

 | Congenital Heart Disease |  |
DOI: 10.32604/chd.2022.019943
ARTICLE
Perpulmonary Device Closure of Patent Ductus Arteriosus with Minimum Diameter More Than 4 mm in Infants
1Department of Cardiovascular Surgery, Shandong Qianfoshan Hospital, Cheeloo College of Medicine, Shandong University, Jinan, China
2Department of Cardiovascular and Thoracic Surgery, The National Heart Hospital, Lusaka, Zambia
*Corresponding Author: Hongxin Li. Email: hongxinli@hotmail.com
#The first two authors contributed equally and must be considered as the first author
Received: 22 November 2021; Accepted: 13 April 2022
Abstract: Background: Closure of large patent ductus arteriosus (PDA) in older children has been accomplished using surgical and percutaneous techniques with remarkable outcomes. However, outcomes amongst infants have been variable with several drawbacks. Here we describe a novel minimally invasive technique, a product of mini-thoracotomy and traditional percutaneous technique skills, accomplished exclusively under echocardiography guidance. Methods: Symptomatic infants with a significant left-to-right shunt from PDA measuring more than 4 mm were selected. The symptoms were varying degrees of tachypnea, tachycardia, heart failure, failure to thrive, recurrent respiratory tract infections, or intensive care unit treatment for a longer duration. Through a left parasternal mini-thoracotomy, two parallel purse-string sutures were placed on the pulmonary trunk. After purse-string circle puncture, under exclusively transesophageal echocardiography guidance, a device secured to the safety-suture was implanted on the ascending aorta via pulmonary trunk using a specially designed set. The safety-suture prevented device migration in case of dislocation. The basic demographics, PDA size, device size and type, intrapulmonary manipulation time, operation time, PDA parameters (length, diameter, type of duct), redeployment of the device, residual shunt, and retention of safety-suture were all recorded and analyzed. The follow-up was done with transthoracic echocardiography on the 2nd postoperative day, 1, 3, 6, and 12 months, and yearly thereafter. Results: Fifty-two infants with a mean age of 8 months ± 2.8 months (Interquartile range = 0) underwent Perpulmonary device closure of PDA. Successful PDA occlusion was accomplished event-free in all subjects. The mean PDA, mean device, and mean operation time were 5.6 mm ± 1.4 mm, 7.9 mm ± 1.7 mm, and 61.2 min ± 12.9 min, respectively. The immediate acceptable residual shunt was noted among 3 subjects and disappeared at a 1-month follow-up. Eighteen infants had retained safety-suture for added safety. There were no reports of the device or procedure-related complications. Conclusion: Perpulmonary device closure is an effective and safe approach to PDA with a diameter measuring > 4 mm among infants. The safety-suture, in case of dislocation, prevents migration and associated complications.
Keywords: Patent ductus arteriosus; perpulmonary device closure; transesophageal echocardiography; infant; minimally invasive surgery
Following the recent developments in medical imaging and technology [1,2], a plethora of congenital heart defects including patent ductus arteriosus (PDA) can now be effectively treated using several approaches such as surgical ligation [3,4], video-assisted thoracoscopic surgery, robotic surgery [5,6], and transcatheter occlusion. Transcatheter occlusion may be the most common method among infants weighing > 3 kg, and less common for infants weighing < 2 kg [7,8]. It provides the most minimal invasiveness and enables early rehabilitation. However, in infants, the technique presents drawbacks including puncture difficulties due to small-caliber vessels, high potential for vascular trauma with possibly catastrophic bleeding, and device dislocation remains a complex challenge, especially for large-sized PDAs [7,9].
Minimally invasive trans-thoracic device closure is a novel technique that is in practice for more than a decade in our center [10]. Following the introduction of this technique by our center, other centers have followed suit, such as Fuwai Hospital and Huaxi Hospital in China. Perpulmonary device closure (PPDC) is a product of surgical (minimally invasive surgery; mini thoracotomy) and transcatheter technique skills [10]. Device deployment is accomplished exclusively under transesophageal echocardiography (TEE) guidance via a left parasternal approach instead of peripheral [10,11]. The study aimed to demonstrate that PPDC is a safe and effective approach for infants with PDA measuring > 4 mm. The salient technical steps of PPDC in infants are discussed and well-illustrated in the sections below.
From February 2009 to November 2020, 600 patients with PDA underwent transcatheter occlusion or PPDC, successfully in our center. Among them, 300 were PPDC occlusions. All of the patients were clinically indicated for closure. All subjects exhibited symptoms from a significant left-to-right shunt: varying degrees of tachypnea, tachycardia, heart failure, failure to thrive, recurrent respiratory tract infections, or intensive care unit treatment for a longer duration. Diagnosis of PDA was made using transthoracic echocardiography as an outpatient or inpatient. The severity of pulmonary hypertension is evaluated commonly using the pulmonary-to-systemic arterial pressure ratio, as mild < 0.5, moderate ≥ 0.5 but < 0.75, severe ≥ 0.75. However, this series focused on the outcomes of the 52 infants who were low weight, diagnosed with large PDA measuring > 4 mm in diameter, or with Krichenko Type B and C [12]. These infants underwent PPDC especially. Patients with a right to left shunt or coexisting other cardiac anomalies which needed to be repaired under cardiopulmonary bypass were not included in the original series of 600 patients.
The selections of surgical ligation, transcatheter occlusion, and PPDC were elaborated to the parents or guardians. The informed consent was obtained from parents or guardians. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the Research and Ethics Committee of Shandong Qianfoshan Hospital (Approval No. S474).
Based on age, patients were divided into two groups: patients aged 0∼6 months were in a group and those 7∼12 months were in another group. And further divided according to the type of device used as a ventricular septal defect (VSD-occluder) and ductal device (ductal-occluder) groups.
The procedure is performed stepwise under general anesthesia with an airway appropriately secured to a ventilation machine. Prior procedure TEE is conducted to reassess PDA dimensions including aortic and pulmonary ampulla, diameter, length, and ductal location. The device was selected in advance and in this series two types:1) ductal occluder; a specially designed plug device suitable for large or Type B PDA with the pulmonic waist/aortic waist diameters ranging from 6/8 to 8/10 mm [12], and 2) concentric VS-occluder; a modified version with following dimensions; waist length (3 or 4 mm), waist diameter (6 to 12 mm), with the smallest diameter being 2 or 3 mm larger than the minimum ductal diameter were utilized. Technically, a parasternal mini thoracotomy measuring about 2 cm is made in the left second intercostal space without injury to the pleura (Fig. 1).

Figure 1: Intra- and postoperative incision. (A) Left parasternal second intercostal incision, a delivery sheath can be seen (arrow); (B) Incision with a drain on 1st postoperative day
Further, pericardiotomy is made to expose the pulmonary trunk. After which, anticoagulation (Heparin; 100 units/kg) is given to achieve activated clotting time (ACT) just above 250 s. Under TEE guidance, the pulmonary trunk is gently compressed using a peanut sponge to locate a suitable puncture spot. After spot identification, two parallel purse-string sutures are placed on it. Then, a puncture within the purse-string circle is made. A safety-suture was used for added security. It was passed under the delivery cable screw, through the nitinol meshwork, just in the middle of the device. The safety-suture was then passed back into the delivery sheath. It was held on the side of the sheath when passing the delivery cable through. The delivery cable was then fastened with the device while keeping the safety-suture in a fixed position, this maneuver avoided the spiraling of the safety-suture around the delivery cable (Fig. 2).

Figure 2: Safety-Suture. (A) The safety-suture (polydioxanone) is passed under the screw just in the middle of the device through the nitinol meshwork (arrow), the delivery cable is seen screwed; (B–C) The safety-suture is passed through the introducer then the device is loaded (arrow). (D) The safety-suture is held in a fixed position by the assistant while allowing the operator to deploy the device
An appropriate device after being washed in heparin was loaded into the delivery system. Under continuous TEE monitoring, the chief surgeon introduced the loaded delivery system into the pulmonary trunk and advances it into descending aorta via PDA. The assistant tightened the two purse-string sutures to avoid bleeding and air entry. Before the device is released, arterial blood is allowed through the delivery sheath arm, thus, a deairing maneuver. The safety-suture is kept still while unscrewing. Satisfactory device-PDA assessment is followed by delivery sheath retraction with subsequent opening and fitting of retention disc against the aortic end of the ampulla, thus, the PDA is occluded (Fig. 3). The closure was done in layers with a drainage tube for 24 to 48 h. The pictorial steps of the PPDC technique are well illustrated in our previous project [10].

Figure 3: Intraoperative transesophageal echocardiography. (A) The diameter of the PDA with color flow doppler; (B) The delivery sheath crossing the PDA into the aorta (arrow); (C) The aortic end of the device is deployed; (D) The Occluder device can be seen in position with both disks deployed and no residual shunt was seen
However, if the PDA proves difficult to penetrate, we could cross it using the probe-assisted delivery system (PADS) (Fig. 4) is used. The probe is a malleable metallic instrument, about 20 cm in length, with a glossy olive tip and retaining a channel throughout. It can be molded into various angles to suit PDA’s position and size. Aided by the PADS, a flexible guidewire can be introduced into descending aorta lumen with subsequent PDA occlusion as aforementioned.

Figure 4: The probe assisted delivery system. (a) A J-Shaped malleable probe; (b) The guidewire; (c) The delivery sheath; (d) The introducer; and (e) The delivery cable
Following device implantation, doppler interrogation is performed to assess possible residual shunt, vessel obstruction, and device transposition. Unsatisfactory assessment compelled device redeployment with the correct size. If the TEE assessment proved satisfactory, the deployment set was withdrawn, the purse-string sutures were knotted tied together with the safety-suture kept in situ, which got absorbed after several months. The supplemental https://Video.1 and https://Video.2 show the procedure being performed in TEE.
Pre- and post-procedure prophylactic antibiotics were given within 48 h. Follow-up transthoracic echocardiography was performed on the 2nd postoperative day along with a chest x-ray (Fig. 5). Most patients were discharged 3 days after the procedure.

Figure 5: Chest X-ray. Lateral chest X-ray demonstrating correct positioning of the device on 1st postoperative day
Patients were reviewed at 1, 3, 6, and 12 months and yearly after the procedure. Echocardiography and electrocardiography were the routine examinations in each review. Subjects were followed up for a minimum of 5 years. The dislodgement/embolization, erosion, residual shunt, hemolysis, and narrowing or stenosis of the pulmonary trunk or aorta were considered device-related complications. The wound infection, bleeding, deep venous thrombosis, and pulmonary embolism were considered procedure-related complications.
Data were analyzed using IBM SPSS Statistics for Windows, Version 27.0 (Armonk, NY, IBM Corp). Further, data are expressed as mean ± standard deviation or median and Interquartile range (IQR) for continuous variables or frequency or percentage for categorical variables. Statistical comparisons of proportions were analyzed using Fisher’s Exact Probability Test and Chi-square test (Stata10.0 software; StataCorp LP, College Station, TX), and the p-value (<0.05) was deemed significant.
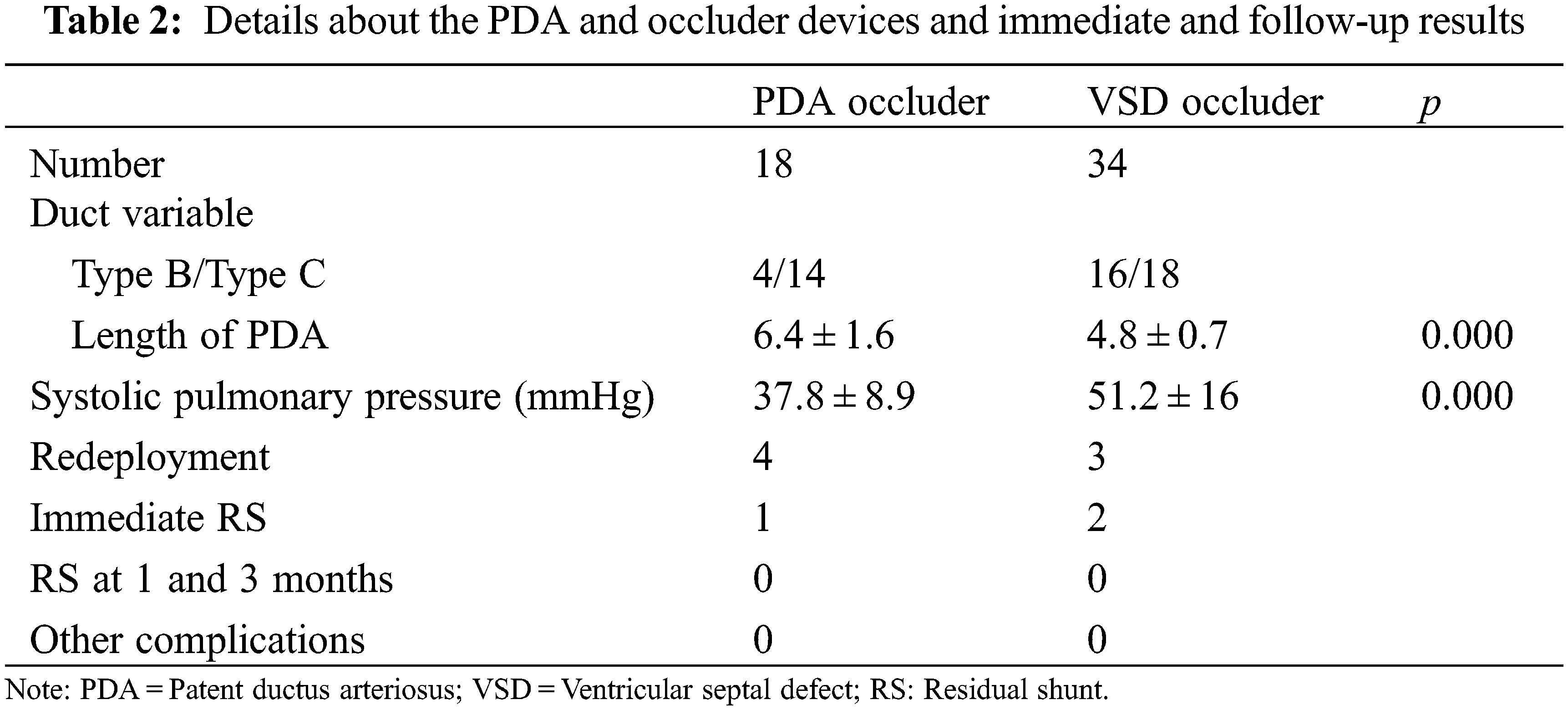
The subjects’ demography, procedural duration, and device dimensions are tabulated in (Table 1). PDA occlusion was successfully conducted in all subjects without complications. Sixteen (31%) of the 52 subjects were male with overall mean age and weight of 8 months ± 2.8 months (IQR = 0) and 7.2 kg ± 1.7 kg (IQR = 3.8), respectively. Of the 52 PDA’s, 18 (35%) were Type B and the remaining Type C [12]. The PADS was utilized in only 8 subjects. The mean intrapulmonary manipulation and operation times were 9.2 min ± 8.9 min and 59.5 min ± 11.2 min, respectively. The VSD-type and ductal occluders were implanted in 32 (62%) and 20 subjects respectively. The occluder redeployment due to inappropriate device size (being either small or large due to stability or shape issues) was performed in 7 patients (13%). There were five patients with coexisting atrial septal defects (ASD), 2 underwent simultaneous device occlusion, whereas the remaining 3 were unattended due to their hemodynamic insignificance. Twenty-five subjects exhibited pulmonary hypertension; 15 were severe and 10 moderate. One subject, dextrocardiac, underwent a ‘mirror image’ approach. Fig. 2 demonstrates the pre-and post-device implantation outcomes. Follow-up echocardiography on the second day uncovered acceptable (<1 mm) RS among 3 subjects, which disappeared on the third postoperative day (Table 2). Eighteen (35%) subjects had retained safety-suture. During the follow-up period, all subjects recovered fully. The symptoms disappeared gradually in all patients. No technique or device-related complications were reported. The costs of surgical ligation, transcatheter occlusion, and PPDC in our center on average are $1570, $3460, and $3930, respectively.

The importance of patent ductus arteriosus (PDA) in an unborn fetus as a source of systemic blood flow cannot be overemphasized [11,13]. Upon birth, among term babies, closure of PDA takes place within 24∼48 h, and its complications if left open are well documented [14]. Traditionally, closure of PDA can be spontaneous or accomplished using pharmacologic, surgical, robotic, video assisted thoracic surgery (VATS), and percutaneous approaches [4,5,7]. Nevertheless, amongst infants, surgical, VATS, and percutaneous retain several drawbacks including trauma, surgical complications, laryngeal nerve injury, ductal lacerations, coarctation of the aorta, and vessel caliber limitation. Others include device dislocation and embolization, procedural failure, catastrophic bleeding, high cost of catheterization sets, and a long learning curve [9,15,16]. There is a notable difference in the management of PDA in low-weight infants, where neonatologists prefer watchful waiting vs. cardiologists in the favor of intervention [17]. The surgical ligation still holds the edge in preterm infants against transcatheter occlusion [18]. A study shows that the trend of PDA closure has decreased in infants treated in neonatal intensive care units. Whereas the trend in transcatheter occlusion has increased as compared to surgical ligation [19]. But the introduction of a new technique is never out of question.
We combined the advantages of mini thoracotomy with traditional percutaneous intervention to present a novel alternative to surgical, VATS, and percutaneous procedures amongst infants. From our experience, PPDC has several advantages:
(1) PPDC requires a 2 cm parasternal incision which avoided the large trauma on the chest wall and the pleural space entry.
(2) Compared with the percutaneous approach, the PPDC approach has a shorter path, a better angle, and can be guided by the probe system, and the operation might be easier. The puncture position can be accurately located with a peanut sponge where it forms a direct angle towards the ductus by the guidance of the TEE. The device kept no tension during the deployment process and kept a better coaxiality with the ductus.
(3) For the infant, the femoral vessel caliber may pose access hurdles, thus increasing the difficulty of access and the risk of adverse events [20]. PPDC can reveal the pulmonary artery trunk easily, reducing the complications of peripheral blood vessels. Device closure of a doubly committed juxtaarterial ventricular septal defect is also operational from this approach [21].
(4) PPDC can retain the ‘safety-suture’ attached to the device during deployment, therefore, preventing catastrophic consequences in case of dislodgement which is 2.6% for occluders [20]. The ‘safety-suture’ is absorbed after 3-month, hence may not cause pulmonary stenosis from the pull on the safety-suture when tied along with the purse string-suture.
Although the cost of PPDC is marginally higher than transcatheter occlusion. These days, we mainly choose this technique in infants owing to; large PDA especially Type B, shallow approach to the pulmonary trunk, less trauma than surgery, and more appealing to locals due to cosmetic and safety profiles.
The PPDC has certain disadvantages: The need for thoracotomy or sternotomy to manage catastrophic bleeding. The expertise in interventional catheterization techniques. The mandatory presence of an expert echocardiographer to guide the procedure. It is relatively traumatic when compared to transcatheter. The operative time is relatively longer than surgical ligation and transcatheter occlusion.
It is not a comparative study. The number of patients is limited. There are no reported complications to date, this might result in biased thoughts in readers.
The PPDC of PDA in infants through a parasternal approach is simple, safe, and efficacious. All subjects recovered fully. A favorable decrease in residual shunt and absence of catastrophic device dislocation was noted. We henceforth, share our experience with Perpulmonary device closure of PDA in infants.
Authorship: Shibin Sun and Geoffrey J. Changwe: Conception, Data collection. Data analysis and interpretation. Drafting the article. Revision. Final approval; Zeeshan Farhaj and Hongxin Li: Conception, Drafting the article, Critical Revision, Final Approval; Yuekun Sun and Zhongzheng Kong: Data collection, Data analysis and interpretation.
Acknowledgement: Authors appreciate the support from members of staff from the two hospitals and the Cheeloo College of Medicine of Shandong University. The language revision assistance provided by Bernard Alejo was greatly appreciated.
Availability of Data and Materials: Data and materials are available upon request.
Funding Statement: The authors received no specific funding for the project, the APC fee is financed by the authors.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Jain, A., Shah, P. S. (2015). Diagnosis, evaluation, and management of patent ductus arteriosus in preterm neonates. JAMA Pediatrics, 169(9), 863–872. DOI 10.1001/jamapediatrics.2015.0987. [Google Scholar] [CrossRef]
2. Arlettaz, R. (2017). Echocardiographic evaluation of patent ductus arteriosus in preterm infants. Frontiers in Pediatrics, 5, 147. DOI 10.3389/fped.2017.00147. [Google Scholar] [CrossRef]
3. Avsar, M. K., Demir, T., Celiksular, C., Zeybek, C. (2016). Bedside PDA ligation in premature infants less than 28 weeks and 1000 grams. Journal of Cardiothoracic Surgery, 11(1), 146. DOI 10.1186/s13019-016-0539-3. [Google Scholar] [CrossRef]
4. Reese, J., Scott, T. A., Patrick, S. W. (2018). Changing patterns of patent ductus arteriosus surgical ligation in the United States. Seminars in Perinatology, 42(4), 253–261. DOI 10.1053/j.semperi.2018.05.008. [Google Scholar] [CrossRef]
5. Lukish, J. R. (2009). Video-assisted thoracoscopic ligation of a patent ductus arteriosus in a very low-birth-weight infant using a novel retractor. Journal of Pediatric Surgery, 44(5), 1047–1050. DOI 10.1016/j.jpedsurg.2009.02.002. [Google Scholar] [CrossRef]
6. Suematsu, Y., Mora, B. N., Mihaljevic, T., del Nido, P. J. (2005). Totally endoscopic robotic-assisted repair of patent ductus arteriosus and vascular ring in children. The Annals of Thoracic Surgery, 80(6), 2309–2313. DOI 10.1016/j.athoracsur.2005.05.078. [Google Scholar] [CrossRef]
7. Backes, C. H., Cheatham, S. L., Deyo, G. M., Leopold, S., Ball, M. K. et al. (2016). Percutaneous patent ductus arteriosus (PDA) closure in very preterm infants: Feasibility and complications. Journal of the American Heart Association, 5(2), e002923. DOI 10.1161/JAHA.115.002923. [Google Scholar] [CrossRef]
8. Choi, G. J., Song, J., Kim, Y. S., Lee, H., Huh, J. et al. (2018). Outcomes of transcatheter closure of ductus arteriosus in infants less than 6 months of age: A single-center experience. Korean Journal of Pediatrics, 61(12), 397–402. DOI 10.3345/kjp.2018.06548. [Google Scholar] [CrossRef]
9. Jang, G. Y., Son, C. S., Lee, J. W., Lee, J. Y., Kim, S. J. (2007). Complications after transcatheter closure of patent ductus arteriosus. Journal of Korean Medical Science, 22(3), 484–490. DOI 10.3346/jkms.2007.22.3.484. [Google Scholar] [CrossRef]
10. Li, H., Guo, W., Mei, Z., Fei, L., Zou, C. et al. (2012). New minimally invasive technique of perpulmonary device closure of patent ductus arteriosus through a parasternal approach. The Annals of Thoracic Surgery, 93(3), 862–868. DOI 10.1016/j.athoracsur.2011.12.017. [Google Scholar] [CrossRef]
11. Gournay, V. (2011). The ductus arteriosus: Physiology, regulation, and functional and congenital anomalies. Archives of Cardiovascular Diseases, 104(11), 578–585. DOI 10.1016/j.acvd.2010.06.006. [Google Scholar] [CrossRef]
12. Krichenko, A., Benson, L. N., Burrows, P., Möes, C. A., McLaughlin, P. et al. (1989). Angiographic classification of the isolated, persistently patent ductus arteriosus and implications for percutaneous catheter occlusion. The American Journal of Cardiology, 63(12), 877–880. DOI 10.1016/0002-9149(89)90064-7. [Google Scholar] [CrossRef]
13. Schneider, D. J., Moore, J. W. (2006). Patent ductus arteriosus. Circulation, 114(17), 1873–1882. DOI 10.1161/CIRCULATIONAHA.105.592063. [Google Scholar] [CrossRef]
14. Fortescue, E. B., Lock, J. E., Galvin, T., McElhinney, D. B. (2010). To close or not to close: The very small patent ductus arteriosus. Congenital Heart Disease, 5(4), 354–365. DOI 10.1111/j.1747-0803.2010.00435.x. [Google Scholar] [CrossRef]
15. Qasim, A., Dasgupta, S., Jain, S. K., Jiwani, A. K., Aly, A. M. (2017). Coarctation of the aorta as a complication of surgical ligation of patent ductus arteriosus in a premature infant. Case Reports in Pediatrics, 2017, 2647353. DOI 10.1155/2017/2647353. [Google Scholar] [CrossRef]
16. Amoozgar, H., Salehi, S., Farhadi, P., Edraki, M. R., Borzoee, M. et al. (2016). Follow-up results of device occlusion of patent ductus arteriosus. Iranian Journal of Pediatrics, 26(3), e3621. DOI 10.5812/ijp.3621. [Google Scholar] [CrossRef]
17. Sathanandam, S., Whiting, S., Cunningham, J., Zurakowski, D., Apalodimas, L. et al. (2019). Practice variation in the management of patent ductus arteriosus in extremely low birth weight infants in the United States: Survey results among cardiologists and neonatologists. Congenital Heart Disease, 14(1), 6–14. DOI 10.1111/chd.12729. [Google Scholar] [CrossRef]
18. Kim, H. S., Schechter, M. A., Manning, P. B., Eghtesady, P., Balzer, D. T. et al. (2019). Surgical versus percutaneous closure of PDA in preterm infants: Procedural charges and outcomes. The Journal of Surgical Research, 243, 41–46. DOI 10.1016/j.jss.2019.04.069. [Google Scholar] [CrossRef]
19. O’Byrne, M. L., Millenson, M. E., Grady, C. B., Huang, J., Bamat, N. A. et al. (2019). Trends in transcatheter and operative closure of patent ductus arteriosus in neonatal intensive care units: Analysis of data from the pediatric health information systems database. American Heart Journal, 217, 121–130. DOI 10.1016/j.ahj.2019.08.009. [Google Scholar] [CrossRef]
20. Backes, C. H., Rivera, B. K., Bridge, J. A., Armstrong, A. K., Boe, B. A. et al. (2017). Percutaneous patent ductus arteriosus (PDA) closure during infancy: A meta-analysis. Pediatrics, 139(2), e20162927. DOI 10.1542/peds.2016-2927. [Google Scholar] [CrossRef]
21. Guo, H. X., Fei, W. B., Liang, F., Zhang, H. Z., Zhu, M. et al. (2015). Perventricular device closure of a doubly committed juxtaarterial ventricular septal defect through a left parasternal approach: Midterm follow-up results. Journal of Cardiothoracic Surgery, 10, 175. DOI 10.1186/s13019-015-0376-9. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |