

 | Congenital Heart Disease |  |
DOI: 10.32604/chd.2022.019973
ARTICLE
Mid-Term Preliminary Results for Safety and Patency of the Occlutech Atrial Flow Regulator in an Animal Model
1Division of Cardiology, Department of Pediatrics, Stead Family Children’s Hospital, University of Iowa Hospitals and Clinics, Iowa City, USA
2Division of Cardiology, Department of Pediatrics, Medical College of Wisconsin, Milwaukee, USA
3Division of Cardiology, Heart Institute, Children’s Hospital of Colorado, University of Colorado at Denver, Aurora, USA
*Corresponding Author: Gareth Morgan. Email: drgarethjmorgan@gmail.com
Received: 27 October 2021; Accepted: 24 March 2022
Abstract: Objective: The Atrial Flow Regulator (AFR) is a double disc device made of self-expanding Nitinol wire mesh, structured around a central lumen. Once deployed via the transfemoral route, the device stents the atrial septum leaving a preselected fixed diameter atrial communication. We sought to evaluate the mid-term performance of the AFR by implanting the device in 5 healthy porcine hearts to assess safety and patency of the device fenestration over a period of 150 days. Method: Five AFR devices were implanted in 5 female Yucatan adult minipigs. The animals were survived to 150 days with periodic assessments at days +3, +30, +60, +90, +120, and +150. These assessments consisted of transesophageal echocardiography and fluoroscopic evaluation. The animals were sacrificed at day +150. Histological and pathological assessments were carried out to characterize neointimal tissue growth, inflammation, thrombus formation, endothelial coverage, endothelial maturity, and the presence of any luminal thrombus. Result: There were no unscheduled deaths. Patency was maintained in all 5 animals across the 150-day study. There was no statistically significant difference in the lumen diameter over the study duration. Neointimal growth was mild to moderate in all specimens and occurred mostly on the surfaces of the device in direct contact with the atrial septum. There was no evidence of any significant inflammatory response on routine blood work or by imaging or histological assessment. Scanning electron microscope (SEM) examination showed nearly complete surface coverage with endothelial tissue. The animals were in a healthy condition for the duration of the study with no attributable pathology and no adverse effects noted on distant organs in any of the 5 animals. Conclusion: As a continuation of our earlier work, this 150-day midterm animal study provides important safety and feasibility information. Our preliminary results show that the AFR is both safe and effective in maintaining a sustainable atrial level communication for the duration of the study.
Keywords: Atrial flow regulator; animal model; atrial septal defect; interventional cardiology; device
There are several instances where the creation of a stable atrial level communication provides substantial benefit to a patient. Significant pulmonary arterial hypertension (PAH), diastolic ventricular dysfunction, and failure of a Fontan circuit are a few such examples. In such instances, elevated pressures in the left or right atria can compromise baseline cardiac output or retard the ability to augment cardiac output during physiological stress. An atrial level communication can decompress the affected atrium and facilitate improved cardiac output homeostasis. There already exists a large collection of literature delineating the benefit of atrial septostomy (AS) patients with PAH [1,2]. AS performed in patients on PAH-specific pharmacotherapy was shown to have beneficial hemodynamic effects and results in symptomatic improvement, all while increasing long-term survival.
The use of AS in patients with PAH, however, is far from being an exact science. The creation of an atrial communication that is neither too small nor too large has proven to be a challenge. Atrial communications that are too large are associated with early mortality and profound cyanosis. Communication that are too small, on the other hand, are more likely to close spontaneously [1]. Although atrial septal stenting may yield a more physiologically predictable communication, it is a more complex procedure bringing a different range of technical and follow-up issues [3].
The Atrial Flow Regulator (AFR) (Occlutech, Istanbul, Turkey) is a double disc device made of self-expanding Nitinol wire mesh. Its design is an amalgamation of a self-expanding stent, a vascular plug and a fenestrated ASD occluder. The device is structured around a central lumen intended to maintain a patent communication. Once deployed, the central portion stents the septum leaving a preselected fixed diameter atrial communication.
The AFR can be used to improve cardiac output and systemic oxygen transport while decreasing right atrial pressure when implanted in patients with PAH [4]. Recognition of the utility of the AFR has led to a host of novel and inventive uses in patients. These have included its use in failing Fontan circuits to establish a stable fenestration between the pulmonary artery and the left atrium [5,6].
In an attempt to assess the efficacy, safety, and short-term patency of the AFR, we previously carried out a study in 11 adolescent pigs [7]. That study, which survived the pigs to 90 days, showed that the AFR was safe and easy to implant. It demonstrated good short-term results with no histological or gross pathological abnormalities noted.
Building on our prior work, we sought to evaluate the mid-term performance of the AFR. To that end, we implanted an AFR device in the atrial septum of 5 healthy pigs with the primary end points being (1) an assessment of safety and (2) patency of the device fenestration over a period of 150 days.
Five AFR devices were implanted in 5 adult Yucatan female minipigs who were treated with aspirin throughout the study starting at 1 day prior to device implantation, defined as day –1. The animals were then followed over a period of 150 days, during which time they underwent regular evaluation as follows. At day 0, defined as day of implantation, the animals underwent AFR implantation. At day +3, the animals underwent the first follow-up imaging study that consisted of a transesophageal echocardiogram (TEE) and fluoroscopic examination. This was done to look for dislodgment, patency of the fenestration, direction, and qualitative assessment of blood flow across the device and any evidence of thrombosis within the AFR or cardiac chambers. The same evaluation was carried out at day +30, +60, +90, and +120. At day +150, the animals underwent their final TEE and fluoroscopic assessment and where subsequently sacrificed. Four of the 5 explanted hearts underwent histological assessment with 1 heart being set aside for evaluation using a scanning electron microscope (SEM). All the euthanized animals underwent gross pathological examination.
The AFR is a self-expanding nitinol wire mesh device consisting of a short central stent between two circular discs. The device is intended for transfemoral deployment, followed by trans-septal perforation and static balloon dilation to aid delivery and minimize compression of the central stent portion (Fig. 1).

Figure 1: Atrial flow regulator device
A spherical ball-and-socket type connector is located off-center on the device’s right atrial disc to connect the delivery system for deployment. After implantation, the AFR is designed to conform flush to either side of the atrial septum leaving an interatrial communication with a preselected fixed diameter. The device self-centers following deployment and is retrievable prior to release. Following release, retrieval of the device is feasible in a similar manner to other Occlutech devices. Of the 5 implanted devices, 2 had a 10 mm fenestration with a 10 mm waist height, 1 had a 10 mm fenestration with a 5 mm waist height, and 2 had an 8 mm fenestration with a 5 mm waist height (Fig. 2).
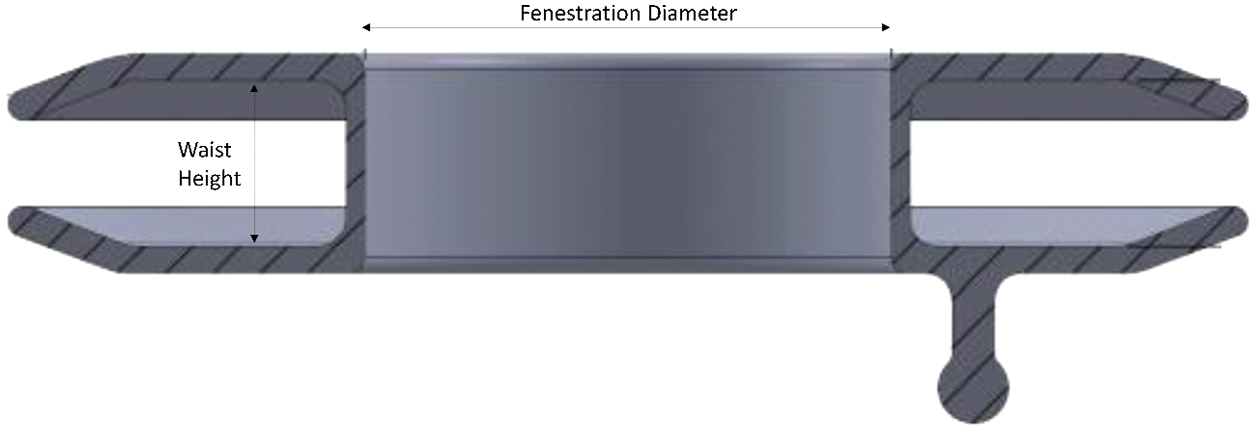
Figure 2: Visual of fenestration diameter and waist height
Device implantation was performed under general anesthesia. The left and right femoral veins were accessed through a cutdown or modified Seldinger technique. An AcuNav Intracardiac Echocardiography (ICE) probe (Manufacturer: Siemens Medical Solutions, Model number: 10135910) was used to assist with procedural guidance along with fluoroscopy. An 8Fr SL-1 Schwartz Braided Trans septal sheath (St Jude Medical, Minneapolis, MN, USA) was placed in the right femoral vein. Through this a BRK 1 extra sharp transseptal needle (St Jude Medical, Minneapolis, MN, USA) was fed and an atrial septal puncture was made. A 0.035 inch Amplatz Super Stiff wire (Boston Scientific, Natick, MA, USA) was placed through the sheath and into one of the left pulmonary veins, or if this was not easily achieved, the wire was curled in the left atrium. A Conquest angioplasty balloon (Bard Peripheral Vascular, Inc., Tempe, AZ, USA) with diameter equal to or 2 mm larger than the planned AFR was inserted and used to predilate the septum. The SL-1 was then removed and exchanged for a proprietary 12Fr delivery sheath. The AFR was loaded into the delivery system and deployed in the usual manner for an Occlutech device as described previously [7]. ICE was then used to evaluate flow through the AFR and to assess position and relative anatomy.
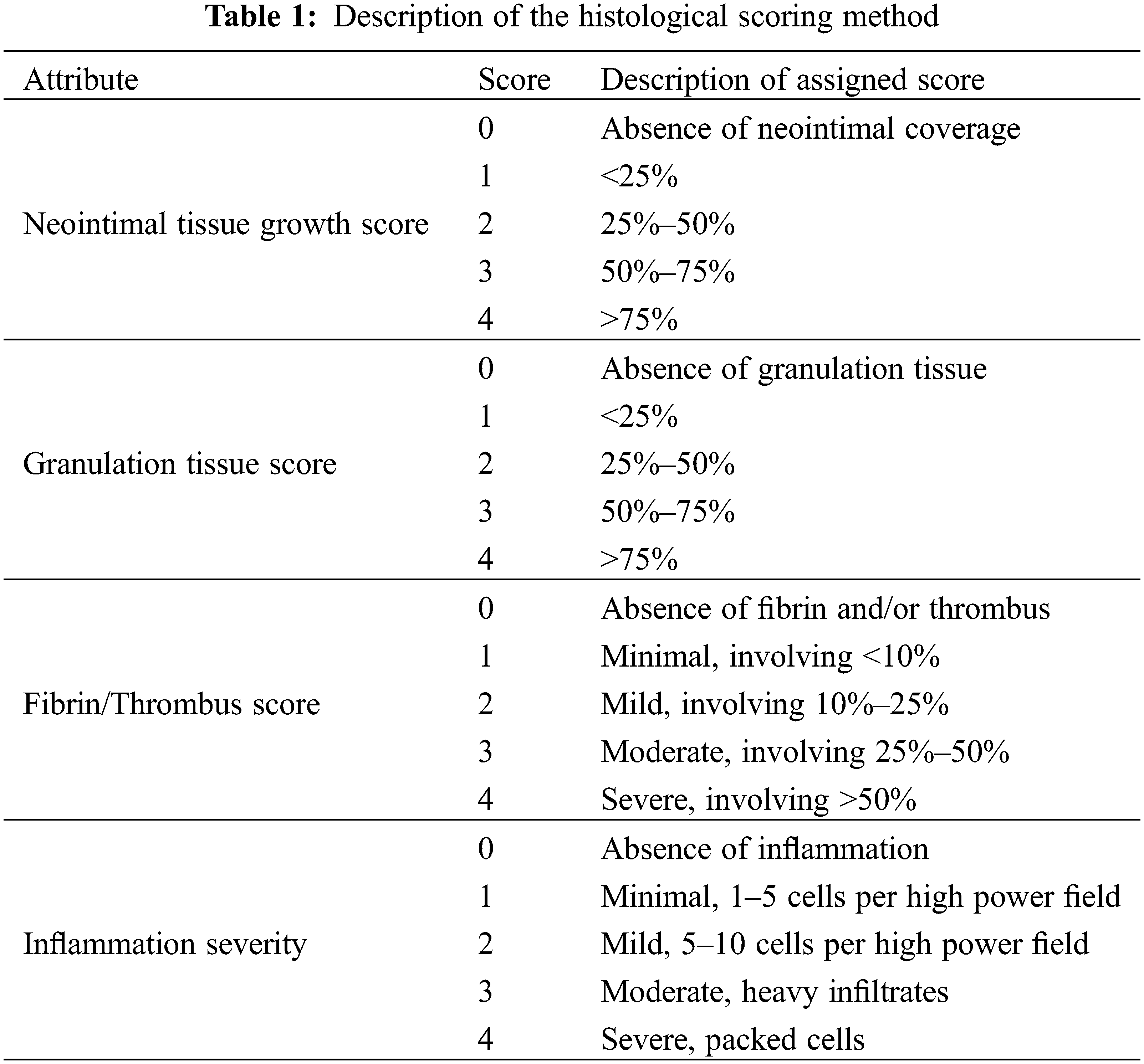
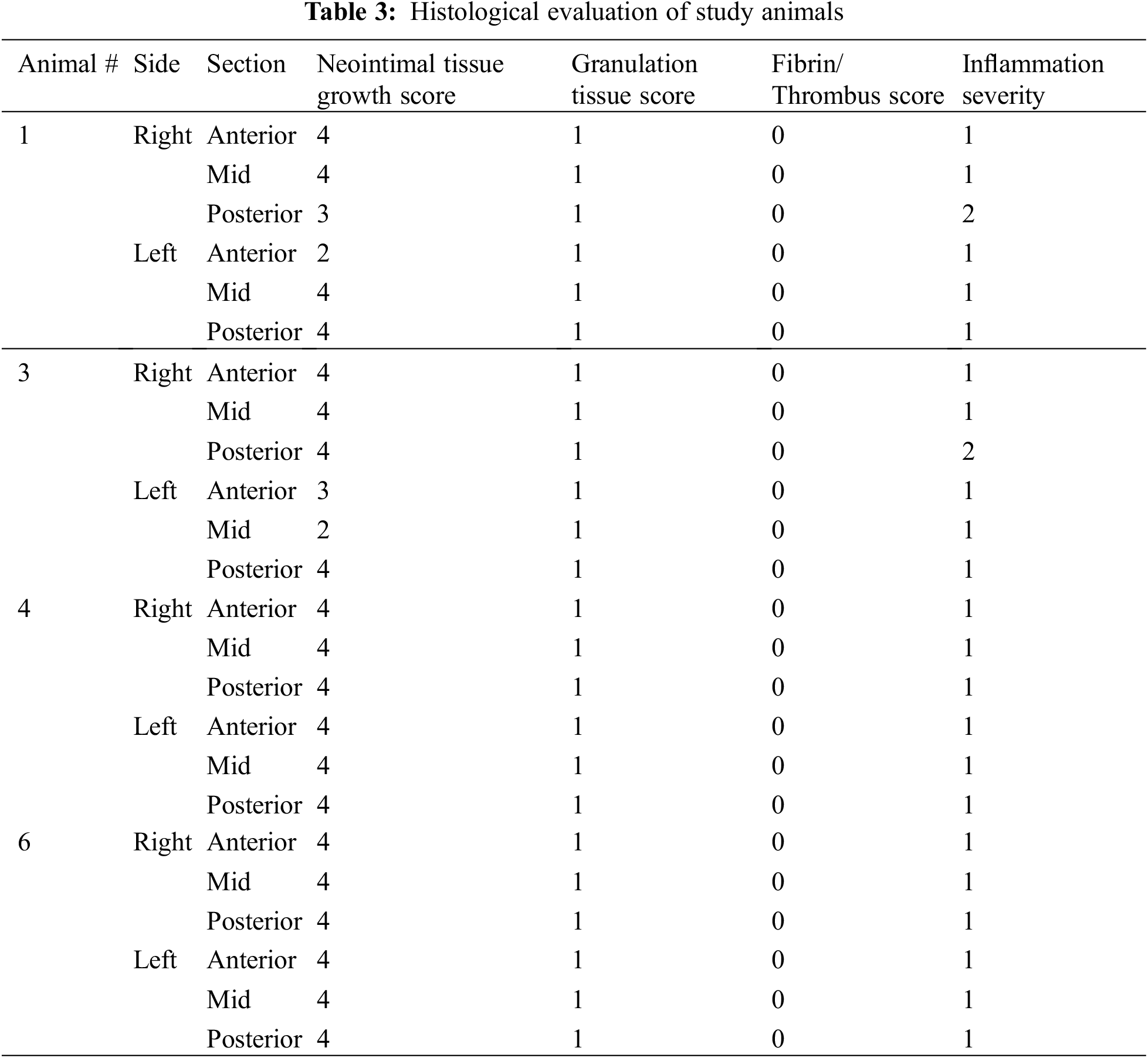
All animals underwent scheduled necropsy with gross examination of heart, lung, spleen, liver, kidney, and brain. Lesions identified during necropsy were also sent for pathological examination. In four of the five animals, the AFR device underwent further evaluation as follows. Histopathology was performed to assess neointimal tissue growth, inflammation, granulation tissue and fibrin and thrombus formation. The scoring method is highlighted in Table 1. In the remining animal, the atrial septum and AFR underwent SEM evaluation. This was done to assess endothelial coverage, endothelial maturity, luminal thrombus formation, and leukocyte adhesion on the right and left atrial surfaces of the device.
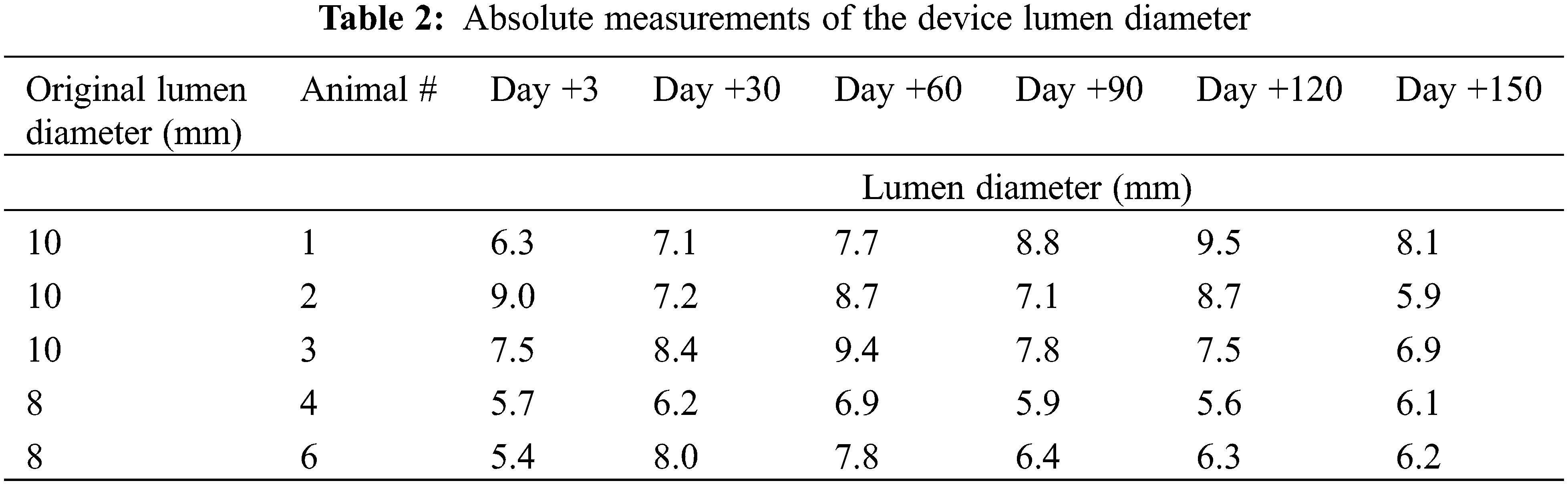
Repeated-Measures ANOVA was carried to assess the % change of the lumen diameter over time. Using TEE, lumen diameter was measured at each time point of +3, +30, +60, +90, +120, and +150 as noted above. Each measurement obtained was indexed by the original device fenestration size, such that the variables presented represent the % change over time. Repeated-Measures ANOVA was then used to compare % change over time. Throughout the manuscript, statistical significance is defined as p < 0.05.
One of the animals was sacrificed during the procedure but prior to device deployment after initial ICE assessment revealed the presence of an atrial septal defect. This animal (labeled as animal #5) was not included among the 5 that underwent the +150-day study. The 5 animals that did undergo AFR device deployment were survived until day +150 +/−2 days post-implantation. There were no unscheduled deaths.
3.1 Patency of Device Fenestration and Gross Pathologic Examination
Patency was maintained in all 5 animals across the 150-day study. This was established at scheduled evaluations by TEE and fluoroscopy at days +30, +60, +90, and +120. Patency was further confirmed by scheduled necropsy and gross evaluation at +150 days. At all said timepoints, radiographic evaluation revealed a patent device with flow across the fenestration. There was no evidence on fluoroscopic assessment of fracture or calcification in any device at any timepoint in the study (Fig. 3).
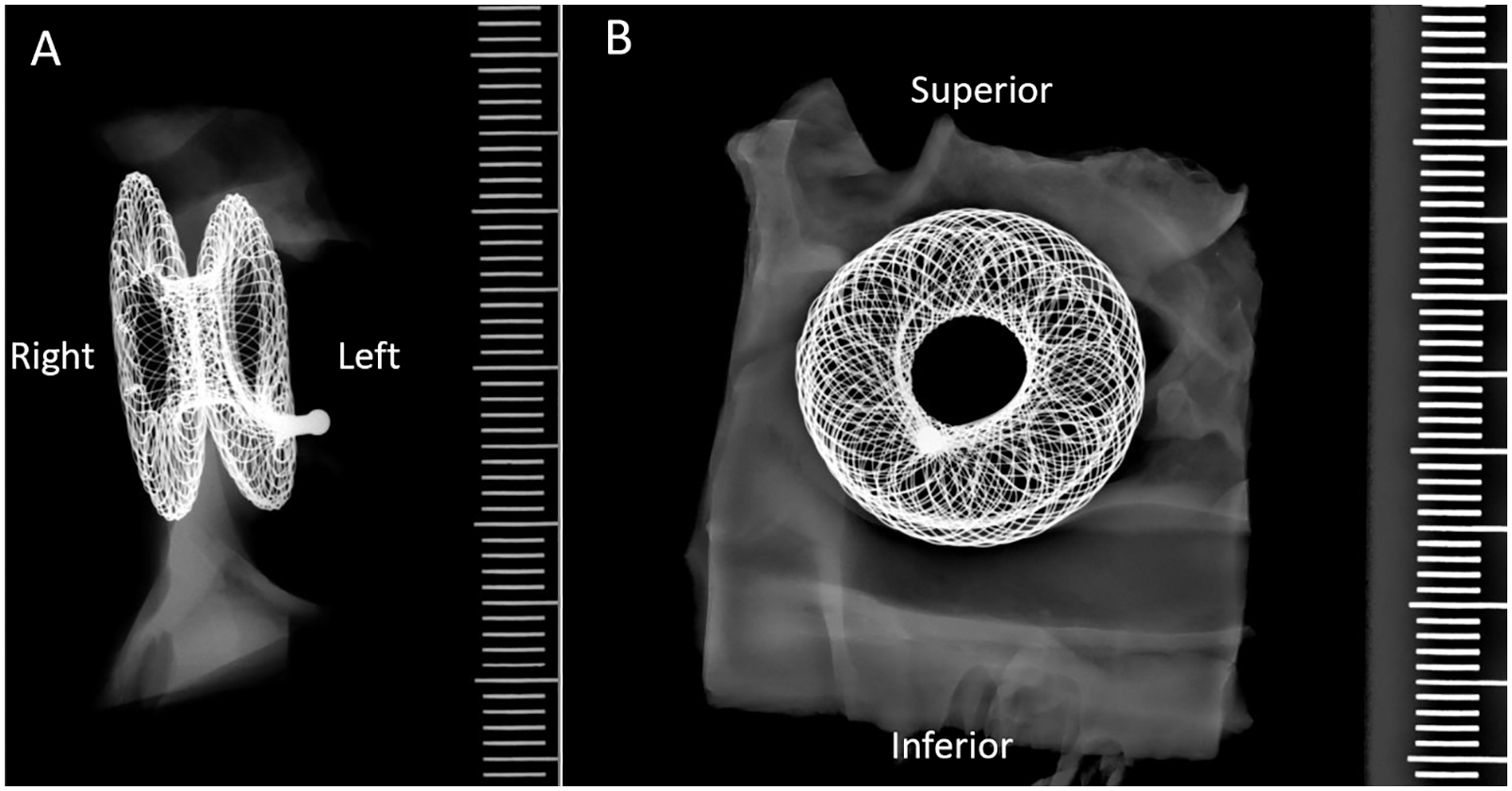
Figure 3: Example of radiograph of the AFR in animal #6 showing no signs of fracture or calcification
Gross pathological assessment of all 5 specimens showed a patent device fenestration (Fig. 4A). In animal #4, the device was found to have a circumferential membrane at the right atrial aspect of the AFR, which decreased the lumen to 4 mm. The rest of the device lumen was fully patent (device fenestration measured 8 mm at insertion) (Fig. 4B). Another device had a diminutive circumferential membrane that did not have an appreciable impact on the fenestration size (Fig. 4A–animal #1). In all devices, gross pathological examination showed an unobstructed coronary sinus, unremarkable left and right atrial walls, and unremarkable mitral and tricuspid leaflets. There was no thrombus grossly noted on any device, though there was a 5 mm area of red discoloration on the left atrial wall at the inferior edge of the device in animal #3 (Fig. 4A–animal #3–black arrow).
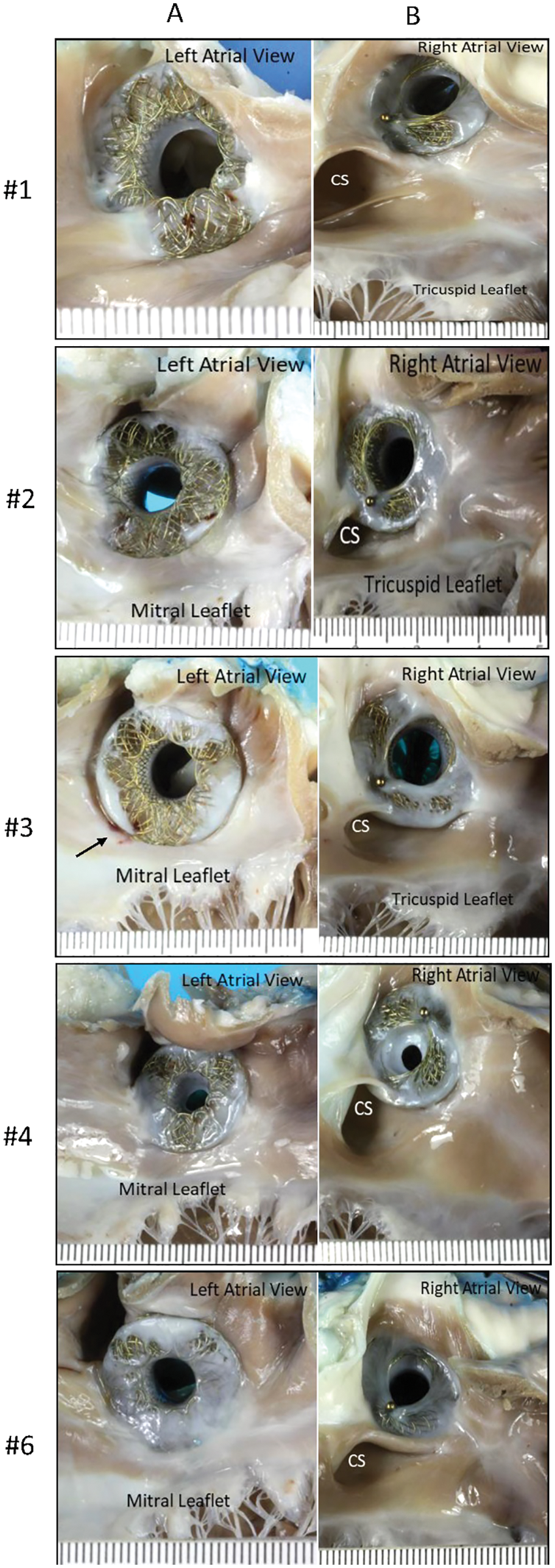
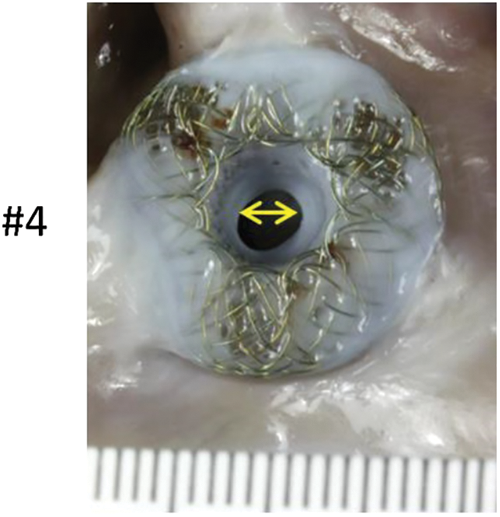
Figure 4: (A) Panel A shows pictures of the AFR as seen from the left atrial view. The black arrow in animal #3 indicates a possible attached tan-brown thrombus on the left atrial wall at the inferior edge of the device. Panel B shows the AFR as seen from the right atrial view (B) A left atrial view shows a circumferential membrane noted in animal #4. The yellow double headed arrow highlights the 4 mm patent fenestration
In 4 of the 5 animals, % change of the lumen diameter showed an increase in lumen size between days 3–60. This was followed by a slight decrease in lumen size between days 60–90 and a stable lumen size between days 90–150. When analyzing all 5 animals using repeated-measures ANOVA, there appears to be no statistically significant difference in the % change of the lumen diameter over time [F = 0.0179 (df = 5, 20)] (Fig. 5, Table 2).
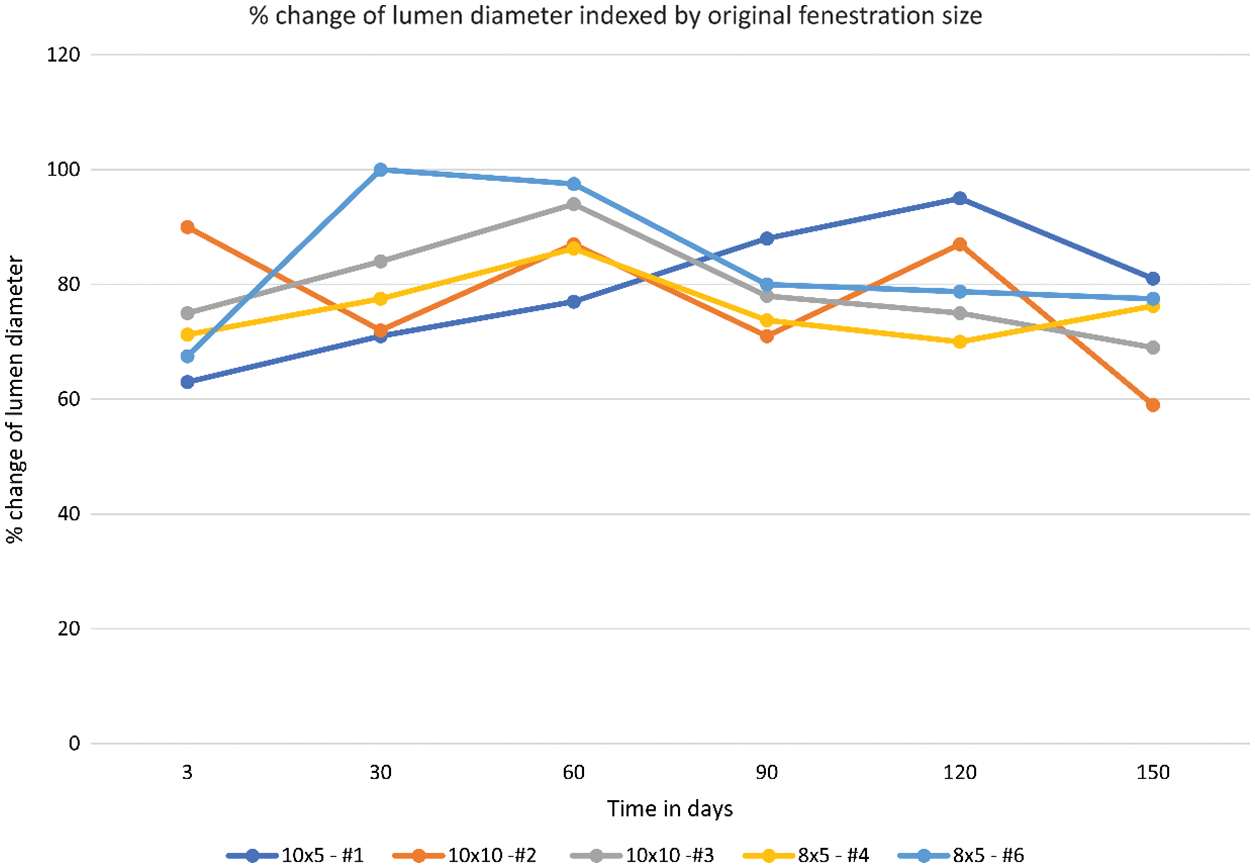
Figure 5: % change of the lumen diameter over time including all animals in the study
Neointimal growth was mild to moderate in all specimens, partially covering both the right and left surfaces of the AFR device (Table 3). Neointimal tissue incorporation was greater in areas where the device came in direct contact with the atrial septal endocardial surface, cranially and caudally. The neointimal coverage consisted of minimal granulation tissue with areas of smooth muscle cell proliferation and minimal fibrin and proteoglycan matrix deposition. Surface blood clots, consisting of an admixture of fibrin and platelets, were not observed on the surface or within the areas of the mesh by light microscopy. The inflammatory response was overall minimal to mild with scores of 1–2. It was chronic in nature and composed of lymphocytes, histiocytes and occasional multinucleated giant cells around struts. It was similar on both the right and left atrial surfaces. In addition, no clots or debris were seen in relation to the implantation sheaths or the delivery system.
3.3 Scanning Electron Microscopy
Animal #2 was randomly designated for scanning electron microscopy evaluation. SEM showed the nitinol wires partially covered by neo-endocardial tissue at the cranial and caudal edges. These were the parts of the device in contact with the atrial endocardial surface. Rare nitinol wires were surrounded by organized clots consisting of mostly platelets with focal inflammatory cells and organized fibrin strands. The central lumen was patent and nearly completely endothelialized. Scattered inflammatory cells adhering to inter-endothelial junctions were also observed.
3.4 Overall Growth and Non-Cardiac Organ Evaluation
The animals were in a healthy condition for the duration of the study, with normal weight gain. Organ evaluation did not show evidence of distant embolic phenomenon. There was no attributable pathology and no adverse effects noted on distant organs in any of the 5 animals.
This was a midterm animal study of the AFR that primarily assessed safety of deployment and patency of the device at 150 days. The study used interval echocardiography and fluoroscopy for assessment of the device at predetermined time points. At the end of the study, the animals were sacrificed for gross and histopathological examination of the AFR, the heart itself, and various other organs.
The results of the study indicate a device that can be placed feasibly and safely. A patent atrial level communication was maintained for at least 150 days in all tested animals. All deployed AFR devices had good to excellent performance without any sign of thrombosis after implantation. At days +3, +30, +60, +90, +120, and on the day of euthanasia, echocardiography and fluoroscopy were performed on all implanted animals and showed no major abnormalities.
Gross examination showed that the implanted devices were intact and well-seated in the interatrial septum. The devices were partially covered by neo-endocardial tissue. The adjacent structures were intact and unobstructed.
Our study showed that the communication at the end of +150 days was on average 72.5% the size of the of the original fenestration. Analysis of % change of the lumen diameter showed that the patency of the device fenestration was not significantly changed over time between day +3 to day +150. This highlights the device’s ability to maintain a predictable fenestration size and underpins the utility of the AFR when compared to other methods of creating an atrial level communication, such as atrial septostomy.
Histopathology revealed mild to moderate neointimal growth lining the device. Neointimal tissue incorporation occurred more in areas where the device sat flush against the atrial septal surface, both cranially and caudally. Similar to the results seen during the acute study [7], areas that were not in direct contact with the interatrial septum showed minimal neointimal growth. It is worth noting that the surface area not covered by neointimal growth was significantly less at 150 days than at 28 days. Also worth noting is the fact that the area not covered by neointimal growth was comparable between animals sacrificed at 90 days and 150 days. In general, the lack of neointimal proliferation in areas without direct contact with the atrial septum comes as no surprise as inflammation is a potent driver of neointimal proliferation [8]. Areas of direct contact will result in a larger inflammatory response that can be expected to drive further neointimal proliferation. It stands to reason that the larger atria in human subjects may allow for improved contact between the interatrial septum and the device, leading to greater inflammation and greater neointimal coverage. The inflammatory response observed in our animal model was overall minimal to mild and was chronic in nature. It was composed of lymphocytes, histiocytes and occasional multinucleated giant cells and was similar in both the right and left atrial surfaces.
There was almost complete endothelialization of the device lumen as seem by SEM. In our prior study, we had noted a progressive increase in endothelialization from 28 days to 90 days, a trend which we now observe through 150 days. We had shown that by 90 days, the lumen of the device had almost complete coverage with the discs being partially covered. This trend continued at 150 days with almost complete endothelialization of the device lumen and significant, though incomplete, coverage of the device discs. This timeline is consistent with postmortem SEM studies done on human coronary arteries after stenting. Three phases of stent integration occured [9]. An acute phase (<6 weeks) with no endothelial coverage, an intermediate phase (6–12 weeks) with increasing numbers of endothelial cells noted on the luminal surface of the stent, and a chronic phase (3 months +) with complete endothelialization of the stent. This is consistent with our observations of minimal coverage at 28 days, and almost complete coverage by 90–150 days. The lack of complete adherence between the AFR disk and the septal walls of the right and left atria likely contributed to the lack of complete endothelialziation of the device disks. The lumen, on the other hand, was in direct contact with the atrial septum and therefore underwent almost complete endothelialization.
Examination at necropsy revealed that the central lumen of all devices were patent. In one animal, we noted narrowing of the central lumen from 8 mm to 4 mm secondary to excess neointimal growth. Examination by light microscopy did not reveal blood clots on the device surface or within the areas of the mesh. It is interesting to note that the measurement of the device lumen by TEE showed that in 4 of the 5 implanted devices, the lumen at 150 days was similar to, or larger than, the lumen as measured at 3 days post implantation. This could be explained by the residual expansion of the nitinol device after a short period of accommodation into the septum.
There were three notable limitations with this study. The first limitation is the morphological difference in the atrial anatomy and atrial septum between pigs and humans. In pigs, the septum tends to be smaller and more muscular. This could potentially affect that way the AFR behaves and could affect the amount of contact made with the atrial septum. This in turn can alter the amount of endothelialization/neointimal proliferation noted on histological examination. The second limitation is the physiology of the hearts within which the AFR was implanted. All animals used had healthy hearts with no pathology prior to the AFR placement. This device is designed to be used in patients with high atrial pressure gradients, which none of our pigs had. The third limitation of the study is the small sample size used. The small sample size means that the results, while encouraging, are preliminary results and need to be replicated in a larger study sample.
In a continuation of our earlier work, this 150-day midterm safety and feasibility assessment showed that the AFR is both safe and effective at maintaining a sustainable atrial level communication for the duration of the study. Our preliminary results show that there was partial incorporation within the atrial septum, minimal inflammation, no embolization, no adverse effects in any other organ, and no significant thrombogenicity. This held true for implanted AFRs with a 10 mm fenestration and those with an 8 mm fenestration. Further studies need to be carried out to define the behavior of the AFR at longer timepoints. Endothelialization and neointimal proliferation, in particular, need further assessment via long-term studies with a larger sample size. It is believed that delayed endothelialization begets thrombosis [10], so it is encouraging that despite the lack of complete endothelial coverage of the device discs the device did not lead to significant thrombosis. However, it would behoove us to better characterize the endothelialization and neointimal-proliferation characteristics of the AFR beyond 150 days. Furthermore, considering that one of our devices developed an intra-luminal narrowing, long term studies with a larger sample size would help identify what, if any, factors contribute to aggressive intraluminal neointimal proliferation.
Authorship: All listed authors should have substantially contributed to the manuscript and have approved the final submitted version, which should include a description of each author’s specific work and contributor ship. The authors confirm contribution to the paper as follows: study conception and design: Ivy, D., Morgan, G.; data collection: McLennan, D.; analysis and interpretation of results: Shibbani, K., McLennan, D., Ivy, D., Morgan, G.; draft manuscript preparation: Shibbani, K. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: All data is available by emailing the corresponding author.
Ethics Approval: The study was reviewed and approved by the Institutional Animal Care and Use Committee. The review ensured compliance with the Canadian Council on Animal Care regulations. The testing facility where the study took place (Charles River Laboratories, Quebec, Canada) is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) and the Canadian Council on Animal Care (CCAC). The testing facility study number is 2907–232G, and the sponsor reference number is RD20_002.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Sandoval, J., Gaspar, J., Peña, H., Santos, L. E., Córdova, J. et al. (2011). Effect of atrial septostomy on the survival of patients with severe pulmonary arterial hypertension. The European Respiratory Journal, 38(6), 1343–1348. DOI 10.1183/09031936.00072210. [Google Scholar] [CrossRef]
2. Allcock, R. J., O’Sullivan, J. J., Corris, P. A. (2003). Atrial septostomy for pulmonary arterial hypertension. Heart, 89(11), 1344–1347. DOI 10.1136/heart.89.11.1344. [Google Scholar] [CrossRef]
3. Rajeshkumar, R., Pavithran, S., Sivakumar, K., Vettukattil, J. J. (2017). Atrial septostomy with a predefined diameter using a novel occlutech atrial flow regulator improves symptoms and cardiac index in patients with severe pulmonary arterial hypertension. Catheterization and Cardiovascular Interventions: Official Journal of the Society for Cardiac Angiography & Interventions, 90(7), 1145–1153. DOI 10.1002/ccd.27233. [Google Scholar] [CrossRef]
4. Sivakumar, K., Rohitraj, G. R., Rajendran, M., Thivianathan, N. (2021). Study of the effect of occlutech atrial flow regulator on symptoms, hemodynamics, and echocardiographic parameters in advanced pulmonary arterial hypertension. Pulmonary Circulation, 11(1), 1–10. DOI 10.1177/2045894021989966. [Google Scholar] [CrossRef]
5. Pascall, E., Jones, M. I., Savis, A., Rosenthal, E., Qureshi, S. A. (2021). Transcatheter creation of a pulmonary artery to left atrial fenestration in a failing fontan circulation using the atrial flow regulator (AFR). Cardiology in the Young, 31(8), 1376–1379. DOI 10.1017/S1047951121000731. [Google Scholar] [CrossRef]
6. O’Callaghan, B., Zablah, J., Vettukattil, J., Levi, D., Salem, M. et al. (2022). Multi-institutional US experience of the Occlutech© AFR device in congenital and acquired heart disease. Congenital Heart Disease, 17(1), 107–116. DOI 10.32604/CHD.2022.018590. [Google Scholar] [CrossRef]
7. McLennan, D., Ivy, D., Morgan, G. J. (2019). Transvenous implantation of the occlutech atrial flow regulator: Preliminary results from swine models. Congenital Heart Disease, 14(5), 819–831. DOI 10.1111/chd.12816. [Google Scholar] [CrossRef]
8. Shah, P. K. (2003). Inflammation, neointimal hyperplasia, and restenosis: As the leukocytes roll, the arteries thicken. Circulation, 107(17), 2175–2177. DOI 10.1161/01.CIR.0000069943.41206.BD. [Google Scholar] [CrossRef]
9. Kipshidze, N., Dangas, G., Tsapenko, M., Moses, J., Leon, M. B. et al. (2004). Role of the endothelium in modulating neointimal formation: Vasculoprotective approaches to attenuate restenosis after percutaneous coronary interventions. Journal of the American College of Cardiology, 44(4), 733–739. DOI 10.1016/S0735-1097(04)01083-6. [Google Scholar] [CrossRef]
10. Inoue, T., Croce, K., Morooka, T., Sakuma, M., Node, K. et al. (2011). Vascular inflammation and repair: Implications for re-endothelialization, restenosis, and stent thrombosis. JACC: Cardiovascular Interventions, 4(10), 1057–1066. DOI 10.1016/j.jcin.2011.05.025. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |