sudden cardiac arrest later in life. Therefore, we aimed to assess abnormal findings of coronary arteries in post-ASO patients. Methods: Coronary computed tomography angiography (CCTA) is performed on post-ASO patients who meet institutional criteria. Intraoperative findings of coronary artery patterns were retrospectively reviewed and categorized using the Leiden classification system. Coronary artery anomalies were detected by CCTA and associations with coronary artery compromise were explored. Results: Forty-three patients who had CCTA with a median age of 15.6 years (12–21.3 years) were included in the study. Unusual coronary patterns were identified in 20 (46%) patients before ASO. CCTA identified 25 CAA in 22 patients (eleven with prepulmonic course, nine with interarterial course, three with acute take-off angle, and two with significant stenosis). Postoperative CAA was more common in patients with unusual coronary patterns (90% vs. 17.4%; p < 0.001). Nine patients experienced chest pain and two patients required coronary artery bypass graft. A common ostium of RCA and LAD or LMCA were associated with significant chest pain (OR 14.3%, 95% CI 2.5 to 82.3). Conclusions: Coronary artery anomalies in post-ASO are common. All post-ASO patients should have coronary artery imaging before participating in competitive sport and when they reach adolescence. Patients with unusual preoperative coronary artery patterns should undergo coronary artery imaging when feasible. Follow-up imaging studies are indicated in patients with post-operative coronary artery abnormalities.
Keywords: Coronary artery anomalies; arterial switch operation; d-transposition of great arteries
Abbreviations
| Ao | aorta |
| AOR | adjusted odds ratio |
| ASO | arterial switch operation |
| AR | aortic regurgitation |
| BMP | beat per minute |
| CAA | coronary artery anomalies |
| CABG | coronary artery bypass graft |
| CCTA | coronary computed tomography angiography |
| CI | 95% confidence interval |
| COR | crude odds ratio |
| CMR | cardiac magnetic resonance |
| Cx | circumflex artery |
| d-TGA | d-transposition of the great artery |
| ECG | electrocardiogram |
| EST | exercise stress test |
| IMP | index of myocardial performance |
| IQR | interquartile range |
| IVS | intact ventricular septum |
| LAD | left anterior descending artery |
| LCA | left coronary artery |
| LM | lecompte maneuver |
| LMCA | left main coronary artery |
| LV | left ventricle |
| LVEF | left ventricular systolic function |
| METs | metabolic equivalent |
| MI | myocardial ischemia |
| MRI | magnetic resonance imaging |
| MRA | magnetic resonance angiography |
| NCC | noncoronary cusp |
| ND | not done |
| NF | nonfacing sinus |
| NSR | normal sinus rhythm |
| PBPV | percutaneous balloon pulmonary valvuloplasty |
| PS | pulmonary stenosis |
| PT | pulmonary trunk |
| PTA | percutaneous transluminal angioplasty |
| PVC | premature ventricular complex |
| RBBB | right bundle branch block |
| RCA | right coronary artery |
| RV | right ventricle |
| VSD | ventricular septal defect |
The d-transposition of the great arteries (d-TGA) accounts for 5%–7% of all congenital heart defects. In 1975, Adib Jatene introduced the arterial switch operation (ASO) to replace the atrial switch operation to anatomically and physiologically correct d-TGA. This improved the 10-year survival rate to 97.7% and resulted in excellent long-term outcomes [1–5]. While late mortality from myocardial infarction occurs in less than 2% of post-ASO survivors [6–12], 3%–9% of this group of patients experience late, nonfatal myocardial infarction and significant coronary artery stenosis [1,11,13–18]. Coronary artery events after ASO are reported to have a bimodal pattern where about 90% of coronary events occur within the first three months, then decrease to almost zero, and slowly increase again after six years with an overall prevalence of up to 11% [13]. Few reports have described myocardial ischemic events in follow-up periods of ten years or longer [6,8,13,19]. Long-term coronary obstruction is due to progressive proximal eccentric intimal thickening, flow abnormalities leading to increased shear stress with progressive fibrocellular intimal thickening, ostial fibrosis at the suture line, and kinking or stretching with reactive injury from surgical manipulation [20].
Several studies have reported serious cardiac events including sudden cardiac death in post-ASO patients without prior chest pain or symptoms that suggest the presence of coronary stenosis [3,11,13,21]. Routine cardiac tests such as electrocardiogram (ECG), echocardiography, exercise stress test (EST), and myocardial scintigraphy have low sensitivity to detect myocardial ischemia. The sensitivity of each of these tests has been reported to be less than 50% and a combination of all tests increased the sensitivity to only 70% [13]. Some studies have suggested that coronary computed tomography angiography (CCTA) may be used to detect coronary artery anomalies [15,17,22]. The 2020 Appropriate Use Criteria on follow-up imaging in patients with congenital heart disease [23] recommends that asymptomatic post-ASO adults and symptomatic patients that experience changes in clinical status and/or new concerning signs and symptoms of myocardial ischemia should be evaluated for coronary imaging. However, there is no strong evidence suggesting when and how the coronary arteries of post-ASO pediatric patients should be evaluated.
Therefore, we aimed to assess coronary artery anatomy, its patency, and abnormal findings in pediatric patients who underwent ASO in early infancy. We used CCTA to evaluate coronary arteries because of its advantages in providing information on the underlying mechanism of coronary luminal narrowing whether from stretching, compression, or intramural course.
The protocol was approved by the Institutional Review Board of Siriraj Hospital (Si 351/2019). Between 1992 and 2019, 170 patients with d-TGA underwent ASO in the Faculty of Medicine Siriraj Hospital, a tertiary care center in Bangkok, Thailand. Of those who underwent ASO (170 cases), seventeen patients follow up at other hospitals. Fifteen patients were peri-operative dead. Ninety-five patients did not meet institutional inclusion criteria as the majority of them are still under 16 years of age.
Our institutional inclusion criteria are 1) patients in early adolescence, 2) experience any symptomatic chest pain or changes in clinical status, 3) innate (preoperative) abnormal coronary patterns and intend to participate in competitive sport. Therefore, only forty-three patients were included to this study. Therefore, data were reviewed from forty-three patients who had undergone ASO and received CCTA based on the criteria for imaging after ASO.
Preoperative coronary artery patterns were identified and addressed by both preoperative echocardiography and operative note (direct inspection by surgeon).
All clinical data including ECG, echocardiography, exercise stress test (EST), CCTA, cardiovascular risk factors such as hypertension and obesity, medications and reoperation during the follow-up period were collected from the Pediatric Cardiology Division database.
Individual operative notes were reviewed to obtain initial coronary artery patterns visualized during surgery. The Leiden classification was used to describe coronary artery patterns [24]. A usual coronary artery pattern was defined as left anterior descending artery (LAD) and circumflex coronary artery (LCx) arising from sinus 1 and right coronary artery (RCA) from sinus 2 (1LCx-2R) (Fig. 1). Clinical data from the Cardiology clinic database and telephone interviews regarding myocardial ischemia-related symptoms such as chest pain during exertion and relieved by rest, recurrent episodes of typical and atypical angina chest pain without improvement by medical treatment of musculoskeletal and gastrointestinal tract problems, and dyspnea on exertion were obtained and recorded.
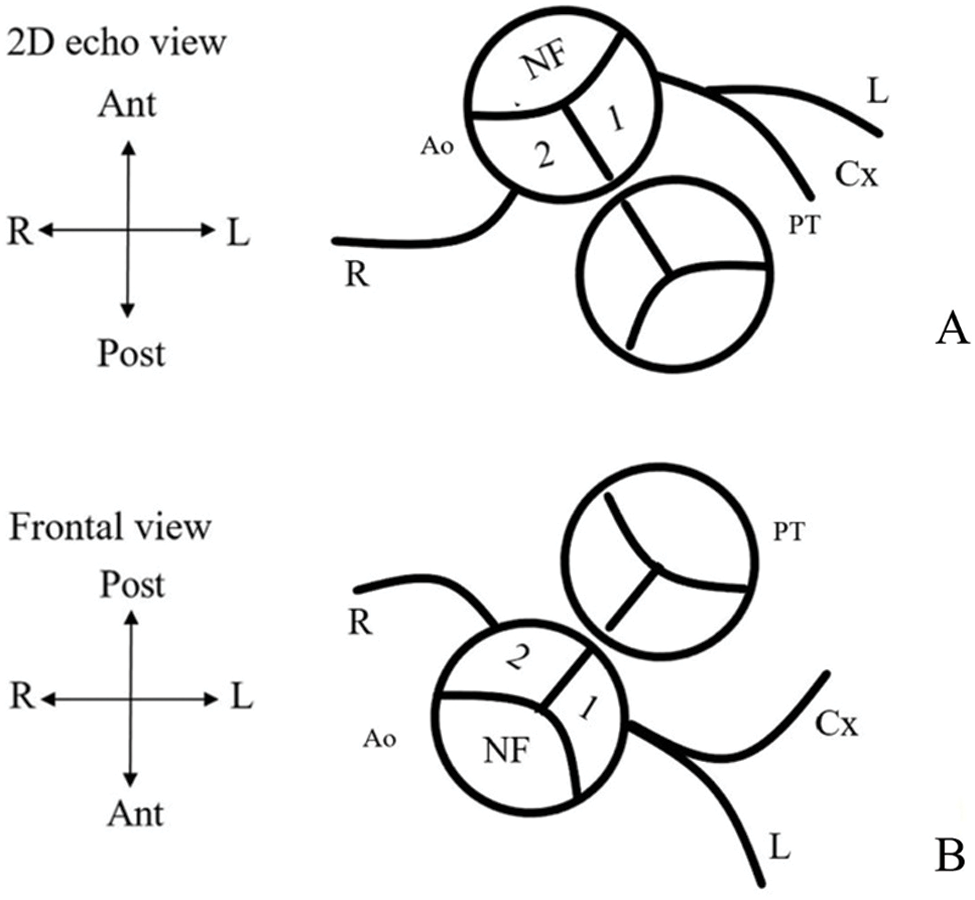
Figure 1: Preoperative usual coronary artery patterns in d-TGA. (A) Coronary artery pattern from a 2D echocardiographic view. (B) Frontal view of coronary pattern using the leiden classification. Clinicians sit in nonfacing sinus of the aorta and looking towards the pulmonic valve. The right-hand facing sinus is sinus 1 and left-hand facing sinus is sinus 2. Thus, a usual coronary artery pattern would be 1LCx-2R (NF = nonfacing sinus; 1 = facing sinus 1; 2 = facing sinus 2; PT = pulmonary trunk; Ao = Aorta; L = left anterior descending artery (LAD); Cx = left circumflex artery (LCx); R = right coronary artery (RCA); Ant = anterior; Post = posterior; R = right; L = left)
All CCTA was performed by using a contrast-enhanced, dual-source, 256 slices CT system (Somatom Definition, Siemens Healthcare, Germany) according to established guidelines [17,25]. Images in post-ASO patients were performed to assess coronary artery courses, neo-aorta and neo-pulmonary vessels, and myocardial function. An ECG-gated CCTA of the entire chest was acquired. Prior to CCTA, laboratory tests and ECG were performed. Patients who had a heart rate >70 beats per min (bpm) usually required pre-medication, such as a single dose oral beta-blocker under the supervision of attending cardiologists to obtain an optimal heart rate during the examination. In patients with a heart rate below 70 bpm, a prospective triggering was applied to reduce the dose of radiation. However, in patients with a heart rate above 70 bpm, retrospective gating or adaptive sequential scan was considered. Acquisition parameters were selected based on the patient’s size to reduce radiation dose. The acquisition was performed after intravenous administration of contrast media with a concentration of iodine 350–370 mg/ml and a flow speed of 1.2–1.8 ml/s (depending on the patient’s body weight and intravenous access size). The acquisition was automatically triggered after reaching the threshold of saturation in the descending aorta. Reconstructions were performed with an interval of 10% of the R-R interval for the phase from 0% to 70% using retrospective gating (diastole) and with the same intervals with prospective triggering in the range automatically selected by the scanner. The images were then reconstructed in the workstation and interpreted by two cardiologists (CV, PWC) and a radiologist (KP) trained in cardiac imaging.
Two cardiologists analyzed coronary artery anatomy. Coronary artery anomalies (CAA) included, 1) significant coronary stenosis (visual grading ≥ 50% luminal narrowing), 2) acute take-off angle (angle between the proximal coronary artery and the aortic wall <45°), 3) interarterial course, and 4) prepulmonic course as described in the literature [25–28].
Analysis was performed using SPSS for Windows, version 22 (released 2013. IBM Corp, Armonk, NY, USA). Mean with standard deviation or median with interquartile range were used to describe continuous data, while percentages were used to describe categorical data. Patients were divided according to the presence of either usual coronary artery pattern (1LCx-2R), or unusual coronary pattern (other than 1LCx-2R pattern). Univariate analyses were initially performed to compare findings between the two groups. Predictive models for significant chest pain symptoms were analyzed using binary logistic regression. All statistical analyses were two-tailed, and a significance level of 0.05 was used to calculate the adjusted odds ratio and 95% confidence interval.
We analyzed 43 cases (67% male) that received coronary CTA after ASO. The median age at CCTA was 15.6 years (interquartile range; IQR, 12–21.3 years). There were 14 (33%) cases of simple d-TGA, 24 (56%) d-TGA with ventricular septal defect, and five (11%) Taussig-Bing anomalies. The median age at surgery was 16.5 days (IQR, 12–58 days) in simple d-TGA, and three months (IQR, 1–6 months) in d-TGA with ventricular septal defect (VSD) and Taussig Bing anomaly patients. Preoperatively, a usual coronary artery pattern was observed in 23 of 43 (53%) patients. During the follow-up period, five cases required percutaneous balloon pulmonary valvuloplasty (PBPV) due to severe pulmonic valve stenosis. Three patients underwent right ventricle to pulmonary valve conduit replacement due to severe subvalvular and valvular pulmonary stenosis from a small annulus. One patient had significant aortic root dilatation and needed aortic root replacement. Two patients underwent coronary artery bypass graft (CABG) surgery. All patients, except the two post-CABG patients, had good left ventricular systolic function (left ventricular ejection function (LVEF) 65.5% ± 7.9%).
3.2 Coronary Computed Tomography Angiography Results and Symptoms
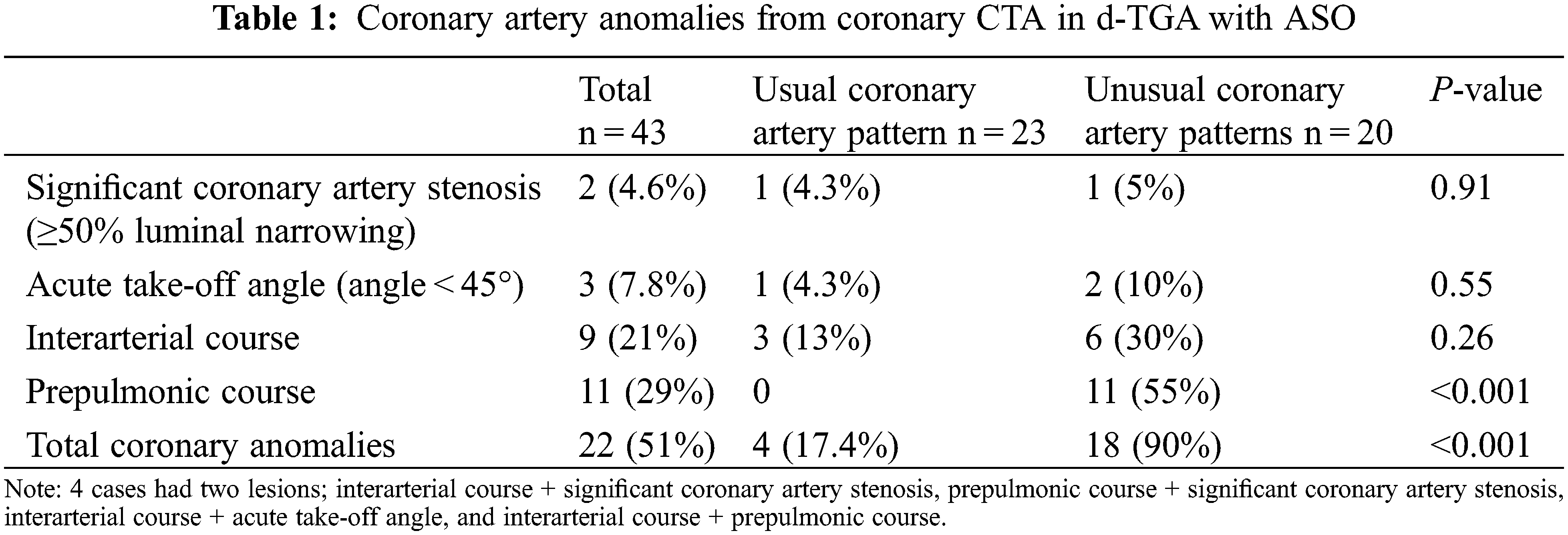
The most common types of unusual coronary patterns were 2LCxR and 1L-2CxR (5 patients in each group), each accounting for 11.6% (Fig. 2). Twenty-two (51%) cases were found to have CAA from CCTA. Twelve patients had acquired abnormal coronary patterns whereas, ten patients had CAA (prepulmonic course) related to innate abnormal coronary patterns. Postoperative CAA were more often observed in patients with unusual coronary patterns (90% vs. 17.4% P < 0.001, Table 1). Of those who had CAA, fourteen patients were asymptomatic, and eight patients experienced chest pain or change in functional class.
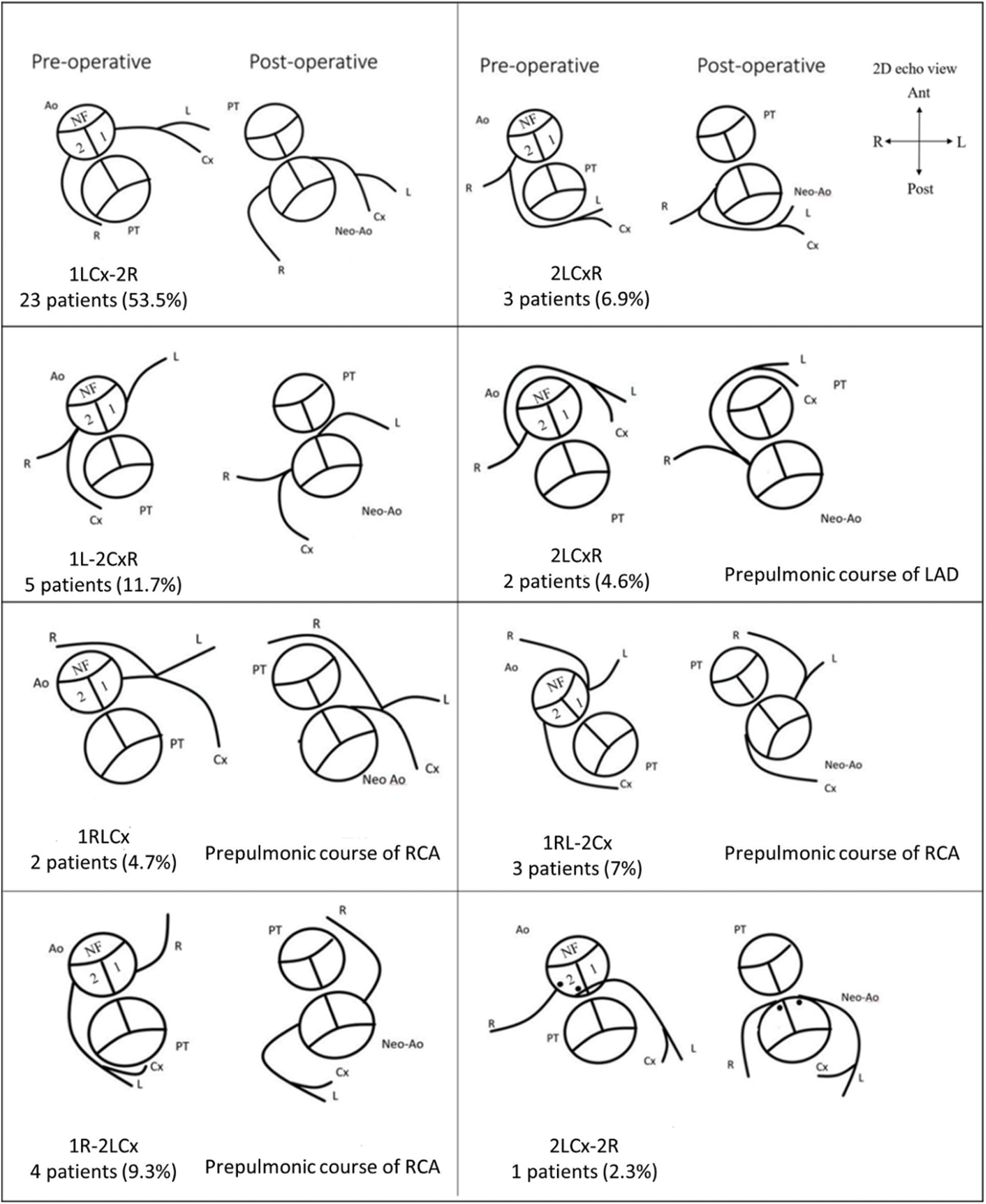
Figure 2: Leiden classification and coronary artery pattern as depicted by 2D echocardiography (NF = nonfacing sinus; 1 = facing sinus 1; 2 = facing sinus 2; PT = pulmonary trunk; Ao = Aorta; L = left anterior descending artery (LAD); Cx = left circumflex artery (LCx); R = right coronary artery (RCA); Ant = anterior; Post = posterior; R = right; L = left)
Characteristics of symptomatic patients are shown in Tables 2 and 3. Two of these patients had significant coronary artery stenosis and the evidence of myocardial ischemia from adenosine stress cardiovascular magnetic resonance (CMR) and underwent CABG surgery. The first patient (#1 in Tables 2 and 3) with a usual coronary pattern experienced dyspnea on exertion and a decline in functional class I to functional class II 24 years after ASO. An exercise stress test showed depression in ECG lead II, III, aVF at less than 7 METs. Adenosine stress CMR showed myocardial ischemia at basal to apical anterior and anteroseptal wall. Coronary CTA showed an interarterial course of LMCA that was significantly compressed by the left pulmonary artery (LPA) and aorta. The LVEF improved from 36% five months after CABG surgery to 63% one year postoperatively. The second patient (#2 in Tables 2 and 3) with an unusual coronary artery pattern (1RL-2Cx) experienced typical angina pain and was admitted to the hospital due to cardiogenic shock one year after ASO. Myocardial perfusion imaging with thallium-201 was performed showing a large area of infarction at the anteroseptal and posteroinferior ventricular wall. The intraoperative finding was 80% stenosis of the coronary ostial at the implantation site that supplied blood to the RCA and LAD. The patient underwent coronary artery bypass graft (CABG) and suffered long-term residual myocardial dysfunction.
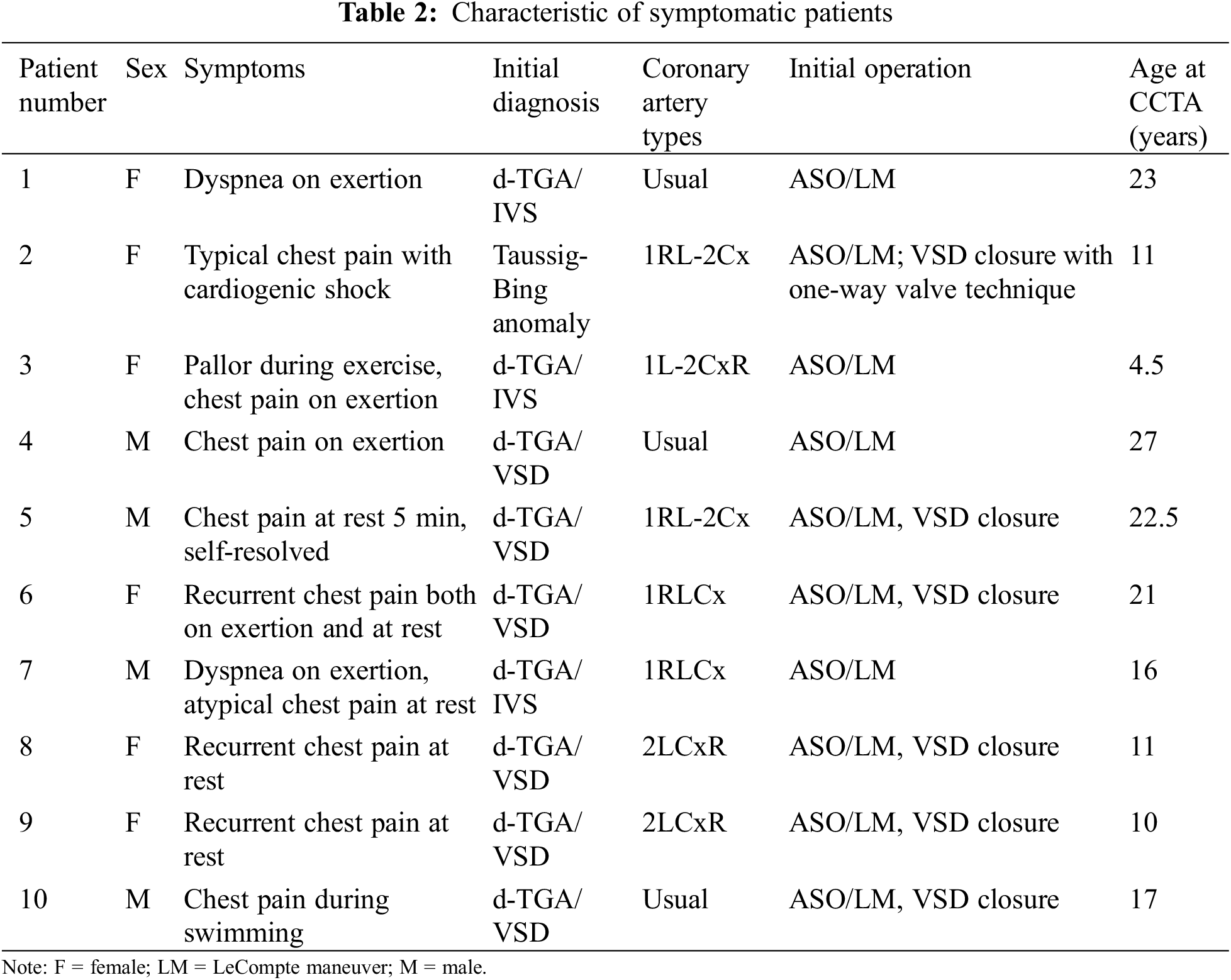
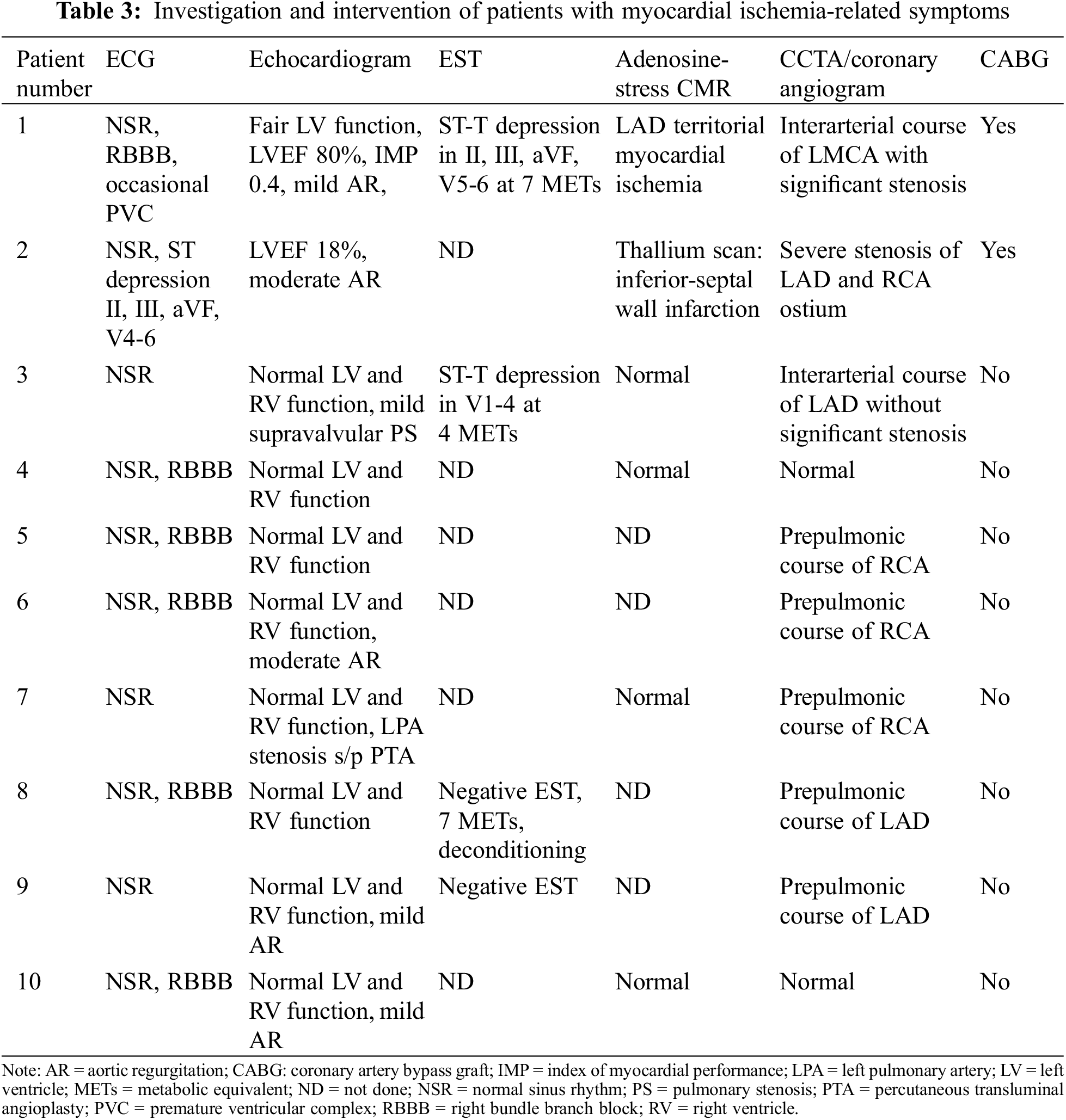
Apart from two patients who have myocardial ischemia and underwent CABG, the remaining of symptomatic patients (Tables 2 and 3) did not have the evidence of myocardial ischemia from adenosine stress CMR or EST. Although there was no evidence of myocardial ischemia, six out of eight had CAA from CCTA. All the remaining symptomatic patients did not required surgery.
Among subjects with a single coronary artery and common ostium of the RCA and LAD 60% (6 out of 10 patients) reported multiple episodes of chest pain (all symptomatic patients also had prepulmonic course of coronary artery after ASO), compared with 9% (3 of 33 cases) of patients with other coronary artery patterns (adjusted OR = 14.3%, 95% CI (2.5–82.3); P = 0.003) (Table 4). Of those who have preoperative unusual coronary patterns, prepulmonic course was found in 11 (55%) patients including 1R-2LCx, 1RL-2Cx, 1RLCx, and 2LCxR (Figs. 2 and 3). Six of 11 (54%) patients in this group experienced significant chest pain (crude OR = 11.6%, 95% CI (2.1–62.2), P = 0.001). Interarterial courses were seen in nine patients (20%), and two of those patients reported significant chest pain (22%). Among them, seven patients had an interarterial course of LAD or LMCA, while two patients had an interarterial course of RCA (Fig. 4). Acute take-off angle occurred in three patients (6.9%); each had high take-off (coronary ostia located above the sinotubular junction) [29], but none had significant coronary stenosis (Fig. 5). The mean aortic root z score in patients post-ASO was 4.71 ± 2.4 [30].
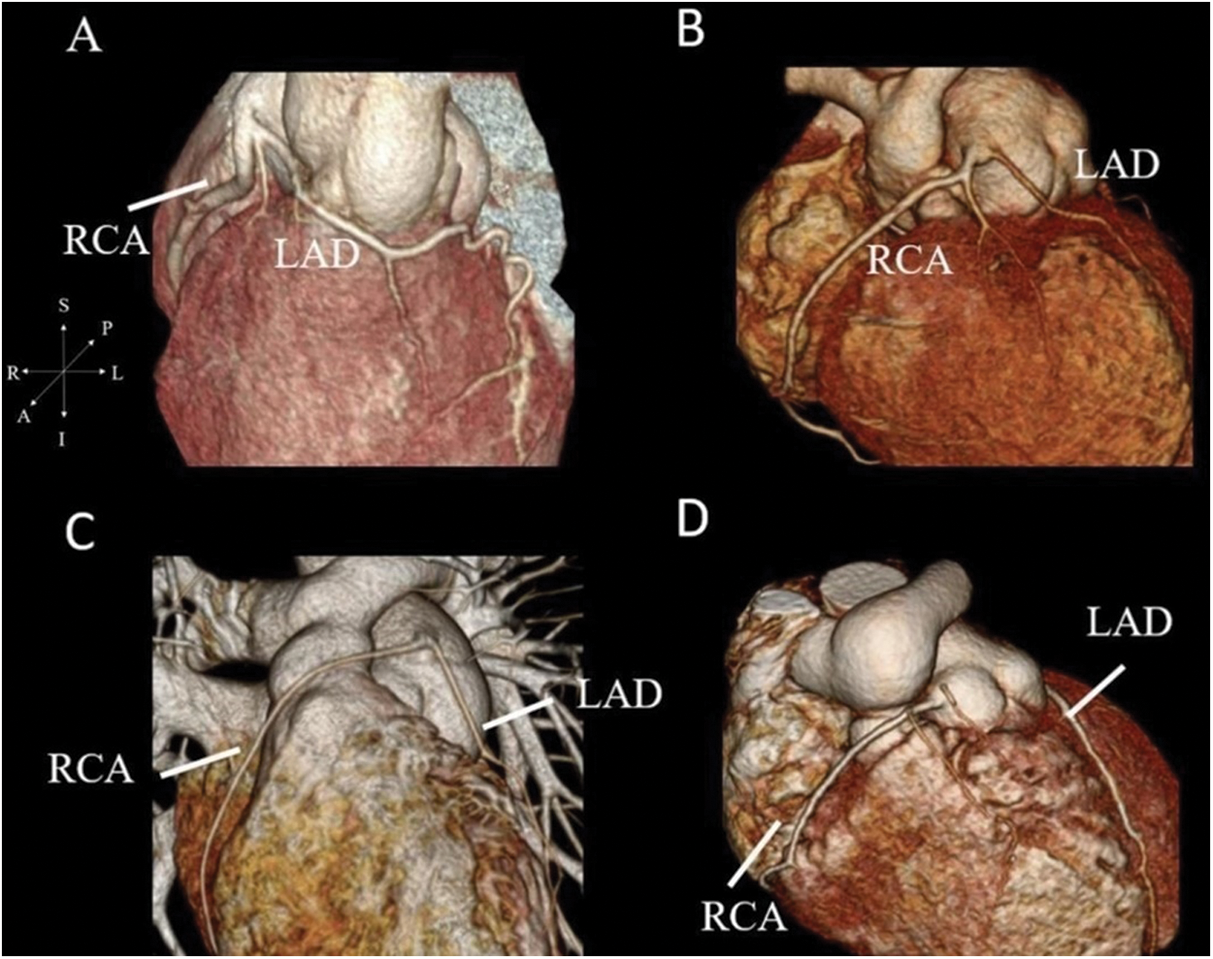
Figure 3: Coronary CTA with 3-dimensional reconstruction. (A) shows the origin of LAD from the right coronary cusp with prepulmonic course in patients with 2LCxR. (B–D) show the prepulmonic course of RCA in 1RL-2Cx, 1RLCx and 1R-2LCx, respectively
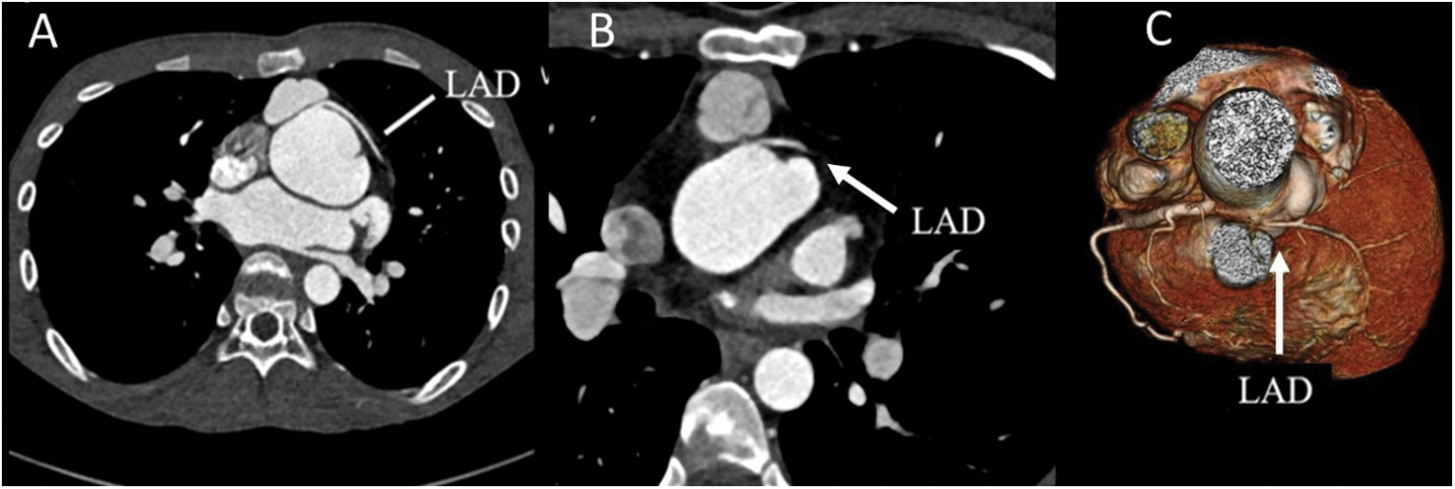
Figure 4: Coronary CTA with selected coronary features. (A) Interarterial course of the LAD without significant stenosis in the axial. (B) Interarterial course of LAD with significant compression by LPA and neoaorta. (C) 3-dimensional reconstruction of significant narrowing of interarterial course of LAD
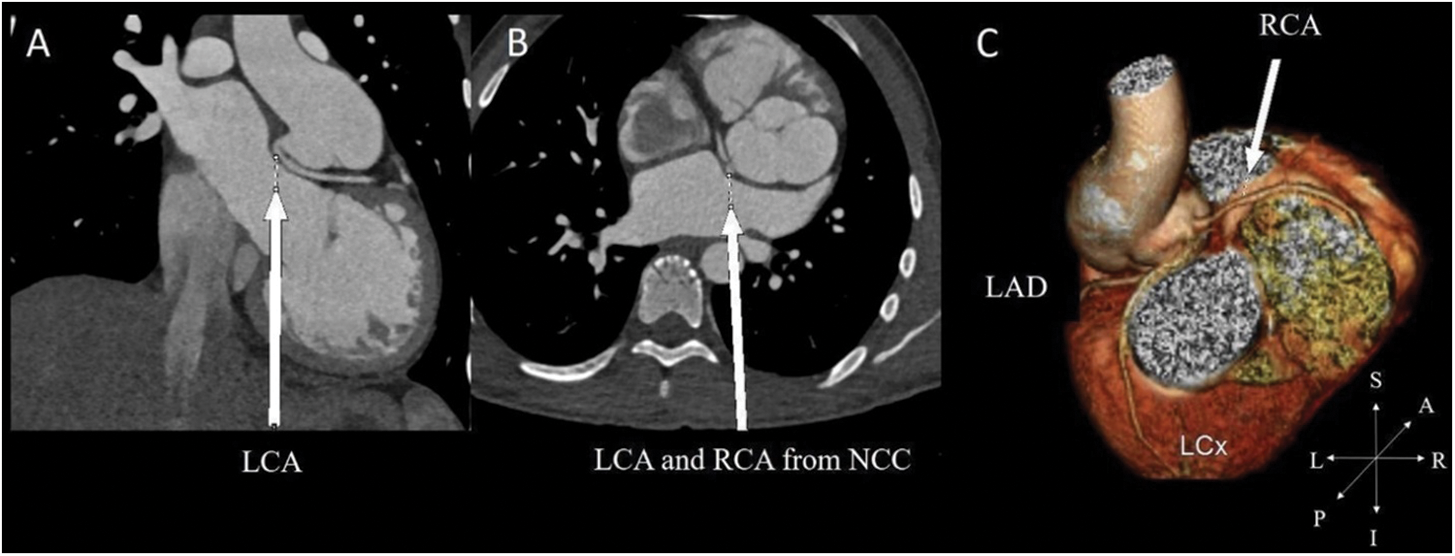
Figure 5: In a patient with preoperative 2LCxR pattern. (A) Acute take-off angle of LCA in the coronal. (B) The LCA and RCA arise from noncoronary cusp (NCC) as reimplantation site in the axial view. (C) 3-dimensional reconstruction of coronary distribution
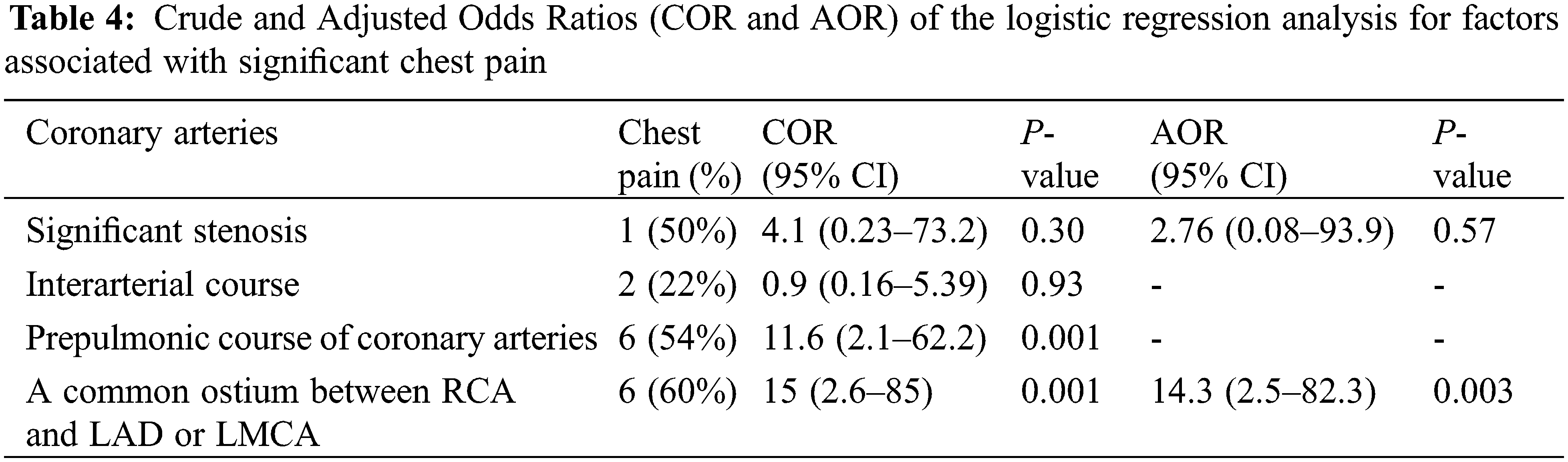
Coronary artery anomaly was observed in approximately 50% of patients that underwent CCTA according to our institutional criteria. These findings were consistent with previous studies that reported the incidence of coronary artery anomalies to be between 20% and 60% [14,16–18]. Two previous studies reported that up to 80% of patients had usual preoperative coronary artery patterns [17,18]. In contrast, nearly half of our patients had unusual preoperative coronary artery patterns. This could explain the high incidence of prepulmonic course (25%) in our study, compared to 6%–11% in other reports [16,21,31,32]. We identified late significant coronary artery stenosis (≥50% narrowing of coronary arteries) in two of 43 (4.6%) of cases, which is consistent with previous studies that reported late coronary stenosis in 3% to 7.1% [14,16–18,21]. Our first case with significant coronary artery stenosis developed dyspnea on exertion. The adenosine-stress CMR imaging showed evidence of myocardial ischemia in the LAD region due to interarterial course and significant compression of the LAD coursing between the neo-aorta and the LPA. Changes during growth and development may cause the transplanted coronary arteries to shift to a more anterior position. This can lead to excessive anterior positioning of the LCA or LAD (between 12 and 1 o’clock on the neo-aorta), leading to an interarterial course of the coronary artery. This artery may then become compressed between the two great vessels, resulting in late stenosis and myocardial ischemia [4]. Although seven of our nine (89%) patients with an interarterial course did not experience myocardial ischemia-related symptoms or significant stenosis on CCTA, they are predisposed to develop significant stenosis later in life and this could lead to sudden cardiac death. According to the 2018 AHA/ACC Guidelines, surgery is recommended for the anomalous aortic origin of the left coronary artery from the right sinus, even in asymptomatic patients [33]. However, there is no recommendation for an interarterial course of the left coronary artery for post-ASO patients without evidence of myocardial ischemia. For these reasons, patients are restricted from all competitive sports and undergo stress imaging and serial coronary imaging to prevent serious cardiac events. The presumptive mechanism of obstruction in the second case with significant coronary artery stenosis patient was an unusual surgical technique using autologous graft to lengthen coronary artery ostia to prevent coronary artery stretching that may have resulted in a small caliber of the constructed vessel and coronary obstruction.
Three of our patients had an acute take-off angle of the coronary artery; one had severe aortic root dilation (sinus of Valsalva diameter was 44 mm, z score = 8), and two had aortic root z scores ≥ 3. Previous studies have reported that more than 50% of adults with sudden cardiac death had acute take-off angle of the coronary artery and developed late coronary compression from progressive dilation of the aortic root dilatation [28,34,35]. Although all patients with acute take-off angle of coronary artery in our study might risk of coronary compression later due to severe aortic root dilatation, they had high take-off coronary artery created from surgical procedure as a protective mechanism to prevent significant compression due to the long distance between coronary ostium and aortic root.
We identified a statistically significant correlation between angina or significant, repeated chest pain and a single coronary artery or a common ostium of RCA and LAD, despite no significant coronary obstruction on CCTA. This might be because single coronary arteries or a common ostium of two major coronary arteries (RCA and LAD) were vulnerable to inadequate blood flow during strenuous exercise or heavy exertion. Furthermore, chest-related symptoms can be compounded by a prepulmonic course of coronary arteries in patients with a single coronary ostium. Sanford et al. reported a patient who had a single coronary artery with a prepulmonic course of LCA and was diagnosed with Prinzmetal’s angina. Intravenous ultrasound demonstrated 50% stenosis of the stretching part of the LCA without evidence of atherosclerosis and worsening to 80% stenosis after inducing spasm by acetylcholine [36]. This could also be explained by looping around the great vessels in the prepulmonic course leading to stretching and possibly later stenosis, especially with the geometric changes that occur during growth and development [37].
Tsuda et al. reported that some patients were found to have almost total obliteration of the coronary artery ostium in the second imaging despite a normal coronary artery on previous coronary angiography [11,12]. Several studies have reported a strong correlation between isolated congenital CAA such as acute take-off angle, interarterial course, intramural or muscular bridge, and sudden cardiac death [28,35,38]. Hence, the main goal for patients with postoperative coronary artery translocation, whether symptomatic or asymptomatic, is to prevent life-threatening events, even in the absence of exercise-induced myocardial ischemia. It is crucial to detect coronary artery anomalies before myocardial infarction or severe cardiac events occur.
Our study was a single-center retrospective review which have a limited number of participants. Additionally, according to our institution criteria of being performed CCTA to evaluate coronary anatomy might be selection bias because we enrolled all patients with innate abnormal coronary patterns before they participate in competitive sports, therefore, there was a significant high prevalence of preoperative unusual coronary patterns. This might be the reason why we found high prevalence of CAA from CCTA. Another point to concern is we did not perform CCTA in patients with usual coronary pattern who have not reach adolescence or asymptomatic. Therefore, the prevalence of CAA in this report might be underestimated.
Coronary artery anomalies such as prepulmonic course, interarterial course, acute take-off angle, and significant coronary stenosis are common in d-TGA patients after ASO. We propose that all patients require coronary artery imaging after ASO at least once before participating in sports and when they reach adolescence. Patients with unusual coronary patterns should have coronary artery imaging as soon as feasible. Patients who undergo ASO with unusual surgical technique or experience any unexplained cardiac symptoms should be evaluated promptly with coronary artery imaging. Serial imaging should be considered, especially in cases with coronary artery anomalies detected in the prior CCTA. Long-term data are needed to determine the optimal interval for serial coronary imaging.
Authorship Contribution: JS created study conception and design. PWC, CV analyzed coronary CTA results. TP participated in the acquisition, analysis and interpretation of data. TP wrote the manuscript, provided data and conducted all statistical analyses. All authors reviewed the final manuscript.
Acknowledgement: The authors gratefully thank Mr. Prajak Tanapibunpon, MSc and Miss Supaporn Nakyen, MSc, our radiology technicians who greatly assisted in CCTA examination and post-processing image reconstruction in the imaging laboratory of Her Majesty Cardiac Center, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand. We also acknowledge Prof. Somchai Sriyoschati MD, our senior cardiovascular thoracic surgeon who initiated ASO in our center and gave meaningful suggestions for the manuscript preparation.
Data Availability: The data underlying this article will be shared on reasonable request to the corresponding author.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Angeli, E., Formigari, R., Napoleone, P. C., Oppido, G., Ragni, L. et al. (2010). Long-term coronary artery outcome after arterial switch operation for transposition of the great arteries. European Journal of Cardio-Thoracic Surgery, 38(6), 714–720. DOI 10.1016/j.ejcts.2010.03.055. [Google Scholar] [CrossRef]
2. Fricke, T. A., d’Udekem, Y., Richardson, M., Thuys, C., Dronavalli, M. et al. (2012). Outcomes of the arterial switch operation for transposition of the great arteries: 25 years of experience. The Annals of Thoracic Surgery, 94(1), 139–145. DOI 10.1016/j.athoracsur.2012.03.019. [Google Scholar] [CrossRef]
3. Villafane, J., Lantin-Hermoso, M. R., Bhatt, A. B., Tweddell, J. S., Geva, T. et al. (2014). D-transposition of the great arteries: The current era of the arterial switch operation. Journal of the American College of Cardiology, 64(5), 498–511. DOI 10.1016/j.jacc.2014.06.1150. [Google Scholar] [CrossRef]
4. Baruteau, A. E., Vergnat, M., Kalfa, D., Delpey, J. G., Ly, M. et al. (2016). Long-term outcomes of the arterial switch operation for transposition of the great arteries and ventricular septal defect and/or aortic arch obstruction. Interactive Cardiovascular and Thoracic Surgery, 23(2), 240–246. DOI 10.1093/icvts/ivw102. [Google Scholar] [CrossRef]
5. Moe, T. G., Bardo, D. M. E. (2018). Long-term outcomes of the arterial switch operation for d-transposition of the great arteries. Progress in Cardiovascular Diseases, 61(3–4), 360–364. DOI 10.1016/j.pcad.2018.08.007. [Google Scholar] [CrossRef]
6. Yamaguchi, M., Hosokawa, Y., Imai, Y., Kurosawa, H., Yasui, H. et al. (1990). Early and midterm results of the arterial switch operation for transposition of the great arteries in Japan. The Journal of Thoracic and Cardiovascular Surgery, 100(2), 261–269. DOI 10.1016/S0022-5223(19)35566-7. [Google Scholar] [CrossRef]
7. Tanel, R. E., Wernovsky, G., Landzberg, M. J., Perry, S. B., Burke, R. P. (1995). Coronary artery abnormalities detected at cardiac catheterization following the arterial switch operation for transposition of the great arteries. The American Journal of Cardiology, 76(3), 153–157. DOI 10.1016/S0002-9149(99)80048-4. [Google Scholar] [CrossRef]
8. Brown, J. W., Park, H. J., Turrentine, M. W. (2001). Arterial switch operation: Factors impacting survival in the current era. The Annals of Thoracic Surgery, 71(6), 1978–1984. DOI 10.1016/S0003-4975(01)02529-2. [Google Scholar] [CrossRef]
9. Lalezari, S., Bruggemans, E. F., Blom, N. A., Hazekamp, M. G. (2011). Thirty-year experience with the arterial switch operation. The Annals of Thoracic Surgery, 92(3), 973–979. DOI 10.1016/j.athoracsur.2011.04.086. [Google Scholar] [CrossRef]
10. Khairy, P., Clair, M., Fernandes, S. M., Blume, E. D., Powell, A. J. et al. (2013). Cardiovascular outcomes after the arterial switch operation for D-transposition of the great arteries. Circulation, 127(3), 331–339. DOI 10.1161/CIRCULATIONAHA.112.135046. [Google Scholar] [CrossRef]
11. Tsuda, T., Bhat, A. M., Robinson, B. W., Baffa, J. M., Radtke, W. (2015). Coronary artery problems late after arterial switch operation for transposition of the great arteries. Circulation Journal, 79(11), 2372–2379. DOI 10.1253/circj.CJ-15-0485. [Google Scholar] [CrossRef]
12. Tsuda, T., Baffa, J. M., Octavio, J., Robinson, B. W., Radtke, W. et al. (2019). Identifying subclinical coronary abnormalities and silent myocardial ischemia after arterial switch operation. Pediatric Cardiology, 40(5), 901–908. DOI 10.1007/s00246-019-02085-4. [Google Scholar] [CrossRef]
13. Legendre, A., Losay, J., Touchot-Kone, A., Serraf, A., Belli, E. et al. (2003). Coronary events after arterial switch operation for transposition of the great arteries. Circulation, 108(10_suppl_1), II-186–II-190. DOI 10.1161/01.cir.0000087902.67220.2b. [Google Scholar] [CrossRef]
14. Michalak, K. W., Sobczak-Budlewska, K., Moll, J. J., Szymczyk, K., Moll, J. A. et al. (2019). Can we predict potentially dangerous coronary patterns in patients with transposition of the great arteries after an arterial switch operation? Cardiology in the Young, 29(11), 1350–1355. DOI 10.1017/S104795111900204X. [Google Scholar] [CrossRef]
15. Ou, P., Celermajer, D. S., Marini, D., Agnoletti, G., Vouhé, P. et al. (2008). Safety and accuracy of 64-slice computed tomography coronary angiography in children after the arterial switch operation for transposition of the great arteries. JACC: Cardiovascular Imaging, 1(3), 331–339. DOI 10.1016/j.jcmg.2008.02.005. [Google Scholar] [CrossRef]
16. Öztunç, F., Barış, S., Adaletli, I., Önol, N. Ö., Olgun, D. C. et al. (2009). Coronary events and anatomy after arterial switch operation for transposition of the great arteries: Detection by 16-row multislice computed tomography angiography in pediatric patients. Cardiovascular and Interventional Radiology, 32(2), 206–212. DOI 10.1007/s00270-008-9432-3. [Google Scholar] [CrossRef]
17. Szymczyk, K., Moll, M., Sobczak-Budlewska, K., Moll, J. A., Stefańczyk, L. et al. (2018). Usefulness of routine coronary CT angiography in patients with transposition of the great arteries after an arterial switch operation. Pediatric Cardiology, 39(2), 335–346. DOI 10.1007/s00246-017-1761-z. [Google Scholar] [CrossRef]
18. Veltman, C. E., Beeres, S. L. M. A., Kalkman, D. N., Kelder, T. P., Kiès, P. et al. (2013). Variation in coronary anatomy in adult patients late after arterial switch operation: A computed tomography coronary angiography study. The Annals of Thoracic Surgery, 96(4), 1390–1397. DOI 10.1016/j.athoracsur.2013.05.004. [Google Scholar] [CrossRef]
19. Pasquali, S. K., Hasselblad, V., Li, J. S., Kong, D. F., Sanders, S. P. (2002). Coronary artery pattern and outcome of arterial switch operation for transposition of the great arteries: A meta-analysis. Circulation, 106(20), 2575–2580. DOI 10.1161/01.cir.0000036745.19310.bb. [Google Scholar] [CrossRef]
20. Sarris, G. E., Balmer, C., Bonou, P., Comas, J. V., da Cruz, E. D. et al. (2017). Clinical guidelines for the management of patients with transposition of the great arteries with intact ventricular septum. European Journal of Cardio-Thoracic Surgery, 51(1), e1–e32. DOI 10.1093/ejcts/ezw360. [Google Scholar] [CrossRef]
21. Moll, M., Michalak, K. W., Sobczak-Budlewska, K., Moll, J. A., Kopala, M. et al. (2017). Coronary artery anomalies in patients with transposition of the great arteries and their impact on postoperative outcomes. The Annals of Thoracic Surgery, 104(5), 1620–1628. DOI 10.1016/j.athoracsur.2017.03.078. [Google Scholar] [CrossRef]
22. Han, B. K., Rigsby, C. K., Hlavacek, A., Leipsic, J., Nicol, E. D. et al. (2015). Computed tomography imaging in patients with congenital heart disease part I: Rationale and utility. an expert consensus document of the society of cardiovascular computed tomography (SCCTEndorsed by the society of pediatric radiology (SPR) and the north American society of cardiac imaging (NASCI). Journal of Cardiovascular Computed Tomography, 9(6), 475–492. DOI 10.1016/j.jcct.2015.07.004. [Google Scholar] [CrossRef]
23. Sachdeva, R., Valente, A. M., Armstrong, A. K., Cook, S. C., Han, B. K. et al. (2020). ACC/AHA/ASE/HRS/ISACHD/SCAI/SCCT/SCMR/SOPE 2020 appropriate use criteria for multimodality imaging during the follow-up care of patients with congenital heart disease: A report of the American college of cardiology solution set oversight committee and appropriate use criteria task force, American heart association, American society of echocardiography, heart rhythm society, international society for adult congenital heart disease, society for cardiovascular angiography and interventions, society of cardiovascular computed tomography, society for cardiovascular magnetic resonance, and society of pediatric echocardiography. Journal of the American College of Cardiology, 75(6), 657–703. DOI 10.1016/j.jcct.2015.07.004. [Google Scholar] [CrossRef]
24. Gittenberger-de Groot, A. C., Koenraadt, W. M. C., Bartelings, M. M., Bökenkamp, R., DeRuiter, M. C. et al. (2018). Coding of coronary arterial origin and branching in congenital heart disease: The modified Leiden convention. The Journal of Thoracic and Cardiovascular Surgery, 156(6), 2260–2269. DOI 10.1016/j.jtcvs.2018.08.009. [Google Scholar] [CrossRef]
25. Cheezum, M. K., Liberthson, R. R., Shah, N. R., Villines, T. C., O’Gara, P. T. et al. (2017). Anomalous aortic origin of a coronary artery from the inappropriate sinus of valsalva. Journal of the American College of Cardiology, 69(12), 1592–1608. DOI 10.1016/j.jacc.2017.01.031. [Google Scholar] [CrossRef]
26. Frescura, C., Basso, C., Thiene, G., Corrado, D., Pennelli, T. et al. (1998). Anomalous origin of coronary arteries and risk of sudden death: A study based on an autopsy population of congenital heart disease. Human Pathology, 29(7), 689–695. DOI 10.1016/s0046-8177(98)90277-5. [Google Scholar] [CrossRef]
27. Hirono, K., Hata, Y., Miyao, N., Nakaoka, H., Saito, K. et al. (2016). Anomalous origin of the right coronary artery evaluated with multidetector computed tomography and its clinical relevance. Journal of Cardiology, 68(3), 196–201. DOI 10.1016/j.jjcc.2015.12.010. [Google Scholar] [CrossRef]
28. Taylor, A. J., Rogan, K. M., Virmani, R. (1992). Sudden cardiac death associated with isolated congenital coronary artery anomalies. Journal of the American College of Cardiology, 20(3), 640–647. DOI 10.1016/0735-1097(92)90019-j. [Google Scholar]
29. Rosenthal, R. L., Carrothers, I. A., Schussler, J. M. (2012). Benign or malignant anomaly? Very high take-off of the left main coronary artery above the left coronary sinus. Texas Heart Institute Journal, 39(4), 538–541. [Google Scholar]
30. Kaiser, T., Kellenberger, C. J., Albisetti, M., Bergsträsser, E., Buechel, E. R. V. (2008). Normal values for aortic diameters in children and adolescents–assessment in vivo by contrast-enhanced CMR-angiography. Journal of Cardiovascular Magnetic Resonance, 10(1), 1–8. DOI 10.1186/1532-429X-10-56. [Google Scholar] [CrossRef]
31. Hutter, P. A., Bennink, G. B., Ay, L., Raes, I. B., Hitchcock, J. F. et al. (2000). Influence of coronary anatomy and reimplantation on the long-term outcome of the arterial switch. European Journal of Cardio-Thoracic Surgery, 18(2), 207–213. DOI 10.1016/S1010-7940(00)00494-2. [Google Scholar] [CrossRef]
32. Wernovsky, G., Mayer Jr, J. E., Jonas, R. A., Hanley, F. L., Blackstone, E. H. et al. (1995). Factors influencing early and late outcome of the arterial switch operation for transposition of the great arteries. The Journal of Thoracic and Cardiovascular Surgery, 109(2), 289–302. DOI 10.1016/S0022-5223(95)70391-8. [Google Scholar] [CrossRef]
33. Stout, K. K., Daniels, C. J., Aboulhosn, J. A., Bozkurt, B., Broberg, C. S. et al. (2019). 2018 AHA/ACC guideline for the management of adults with congenital heart disease: A report of the American college of cardiology/American heart association task force on clinical practice guidelines. Journal of the American College of Cardiology, 73(12), e81–e192. DOI 10.1016/j.jacc.2018.08.1029. [Google Scholar] [CrossRef]
34. Niwa, K. (2018). Aortic dilatation in complex congenital heart disease. Cardiovascular Diagnosis and Therapy, 8(6), 725–738. DOI 10.21037/cdt. [Google Scholar] [CrossRef]
35. Virmani, R., Chun, P. K., Goldstein, R. E., Robinowitz, M., Mcallister, H. A. (1984). Acute takeoffs of the coronary arteries along the aortic wall and congenital coronary ostial valve-like ridges: Association with sudden death. Journal of the American College of Cardiology, 3(3), 766–771. DOI 10.1016/S0735-1097(84)80253-3. [Google Scholar] [CrossRef]
36. Sanford, G. B., Molavi, B., Sinha, A. K., Garza, L., Angelini, P. (2007). Single coronary artery with prepulmonic coursing left main coronary artery manifesting as prinzmetal’s angina. Texas Heart Institute Journal, 34(4), 449–452. [Google Scholar]
37. Ou, P., Khraiche, D., Celermajer, D. S., Agnoletti, G., Le Quan Sang, K. H. et al. (2013). Mechanisms of coronary complications after the arterial switch for transposition of the great arteries. The Journal of Thoracic and Cardiovascular Surgery, 145(5), 1263–1269. DOI 10.1016/j.jtcvs.2012.06.009. [Google Scholar] [CrossRef]
38. Catanzaro, J. N., Makaryus, A. N., Catanese, C. (2005). Sudden cardiac death associated with an extremely rare coronary anomaly of the left and right coronary arteries arising exclusively from the posterior (noncoronary) sinus of valsalva. Clinical Cardiology, 28(11), 542–544. DOI 10.1002/clc.4960281111. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |