

 | Energy Engineering |  |
DOI: 10.32604/EE.2021.014870
ARTICLE
Emission Behaviors of Submicron Particles (PM1) Generated by the Combustion of Sesame Stalk after Combined Water Washing and Carbonization Pretreatment
1State Key Laboratory of Coal Combustion, School of Energy and Power Engineering, Huazhong University of Science and Technology, Wuhan, 430074, China
2Department of New Energy Science and Engineering, School of Energy and Power Engineering, Huazhong University of Science and Technology, Wuhan, 430074, China
3China-EU Institute for Clean and Renewable Energy, Huazhong University of Science and Technology, Wuhan, 430074, China
4State Grid Jiangxi Electric Power Science Research Institute, Nanchang, 330096, China
*Corresponding Author: Chang Wen. Email: wenchang@hust.edu.cn
Received: 04 November 2020; Accepted: 16 December 2020
Abstract: Pretreatment before biomass combustion is significant for its efficient utilization and that combined water washing and carbonization can be efficient. An agricultural processing residues sesame stalk was selected and carried out two pretreatments separately, i.e., water washing-torrefaction (W-T) and torrefaction-water washing (T-W), to explore the effect on the fuel properties, combustion characteristics and particulate matter (PM) emission. The obtained biochar was also combusted under air and oxy50 (CO2:O2 = 50:50) conditions for the sake of investigating the effect of pretreatment and combustion atmosphere. The results indicate that, W-T and T-W both not only have great effect on the improvement of fuel properties but also reduce the content of water-soluble elements like K, Cl, etc. Due to the difference in hydrophobicity, the biochar obtained by W-T have the optimal fuel properties. At the same time, the pretreatment also hinder the combustion in a certain extent in which the comprehensive combustion characteristics (SN) show a downward trend. Furthermore, both two pretreatments have obvious benefit on the reduction of PM1 emission and W-T have the best effect related to the higher removal efficiency of inorganic elements (especially K + Na + Cl + S). Under oxy50 condition, the oxygen concentration and combustion temperature is higher, improving the sulfation of K and vaporization of Ca, P and Mg which result in weakening in the pretreatment reduction effect on PM1 emission.
Keywords: Biomass; torrefaction; water washing; oxy-fuel; PM1
As the clean and renewable energy, the utilization of biomass has aroused extensive attention in which combustion power generation become a research focus [1]. China is a traditional agricultural country, the annual harvestable resources for crops of China are about 690 million tons and agricultural product processing residues are about 120 million tons in which 60 million tons are available for energy utilization per year [2]. Sesame is one of the main oil crops in China and its seed oil content is as high as 55% with high application value. Sesame stalk, as its processing residue, has a large yield and potential for combustion. While some inherent defects of biomass like low calorific value, high moisture content, poor hydrophobicity and low grindability obstruct its widespread combustion utilization [3]. Moreover, the ash-related problems during biomass combustion such as fouling, slagging, corrosion and the emission of particulate matter (PM) are serious because of the high content of alkali and alkaline earth metals (AAEMs) in biomass [4]. In order to alleviate the above-mentioned ash-related problems and make it a better fuel for combustion, thermochemical pretreatment before combustion can be useful.
Torrefaction, one of the typical thermochemical pretreatment which can significantly improve the energy density of raw biomass, is a mild pyrolysis process under low temperature (200–300°C) and inert atmosphere [5]. In this process, the moisture and volatiles in the biomass released successively and biochar formed finally. Biochar has higher calorific value compared with raw biomass due to the loss of hydroxyl groups and the disappearance of fiber structure, it has better hydrophobicity and grindability [1]. However, considering that biomass volatiles released in process lead to mass reduction while the ash content remains the same, the relative ash content in biochar increased and the damage of AAEMs still exists. Therefore, the ash-related problems remains serious especially the emission of PM10 [6,7]. Some scholars have studied the emission characteristics of PM10 during biochar combustion. Yani et al. [8] found that the combustion of the torrefied mallee leaf leads to considerably higher yields of PM10 than that of the raw biomass. Hu et al. [9] found that the combustion of biomass pretreated at 250–500°C presents 1.5–2 times of PM1 emissions than raw biomass, which alerts that biomass upgrading at moderate temperatures result in aggravated fine particle emissions.
Introducing water washing seems to be a feasible solution. The combined two-stage pretreatment of carbonization and water washing can simultaneously combine the advantages of demineralization and energy density enhancement, improving the calorific value of biomass and alleviating severe PM10 emission [10]. According to the sequence, the combined pretreatment of torrefaction and water washing can be divided into two types: water washing after torrefaction (T-W) and torrefaction after water washing (W-T). Relevant researches have also been carried out on the two types of pretreatment. Abelha et al. [11] found that up to 90% of Cl and 60–80% of K can be removed by water washing and then torrefaction, fine PM formation was also strongly reduced. Zhang et al. [12] investigated the influence of water washing-torrefaction pretreatments on microwave pyrolysis of rice husk. The results indicated that the process of W-T could effectively remove a large portion of inorganics and improve the fuel characteristics to a certain extent. For water washing after torrefaction (T-W), Liu et al. [13] assessed the technical feasibility and concluded that washing treatment significantly improved fuel qualities of pyrolytic biochars and more than 72% of AAEMs were removed. Wang et al. [14] studied the effect of torrefaction-water washing on PM10 emissions during the combustion of straw. The results showed that combined pretreatment effectively removes inorganic elements (Cl, K, P), thereby reducing the emission of PM10. The above researches show that combined pretreatment of torrefaction and water washing can effectively improve the combustion characteristics of the raw biomass and remove some inorganic elements, which theoretically alleviating the ash-related problems during biomass combustion. However the existing researches have only conducted separate studies on torrefaction and water washing pretreatment. There is no study to compare the influence of the sequence of these two pretreatments on biomass combustion and ash-related problems, especially the influence of submicron particles (PM1) emissions.
On the other hand, oxy-fuel combustion is one of the promising combustion technology to reduce CO2 emissions, which is considerable to be commercially viable and economically competitive [15]. Biomass energy can achieve “CO2 negative emission” under oxy-fuel conditions due to its own carbon neutral characteristics. Predecessors have conducted relevant studies on PM10 emissions from biomass combustion under oxy-fuel conditions. Ruscio et al. [16] studied the characterization of particulate matter emitted from combustion of three pulverized biomass residue under air/oxy-fuel atmosphere and concluded that the submicrometer ash particle yields were lower in oxy-fuel conditions than air. Wang et al. [17] combust the pulverized rice husks in a 100 kW down-fired oxy-fuel combustor with natural gas and find that there are more sub-micrometer aerosols generated in the high flame temperature case. The above works indicated that the emission characteristics of PM10 during biomass combustion under oxy-fuel conditions are very different from those under air conditions. And previous works mostly focused on the raw biomass combustion but there are few studies focused on the biochar, especially the biochar produced by the combined pretreatment of torrefaction and water washing.
In view of above reasons, sesame stalk, an agricultural processing residues, was selected and carried out two pretreatments of water washing-torrefaction (W-T) and torrefaction-water washing (T-W) separately. The fuel properties, combustion characteristics, and submicron particles (PM1) emission during biochar combustion are explored. At the same time, the obtained biochar was combusted under air and oxy-fuel conditions for the sake of exploring the effects of pretreatment sequence and combustion atmosphere conditions to evaluate the potential of practical application.
The studied sesame stalk was collected from Henan Province, China. The samples were broken and sieved to less than 500 μm. The combined torrefaction and water washing pretreatment can divided into two steps of torrefaction and water washing. The sample was stirred in a beaker for washing with solid-liquid ration of 1:40 (wt:wt) and the duration time of 2 h. Torrefaction conditions were chosen for receiving the optimal energy yield. The preparation temperature was 270–280°C and the raw biomass was placed in an electric fixed bed reactor with an inert atmosphere of N2 (3 L/min) and a heating rate of 10 °C/min, followed by holding for 30 min. The obtained biochars were named as “SS W-T” for water washing-torrefaction pretreatment and “SS T-W” for torrefaction-water washing pretreatment, respectively.
The combustion characteristics of upgraded samples were compared using thermogravimetric analysis (TGA, Labsys Evo 115, France). Experiments were carried out with a simulated air at a flow rate of 50 ml/min. The samples were heated at a rate of 20 °C/min to the final temperature at which the fuel mass no longer decreased (approximately 800°C). The samples were also combusted in a high-temperature drop-tube furnace (DTF) at 1400°C. The reaction tube in the furnace has a constant temperature section of about 500 mm with the details described in previous study [18,19]. This study considered two types of combustion atmosphere: Air and oxy50 (CO2:O2 = 50:50). All samples were fed at a rate of about 0.1 g/min and then entrained with air at a flow rate of 10 L/min, with a residence time of less than 1.5 s which is sufficient for complete combustion proved by the reported method [20]. The generated PM was collected using a 13 stages Dekati low pressure impactor (DLPI) and the first seven stages belong to submicron particles (PM1). The entire sampling system was maintained at 130°C to avoid any acid gas condensation during sampling.
2.3 Sample Analysis and Characterization
Proximate analysis for biomass and biochar was based on NY/T 1881-2010 standard. Ultimate analysis was measured using an Elementary Vario Micro Cube. The ash composition of samples was detected using EDAX EAGLE III X-ray fluorescence (XRF). The lower heating value (LHV) was measured and calculated from the bomb calorimetric method. As for the contents of inorganic elements (such as K, Ca, Mg and P) in samples and the distribution of their forms of occurrence, the samples were dissolved in the solution by various solvents (such as H2O, NH4Ac, HCl and HF-H2O2-HNO3) and then quantitatively analysed by an inductively coupled plasma optical emission spectrometer (ICP-OES, Prodigy Plus, LEEMAN LABS). The quantification of Cl was conducted by referring to GB/T 3558-2014 of China. The PM1 sample was collected through the aluminium film and polycarbonate membrane and then weighed using a microbalance with accuracy of 0.001 mg (Sartorius M2P) to require the particle size distributions (PSDs) and yields of PM1. Then its inorganic composition was analyzed through XRF.
3.1 Fuel Properties of the Materials
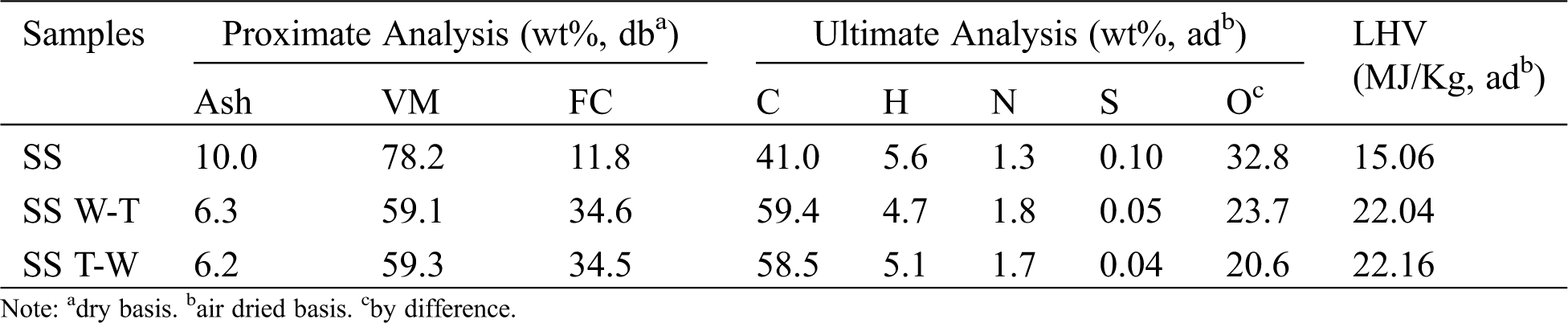
As shown in Tab. 1, the combined pretreatment of torrefaction and water washing improved the fuel characteristics significantly. The ash content of both two samples were reduced visibly, which is predicted to effectively alleviate a series of ash-related problems. At the same time, the volatile content showed a downward trend, while the fixed carbon content increased which is due to the decomposition of a large amount of hemicellulose and partial depolymerisation of cellulose and lignin in process of torrefaction pretreatment [21]. And during the decarboxylation and dehydration reaction, the increase of C content and reduction of O and H content lead to a higher LHV because the energy of C-C bond is higher than that of C-H and C-O bond [22]. For the two pretreatments, the LHV was 46% higher than that of the raw biomass, which is close to lignite and has the potential of independent combustion.
Table 1: Proximate analysis and ultimate analysis of sesame stalk and biochar
A Van Krevelen diagram is adopted to more intuitively express the change in H/C and O/C ratios of samples. As shown in Fig. 1, both two biochars have lower H/C and O/C ratios compared with raw one, moving towards the lower left corner of the figure and their fuel properties are more similar to lignite. Compare the effects of these two pretreatments, it can be found two biochars have similar H/C and O/C ratios which means two pretreatments have equivalent improvement to the biomass properties. That is because the change in the fuel properties compared with raw biomass was mainly caused by the torrefaction process and water washing process mainly play a role in the change of inorganic composition. The changes in the inorganic components of the samples were further explained.
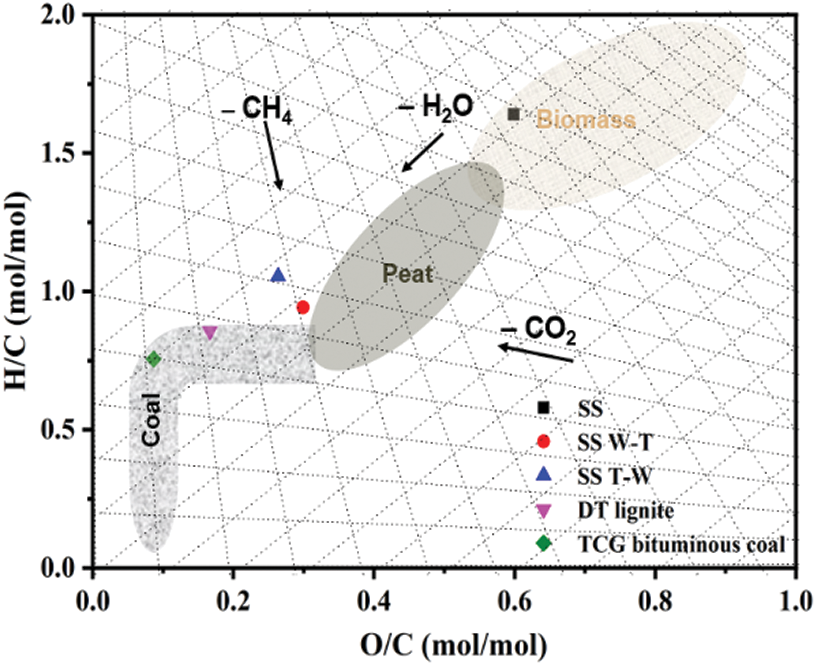
Figure 1: H/C and O/C molar ratios of sesame stalk and biochar in the Van Krevelen diagram
According to the ash composition analysis in Tab. 2, sesame stalk ash is mainly composed of K and Ca. After pretreatment, the content of K decrease significantly, while Ca increased a lot. Among them, the SS W-T sample has the lowest K but highest Ca content. The specific contents of inorganic elements in samples are shown in Fig. 2 and it can be found that in the raw sesame stalk, most of K exists in the forms of water-soluble state, while Ca mainly exists in the form of ion-exchangeable state. Due to the introduction of water washing, a large amount of water-soluble K was removed and the content was significantly reduced. Compared two pretreatments, water washing-torrefaction had a more efficient removal effect on K, mainly because the hydrophobicity of biochar was better than that of raw biomass. Therefore, the removal efficiency of inorganic elements on biochar after water washing was worse than that of biomass, leading to a better effect of water washing-torrefaction pretreatment than torrefaction-water washing. For the Ca with low water solubility, the enrichment of torrefaction was greater than the removal of water washing which causing the increased content. Similarly, due to the water washing effect, the content of Cl after pretreatment also decreased slightly.
Table 2: Chemical composition of sesame stalk and biochar ash (wt%)
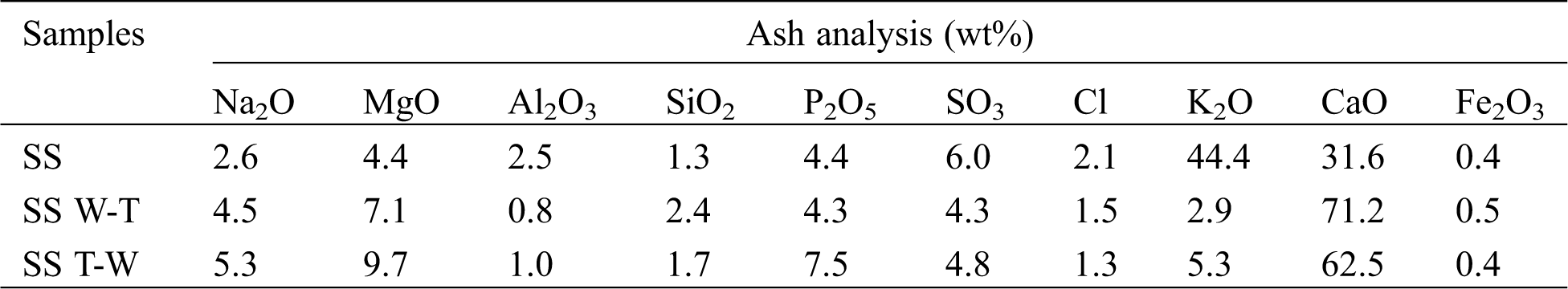
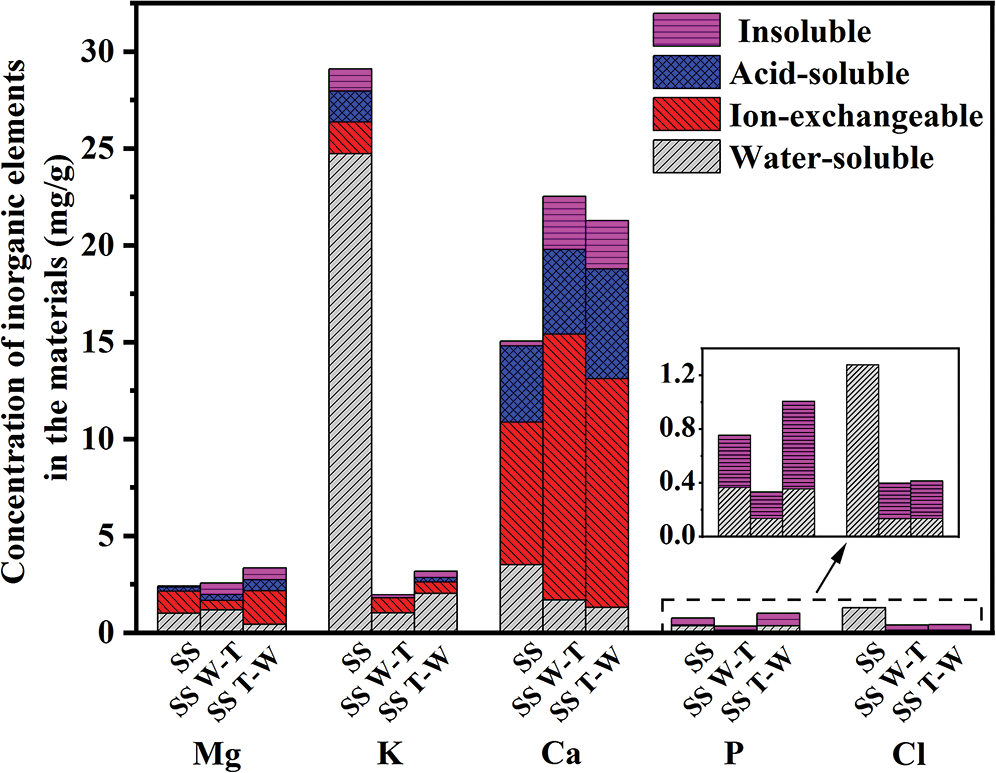
Figure 2: Concentration of inorganic elements in samples (including the contents of water-soluble, ion-exchangeable, and acid-soluble Mg, K, and Ca and only the contents of water-soluble and water-insoluble P and Cl)
3.2 Combustion Characteristics and Kinetic Analysis
The combustion characteristics of the samples were studied by TGA, with Fig. 3 presenting the DTG curves. As seen from the DTG graph, the weight loss curves of all the samples present bimodal distribution, corresponding to the release of volatiles and the combustion of fixed carbon. After pretreatment, the peak positions of the two peaks migrate to a higher temperature, and the intensity of the first peak weakens. This is mainly because volatile content of the sample reduced after W-T and T-W pretreatment, and the weight loss rate at this stage is weakened.
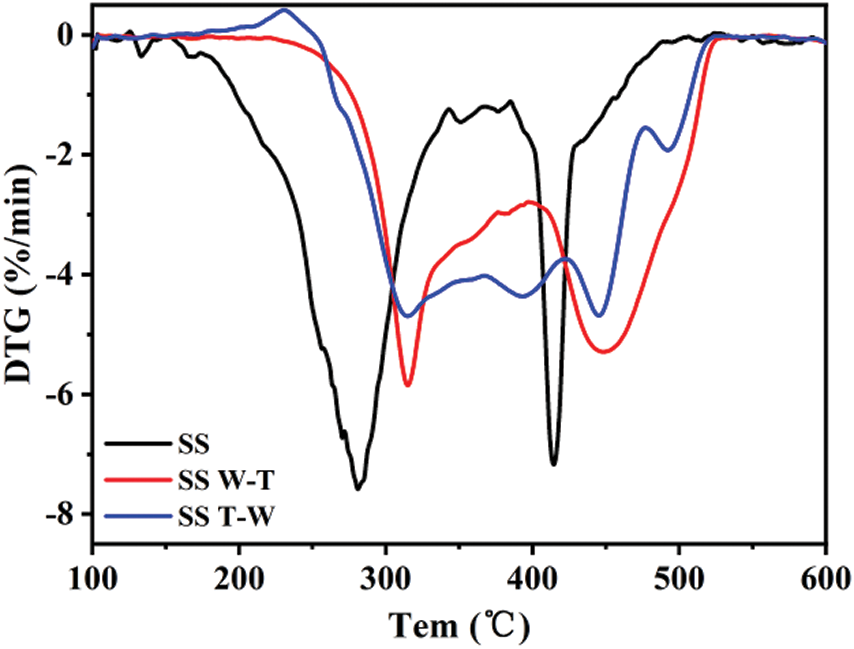
Figure 3: Differential thermogravimetric (DTG) curves of sesame stalk and biochar
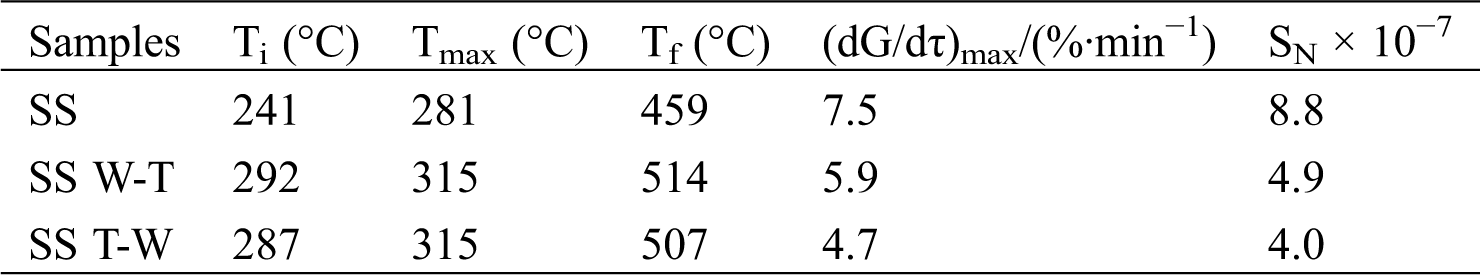
The following ignition temperature Ti, maximum weight loss peak temperature Tmax, burnout temperature Tf and comprehensive combustion characteristic index SN are used to evaluate the combustion characteristics of the samples. The definitions of various parameters were described in the literature [23]. The specific data are shown in Tab. 3. It can be seen that the maximum weight loss peak temperature Tmax of sesame stalk is 291°C which is the lowest, corresponding to the peak of the volatile release. This can be rationalized by the fact that biomass combusted mainly in the form of volatiles, so the biomass will be ignited quickly at a lower temperature then reaches the maximum weight loss rate during this combustion process [24]. After the W-T and T-W pretreatment, the ignition temperature Ti and burnout temperature Tf both increased which is due to the reduction of highly reactive substance after carbonization, resulting in an increase in the temperature required for combustion. And the maximum weight loss peak temperature Tmax also increased due to the release of the volatiles during the pretreatment process. At the same time, the maximum weight loss rate decreased after pretreatment which can be attributed to the lower volatile content. Considering the comprehensive combustion characteristic index SN, it presents a downward trend for both SS W-T and SS T-W and this can be explained by the above lower volatile content after pretreatment delay the combustion and the reduction of highly reactive substance after carbonization reduce the intensity of combustion. Compared with SS T-W, the SS W-T has higher maximum weight loss rate and comprehensive combustion characteristic index and this is because the hydrophobicity of the raw biomass is worse than biochar. After water washing, the raw biomass has a better ash removal efficiency, less impact on subsequent combustion and higher combustion rate leading to a better combustion characteristics.
Table 3: Combustion characteristics parameters of sesame stalk and biochar
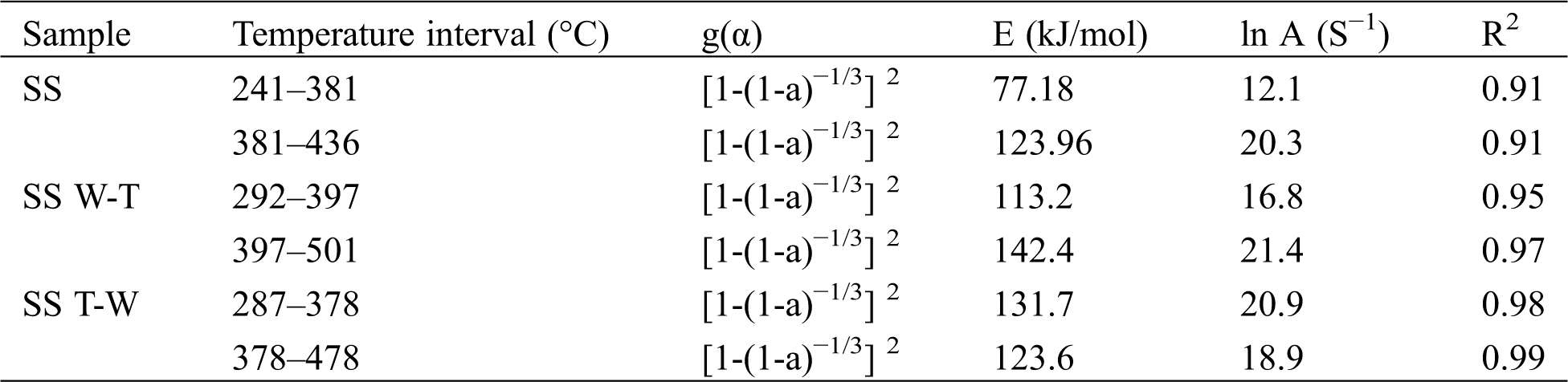
According to the Coats-Redfern integral method, dynamic fitting was carried out for the main reaction stage of the samples, model functions with good linearity in common gas-solid reaction mechanism functions were selected to describe the combustion reaction process, and the apparent activation energy E and pre-exponential factor A of the corresponding stage were obtained [25], as shown in Tab. 4. The correlation coefficients R2 of linear fitting by this method are all above 0.9, indicating that the above kinetic model can be used to study combustion dynamics. According to the DTG curve in Fig. 3, there are two main combustion stages in sesame stalk and thus the kinetic integral function fitting interval of the combustion process is also two-stage. The E values in the first stage, namely the volatile release stage, are 77.18 kJ/mol for sesame stalk, while in the second stage, namely the fixed carbon combustion stage, are 123.96 kJ/mol. Compared with the fixed carbon combustion stage, the E value of the volatile release stage is smaller, which may be determined by the higher volatiles content in biomass and the lower fixed carbon content. The larger value of E indicates that the energy barrier required for the reaction is higher and the reaction is harder to proceed. After pretreatment, the activation energy during the first combustion stage of SS W-T and SS T-W both increased, especially the SS T-W. That is due to the release of the volatiles and decomposition of active components during pretreatment process. For the second combustion stage, the activation energy of SS W-T increased significantly while that of SS T-W remained almost the same, which can be attributed to the pretreatment sequence of water washing and torrefaction and different hydrophobicity of the raw biomass and biochar. Based on comprehensive consideration, it can be found that after both two pretreatments the combustion characteristics of biochar is relatively worse than the raw sesame stalk which is due to the release of the volatiles.
Table 4: Combustion reaction kinetic parameters of sesame stalk and biochar
3.3 PSDs, Yields, and Inorganic Compositions of Submicron Particles
Fig. 4 is the particle size distributions (PSDs) and yields of submicron particles (PM1) generated by combustion in DTF under air/oxy50 atmosphere respectively and the results are normalized to the same fuel input. Under air combustion condition, the PM1 curve of sesame stalk presents a unimodal distribution, and the peak of fine mode submicron particles appears near 0.2 μm. After pretreatment, the fine modal peak value drops, and the peak position of it moves toward a finer particle size, appearing near 0.07 μm. Under oxy50 combustion condition, compared with the air atmosphere, the peak value of the fine mode submicron particles of all samples both increased, and the peak position moves to a larger particle size, appearing near 0.3 μm for SS and SS T-W and 0.2 μm for SS W-T. From PM1 yields of Fig. 4, under air atmosphere, the PM1 yield of sesame was 2.06 mg/g. After pretreatment, the PM1 yield of SS W-T and SS T-W was reduced to 0.33 mg/g and 0.54 mg/g which reduced by 84% and 74% respectively, showing a good emission reduction effect. This can be interpreted as the removal of water-solute elements such as Na, K and sequence of pretreatment which affects the washing efficiency. Under oxy50 atmosphere, the PM1 yield of sesame stalk is 2.69 mg/g which was increased compared with air combustion condition. After pretreatment, the PM1 yields decreased by 52% and 38% for SS W-T and SS T-W respectively in which the effect of emission reduction has declined compared with air atmosphere due to the higher oxygen concentration and combustion temperature under oxy50 conditions. The following inorganic components and particle size distributions of main elements diagram were used to further discuss the reduction mechanism of PM1.
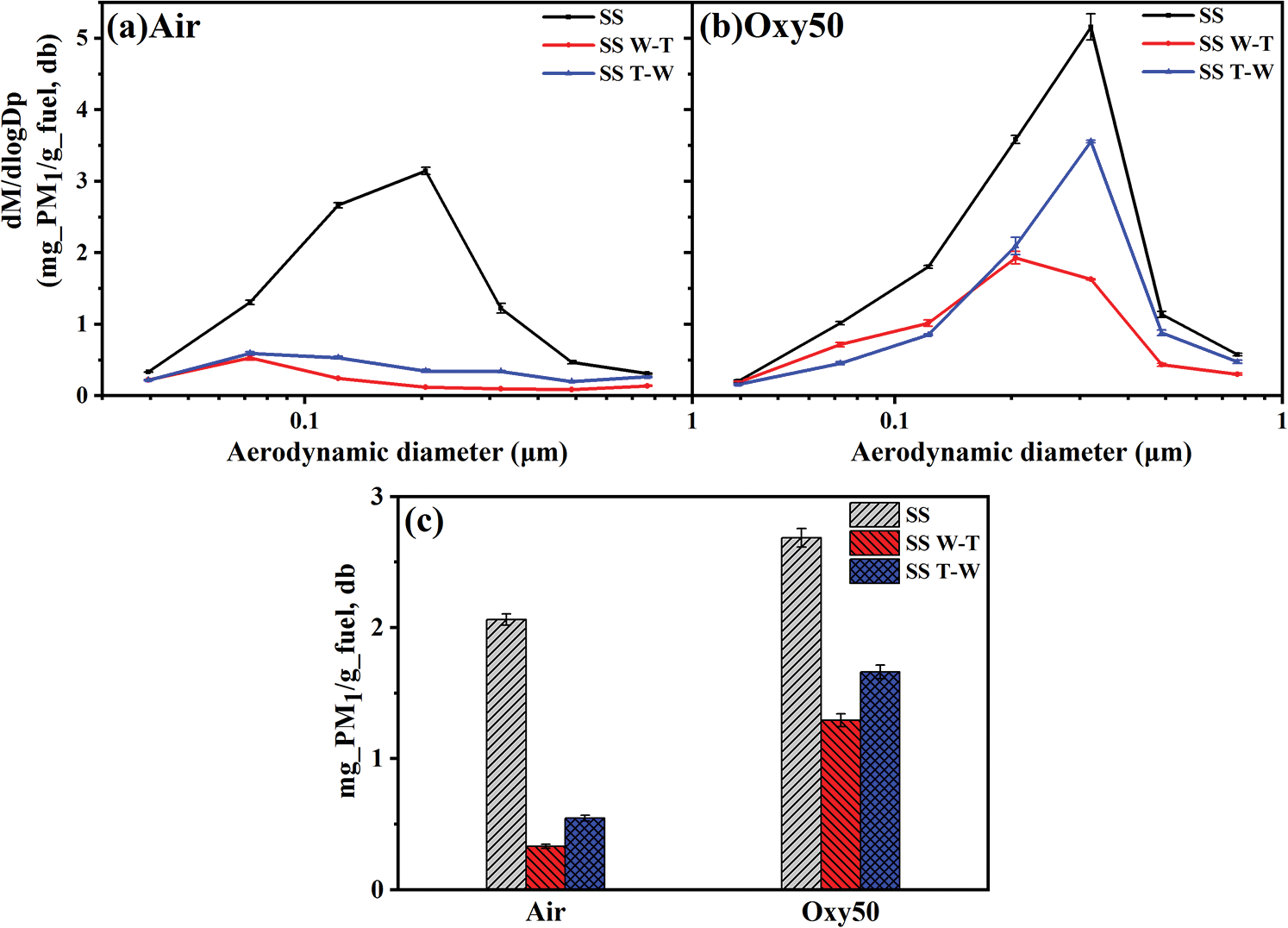
Figure 4: (a, b) Mass-based particle size distributions and (c) Yields of submicron particles generated from combustion of sesame stalk and biochar under air and oxy50 conditions
Fig. 5 presents the inorganic components of submicron particles (PM1). The PM1 generated by sesame stalk combustion under air atmosphere was mainly composed of K, Cl and S. After combined water washing and torrefaction pretreatment, the inorganic composition of PM1 changed greatly, in which the proportion of K and Cl was significantly reduced and Ca increased markedly. It was reported that alkali metals mainly released into the gas phase during the formation of alkali chlorides in the presence of Cl. Most of them are the precursor of fine mode particles owing to their chemical stability [26,27]. Due to the release of Cl and K during pretreatment, the amount of KCl released into gas phase during combustion decreased, resulting in weakening of homogeneous and heterogeneous condensation and therefore the release of PM1 reduced [28]. Under oxy50 atmosphere, compared with air, the proportion of K in PM1 increased, while the proportion of Cl showed a downward trend. After pretreatment, the proportion of K in PM1 decreased, while Ca, Mg and P increased significantly. Attributed to the higher oxygen concentration and combustion temperature of oxy50, the formation of sulfate and the gasification of Ca, P and Mg are promoted, resulting in higher PM1 emission than air atmosphere. In addition, as a properties of itself, it is worth noting that the content of Ca in sesame stalk is high which will contribute to the formation of coarse particles due to the self-sintering of CaO. So the content change of Ca caused by pretreatment can be predicted to have a great impact on the emission of PM1-10 although this is not the focus of this study.
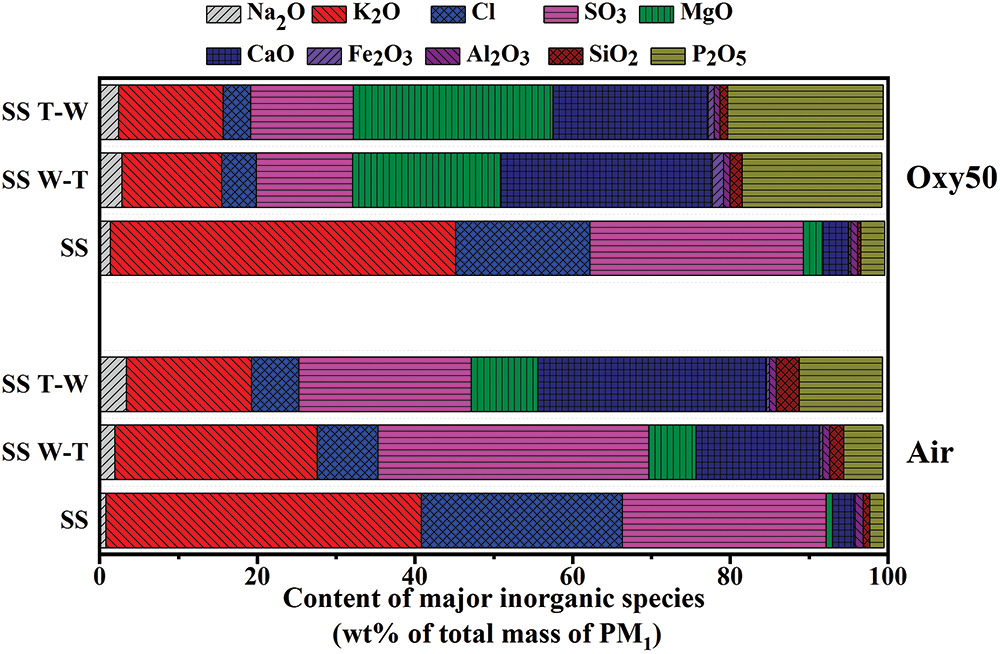
Figure 5: Inorganic compositions of submicron particles generated from combustion of sesame stalk and biochar under air and oxy50 conditions
In order to determine the influence of pretreatment and atmosphere on the emission of PM1, the mass particle size diagram of the main elements is shown in Fig. 6. Firstly, the influence of water washing and torrefaction pretreatment was investigated. It can be seen from the figure that the K, Cl and S content of the biochar is significantly reduced after pretreatments. Previous research has shown that in most combustion process of biomass, PM1 is mainly composed of chloride/sulfate. Na and K as easy evaporated elements, contribute to the formation of PM1 [29]. For example, K mainly evaporates in the form of KCl/KOH during biomass combustion and after subsequent sulfation and capture by coarse particles, part of KCl/K2SO4 forms most of PM1 through homogeneous nucleation and heterogeneous condensation [30]. Therefore the removal of K, Cl and S during pretreatment reduced the content of chloride and sulfate produced during combustion which decreased the PM1 formed by the mutual nucleation and heterogeneous condensation of chloride (mainly KCl) and sulfate [31], finally leading to the reduction of PM1 emission of the obtained biochar. The two pretreatments have different emission reduction effects on PM1, and the reason is that the hydrophobicity of biochar is stronger than that of biomass. Therefore, the removal efficiency on inorganic elements (especially K + Na + Cl + S) of W-T is significantly higher than that of T-W pretreatment, leading to a better reduction effect on PM1 emissions. This is similar to the conclusion from our previous research on rice straw [14]. In our previous research, we found that the content of Na2O + K2O + Cl + SO3 in PM1 has liner relationship with the content of Cl + S in the samples which means the removal of Cl and S by pretreatment can effectively reduce the emission of submicron particles.
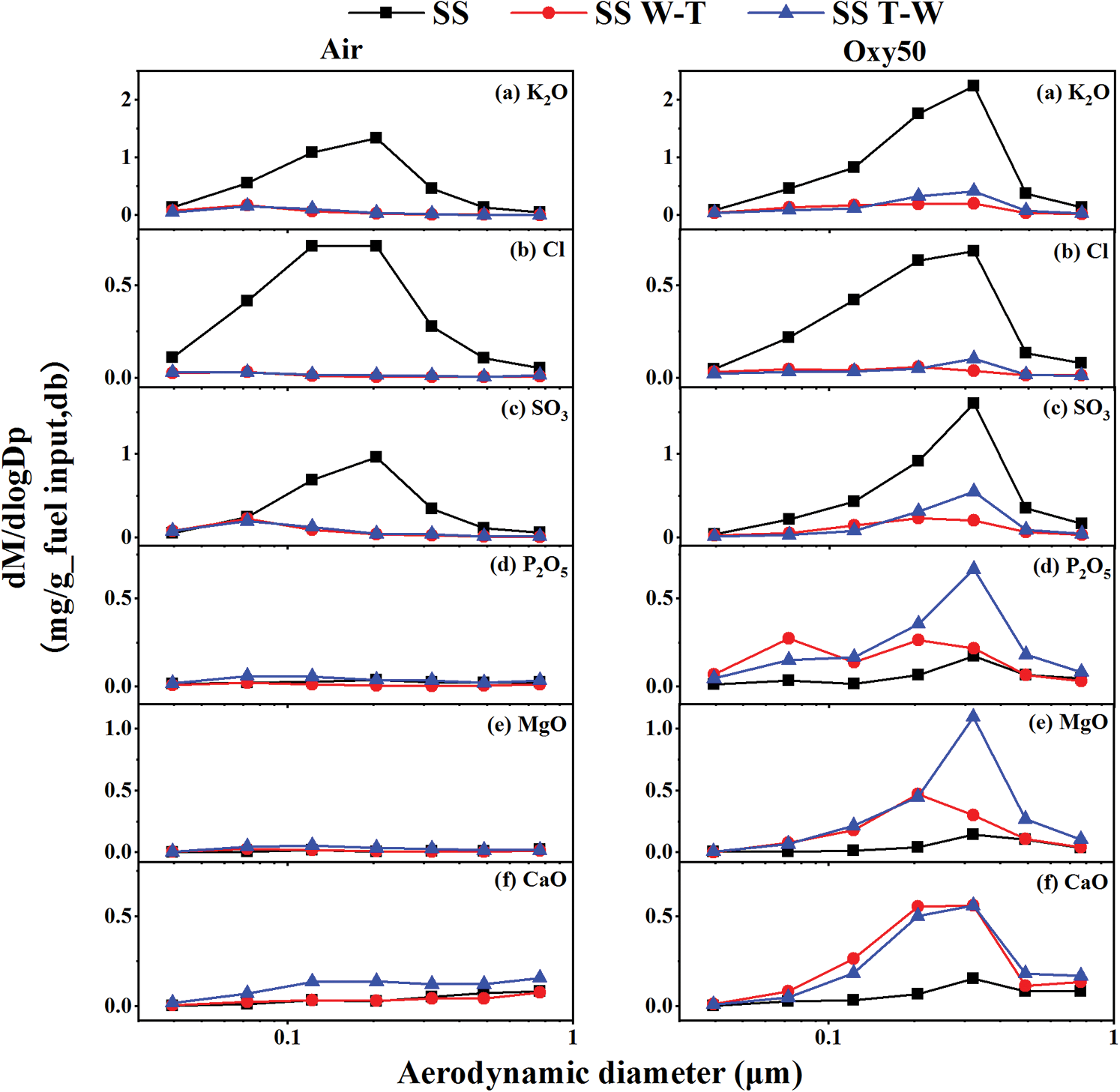
Figure 6: Major elemental mass-based particle size distributions of submicron particles produced from the combustion of sesame stalk and biochar under air and oxy50 conditions
The influence of oxy50 atmosphere is further explored. It can be seen from the figure that the PM1 yields of each sample increased and the content of K and S also raised. This is mainly because the oxygen concentration is higher in the oxy50 atmosphere, and the K released into the gas phase will react with SO2 and O2 to form sulfate which contributes a part of PM1 through condensation. And the main related factors of sulfate is the production of SO3 rate which is closed related to the oxygen concentration. Therefore under high oxygen concentration atmosphere of oxy50, most of K will be sulfated and retained in PM1, resulting in the amount of PM1 released increased. On the other hand, oxy50 atmosphere not only has a higher oxygen concentration but also markedly increase the combustion temperature and promote the vaporization of elements such as Ca. At the same time, intense combustion will lead to a local reducing atmosphere, which will also aggravate the reduction of oxides and subsequent vaporization. Therefore, the Ca, P and Mg are partially retained in PM1 after vaporization, leading to an increased content and also raise PM1 emissions. The enrichment of torrefaction in the pretreatment process leads to a higher content of Ca and Mg in biochar and Ca and Mg migrate to PM1 under oxy50 atmosphere which contributes to the weakening of the pretreatment reduction effect on PM1. However due to the high content of K in the sesame stalk, there are many K-containing gas phase components formed during combustion. And these components are easy to condensate on above mentioned Ca, Mg and P submicron particles in heterogeneous phase and thus agglomerating to form PM1+. So the contents of Ca, Mg and P in PM1 of sesame stalk under oxy50 condition are relatively less.
In this study, sesame stalk, an agricultural processing residues with combustion value, was used as raw materials, and two pretreatments combined carbonization and water washing, namely water washing-torrefaction (W-T) and torrefaction-water washing (T-W), were carried out to explore the influence to the fuel properties, combustion characteristics and PM1 emission on air/oxy50 atmosphere. The main conclusions are as follows:
1. W-T and T-W pretreatment not only make the fuel properties of obtained biochar similar to lignite but also greatly reduce the content of water-soluble elements (K, Cl). Because the hydrophobicity of the raw biomass is better than that of biochar, therefore the effect of W-T pretreatment is better and the obtained biochar has the optimal fuel properties.
2. Due to the lower volatile content and the reduction of active substances during torrefaction process, both pretreatments have hindered the combustion in a certain extent. After pretreatment, the activation energy required for the release of volatiles is increased while has little effect on the kinetic parameters of the fixed carbon combustion stage.
3. W-T and T-W pretreatments can effectively reduce the emission of PM1. And the efficiency of emission reduction reached to 84% and 74% respectively. The reason is that pretreatment can remove K, Cl and S in which W-T have a better removal efficiency on the above elements, its PM1 emission reduction effect is the best. Under oxy50 atmosphere, due to the higher oxygen concentration and combustion temperature, the former increased the sulfation of K, making most of K is sulfated and retained in PM1, the latter and the local reducing atmosphere formed intensify the vaporization of Ca, P and Mg migrating to PM1, leading to an increase in the release of PM1 and weakens the emission reduction effect of pretreatment.
Acknowledgement: Acknowledgements are also given for the supports from the Analytical and Testing Center at Huazhong University of Science and Technology.
Funding Statement: This research was funded by the National Key Research and Development Program of China (No. 2016YFB0600605), Hubei Province Technology Innovation Project (No. 2018AHB017) and National Natural Science Foundation of China (No. 52076091).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Niu, Y. Q., Lv, Y., Lei, Y., Liu, S. Q., Liang, Y. et al. (2019). Biomass torrefaction: Properties, applications, challenges, and economy. Renewable and Sustainable Energy Reviews, 115, 109395. DOI 10.1016/j.rser.2019.109395. [Google Scholar] [CrossRef]
2. Liu, H., Jiang, G. M., Zhuang, H. Y., Wang, K. J. (2008). Distribution, utilization structure and potential of biomass resources in rural China: With special references of crop residues. Renewable and Sustainable Energy Reviews, 12(5), 1402–1418. DOI 10.1016/j.rser.2007.01.011. [Google Scholar] [CrossRef]
3. Baxter, L. (2005). Biomass-coal co-combustion: Opportunity for affordable renewable energy. Fuel, 84(10), 1295–1302. DOI 10.1016/j.fuel.2004.09.023. [Google Scholar] [CrossRef]
4. Niu, Y. Q., Tan, H. Z., Hui, S. (2016). Ash-related issues during biomass combustion: Alkali-induced slagging, silicate melt-induced slagging (ash fusionagglomeration, corrosion, ash utilization, and related countermeasures. Progress in Energy and Combustion Science, 52, 1–61. DOI 10.1016/j.pecs.2015.09.003. [Google Scholar] [CrossRef]
5. Chew, J. J., Doshi, V. (2011). Recent advances in biomass pretreatment–Torrefaction fundamentals and technology. Renewable and Sustainable Energy Reviews, 15(8), 4212–4222. DOI 10.1016/j.rser.2011.09.017. [Google Scholar] [CrossRef]
6. Saddawi, A., Jones, J. M., Williams, A., Coeur, C. L. (2012). Commodity fuels from biomass through pretreatment and torrefaction: Effects of mineral content on torrefied fuel characteristics and quality. Energy & Fuels, 26(11), 6466–6474. DOI 10.1021/ef2016649. [Google Scholar] [CrossRef]
7. Xu, M. H., Yu, D. X., Yao, H., Liu, X. W., Qiao, Y. (2011). Coal combustion-generated aerosols: Formation and properties. Proceedings of the Combustion Institute, 33(1), 1681–1697. DOI 10.1016/j.proci.2010.09.014. [Google Scholar] [CrossRef]
8. Yani, S., Gao, X. P., Wu, H. W. (2015). Emission of inorganic PM10 from the combustion of torrefied biomass under pulverized-fuel conditions. Energy Fuels, 29(2), 800–807. DOI 10.1021/ef5023237. [Google Scholar] [CrossRef]
9. Hu, Z. F., Wang, X. B., Adeosun, A., Ruan, R. H., Tan, H. Z. (2018). Aggravated fine particulate matter emissions from heating-upgraded biomass and biochar combustion: The effect of pretreatment temperature. Fuel Processing Technology, 171, 1–9. DOI 10.1016/j.fuproc.2017.11.002. [Google Scholar] [CrossRef]
10. Wang, W. Y., Wen, C., Chen, L. C., Liu, T. Y., Li, C. K. et al. (2020). Effect of combining water washing and carbonisation on the emissions of PM10 generated by the combustion of typical herbs. Fuel Processing Technology, 200, 106311. DOI 10.1016/j.fuproc.2019.106311. [Google Scholar] [CrossRef]
11. Abelha, P., Vilela, C. M., Nanou, P., Carbo, M., Janssen, A. et al. (2019). Combustion improvements of upgraded biomass by washing and torrefaction. Fuel, 253, 1018–1033. DOI 10.1016/j.fuel.2019.05.050. [Google Scholar] [CrossRef]
12. Zhang, S. P., Dong, Q., Zhang, L., Xiong, Y. Q., Liu, X. Z. et al. (2015). Effects of water washing and torrefaction pretreatments on rice husk pyrolysis by microwave heating. Bioresource Technology, 193, 442–448. DOI 10.1016/j.biortech.2015.06.142. [Google Scholar] [CrossRef]
13. Liu, Z., Hoekman, S. K., Balasubramanian, R., Zhang, F. S. (2015). Improvement of fuel qualities of solid fuel biochars by washing treatment. Fuel Processing Technology, 134, 130–135. DOI 10.1016/j.fuproc.2015.01.025. [Google Scholar] [CrossRef]
14. Wang, W. Y., Wen, C., Liu, T. Y., Li, C. K., Xu, M. H. et al. (2020). Emission reduction of PM10 via pretreatment combining water washing and carbonisation during rice straw combustion: Focus on the effects of pretreatment and combustion conditions. Fuel Processing Technology, 205, 106412. DOI 10.1016/j.fuproc.2020.106412. [Google Scholar] [CrossRef]
15. Sher, F., Pans, M. A., Sun, C., Snape, C., Liu, H. (2018). Oxy-fuel combustion study of biomass fuels in a 20 kWth fluidized bed combustor. Fuel, 215, 778–786. DOI 10.1016/j.fuel.2017.11.039. [Google Scholar] [CrossRef]
16. Ruscio, A., Kazanc, F., Levendis, Y. A. (2014). Characterization of particulate matter emitted from combustion of various biomasses in O2/N2 and O2/CO2 environments. Energy Fuels, 28(1), 685–696. DOI 10.1021/ef401796w. [Google Scholar] [CrossRef]
17. Wang, Y. M., Li, X. L., Went, J. O. (2018). Ash aerosol and deposition formation mechanisms during air/oxy-combustion of rice husks in a 100 kW combustor. Energy & Fuels, 32(4), 4391–4398. DOI 10.1021/acs.energyfuels.7b03127. [Google Scholar] [CrossRef]
18. Wang, C., Liu, X. W., Li, D., Wu, W. C., Xu, Y. S. et al. (2014). Effect of H2O and SO2 on the distribution characteristics of trace elements in particulate matter at high temperature under oxy-fuel combustion. International Journal of Greenhouse Gas Control, 23, 51–60. DOI 10.1016/j.ijggc.2014.01.012. [Google Scholar] [CrossRef]
19. Wen, C., Zhang, P. H., Yu, D. X., Gao, X. P., Xu, M. H. (2017). Loading identical contents of sodium and quartz into different ash-removed coals to elaborately investigate the real effects of coal particle combustion on the emission behavior of PM10. Proceedings of the Combustion Institute, 36(2), 2191–2198. DOI 10.1016/j.proci.2016.08.052. [Google Scholar] [CrossRef]
20. Zhao, M. Y., Han, Z. N., Sheng, C. D., Wu, H. W. (2013). Fates and roles of alkali and alkaline earth metals during the pyrolysis of a Victorian brown coal. Energy & Fuels, 27(2), 898–907. DOI 10.1021/ef301715p. [Google Scholar] [CrossRef]
21. Lv, D. Z., Xu, M. H., Liu, X. W., Zhan, Z. H., Li, Z. Y. et al. (2010). Effect of cellulose, lignin, alkali and alkaline earth metallic species on biomass pyrolysis and gasification. Fuel Processing Technology, 91(8), 903–909. DOI 10.1016/j.fuproc.2009.09.014. [Google Scholar] [CrossRef]
22. Phanphanich, M., Mani, S. (2011). Impact of torrefaction on the grindability and fuel characteristics of forest biomass. Bioresource Technology, 102(2), 1246–1253. DOI 10.1016/j.biortech.2010.08.028. [Google Scholar] [CrossRef]
23. Chen, L. C., Wen, C., Wang, W. Y., Liu, T. Y., Liu, E. Z. et al. (2020). Combustion behaviour of biochars thermally pretreated via torrefaction, slow pyrolysis, or hydrothermal carbonisation and co-fired with pulverised coal. Renewable Energy, 161, 867–877. DOI 10.1016/j.renene.2020.06.148. [Google Scholar] [CrossRef]
24. Li, X. G., Lv, Y., Ma, B. G., Jian, S. W., Tan, H. B. (2011). Thermogravimetric investigation on co-combustion characteristics of tobacco residue and high-ash anthracite coal. Bioresource Technology, 102(20), 9783–9787. DOI 10.1016/j.biortech.2011.07.117. [Google Scholar] [CrossRef]
25. Shen, D. K., Gu, S., Luo, K. H., Bridgwater, A. V., Fang, M. X. (2009). Kinetic study on thermal decomposition of woods in oxidative environment. Fuel, 88(6), 1024–1030. DOI 10.1016/j.fuel.2008.10.034. [Google Scholar] [CrossRef]
26. Yu, D. X., Xu, M. H., Yao, H., Liu, X. W., Zhou, K. (2008). Effective identification of the three particle modes generated during pulverized coal combustion. Chinese Science Bulletin, 53, 1593–1602. [Google Scholar]
27. Wang, W. Y., Wen, C., Li, C. K., Wang, M., Li, X. M. et al. (2019). Emission reduction of particulate matter from the combustion of biochar via thermal pre-treatment of torrefaction, slow pyrolysis or hydrothermal carbonisation and its co-combustion with pulverized coal. Fuel, 240, 278–288. DOI 10.1016/j.fuel.2018.11.117. [Google Scholar] [CrossRef]
28. Yu, D. X., Xu, M. H., Yao, H., Sui, J. C., Liu, X. W. et al. (2007). Use of elemental size distributions in identifying particle formation modes. Proceedings of the Combustion Institute, 31(2), 1921–1928. DOI 10.1016/j.proci.2006.07.115. [Google Scholar] [CrossRef]
29. Huang, Q., Li, S. Q., Li, G. D., Yao, Q. (2017). Mechanisms on the size partitioning of sodium in particulate matter from pulverized coal combustio. Combustion and Flame, 182, 313–323. DOI 10.1016/j.combustflame.2017.04.026. [Google Scholar] [CrossRef]
30. Wang, W. Y., Wen, C., Liu, T. Y., Li, C. K., Chen, L. C. et al. (2020). Effects of various occurrence modes of inorganic components on the emissions of PM10 during torrefied biomass combustion under air and oxy-fuel conditions. Applied Energy, 259, 114153. DOI 10.1016/j.apenergy.2019.114153. [Google Scholar] [CrossRef]
31. Wen, C., Yu, D. X., Wang, J. P., Wu, J. Q., Yao, H. et al. (2014). Effect of the devolatilization process on PM10 formation during oxy-fuel combustion of a typical bituminous coal. Energy & Fuels, 28(9), 5682–5689. DOI 10.1021/ef501264v. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |