

 | Congenital Heart Disease |  |
DOI: 10.32604/chd.2022.019385
ARTICLE
Diagnostic Yield of Non-Invasive Testing in Patients with Anomalous Aortic Origin of Coronary Arteries: A Multicentric Experience
1Department of Cardio-Thoracic, Vascular Sciences and Public Health, University of Padova, Padova, Italy
2Department of Woman and Child’s Health, Pediatric Cardiology, University of Padova, Padova, Italy
3Athens Heart Surgery Institute and Iaso Children’s Hospital, Athens, Greece
4Department for Cardiovascular Surgery, University Medical Center, Leiden, The Netherlands
5Department for Cardiovascular Surgery, University Hospital Bern, Bern, Switzerland
6Department of Congenital Cardiac Surgery, IRCCS Policlinico San Donato, San Donato Milanese, Italy
7Department of Pediatric Cardiology and Congenital Heart Disease, Hôpital Marie Lannelongue, Université Paris-Sud, Le Plessis-Robinson, France
8Deutsch Herz Zentrum, Munich, Germany
9Katholieke Universiteit Leuven, Leuven, Belgium
10Cardiothoracic Surgery Hospital de Santa Marta Rua, Lisbon, Portugal
11Department of Pediatric Cardiothoracic Surgery, Children’s Hospital of Georgia, Georgia, USA
12Department of Cardiac Surgery, University Hospital Ghent, Ghent, Belgium
13Division of Cardiac Surgery, University Hospital Center of Tirana, Tirana, Albania
14Department of Pediatric Cardiac Surgery, Hospital for Children and Adolescents, University of Helsinki, Helsinki, Finland
15Congenital Cardiac Surgery Department, Hospital Universitario La Paz, Madrid, Spain
16Department of Pediatric Cardiac Surgery, National Institute of Cardio-Vascular Diseases-Children’s Heart Center, Bratislava, Slovakia
17Division of Pediatric Cardiology, Ospedale Brotzu, Cagliari, Italy
18Division of Sport Medicine, Ospedale Ca’ Foncello, Treviso, Italy
19Department of Medicine, Radiology Clinic, University of Padua Medical School, Padova, Italy
*Corresponding Author: Massimo A. Padalino. Email: massimo.padalino@unipd.it
#These authors share the first co-authorship
Received: 23 January 2022; Accepted: 15 May 2022
Abstract: Background: Anomalous aortic origin of a coronary artery (AAOCA) is a congenital heart disease with a 0.3%−0.5% prevalence. Diagnosis is challenging due to nonspecific clinical presentation. Risk stratification and treatment are currently based on expert consensus and single-center case series. Methods: Demographical and clinical data of AAOCA patients from 17 tertiary-care centers were analyzed. Diagnostic imaging studies (Bidimensional echocardiography, coronary computed tomography angiography [CCTA] were collected. Clinical correlations with anomalous coronary course and origin were evaluated. Results: Data from 239 patients (42% males, mean age 15 y) affected by AAOCA were collected; 154 had AAOCA involving the right coronary artery (AAORCA), 62 the left (AAOLCA), 23 other anomalies. 211 (88%) presented with an inter-arterial course. Basal electrocardiogram (ECG) was abnormal in 37 (16%). AAOCA was detected by transthoracic echocardiography and CCTA in 53% and 92% of patients, respectively. Half of the patients reported cardiac symptoms (119/239; 50%), mostly during exercise in 121/178 (68%). An ischemic response was demonstrated in 37/106 (35%) and 16/31 (52%) of patients undergoing ECG stress test and stress-rest single positron emission cardiac tomography. Compared with AAORCA, patients with AAOLCA presented more frequently with syncope (18% vs. 5%, P = 0.002), in particular when associated with inter-arterial course (22% vs. 5%, P < 0.001). Conclusion: Diagnosis of AAOCA is a clinical challenge due to nonspecific clinical presentations and low sensitivity of first-line cardiac screening exams. Syncope seems to be strictly correlated to AAOLCA with inter-arterial course.
Keywords: Anomalous coronary arteries; congenital; echocardiography; coronary computed tomography angiography
Anomalous aortic origin of coronary artery (AAOCA) from the inappropriate sinus of Valsalva is an increasingly recognized congenital heart disease (CHD), affecting approximately 0.3%–0.5% of the general population [1,2]. Autoptic and prospective studies performed in high-risk populations report an overall risk of sudden cardiac death (SCD) of 0.17%. Specifically, it is estimated as 0.2% in patients with the anomalous aortic origin of the right coronary artery (AAORCA) and 6.3% in the anomalous aortic origin of the left main coronary artery (AAOLCA) [3]. Diagnosis is often incidental since most patients are primarily asymptomatic or report nonspecific symptoms (palpitations, fatigue, chest discomfort) [4]. Furthermore, first-line screening exams like electrocardiogram (ECG) and ECG stress test are too aspecific for a precise diagnosis, and transthoracic echocardiography (TTE) requires high expertise and good experience for the evaluation of coronary arteries [5–9]. In case of high suspicion of AAOCA, coronary computed tomography angiography (CCTA) and cardiac magnetic resonance (CMR) imaging are currently the gold standards for diagnosis since they are accurate and precise in defining the anatomic details of the coronary anomaly [10–13]. Providing a good visualization of the anomalous coronary origin and course is crucial for the effective management of patients with AAOCA. Indeed, the presence of high-risk anatomical features (e.g., slit-like orifice, the acute angle of take-off, intramural course) or stress-induced myocardial ischemia are considered among the criteria supporting the surgical indication in both symptomatic and asymptomatic patients [14,15].
To date, real-world data regarding clinical presentation and diagnostic work-up of patients with AAOCA are scant and mostly confined to monocentric experiences [16–18]. Herein, we report a multicentric experience whose aim is to investigate the clinical onset and diagnostic pathway in a large series of patients with AAOCA, in order to assess the diagnostic yield of non-invasive testing and to outline possible associations between common cardiac symptoms and high-risk coronary conditions.
This is a multicenter retrospective longitudinal study including all patients diagnosed with AAOCA and followed up in 17 European tertiary care centers since 1991. Patients with isolated high coronary take-off, an anomalous left coronary artery from the pulmonary artery (ALCAPA), and major associated CHD were excluded from this analysis. Patients’ medical history was investigated in-depth, and all diagnostical tests performed before diagnosis were accurately reviewed. Coronary artery anatomical pattern and morphology, symptoms appearance during stress tests and an indication of surgery were examined. Finally, patients were grouped into different cohorts according to the type of anomalous origin (AAORCA vs. AAOLCA) and the presence of an inter-arterial and/or intramural course. Intraoperative and postoperative data were retrieved from a common database. Individual patients were not identified, and the need for patient consent was waived. This study complied with Helsinki’s declaration and was approved by the Local Ethical Committee (Comitato Etico per la Ricerca Clinica della Provincia di Padova) (approval code 4901/AO/20).
Categorical variables were reported as numbers and percentages and compared using the χ2 test or Fisher’s exact test, when appropriate. The only one continuous variable (age) was reported as mean ± standard deviation. Analyses were performed using SPSS (IBM SPSS Statistics for MacOS, Version 23.0. IBM Corp., Armonk, NY).
Overall, we enrolled 239 patients affected by AAOCA who either underwent surgery (172/239, 72%) or did not require surgery for various reasons (lack of indication, contraindication to surgery, denied consent). Surgery consisted mostly of coronary unroofing and reimplantation, with operative mortality <1% (only 2 early deaths occurring in patients with severe preoperative cardiac dysfunction). Surgical results have already been reported elsewhere [19]. Baseline characteristics and complaints during the first visit are summarized in Table 1 and have been partially reported previously [19]. AAORCA was noticed in 154 (64%) and AAOLCA in 62 (26%) patients. Minor anomalies, like a single coronary artery or left circumflex artery origin from the non-coronary sinus, were detected in 23 (10%). In 211 (88%) an inter-arterial course was diagnosed (147 AAORCA, 50 AAOLCA, 14 minor). The mean age at the first evaluation was 15 (±11) years.
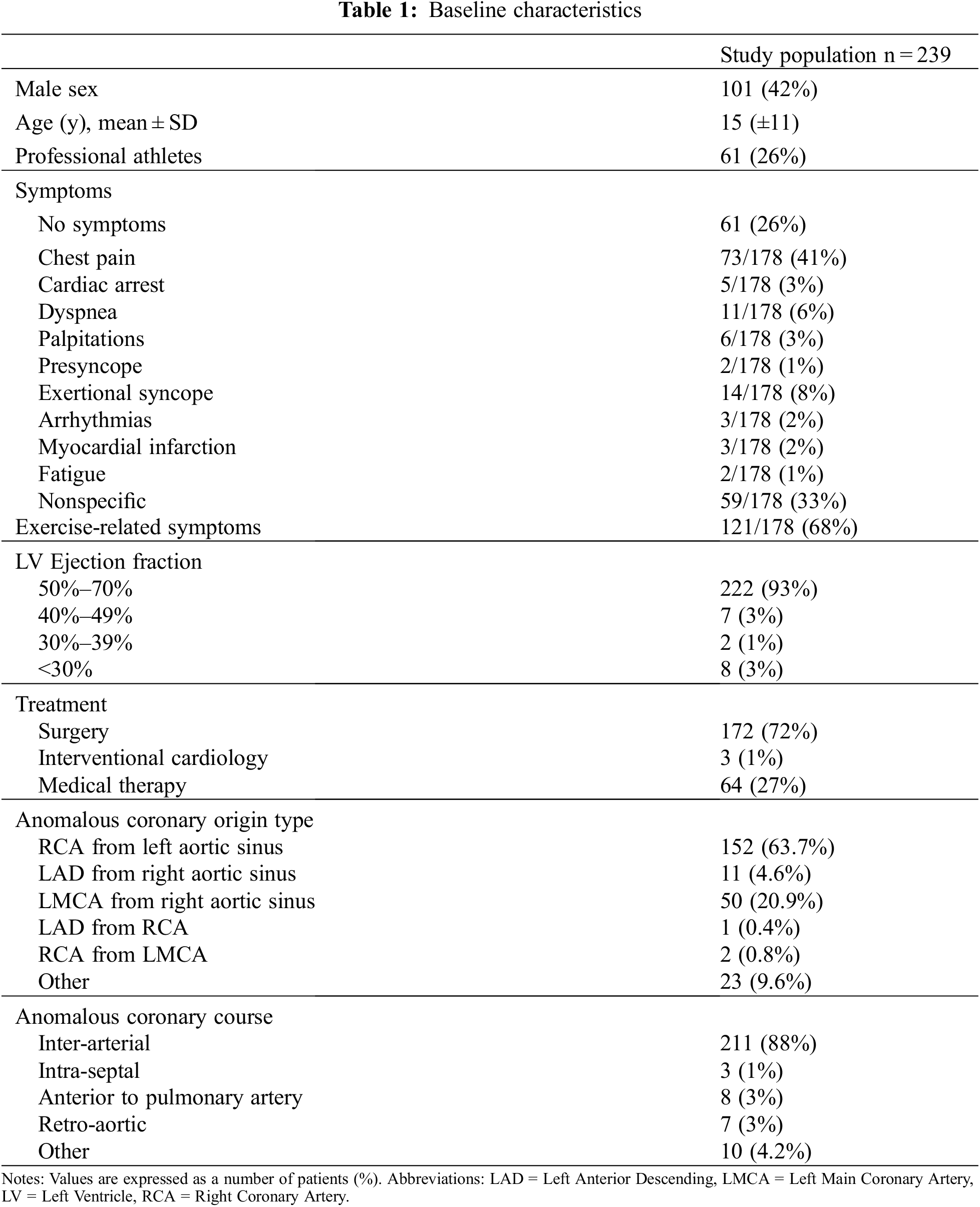
In this series, the diagnosis was incidental in 61 (25.5%) asymptomatic individuals. Conversely, symptoms were reported by 178/239 (74.5%) patients and occurred during physical activity in 121/178 (68%) cases. In particular, 73 (31%) complained of chest pain, while syncope was reported by 14 (6%), dyspnea in 11 (5%), palpitations in 6 (3%), arrhythmias in 3 (1%), presyncope in 2 (1%), and fatigue in 2 (1%). Overall, 59 (25%) reported a sort of undefined discomfort. The diagnosis of AAOCA followed a cardiac arrest as a first symptom requiring cardiopulmonary resuscitation in 5 (2%); these patients had AAORCA in 3 cases and AAOLCA in 2 cases. Although patients with AAOLCA were more frequently asymptomatic (33% vs. 20%; P = 0.05), syncope was significantly more common in these patients than AAORCA (18% vs. 5%, P = 0.002). Tests performed during diagnostic work-up and their results are summarized in Table 2.
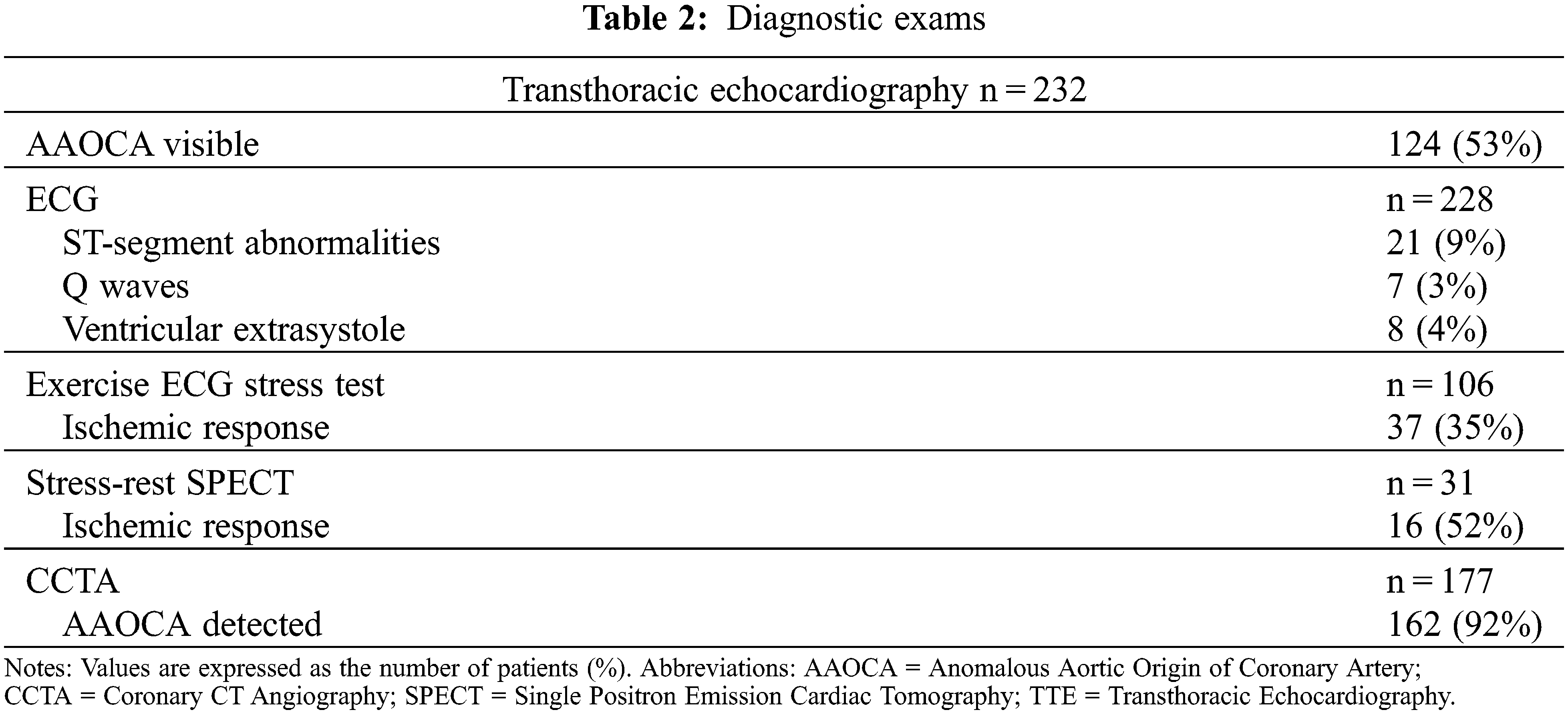
Patients’ basal ECG was abnormal in 37 (16%), presenting Q waves, ST alterations, or ventricular ectopic beats. Transthoracic echocardiography correctly diagnosed AAOCA in 123 (53%) patients, and it did more accurately in AAOLCA than AAORCA (70% vs. 44%, P < 0.001) and in non-inter-arterial course (74% vs. 50%, P = 0.05) (Figure 1). An ischemic response was found in 37/106 (35%) and 16/31 (52%) of patients undergoing ECG stress test and stress-rest single positron emission cardiac tomography (SPECT), respectively. In particular, hypoperfusion suggestive of myocardial ischemia in SPECT was more frequent in AAOLCA rather than AAORCA (80% vs. 42.1%, P = 0.05). Among cardiac imaging procedures, AAOCA was detected by CCTA in 162 (92%) and by CMR or coronary angiography in 62 (26%). Overall, an approach combining echocardiography plus CCTA was adopted in 109 (46%) patients, while echocardiography plus stress test plus CCTA in 48 (20%).
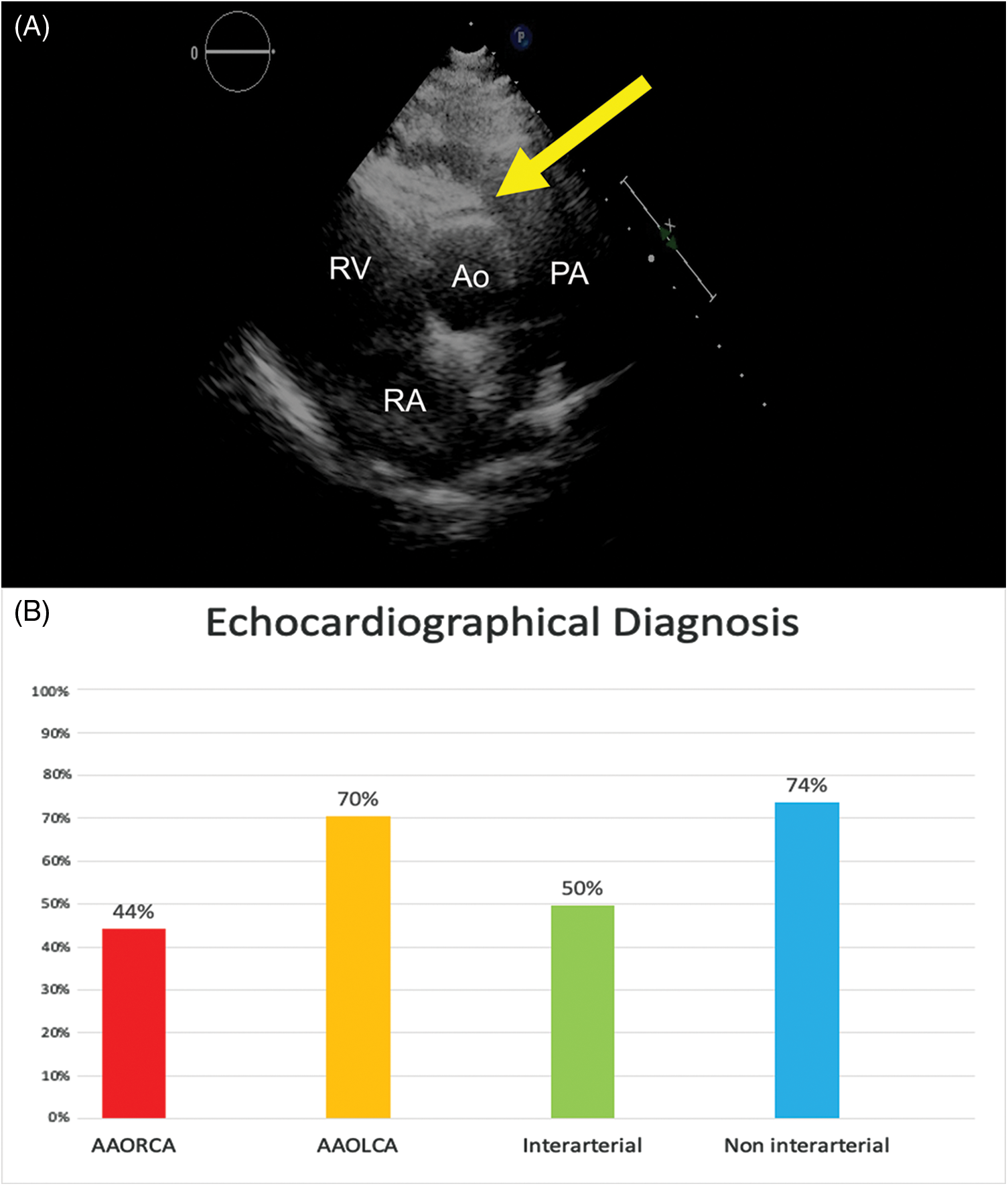
Figure 1: (Panel A) Echocardiographic short axis image showing the AAORCA anomalous course anterior to the aortic valve (yellow arrow; Ao: Aortic Root, PA: pulmonary artery, LA: Left Atrium, RA: Right Atrium). (Panel B) Histograms showing the percentage of positive echocardiographic diagnosis in patients suffering from AAORCA (red), AAOLCA (orange), anomalous origin with an inter-arterial course (green), anomalous origin with a non inter-arterial course (blue)
Differences in symptoms’ arousal and diagnostic test’s results between AAORCA and AAOLCA are summarized in Table 3.
Patients with the inter-arterial course were more frequently symptomatic than those without (78% vs. 58%, P = 0.05). Differences in symptoms’ appearance and diagnostic test’s results between patients suffering from an inter-arterial and a non-inter-arterial course are summarized in Table 4.
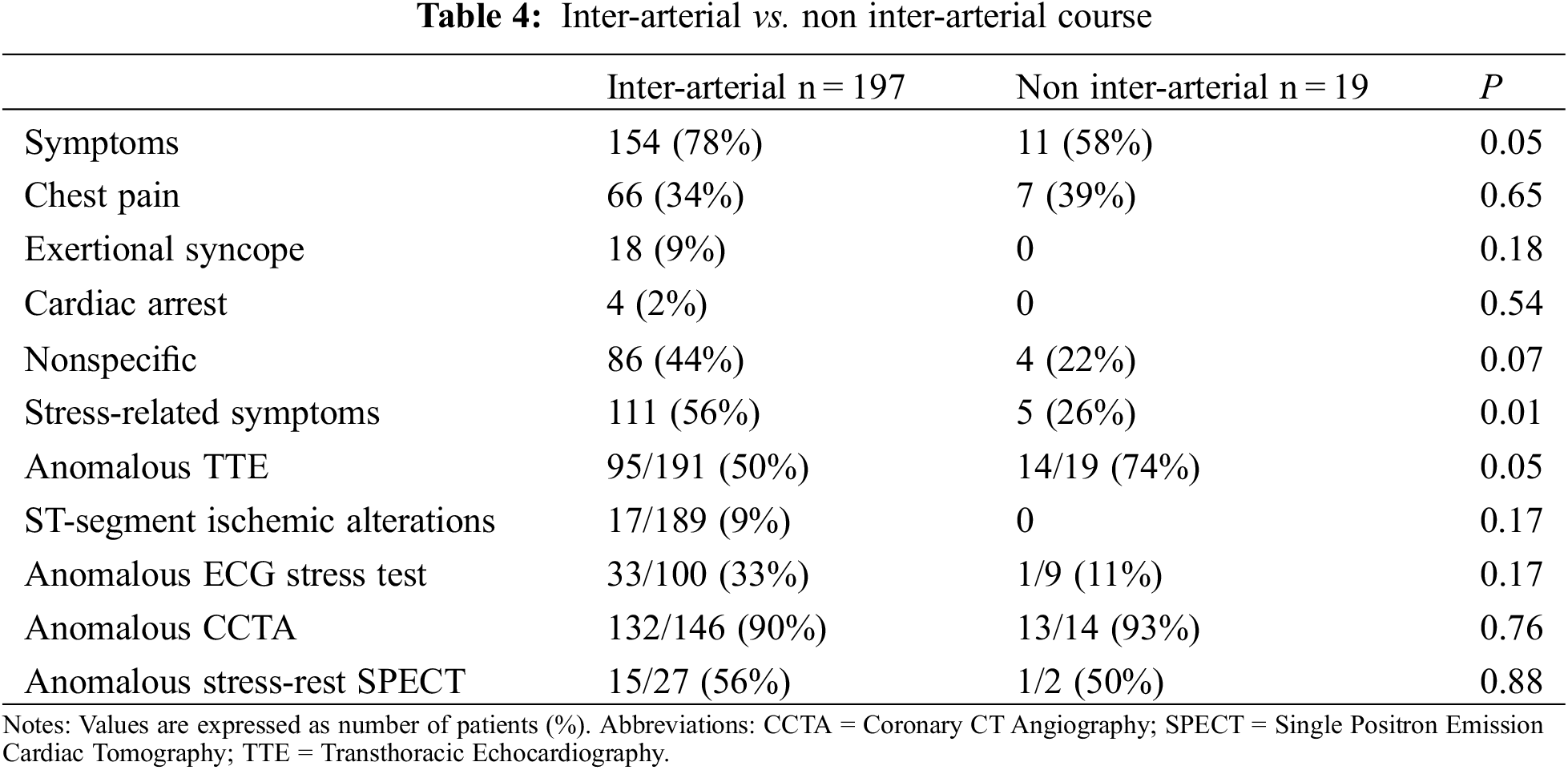
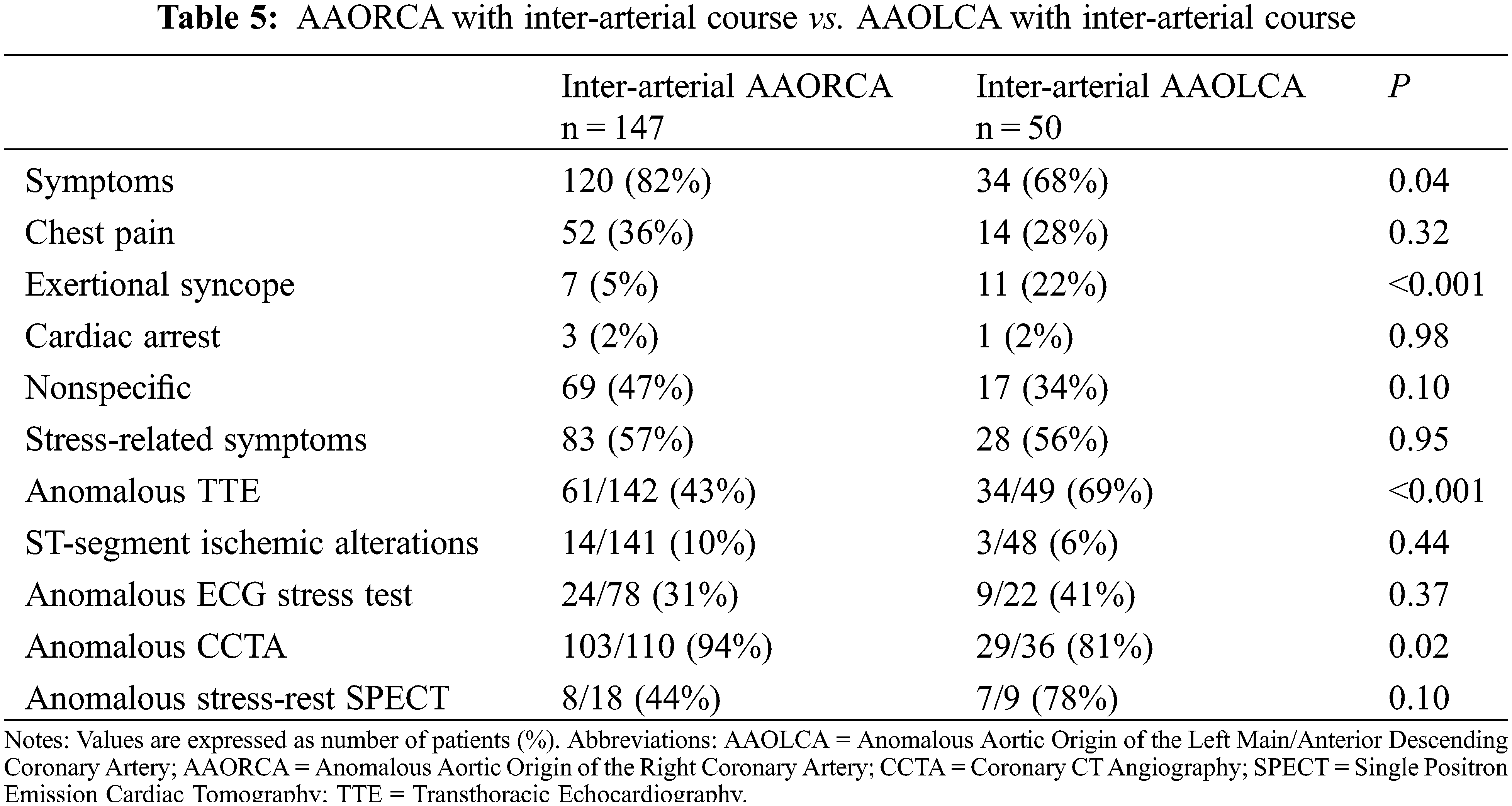
In particular, when comparing AAORCA and AAOLCA with the associated inter-arterial course, symptoms were more common in patients with AAORCA (82% vs. 68%, P = 0.04). However, syncope was again more prevalent in AAOLCA (22% vs. 5%, P < 0.001) (Figure 2). Differences in symptoms’ appearance and diagnostic tests’ results between patients suffering from AAORCA with an Inter-arterial course and AAOLCA with an Inter-arterial course are summarized in Table 5.
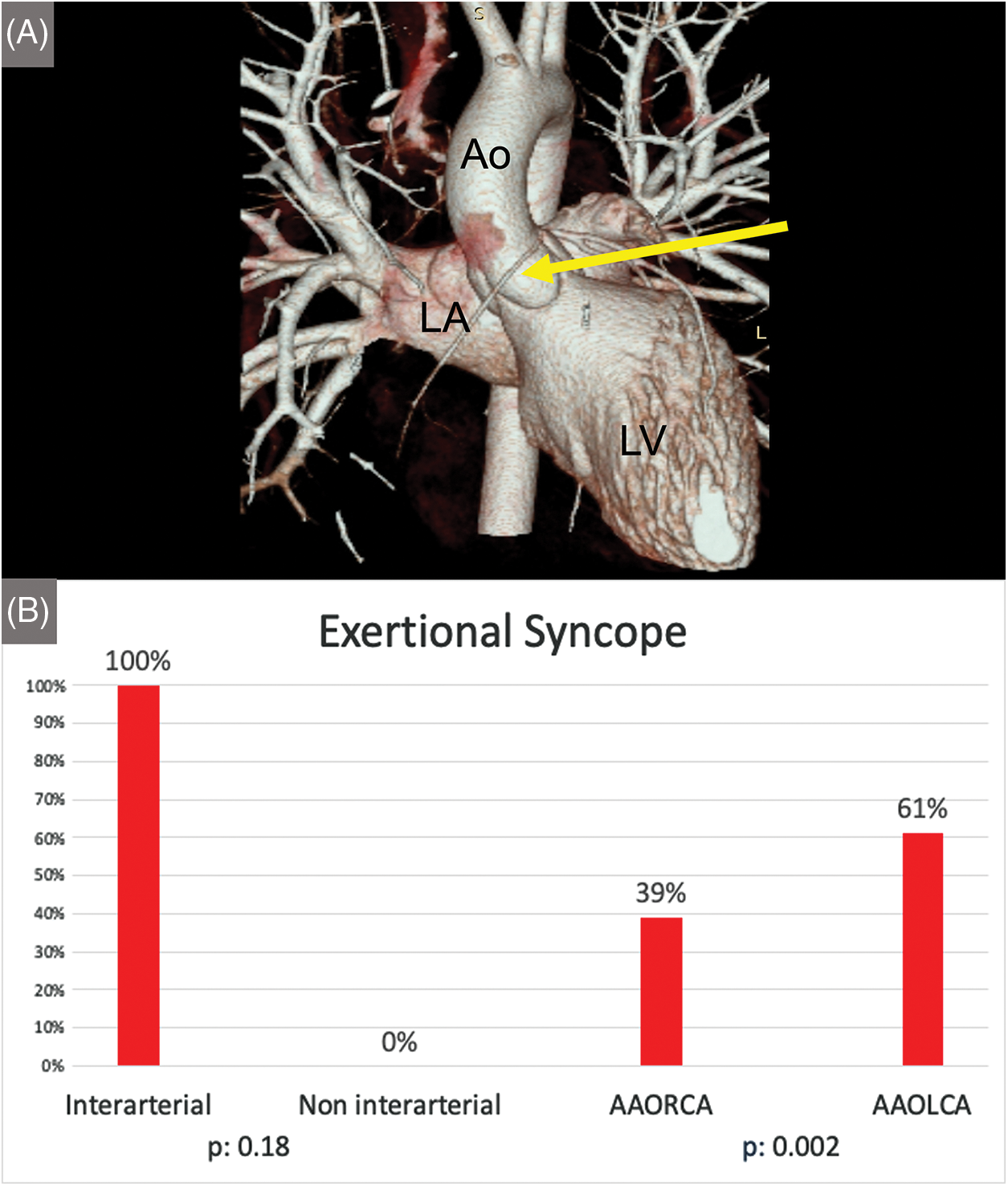
Figure 2: (Panel A) Coronary CT-angiography 3D reconstruction depicting an AAORCA (yellow arrow) originating from the left aortic sinus (Panel A; Ao: Aorta, LV: Left Ventricle, LA Left Atrium). Histograms showing the difference in occurrence of exertional syncope in inter-arterial vs. non-interarterial course and AAOLCA vs. AAORCA (Panel B)
This large retrospective multicenter study aimed to investigate the most common clinical presentations of patients affected by AAOCA and the effectiveness of diagnostic workup in clinical practice. In this cohort of young patients with AAOCA, we observed that the clinical onset can be very heterogeneous, ranging from asymptomatic cases (26%) or nonspecific symptoms (33%) to chest pain (41%) and cardiac arrest (3%). In most patients, first-line cardiac screening exams like basal and stress ECG failed to recognize AAOCA. On the contrary, bidimensional echocardiography effectively diagnosed AAOCA in about 50%, and more invasive cardiac procedures like CCTA detected the anomalies (also incidentally) in 92%. Subsequent analysis showed that the inter-arterial course was significantly associated with the presence of symptoms, ischemic response to provocative tests, and occurrence of arrhythmic events like syncope and malignant arrhythmias.
Our data showed that almost three out of four individuals with AAOCA presented with symptoms. Chest pain was the most frequent (41%) and was equally distributed between right- and left-sided anomalies and between the inter-arterial and non-inter-arterial courses. However, the ischemic etiology was more commonly demonstrated by provocative tests (stress ECG and stress-rest SPECT) in those with the inter-arterial course. These findings encourage further investigations focused on coronary artery imaging even in young age subjects and no other cardiovascular risk factors.
It is of note that 26% of this cohort was asymptomatic and, as such, incidentally diagnosed. This may account for the current significant underdiagnosis of this condition in the general population [1]. Moreover, considering the low sensitivity of basal and stress ECG for detection of AAOCA, we can understand how high the risk of false-negative results may be in young athletes with AAOCA undergoing preparticipation screening programs [4]. Coronary artery anomaly is one of the most prevalent causes of SCD in the athletes (the second in the United States after hypertrophic cardiomyopathy [20]), occurring in 5% to 17% of cases according to post-mortem registries [20,21], and much more frequently in young patients under 18 years old and in AAOLCA than AAORCA) [4,20–22].
Unlike ECG, echocardiography was confirmed to be a fundamental step in the diagnostic work-up of AAOCA. In our study, half of the patients were diagnosed by echocardiography, and higher percentages (71% and 74%, respectively) were reported in the presence of left coronary anomalies and non-inter-arterial (i.e., retro aortic) courses. We believe that the negative results of echocardiogram may be due primarily to incomplete and not-standardized evaluations, not including the routine assessment of right and left coronary arteries ostia, rather than a real low sensitivity. Previous studies have highlighted how echocardiographic examination of coronary artery ostia can be feasible and reliable in high percentages of young athletes, in which the quality of acoustic windows is generally optimal [6–23]. High expertise, broader experience, and dedicated or standardized protocols are therefore required in the evaluation of coronary arteries in echocardiography in order to increase further the diagnostic accuracy for AAOCA [24,25].
As previously reported [10–13], second-level operator-independent cardiac imaging tests, like CCTA, were highly accurate in diagnosing AAOCA. Although CCTA image quality can be hampered by tachycardia and low patient compliance, identifying anomalous coronary arteries and their course is reliable and highly reproducible [26]. It could have been noted that the use of contrast media and exposure to radiation should be avoided in pediatric settings. However, at present, exceptionally low radiation dose scan protocols are available [27], also, non-contrast CCTA has shown promising results in AAOCA evaluation [11]. Therefore, considering that patients presenting with exertional syncope were more frequently associated with dangerous anatomical patterns like AAOLCA and inter-arterial course, our data support the use of CCTA in these patients, especially pediatrics, to rule out with certainty the presence of malignant coronary anomalies.
This study is subject to the constraints of a retrospective multicentric study. Data collection was performed in multiple centers without a pre-specified fixed diagnostic algorithm, and exam choice depended on the physician and single-center experience. Data regarding the missing diagnosis of each diagnostic technique were not collected. The reason for incidental diagnosis was not deeply investigated. CMR imaging results were too scant and heterogeneous to provide evidences regarding its use in AAOCA evaluation. Prospective studies with a standard pre-specified diagnostic algorithm are needed to improve the clinical work-up of patients with AAOCA.
Despite the increase in medical awareness and experience, the diagnosis of AAOCA remains a clinical and diagnostic challenge. Our data showed that these patients’ symptoms are primarily nonspecific, and that first-line screening exam (i.e., basal and stress ECG) are not sensitive in diagnosis and risk stratification.
By contrast, echocardiography may represent a valuable tool for diagnosing AAOCA, particularly with left-sided and non-inter-arterial variants. Second-level cardiac imaging tests like CCTA must be considered in highly suspicious settings, particularly in young patients with effort-related syncope. In fact, this condition is indeed associated with high-risk anatomical features.
Authorship: The authors confirm their contribution to the paper as follows: study conception and design: A. C., D. C. and M. A. P.; data collection: A. C., P. B. D. A., L. M., D. S., G. S., M. H., T. C., A. F., V. S., M. L. R., J. H., R. R., J. C., B. M., J. F., H. T., A. C. P., K. F., A. V., J. S., A. G. R., M. N., E. P., R. T., P. S., C. P., R. M., G. D. S., V. L. V.; analysis and interpretation of results: A. C., P. B. D. A. and M. A. P. draft manuscript preparation: A. C., P. B. D. A., D. C. and M. A. P.. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The data underlying this article will be shared on reasonable request to the corresponding author.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors Dr. Mauro Lo Rito, Dr. Jurgen Horer, Dr. Giovanni Di Salvo, Dr. Vladimiro. L Vida, and Dr. Massimo A. Padalino are board members of Congenital Heart Disease. These authors have completely avoided being involved in the entire review process.
1. Cheezum, M. K., Liberthson, R. R., Shah, N. R., Villines, T. C., O’Gara, P. T. et al. (2017). Anomalous aortic origin of a coronary artery from the inappropriate sinus of valsalva. Journal of American College Cardiology, 69, 1592–1608. [Google Scholar]
2. Angelini, P., Cheong, B. Y., Lenge de Rosen, V. V., Lopez, A., Uribe, C. et al. (2018). High-risk cardiovascular conditions in sports-related sudden death: Prevalence in 5,169 schoolchildren screened via cardiac magnetic resonance. Texas Heart Institute Journal, 45(4), 205–213. [Google Scholar]
3. Brothers, J., Carter, C., McBride, M., Spray, T., Paridon, S. (2010). Anomalous left coronary artery origin from the opposite sinus of Valsalva: Evidence of intermittent ischemia. Journal of Thoracic and Cardiovascular Surgery, 140(2), e27–e29. [Google Scholar]
4. Basso, C., Maron, B. J., Corrado, D., Thiene, G. (2000). Clinical profile of congenital coronary artery anomalies with origin from the wrong aortic sinus leading to sudden death in young competitive athletes. Journal of American College Cardiology, 30, 1493–1501. [Google Scholar]
5. Angelini, P. (2002). Coronary artery anomalies–current clinical issues: Definitions, classification, incidence, clinical relevance, and treatment guidelines. Texas Heart Institute Journal, 29(4), 271–278. [Google Scholar]
6. Pelliccia, A., Spataro, A., Maron, B. J. (1993). Prospective echocardiographic screening for coronary artery anomalies in 1,360 elite competitive athletes. American Journal of Cardiology, 72, 978–979. [Google Scholar]
7. Zeppilli, P., Dello Russo, A., Santini, C., Palmieri, V., Natale, L. et al. (1998). In vivo detection of coronary artery anomalies in asymptomatic athletes by echocardiographic screening. Chest, 114, 89–93. [Google Scholar]
8. Davis, J. A., Cecchin, F., Jones, T. K., Portman, M. A. (2001). Major coronary artery anomalies in a pediatric population: Incidence and clinical importance. Journal of American College Cardiology, 37, 593–597. [Google Scholar]
9. Osaki, M., Mccrindle, B. W., Van Arsdell, G., Dipchand, A. I. (2008). Anomalous origin of a coronary artery from the opposite sinus of valsalva with an interarterial course: Clinical profile and approach to management in the pediatric population. Pediatric Cardiology, 29, 24–30. [Google Scholar]
10. Albrecht, M. H., Varga-Szemes, A., Schoepf, U. J., Nance, J. W., de Cecco, C. N. et al. (2019). Diagnostic accuracy of non-contrast self-navigated free-breathing MR angiography versus CT angiography: A prospective study in pediatric patients with suspected anomalous coronary arteries. Academic Radiology, 26, 1309–1317. [Google Scholar]
11. Jappar, I. A., Chua, T., Htoo, M. M. A., Cheah, F. K., Allen, J. C. et al. (2012). Diagnosis of anomalous original and course of coronary arteries using non-contrast cardiac CT scan and detection features. Journal of Cardiovascular Computed Tomography, 6, 335–345. [Google Scholar]
12. Mikolich, J. R. (2009). Cardiac magnetic resonance imaging and coronary computed tomography angiography in the diagnosis of anomalous coronary artery. Journal of American College of Cardiology, 53, 456. [Google Scholar]
13. Amado, J., Carvalho, M., Ferreira, W., Gago, P., Gama, V. et al. (2021). Coronary arteries anomalous aortic origin on a computed tomography angiography population: Prevalence, characteristics and clinical impact. International Journal of Cardiovascular Imaging, 32, 983–90. [Google Scholar]
14. Pelliccia, A., Sharma, S., Gati, S., Bäck, M., Börjesson, M. et al. (2021). 2020 ESC guidelines on sports cardiology and exercise in patients with cardiovascular disease. European Heart Journal, 42, 17–96. [Google Scholar]
15. Baumgartner, H., de Backer, J., Babu-Narayan, S. V., Budts, W., Chessa, M. et al. (2021). ESC scientific document group. 2020 ESC guidelines for the management of adult congenital heart disease. European Heart Journal, 42, 563–645. [Google Scholar]
16. Labombarda, F., Coutance, G., Pellissier, A., Mery-Alexandre, C., Roule, V. et al. (2014). Major congenital coronary artery anomalies in a paediatric and adult population: A prospective echocardiographic study. European Heart Journal of Cardiovascular Imaging, 15, 761–768. [Google Scholar]
17. Çanga, Y., Güvenç, T. S., Karataş, M. B., Çalık, A. N., Onuk, T. et al. (2017). Congenital coronary artery anomalies in adults: Review of 111 cases from a single-centre experience. Cardiology in the Young, 27, 1041–1050. [Google Scholar]
18. Fabozzo, A., DiOrio, M., Newburger, J. W., Powell, A. J., Liu, H. et al. (2016). Anomalous aortic origin of coronary arteries: A single-center experience. Seminars in Thoracic and Cardiovascular Surgery, 28, 791–800. [Google Scholar]
19. Padalino, M. A., Franchetti, N., Sarris, G. E., Hazekamp, M., Carrel, T. et al. (2019). Anomalous aortic origin of coronary arteries: Early results on clinical management from an international multicenter study. International Journal of Cardiology, 291, 189–193. [Google Scholar]
20. Maron, B. J., Doerer, J. J., Haas, T. S., Tierney, D. M., Mueller, F. O. (2009). Sudden deaths in young competitive athletes: Analysis of 1866 deaths in the United States, 1980–2006. Circulation, 119, 1085–1092. [Google Scholar]
21. Finocchiaro, G., Papadakis, M., Robertus, J. L., Dhutia, H., Steriotis, A. K. et al. (2016). Etiology of sudden death in sports: Insights from a United Kingdom regional registry. Journal of American College of Cardiology, 67(18), 2108–2115. [Google Scholar]
22. Corrado, D., Basso, C., Schiavon, M., Thiene, G. (1998). Screening for hypertrophic cardiomyopathy in young athletes. New England Journal of Medicine, 339, 364–9. [Google Scholar]
23. Witt, C. M., Elvert, L. A., Konik, E. A., Ammash, N. M., Foley, D. A. et al. (2018). The RAC sign: Retroaortic anomalous coronary artery visualization by transthoracic echocardiography. JACC Cardiovascular Imaging, 11, 648–649. [Google Scholar]
24. Thankavel, P. P., Lemler, M. S., Ramaciotti, C. (2015). Utility and importance of new echocardiographic screening methods in diagnosis of anomalous coronary origins in the pediatric population: Assessment of quality improvement. Pediatric Cardiology, 36, 120–125. [Google Scholar]
25. Bianco, F., Colaneri, M., Bucciarelli, V., Surace, F. C., Iezzi, F. V. et al. (2021). Echocardiographic screening for the anomalous aortic origin of coronary arteries. Heart, 8, e001495. [Google Scholar]
26. Ropers, D., Moshage, W., Daniel, W. G., Jessl, J., Gottwik, M. et al. (2001). Visualization of coronary artery anomalies and their anatomic course by contrast-enhanced electron beam tomography and three-dimensional reconstruction. American Journal of Cardiology, 87, 193–197. [Google Scholar]
27. Richards, C. E., Dorman, S., John, P., Davies, A., Evans, S. et al. (2018). Low-radiation and high image quality coronary computed tomography angiography in “real-world” unselected patients. World Journal of Radiology, 10, 135–142. [Google Scholar]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |