| Computers, Materials & Continua DOI:10.32604/cmc.2022.029266 |  |
| Article |
MIoT Based Skin Cancer Detection Using Bregman Recurrent Deep Learning
1Department of Computer Sciences, College of Computer and Information Sciences, Princess Nourah Bint Abdulrahman University, Riyadh, 11671, Saudi Arabia
2Department of Information Systems, College of Computer and Information Sciences, Princess Nourah Bint Abdulrahman University, Riyadh, 11671, Saudi Arabia
*Corresponding Author: Nithya Rekha Sivakumar. Email: NRRaveendiran@pnu.edu.sa
Received: 01 March 2022; Accepted: 09 June 2022
Abstract: Mobile clouds are the most common medium for aggregating, storing, and analyzing data from the medical Internet of Things (MIoT). It is employed to monitor a patient’s essential health signs for earlier disease diagnosis and prediction. Among the various disease, skin cancer was the wide variety of cancer, as well as enhances the endurance rate. In recent years, many skin cancer classification systems using machine and deep learning models have been developed for classifying skin tumors, including malignant melanoma (MM) and other skin cancers. However, accurate cancer detection was not performed with minimum time consumption. In order to address these existing problems, a novel Multidimensional Bregman Divergencive Feature Scaling Based Cophenetic Piecewise Regression Recurrent Deep Learning Classification (MBDFS-CPRRDLC) technique is introduced for detecting cancer at an earlier stage. The MBDFS-CPRRDLC performs skin cancer detection using different layers such as input, hidden, and output for feature selection and classification. The patient information is composed of IoT. The patient information was stored in mobile clouds server for performing predictive analytics. The collected data are sent to the recurrent deep learning classifier. In the first hidden layer, the feature selection process is carried out using the Multidimensional Bregman Divergencive Feature Scaling technique to find the significant features for disease identification resulting in decreases time consumption. Followed by, the disease classification is carried out in the second hidden layer using cophenetic correlative piecewise regression for analyzing the testing and training data. This process is repeatedly performed until the error gets minimized. In this way, disease classification is accurately performed with higher accuracy. Experimental evaluation is carried out for factors namely Accuracy, precision, recall, F-measure, as well as cancer detection time, by the amount of patient data. The observed result confirms that the proposed MBDFS-CPRRDLC technique increases accuracy as well as lesser cancer detection time compared to the conventional approaches.
Keywords: MIoT; skin cancer detection; recurrent deep learning classification; multidimensional bregman divergencive scaling; cophenetic correlative piecewise regression
Skin cancer is the most important type of risky form of cancer to affect the human death rate. Skin cancer is affected by the irregular development of skin cells. Melanoma and non-melanoma are the most common type of skin cancer. Skin cancer cases have enlarged significantly in modern years because of malignant melanoma. In the United States per year, 5.4 million patients are affected by skin cancer. The skin prevents the person’s body from various undesirable impacts of surroundings. Therefore, skin fitness monitoring and early-stage detection are challenging task for effective treatment and it helps to cure it. Manually skin lesions detection is very complex. Besides, due to variants in skin textures and injuries, identification, as well as categorization of skin cancer, was a complex mission. Internet of things (IoT) and deep learning possess high accuracy with flexibility. This helps to detect skin cancer at an early stage for the enhancement of accuracy and time. In addition, the machine learning method is employed for discovering skin cancer to obtain good efficiency in detection.
An Automated Hyper-Parameter Optimized Convolution Neural Net (CNN) was introduced in [1] for detecting the class of skin cancer. The Grey Wolf Optimization algorithm was applied by choosing the applicable hyper-parameters and improving the accuracy. This helps to handle the skin cancer multi-class classification issue. Image preprocessing is performed by applying Bilinear Incorporation (BI) for protecting the training time. The designed method is introduced with higher accuracy and a lesser loss rate. But, the time complexity was not minimized by applying multiclass datasets. A Deep CNN-based structure of instantaneous identification, as well as recognition of skin lesions called Dermo-DOCTOR, was developed in [2]. But the accuracy of detection and recognition of the skin lesions was not improved.
The deep Convolutional Neural Network (DCNN) model was introduced in [3] to achieve the classification rate. But, the classification accuracy was not improved. In [4], machine learning techniques and their application in the diagnosis of skin cancer were studied. Integration of human and artificial intelligence techniques was introduced in [5] for skin cancer detection. But the different process of skin cancer detection was not analyzed with a large dataset.
The support vector machines and convolutional neural networks were developed in [6] for malignant melanoma skin cancer detection. But the designed classifiers models were not efficient to increase their accuracy. Malignant melanoma classification using deep learning classification was introduced in [7]. However, increasing the accuracy rate remains a major challenge. Several deep learning methods were developed in [8] for skin cancer diagnosis by applying the entropy as well as VIKOR approaches. But the designed method was not suitable for designing the diagnostic system.
Deep neural network was introduced in [9] for the skin lesions classification system. The designed model achieved a high accuracy rate but the precision and recall were not estimated. Deep learning-based model-driven construction was designed in [10] for Skin cancer detection. But the performance of time complexity analysis was not performed.
Most of the conventional CNN-based methods have been designed for the Internet of things (IoT). But it failed to consider the cancer detection time. In addition, the precision was not enhanced. Then, the existing CNN-based method failed to offer better accuracy and recall to identify cancer at an earlier stage. To overcome the existing issue, MBDFS-CPRRDLC technique is introduced to enhance the accuracy with lesser cancer detection time. The novelty and contributions are illustrated as given below,
• An MBDFS-CPRRDLC was developed for improving skin cancer detection accuracy in lesser time.
• The Multidimensional Bregman Divergencive Feature Scaling technique is designed in the MBDFS-CPRRDLC technique with the novelty of the Bregman Divergence. It is utilized as a Recurrent Deep Neural learning classifier for determining the important features to find the disease with minimum time consumption.
• A new thought of the cophenetic correlative piecewise regression is applied in the MBDFS-CPRRDLC technique for analyzing the testing and training cancer data. The novel idea of the piecewise regression is useful for partitioning the data dissimilar classes, exhibit different relationships between the testing and training cancer data.
• Cophenetic correlation coefficient is employed to the relationships among the testing and training cancer data. This process is repetitively performed until the error gets minimized. In this way, cancer classification is accurately performed with higher accuracy.
• Extensive experimentation is conducted for calculating the MBDFS-CPRRDLC as well as related works. The obtained result shows that our proposed, MBDFS-CPRRDLC provides better performance in terms of accuracy, precision, recalls, F-measure, and detection time.
The main objective of the MBDFS-CPRRDLC technique is described as follows.
• To enhance the skin cancer detection accuracy, an MBDFS-CPRRDLC technique is introduced based on feature selection and classification.
• To reduce the cancer detection time the Multidimensional Bregman Divergencive Feature Scaling technique is employed in the MBDFS-CPRRDLC technique as a Recurrent Deep Neural learning classifier to find the significant features for disease identification
• To improve the precision and recall, a cophenetic correlative piecewise regression is utilized in the MBDFS-CPRRDLC technique to a hidden layer of Recurrent Deep Neural learning classifier to examine the testing and training cancer data.
The article is organized as follows. Section 2 discusses the related works. Section 3 describes the MBDFS-CPRRDLC by a neat figure. Section 4 explains the simulation settings. Section 5 provides the results and discussion. Section 6 gives the conclusion of the paper.
In this section, we present contemporary pertinent related works to our work that led and motivated us to carry out this research work. The related works are related to deep learning methods along with the review of works conducted in the area Skin cancer detection. An architecture search framework was introduced in [11] for malignant melanoma detection. But it failed to yield accurate classification results. A new transfer learning-driven deep IoHT framework was developed in [12] for the recognition and categorization of skin cancer. However, it failed to improve the classification accuracy.
A deep learning feature fusion as well as extreme learning machine were introduced in [13] for multiclass skin lesion classification. But it failed to consider the recent deep learning models for accurate lesion classification. A novel method consisting of artificial intelligence and cloud-based IoT was introduced in [14] for skin disease classification. But it failed to provide efficient skin disease classification results.
A medical artificial intelligence structure was introduced in [15]. But the accuracy was not enhanced using self-learning scheme. A new hybrid machine learning approach was developed in [16] for various cases of melanoma skin cancer detection. But it failed to improve the model efficiency and performance of melanoma detection.
A Deep Convolutional Neural Networks architecture was designed in [17] for Skin Lesion Classification. But the time consumption of Skin Lesion Classification was not minimized. Transfer Learning with Deep Learning was introduced in [18] based on IoT for the diagnosis of skin lesions. But the designed approach failed to detect the different types of skin lesions with different datasets.
A deep convolutional neural network was developed in [19] for multi-class skin Cancer classification. However, deep learning computer-aided schemes were not applied. A fusion-based Deep learning methodology was developed in [20] for the Detection of different stages of melanoma skin cancer. But it considered every feature of classification as well as consumes greater time. Several machine learning techniques were developed in [21] for skin cancer detection. But the designed technique was not efficient for the large volume of data generated by the healthcare system. Lightweight CNN classification model based on transfer learning was developed in [22] to exactly find the COVID-19 diagnose patients. But the time was higher. Multi-label learning algorithms were introduced in [23] for improving accuracy. However, the relevant features are not selected.
We present contemporary pertinent related works to our work that led and motivated us to carry out this research work. The related works are related to machine learning and deep learning methods along with the review of works conducted in the area of remote monitoring systems. In recent few years, the healthcare industry has overlooked notable coercion owing to the ceaseless upsurge in patients concerning chronic diseases and their long-term treatment given to the patient. Different clinical applications have been proposed to provide solutions to the surge rate of chronic patients in the hospital.
3 Methodology of MBDFS-CPRRDLC for the Deep Learning MIOT
Skin cancer was a risky type of cancer as well as improves the cause of fatality for the need facts. Therefore, early identification of starting stage is essential to prevent the spreading of cancer. Skin diseases were the major widespread problem that needs survey. Diagnosis was reliant on the knowledge of physicians. Misdiagnosis, delayed diagnosis, or inability diagnosis cause life-threatening effects. Therefore, a novel work MBDFS-CPRRDLC improves the diagnostic procedure for skin cancer. MBDFS-CPRRDLC is used for the deep learning MIoT driven structure of skin cancer.
Fig. 1 given below demonstrates the architecture of the Mobile Cloud service provisioning based proposed MBDFS-CPRRDLC technique. The architectural model consists of three major entities namely the number of patients
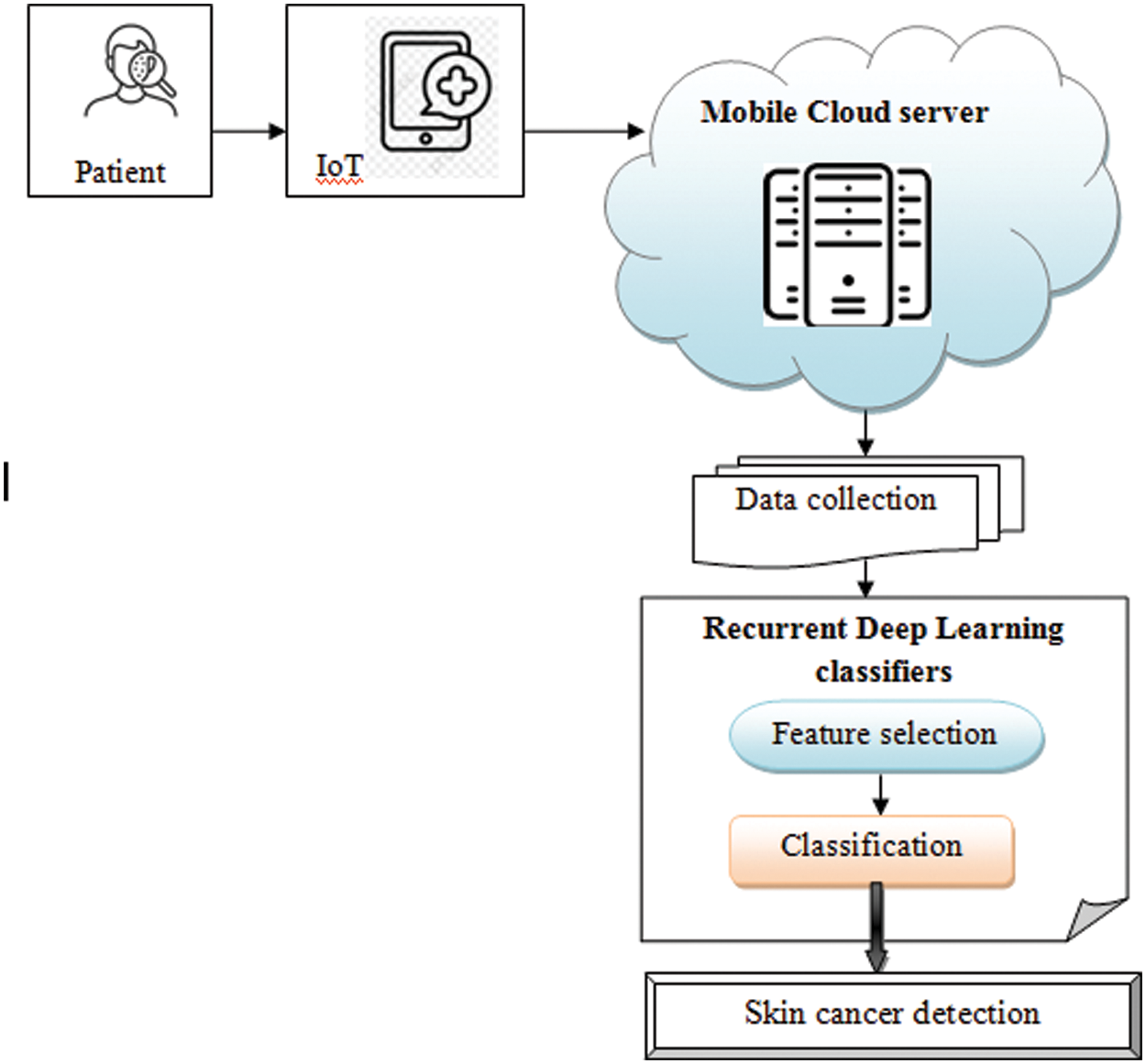
Figure 1: Architecture of proposed MBDFS-CPRRDLC
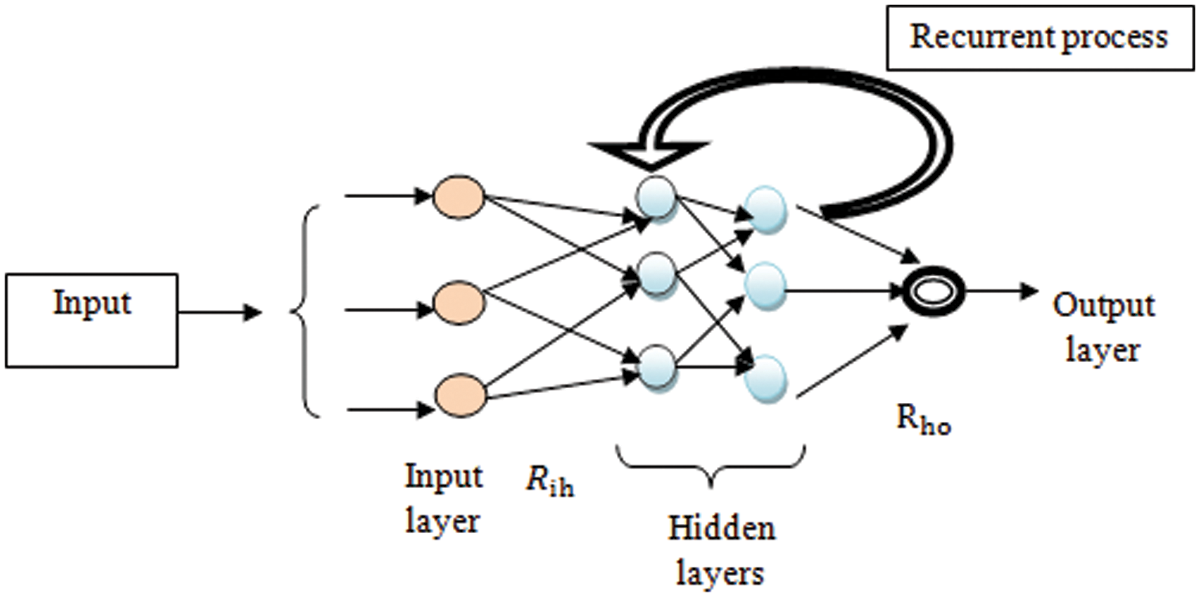
Figure 2: Structural diagram of Recurrent Deep Neural learning
Fig. 2 given above illustrates a structural diagram of adaptive deep Recurrent Deep Neural learning with multiple layers. The deep Recurrent Deep Neural network consists of the neuron-like nodes within various layers and connected within the subsequent successive layers. In one layer, the neuron is completely linked with the next successive layer as well as repeatedly performs deep feature learning it was termed a recurrent deep neural classifier. The input layer receives the number of features within a network by time ‘
From (1),
Initially, the features are arranged into the matrix in the form of row and column
where,
where
Fig. 3 shows the block diagram of Cophenetic correlative piecewise regression-based cancer detection. Piecewise regression function is applied to identify the training as well as testing data on Cophenetic correlation function. Main aim of regression analysis is used to predict types of skin cancer with a specific mathematical criterion. The mathematical criterion is performed using a Cophenetic correlation.
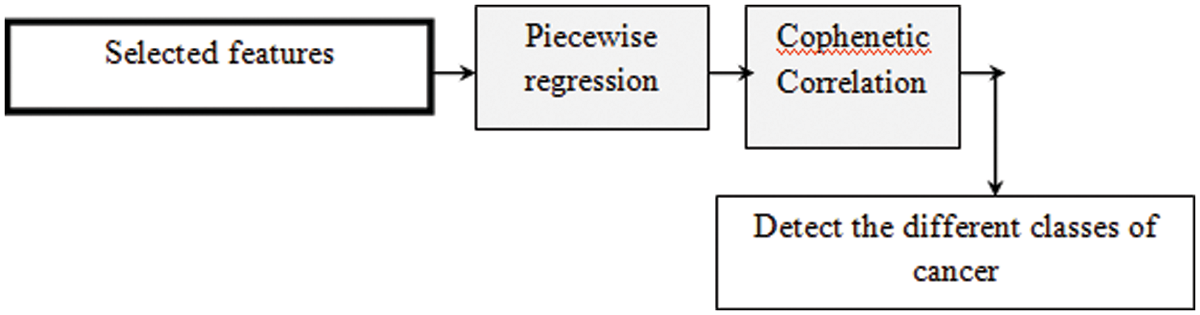
Figure 3: Block diagram of Cophenetic correlative piecewise regression-based cancer detection
The regression function considers the input training data
From (5), ‘
The hidden layer output is given by,
where, ‘
where ‘
From Eq. (7), ‘
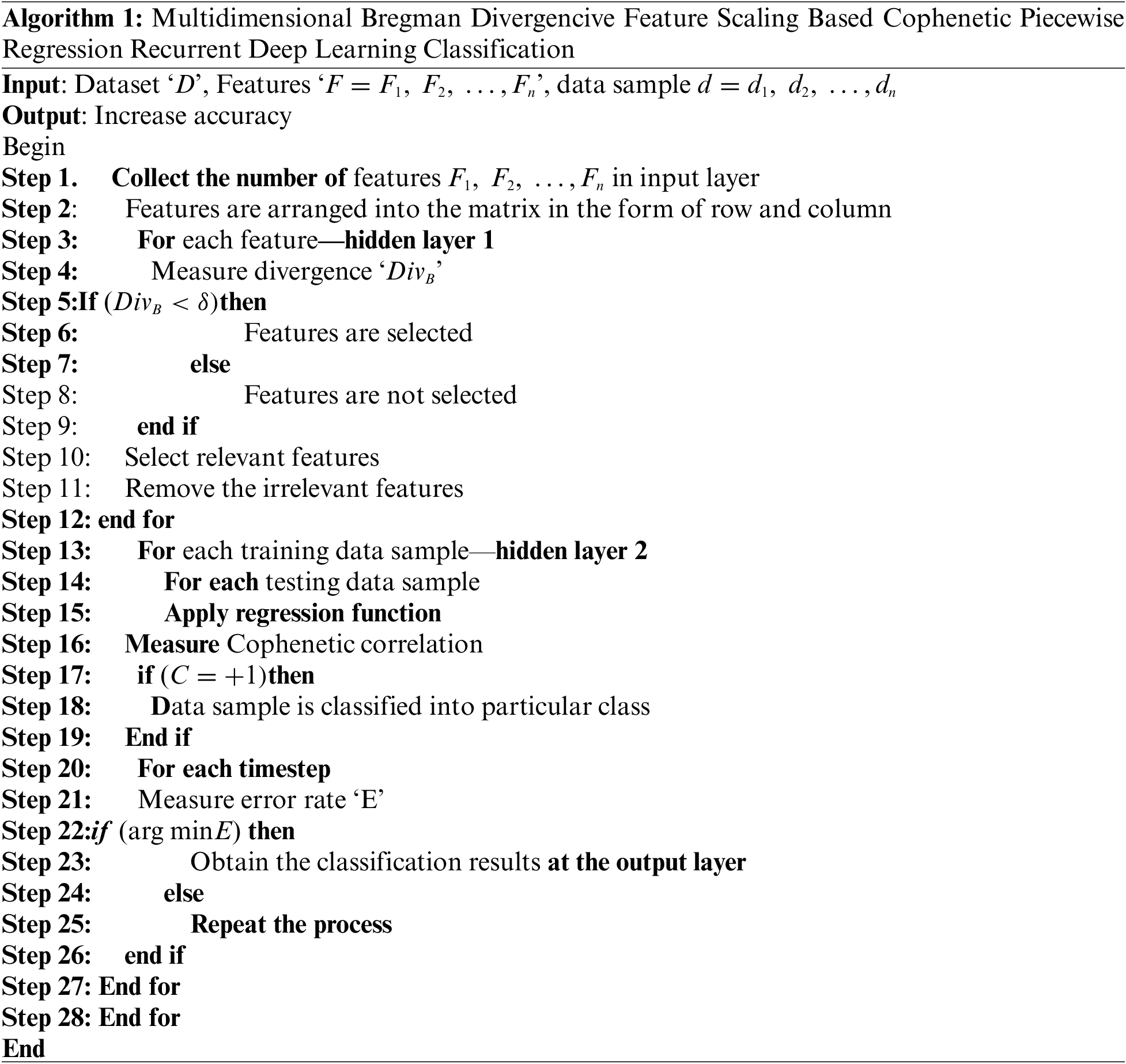
Algorithm 1 given above demonstrates the step-by-step process of skin cancer detection using MBDFS-CPRRDLC. First, the patent data samples are collected from the IoT. Then the Recurrent Deep Learning Classifier uses the different layers for cancer detection. First, the input layer receives the number of features. Then the Multidimensional Bregman Divergence Scaling is applied for finding the significant features in the first hidden layer of Recurrent Deep Learning Classifier. With the selected feature values, the classification is done at the second hidden layer by analyzing the testing and training data using cophenetic correlative piecewise regression. Based on regression analysis, accurate classification is performed and finds the different classes of skin cancer. For each timestep, the error rate is measured. If the error is minimal, then the process gets to stop. Otherwise, the process gets iterated until the minimum error is found. Finally, the classification outcomes were detected on the output layer. This in turn increases the accuracy of cancer prediction with minimum error.
Experimental evaluation of the proposed MBDFS-CPRRDLC technique and existing Automated Hyper-parameter Optimized CNN [1], Dermo-DOCTOR [2], DCNN [3] are implemented in Java using Skin Cancer MNIST: HAM10000 taken from the Kaggle (https://www.kaggle.com/kmader/skin-cancer-mnist-ham10000). Based on the objective of the proposed MBDFS-CPRRDLC technique i.e., focused on improving the skin cancer detection accuracy and detecting cancer at an earlier stage with minimum time the existing methods such as Automated Hyper-parameter Optimized CNN [1], Dermo-DOCTOR [2], and DCNN model [3] are taken as base paper. These two base papers are explained to understand the proposed method. The proposed method concept is derived by considering the problems of these base papers. The drawbacks of these methods are effectively convinced by implementing the proposed method. The Skin Cancer MNIST: HAM10000 dataset is used to accurately identify cancer detection. This dataset consists of two CSV files. The dataset consists of 10015 images of skin legions collected from IoT devices. From the images, different features are extracted for disease identification. There are different files are available corresponding to 28 × 28 colored images and which form a feature vector of 784*3 = 2352 and 8 × 8 colored images which form 192 features and lastly, 8 × 8 gray images or 64 features. The data samples are divided into 7 different classes of skin cancer and the description is shown in Tab. 1. Then, the dataset is selected to provide better performance for skin cancer detection.
5 Performance Results and Discussion
Simulation of MBDFS-CPRRDLC technique and existing Automated Hyper-parameter Optimized CNN [1], Dermo-DOCTOR [2], and DCNN [3] are discussed. Based on the objective of the proposed MBDFS-CPRRDLC technique, experimental parameters such as Accuracy, precision, recall, F-measure and cancer detection time are selected for analyzing the performance of the proposed and existing method. The performances of the proposed and existing methods were explained by the help of tables as well as graphical illustrations.
• Accuracy: It was referred by the number of samples were properly classified within various classes to the entire number of samples collected from the dataset. Therefore, it is expressed by,
• Precision: It is referred to proportion of the number of samples that were properly classified from an entire number of samples collected from the dataset. The precision was formulated by,
where
• Recall: It is measured by the proportion of the amount of samples that were properly classified to the entire amount of samples. Formula for calculating the recall is expressed as follows,
where
• F-measure: It is estimated based on the mean of precision as well as recall. The formula for calculating F-measure is expressed as given below,
where
• Detection time: It was measured by number of time taken with the algorithm for performing the cancer detection on classification. Detection time is measured as follows,
where
Tab. 2 portrays the accuracy by the number of samples taken in the ranges from 1000 to 10000. As shown in the table, the accuracy of four methods namely MBDFS-CPRRDLC and existing Automated Hyper-parameter Optimized CNN [1], Dermo-DOCTOR [2], DCNN [3] are reported. The results noted that the MBDFS-CPRRDLC provides superior results when compared to conventional methods. This is proved through the statistical measure. For example, with the number of 1000samples, the accuracy observed by applying the MBDFS-CPRRDLC technique is 93% and the accuracy was found to be 89%, 84%, 82% by applying existing Automated Hyper-parameter Optimized CNN [1], Dermo-DOCTOR [2], DCNN [3]. Subsequently, different performance results are observed for each method. MBDFS-CPRRDLC was compared with conventional methods. The comparison result demonstrates the proposed MBDFS-CPRRDLC technique enhances the accuracy by 6%, 9% and 11% when compared to [1,2] and [3] respectively. This is due to the application of Cophenetic Piecewise Regression Recurrent Deep Learning Classification. The cophenetic correlative piecewise regression is applied to the Recurrent Deep Learning technique for analyzing the testing and training disease data. Based on regression analysis, different levels of skin cancers such as akiec, bcc, bkl, df, nv, mel and vasc are identified with higher accuracy.
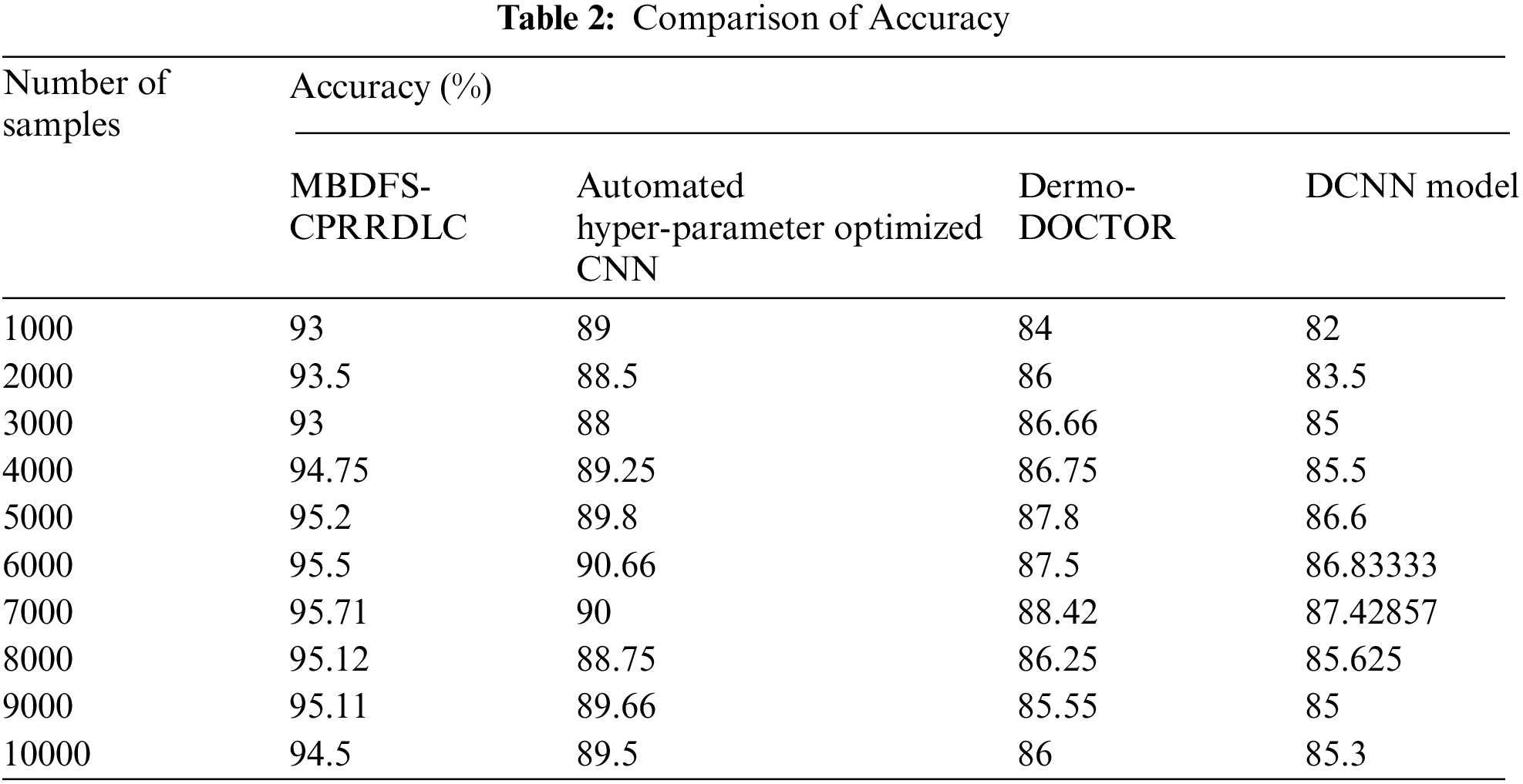
Fig. 4 illustrates the convergence diagram of precision vs. the number of samples taken from the dataset. The observed results indicate that the performance analysis of precision is increased by applying MBDFS-CPRRDLC when compared to conventional methods. This improvement of the proposed MBDFS-CPRRDLC is to apply the correlation-based regression is applied to recurrent deep learning classifier. The regression function analyzes the training and testing cancer data provides the higher true positive and minimizes the false positive. The overall results of precision are increased as 4%, 6% and 7% compared with [1,2] and [3] respectively.
Figure 4: Performance analysis of precision
Tab. 3 demonstrates the performance of recall analysis using four methods and the number of samples collected from the dataset. For each method, ten runs are observed and the equivalent results are reported as a graph. As shown in Tab. 3, the performance of recall is observed using MBDFS-CPRRDLC is comparatively higher when compared to conventional methods. For example, with 1000samples are taken for experimentation, the recall rate using the proposed MBDFS-CPRRDLC was found to be 96.62%. By applying Automated Hyper-parameter Optimized CNN [1], Dermo-DOCTOR [2], DCNN [3], the performance of recall 94.04%, 91.13% and 89.61% was observed. Similarly, different outcomes are observed for each method with respect to a number of samples. Finally, the obtained ten results indicate that the overall performance of recall is considerably improved by 3%, 5% and 6% using MBDFS-CPRRDLC compared with [1,2] and [3] respectively.
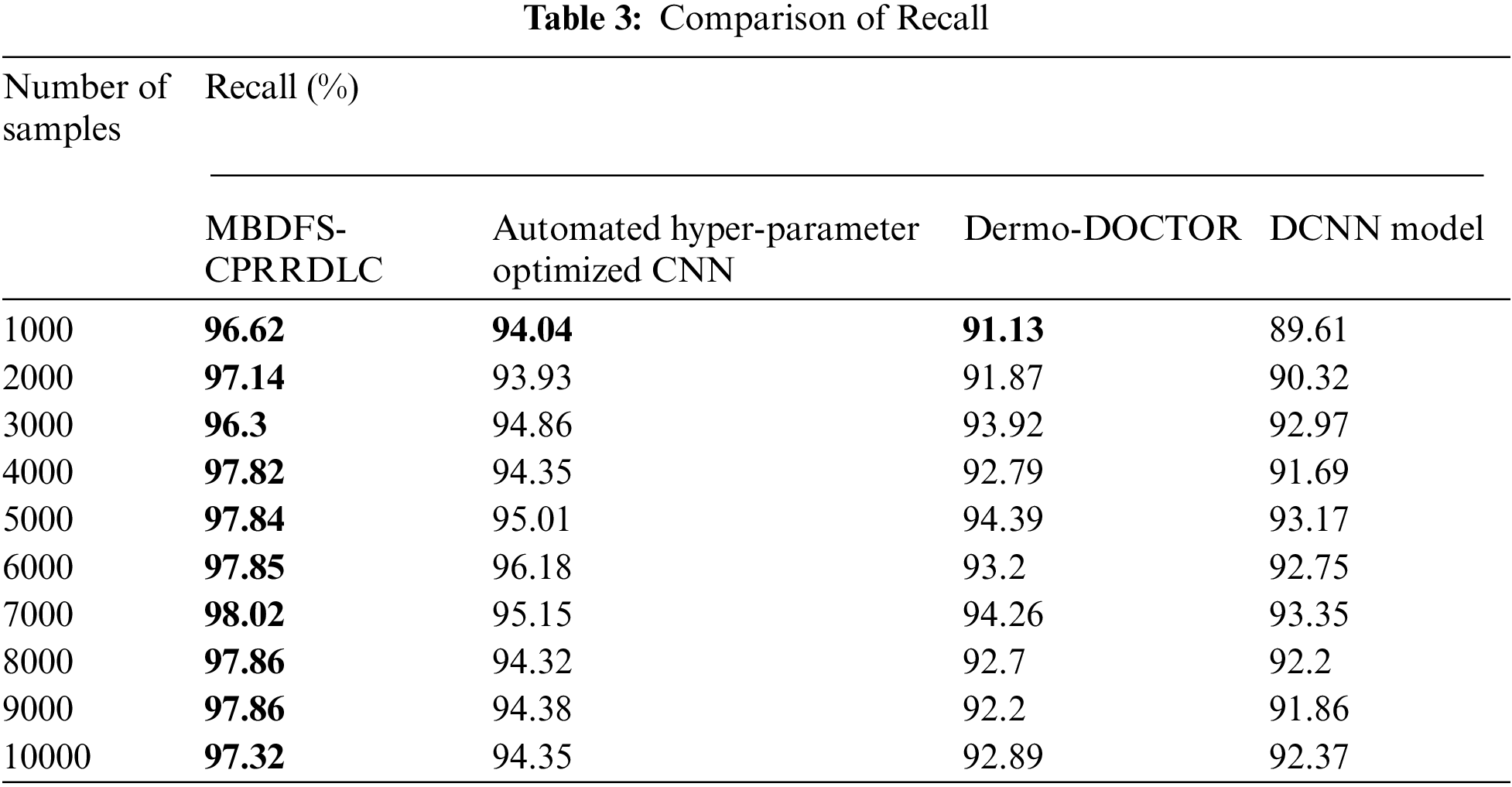
Fig. 5 given above illustrates the results of the F-measure against a number of samples taken from the dataset. The F-measure is calculated based on the performance of precision as well as recall. The observed results indicate that the MBDFS-CPRRDLC provides improved performance when compared to conventional methods. Finally, the MBDFS-CPRRDLC was compared with existing methods. F-measure is significantly improved as 3%, 5% and 6% compared with existing methods.
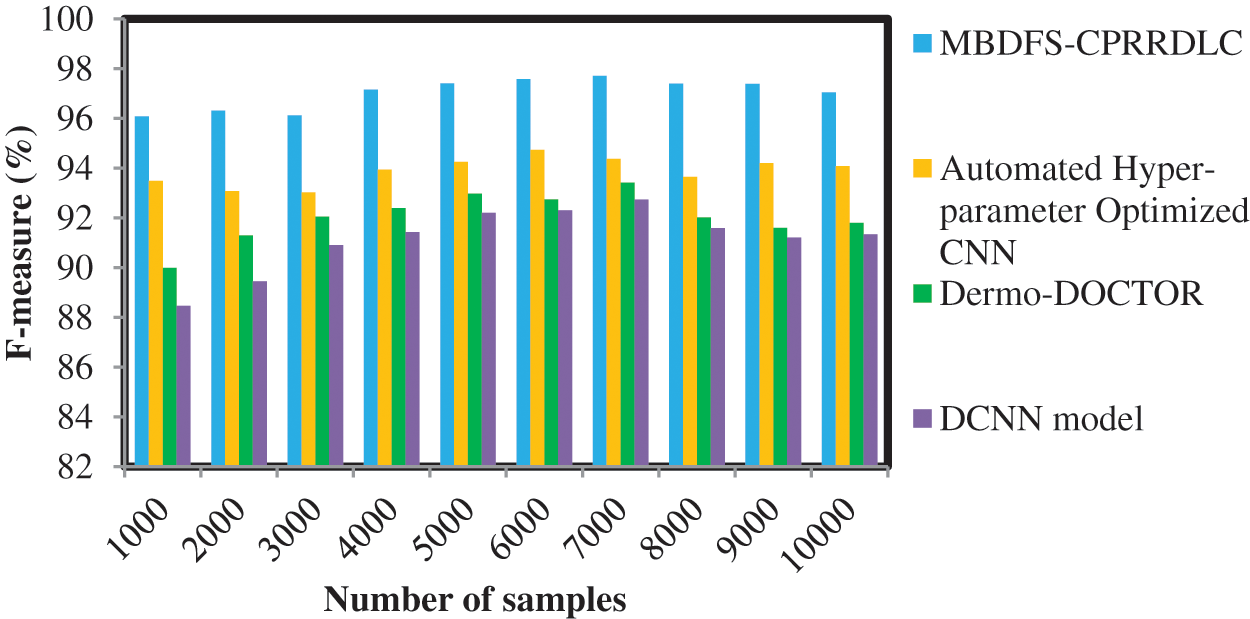
Figure 5: Performance analysis of F-measure
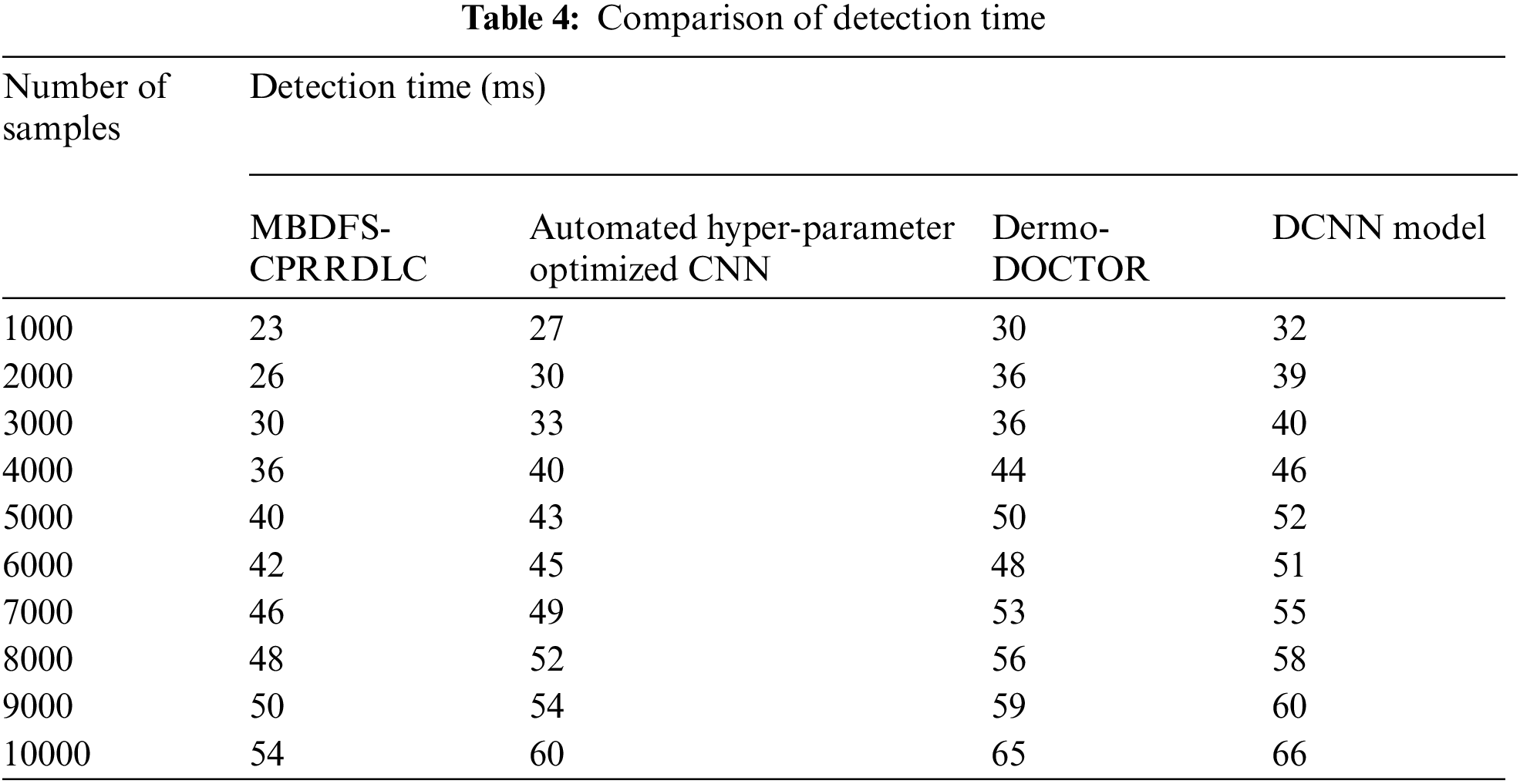
Tab. 4 illustrates the performance of cancer detection time versus the number of samples taken from the dataset. Here, the detection time is measured by number of time for detecting the different types of cancer. The cancer detection time is measured using three different techniques. Among three methods, the proposed MBDFS-CPRRDLC technique minimizes the cancer detection time when compared to conventional methods. Let us consider 1000samples for experimentation. The cancer detection time of the proposed MBDFS-CPRRDLC technique is
An efficient detection model called MBDFS-CPRRDLC for skin cancer is developed by integrating feature selection and classification to improve accuracy. First, the patient skin data are collected from the mobile cloud server. Then applying the Multidimensional Bregman Divergencive Scaling technique is applied to the recurrent deep learning classifier for minimizing the cancer detection time by selecting the significant features from the dataset. In addition, the relevant feature selection process of MBDFS-CPRRDLC minimizes the complexity of cancer detection. Followed by, Cophenetic Piecewise Regression is applied to the Recurrent Deep Learning classifier to find the accurate class of cancer detection by minimizing the error. This helps to improve the accuracy of cancer detection. A comprehensive experimental assessment is carried out with various parameters with respect to number of samples. The performance results in the discussion illustrate that the advantage of MBDFS-CPRRDLC technique achieves exactly identify the class of cancer detection, higher accuracy of cancer detection, precision, recall, F-measure, and perform relevant feature selection process with minimum time than the conventional methods. The results prove that the MBDFS-CPRRDLC technique attains better improvement of accuracy, and precision, recall, F-measure by 9%, 6%, 5%, and 5% and minimize the detection time by 16% as compared to another method. In future work, the proposed technique is further extended to obtain enhanced accuracy with higher precision and minimum detection time with aid of the data preprocessing stage by filling the missing value and removing the duplicate data for Skin Cancer Detection.
Acknowledgement: This research is supported by Princess Nourah Bint Abdulrahman University Researchers Supporting Project number (PNURSP2022R194), Princess Nourah Bint Abdulrahman University, Riyadh, Saudi Arabia for providing a research environment and platform to work.
Funding Statement: This research is funded by Princess Nourah Bint Abdulrahman University Researchers Supporting Project number (PNURSP2022R194), Princess Nourah Bint Abdulrahman University, Riyadh, Saudi Arabia.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. R. Mohakud and R. Dash, “Designing a grey wolf optimization-based hyper-parameter optimized convolutional neural network classifier for skin cancer detection,” Journal of King Saud University-Computer and Information Sciences, Elsevier, vol. 1, pp. 1–12, 2021. [Google Scholar]
2. Md. K. Hasan, S. Roy, C. Mondal, Md. A. Alam and Md. T. E. Elahi, “Dermo-doctor A framework for concurrent skin lesion detection and recognition using a deep convolutional neural network with end-to-end dual encoders,” Biomedical Signal Processing and Control, Elsevier, vol. 68, pp. 1–16, 2021. [Google Scholar]
3. M. S. Ali, M. S. Miah, J. Haque, M. M. Rahman and M. K. Islam, “An enhanced technique of skin cancer classification using deep convolutional neural network with transfer learning models,” Machine Learning with Applications, Elsevier, vol. 5, pp. 1–8, 2021. [Google Scholar]
4. K. Das, C. J. Cockerell, A. Patil, P. Pietkiewicz and M. Giulini, “Machine learning and its application in skin cancer,” International Journal of Environmental Research and Public Health, vol. 18, pp. 1–10, 2021. [Google Scholar]
5. A. Hekler, J. S. Utikal, A. H. Enk, A. Hauschild and M. Weichenthal, “Superior skin cancer classification by the combination of human and artificial intelligence,” European Journal of Cancer, Elsevier, vol. 20, pp. 114–121, 2019. [Google Scholar]
6. C. Ebuka Ojukwu, “Melanoma skin cancer detection using support vector machines and convolutional neural networks,” International Journal of Scientific Research in Computer Science and Engineering, vol. 9, no. 6, pp. 9–12, 2021. [Google Scholar]
7. A. Naeem, M. Shoaib Farooq, A. Khelifi and A. Abid, “Malignant melanoma classification using deep learning: Datasets, performance measurements, challenges and opportunities,” IEEE Access, vol. 8, pp. 110575–110597, 2020. [Google Scholar]
8. B. Al Bander, Q. M. Yas, H. Mahdi and R. Al Hamd, “Benchmarking of deep learning algorithms for skin cancer detection based on a hybrid framework of entropy and VIKOR techniques,” Turkish Journal of Electrical Engineering & Computer Sciences, vol. 29, pp. 2634–2648, 2021. [Google Scholar]
9. K. M. Hosny, M. A. Kassem and M. M. Foaud, “Classification of skin lesions using transfer learning and augmentation with alex-net,” PLoS One, vol. 14, no. 5, pp. 1–17, 2019. [Google Scholar]
10. M. Ali Kadampur and S. Al Riyaee, “Skin cancer detection: Applying a deep learning based model driven architecture in the cloud for classifying dermal cell images,” Informatics in Medicine Unlocked, Elsevier, vol. 18, pp. 1–6, 2020. [Google Scholar]
11. A. Kwasigroch, M. Grotowski and A. Mikołajczyk, “Neural architecture search for skin lesion classification,” IEEE Access, vol. 8, pp. 9061–9071, 2020. [Google Scholar]
12. A. Khamparia, P. K. Singh, P. Rani, D. Samanta and A. Khanna, “An internet of health things-driven deep learning framework for detection and classification of skin cancer using transfer learning,” Transaction on Emerging Telecommunication Technology, Wiley, vol. 32, no. 7, pp. 1–11, 2021. [Google Scholar]
13. F. Afza, M. Sharif, M. Attique Khan, U. Tariq and H. Yong, “Multiclass skin lesion classification using hybrid deep features selection and extreme learning machine,” Sensors, vol. 22, pp. 1–22, 2022. [Google Scholar]
14. S. Juyal, S. Sharma and A. Shankar Shukla, “Smart skin health monitoring using AI-enabled cloud-based IoT,” Materials Today: Proceedings, Elsevier, vol. 46, no. 20, pp. 10539–10545, 2021. [Google Scholar]
15. M. Chen, P. Zhou, D. Wu, L. Hu and M. M. Hassan, “AI-Skin: Skin disease recognition based on self-learning and wide data collection through a closed-loop framework,” Information Fusion, Elsevier, vol. 54, pp. 1–9, 2020. [Google Scholar]
16. S. Zhang, S. Huang, H. Wu, Z. Yang and Y. Chen, “Intelligent data analytics for diagnosing melanoma skin lesions via deep learning in IoT system,” Mobile Information Systems, Hindawi, vol. 2021, pp. 1–12, 2021. [Google Scholar]
17. J. Najeeb Saeed and S. R. M. Zeebaree, “Skin lesion classification based on deep convolutional neural networks architectures,” Journal of Applied Science and Technology Trends, vol. 2, no. 1, pp. 41–51, 2021. [Google Scholar]
18. D. d. A. Rodrigues, R. F. Ivo, S. C. Satapathy, S. Wang and J. Hemanth, “A new approach for classification skin lesion based on transfer learning, deep learning, and IoT system,” Pattern Recognition Letters, Elsevier, vol. 136, pp. 8–15, 2020. [Google Scholar]
19. E. Pérez and S. Ventura, “An ensemble-based convolutional neural network model powered by a genetic algorithm for melanoma diagnosis,” in Neural Computing and Applications, Springer, vol. 1, pp. 1–22, 2021. [Google Scholar]
20. N. M. S. Kumar, K. Hariprasath, S. Tamilselvi, A. Kavinya and N. Kaviyavarshini, “Detection of stages of melanoma using deep learning,” in Multimedia Tools and Applications, Springer, vol. 80, pp. 18677–18692, 2021. [Google Scholar]
21. R. Malladi, P. Vempaty, G. Raju and V. Pogaku, “Advanced machine learning based approach for prediction of skin cancer,” Materials Today: Proceedings, Elsevier, vol. 1, pp. 1–6, 2021. [Google Scholar]
22. X. R. Zhang, J. Zhou, W. Sun and S. K. Jha, “A lightweight CNN based on transfer learning for COVID-19 diagnosis,” Computers, Materials & Continua, vol. 72, no. 1, pp. 1123–1137, 2022. [Google Scholar]
23. J. He, C. Wang, H. Wu, L. Yan and C. Lu, “Multi-label Chinese comments categorization: Comparison of multi-label learning algorithms,” Journal of New Media, vol. 1, no. 2, pp. 51–61, 2019. [Google Scholar]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |