

 | Energy Engineering |  |
DOI: 10.32604/ee.2022.018821
ARTICLE
Stepwise Pyrolysis by LBCR Downstream to Enhance of Gasoline Fraction of Liquid Fuel from MMSW
1Department of Mechanical Engineering Education, Universitas Pendidikan Indonesia, Bandung, 40154, Indonesia
2Postgraduate Student of Doctoral Program, Faculty Agroindustrial Technology, Universitas Padjadjaran, Jatinangor, 45363, Indonesia
3Faculty Agroindustrial Technology, Universitas Padjadjaran, Jatinangor, 45363, Indonesia
4Department of Mechanical Engineering Education, Universitas Sebelas Maret, Surakarta, 57126, Indonesia
*Corresponding Authors: Indra Mamad Gandidi. Email: indra.gandidi@upi.edu; Nugroho Agung Pambudi. Email: agung.pambudi@staff.uns.ac.id
Received: 19 August 2021; Accepted: 22 October 2021
Abstract: Pyrolysis is one of the thermal cracking methods to convert hydrocarbon to liquid fuel. The quantity and quality of the process are dependent on several condition including temperature, reaction time, catalyst, and the type of reactor. Meanwhile, a gasoline fraction was maximum product to be considered in the pyrolisis process. Therefore, this study aims to increase the gasoline fraction in liquid fuel using stepwise pyrolysis with a long bed catalytic reactor downstream (LBCR). The LBCR downstream was equipped with the top and bottom outlet and the fed source was mixed municipal solid waste (MMSW). The activated natural dolomite at 500°C was used to allow the repetition of the secondary cracking. Also, the reactor temperature was setup at around 200°C–300°C and the pyrolizer was 400°C. To analyze the gasoline fraction and physical properties of liquid fuel, Gas Chromatography-Mass Spectroscopy (GC-MS) and ASTM standard were employed. The experimental results showed there was a significant increase in the gasoline fraction of liquid fuels compared to using direct catalytic cracking and absence of catalysts. By using a LBCR at 250°C, the liquid fuel obtained at top outlet (TO) and bottom outlet (BO) have 84.08 and 56.94 percent peak area of gasoline fraction (C5--C12), respectively. The average value (TO and BO) of the fraction at 250°C by LBCR was 70.51 percent peak area and it was increased by about 93.6% and 51.14% compared to without catalyst and direct catalytic, respectively. Furthermore, pyrolytic liquid oils were found to have kinematic viscosity of 2.979 and 0.789 cSt, density of 0.781 and 0.782 g/cm3, and flash point <−5°C for BO-250 and TO-250 liquid fuel, respectively. These results showed BO liquid fuel was comparable to diesel conventional fuel while TO liquid fuel was comparable to gasoline. Evidently, the presence of LBCR made a major contribution to generate multi secondary cracking and to produce more gasoline fraction from mixed MMSW feedstock, as well as to increase the physical properties of liquid fuel.
Keywords: Liquid fuel; gasoline fraction; LBC; MMSW; stepwise pyrolysis; natural dolomite catalysts
Regarding environmental friendly and energy security, the mixed municipal solid waste (MMSW) has been strongly considered to be used as a hydrocarbon resource to produce bioenergy such as liquid fuel due to its abundance and availability [1–4]. In developed countries, the MMSW is used as a feedstock for energy and electricity production through emerging technology [5], and it produces a significant benefit economically [6]. The produced liquid fuel as product can be used as a boiler fuel to power electrical generation, household stove, transportation, as well as small and big scale industries of burner [7]. Furthermore, it has a better combustion efficiency compared to direct combustion of MMSW [8] and it has a very low environmental impact [9,10]. This method has many advantages including minimizing costs of waste management, transportation, vehicle maintenance, labor, and conservation of final disposal.
Pyrolysis is one of the promising thermal degradation methods that is possible to be applied in converting MMSW into liquid fuel. This process involves heating the hydrocarbon at a certain temperature in the absence of oxygen. Furthermore, the long chain hydrocarbon of MMSW change into a short chain [11]. The quantity and quality of the liquid fuel produced from the pyrolysis is highly dependent on many operating conditions including temperature, heating rate, reaction time, isothermal, non-isothermal, and catalyst types. These factors create different influence on the reactions and therefore determine which of the products are favored. An increase in temperature and heating rate will reduce the amount of solids and increase gaseous products [12,13]. The reaction time also has an influence on the distribution of products. The secondary cracking is promoted when the reaction time is increased and result in a decrease of liquid fuel [14]. However, in ordinary thermal pyrolysis, the cracking of long molecular chain occurs randomly. This results in very broad range of hydrocarbon product [15]. Consequently, the complex separation and purification to each of hydrocarbon fraction are necessary and it is unprofitable from an economic point of view.
Due to gasoline selectivity and requirement of high energy consumption, the use of catalysts can be considered to solve these problems. Product distribution is controlled by the selection of a catalyst, which reduces activation energy and facilitate operation at low temperature. In this study, the thermal catalytic cracking was performed by zeolite and dolomite at a temperature range of 200°C–750°C to mixed MMSW under fixed bed reactor [16]. The results showed the presence of calcined dolomite significantly influenced the product yields and gas composition. Furthermore, it increases gas yield and decreases oil and char compared to zeolite and non-catalytic process [16]. Using ZSM-5 catalyst in rice husk pyrolysis increased the product of aromatic hydrocarbons and light phenols fraction, while Al-MCM-41 catalyst reduces the acetic acid product. The method of silica isolation from agricultural waste and its application as a catalyst has been carried out [17]. The catalyzed pyrolysis increased HV and water content while viscosity, density and amount of acid decreased [18]. Meanwhile, the catalyzed pyrolysis process of plastic waste with the presence of Y-zeolite and ZSM-5 catalysts will reduce the C2–C4 hydrocarbon fraction [19]. Also, the presence of catalysts in the pyrolysis process of MMSW and MPW will convert aliphatic hydrocarbons into aromatic and cyclic compounds in liquid fuel products [20]. Ratnasari et al. [21] investigated the effect of stage catalyst process in the plastic pyrolysis by using mesoporous MCM-41 followed by microporous ZSM-5. The results showed using the staged catalysis, a high yield of oil product (83.15 wt%) was obtained from high density polyethylene [21]. The yield of product oil from the mixed plastics waste pyrolyzed was decreased with the addition of catalysts, but the oil was of significantly lower molecular weight range, containing a product slate of premium fuel grade C5–C15 hydrocarbons or gasoline fraction. Also, the content of aromatic compounds in the product oil was increased [22]. Aguado et al., 2007 reported 74.7 wt% liquid fuel obtained by thermal degradation at 425°C of polyethylene in absence of catalyst and by two step thermo catalytic process which obtained a gaseous product of 73.5% over n-HZSM-5 catalyst at 450°C [23]. This implies that the use of catalysts can increase the efficiency of product selectivity. Recently, Saha et al. [24] reported that the temperature and reaction time on secondary reactor in two step pyrolysis play a major in controlling the liquid fuel profile.
Furthermore, the investigations to increase the productivity and selectivity of liquid fuels of MMSW such as mixtures in biomass, various plastics, textiles, papers, and rubber wastes are rarely reported. Therefore, this study aims to increase the selectivity and productivity of liquid fuels products (gasoline fraction) using LBCR (Long Bed Catalytic Reactor) downstream in stage pyrolysis by using natural dolomite catalyst. Furthermore, the bed temperature was varied between 200°C–300°C.
2 Material and Experimental Methods
2.1 Mixed MMSW Sample and Catalyst
Six types of waste materials in MMSW (biomass, HDPE, LDPE, rubber, papers and textiles) were used in this study. These wastes were directly obtained from the final disposal site in Bandung City, Indonesia. The biomass mostly contained vegetables and fruits from households and markets. Subsequently, the MMSW sample was dried by solar drying for 3–5 days prior to being chopped into small pieces between 5–10 cm, which is the appropriate size for the reactor capacity. The composition of mixed waste refer to waste from disposal based air dried including mixed biomass 34%, plastics 52%, papers 9%, rubber 3%, and textiles 2% (Fig. 1). Meanwhile, dolomite is a natural carbonate mineral containing magnesium and calcium nutrients in the form of flour with the chemical formula CaMg(CO3)2. It is widely available in nature and it can be used as a catalyst in the pyrolysis process [25]. Therefore, this study was conducted by using natural dolomite as a catalyst from local source and prior to use in experimental process, natural dolomite was activated thermally at 500°C for 2 h to improve the active site and crystallinity.

Figure 1: The mixed MMSW sample composition and natural dolomite catalyst
There were two experiments carried out, namely stepwise pyrolysis and direct catalytic cracking. The stepwise pyrolysis has been used, whereby the non-isothermal cracking took place in the first reactor, while the secondary reactor (LBCR) was operated to catalytic cracking with natural dolomite as a catalyst (see Fig. 2). Then, the direct catalytic cracking (without LBCR) by non-isothermal in which MMSW sample was mixed directly with catalyst was performed to analyze the results of LBCR improvement. In addition, other comparisons with liquid fuel from pyrolysis process in absence of catalysts have also been attempted [26]. Hereinafter, the first reactor for thermal cracking was made from the stainless steel cylinder with the dimension of 310 mm in height and 160 mm ID and be equipped with an electrical heating jacket around the reactor. The reactor was set vertically and N2 gas was introduced into the reactor at the rate of 500 ml/min from the bottom and passed through the top of the reactor. The flow of nitrogen replaces the air from the reactor and permits the pyrolysis reaction under inert atmospheric condition. Whereas the LBCR downstream as secondary reactor for upgrading of gaseous from the first reactor was make from stainless steel cylinder with the 1000 mm in high and 120.7 mm in ID and also set vertically and placed between condenser and first reactor. Among of first reactor and LBCR downstream, it has been installed a valve to control the reaction time. The shell and tubes condenser type was employed in this study to condense the gaseous fraction that out flow from the LBCR. Thus, a part of gaseous fraction will be condensed into the liquid fuel and other fraction as permanent gas will flow out from condenser and burned off regarding to environmental pollution impact. The char produced was remained in the reactor and collected after the pyrolysis reaction.
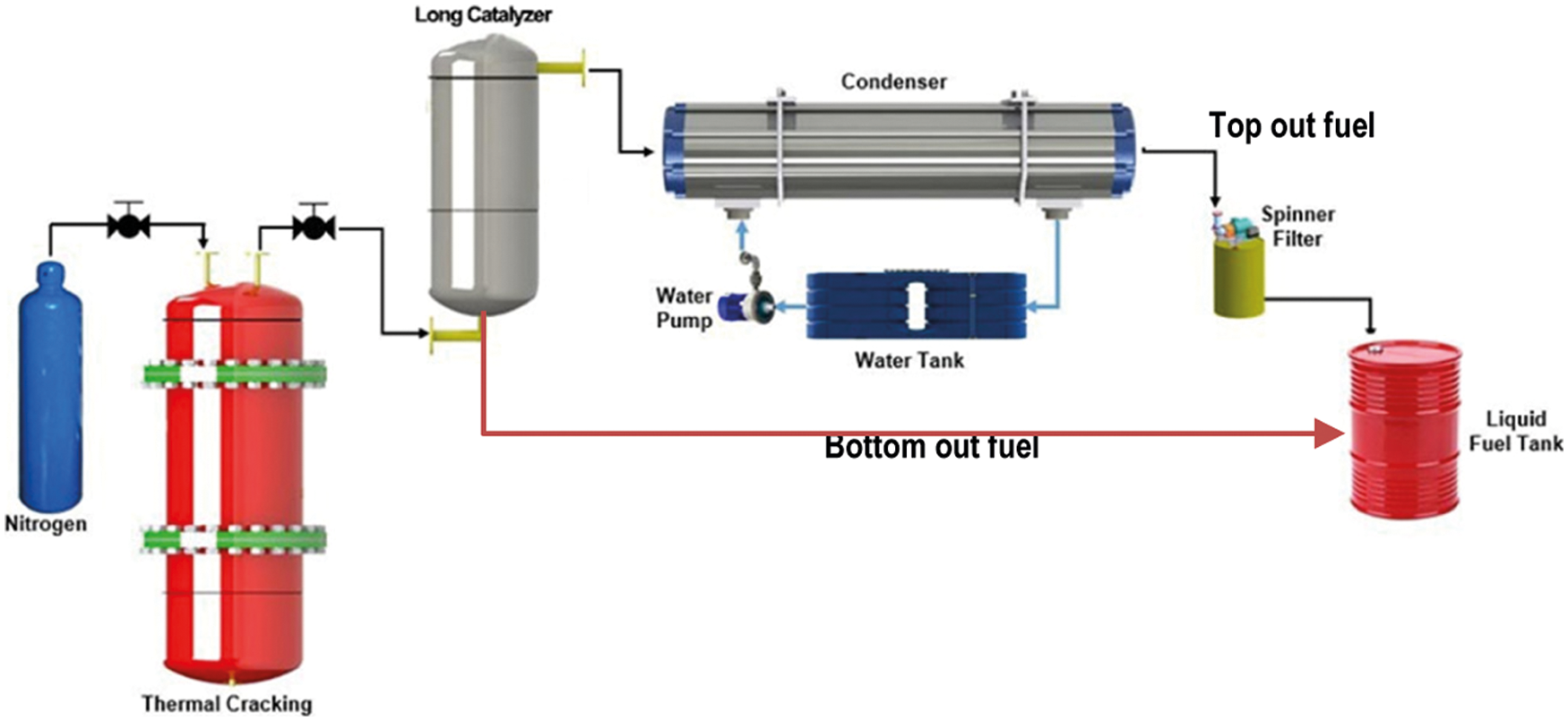
Figure 2: Experimental set up for stepwise pyrolysis
In the experiment process, 500 gram mixed MMSW sample (unwashed) was loaded into the first reactor (initial step) from the top for both of stepwise and direct catalytic pyrolysis (MMSW-catalyst ratio 1:0.5 by weight). In the wake of sample was added, the reactor and its contents were heated at heating rate of 12°C by electrical heater jacket until the reactor's temperature was achieved 400°C and holded for 60 min by thermo controller. However, for the direct catalytic, the first reactor was not connected to LBCR downstream but with condenser directly. Afterward, the temperature of LBCR was set varies from the 200°C–300°C. When the reaction time was held for one experimental, the valve control was opened to discharge the gaseous phase from the reactor to LBCR and then flow to condenser at 20°C of temperature. Anyhow, when the gaseous fraction flows through the LBCR, the amount of gaseous fraction will be condensed due to the point of evaporation temperature and flow out at the bottom of the LBCR (BO) toward to the spinner purification and afterward liquid tank. And liquid fuel fraction from the condenser (TO) was flow to spinner filter and liquid tank. Finally, the mass yield of liquid fuel pyrolytic and solid residue were counted by Tora TR-DS11030 of electronic digital scales and then tabulated.
Chemical composition of the liquid fuel that derived from both of pyrolysis process was studied to determine the influences of LBCR to improve the gasoline range from mixed MMSW. This study was intended to determine the distribution of hydrocarbon coverage in liquid fuels from a mixed MMSW sample namely gasoline fraction (C5–C12), diesel fuel fraction (C13–C20) and heavy weight (>C20) [27]. Gas chromatography-mass spectrometry (GC-MS, QP2010S Shimadzu) was used to determine mass fraction in liquid fuel. The column DB-1 (Agilent J 100% dimethylpolysiloxane) was capillary column having 30 m length, 0.25 mm diameter and 25 μm film thickness. Helium was used as the gas carrier. Initially temperature was set at 60°C for 5 min followed by heating rate of 5 °C/min up to the temperature of 280°C and held for 51 min. In addition, to analyze the physical properties of liquid fuel from mixed MMSW, the ASTM standard was employed. The physical properties are including density, viscosity, flash point, solid and water content in the liquid fuel.
3.1 Productivity of Liquid Fuel
Productivity refers to the amount of liquid fuel obtained from mixed MMSW for one pyrolysis period. Fig. 3 shows the productivity results (mass yield) for various temperatures of LBCR from direct catalytic and without catalyst. Furthermore, LBCR-200, LBCR-250, LBCR-300 were represented of bed temperature at 200°C, 250°C, and 300°C, respectively. Fig. 3 shows that the maximum yield in liquid fuels has been obtained at a bed temperature of 250°C and followed by bed temperature of 200°C and 300°C. The presence of LBCR with natural dolomite catalyst was promoted the multi-secondary cracking of solid fraction to produce more liquid fuel and gaseous fraction. Nevertheless, the large reduction in liquid fuel was taken place at 300°C of temperature in which indicated that this condition was convenient to produce more gaseous product. The similar result of catalyst effect was also reported by [28] with decrease of liquid fuel fraction. Then, liquid fuel product at 200°C was seen as a thick and sticky fluid (wax fraction) on which viscous rapidly at room temperature. It had a high yield in liquid fuel fraction and the next cracking was still necessary for this viscosity process. When the LBCR mass yield, direct catalytic (DC), and absence of catalyst (NC) were compared, a significant increase of liquid fuel was obtained using LBCR and natural dolomite catalyst. The maximum increase of mass yield improvement (MYI) of 87.5 wt% and 50.0 wt% were achieved at 250°C temperature for NC-LBCR and DC-LBCR, respectively (see Fig. 4).
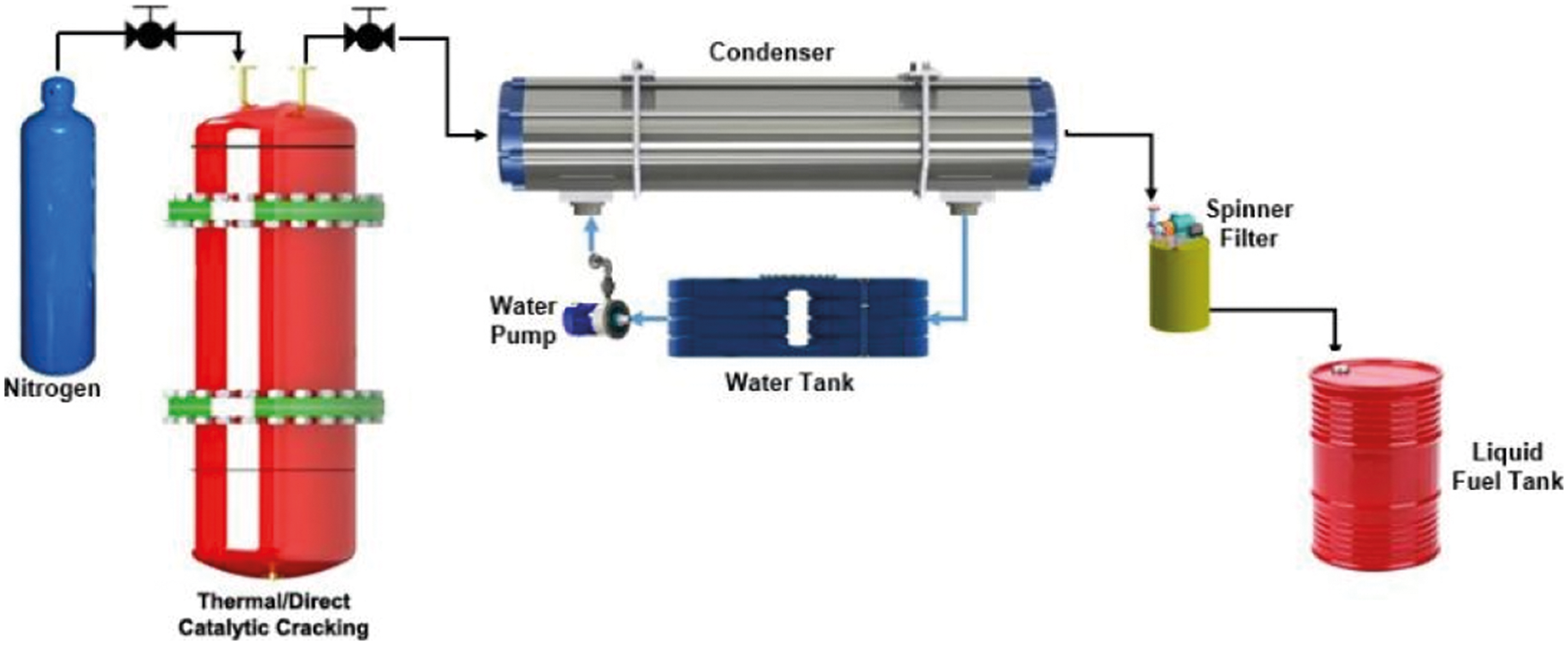
Figure 3: Experimental set up for direct catalytic cracking
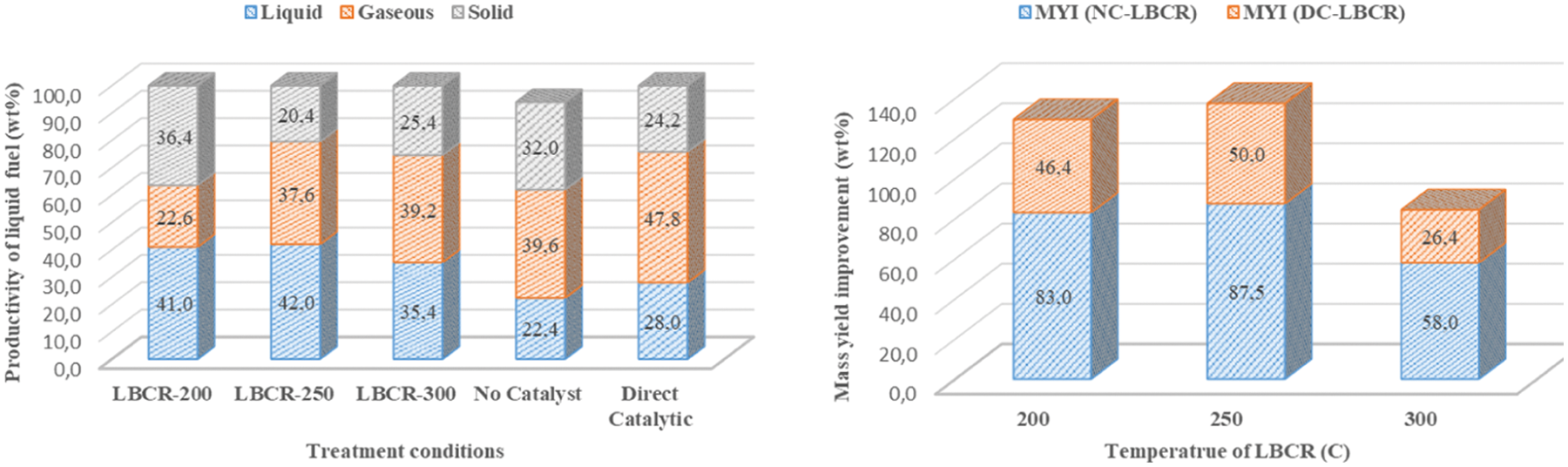
Figure 4: Mass yield of liquid fuel from mixed MMSW at 400°C and mass yield improvement
Further, when the gas phase pass through the LBCR with catalyst dolomite, a part of gas phase will be condensed into liquid fuel and flow out at the bottom of LBCR (BO), and other part will flow out from the top of LBCR (TO) and ride into condenser and condensed. Fig. 5 shows that the gas phase pass through the LBCR is more condensed into liquid fuel flow out on the BO. This indicates that the gas phase is still a long chain hydrocarbon compound and is condensed at high temperatures. Nonetheless, an increase of temperature was the best way to reduce BO liquid fuel and increase of amount TO liquid fuel significantly although liquid fraction will be decreased and an increase in the gaseous fraction as a consequence.
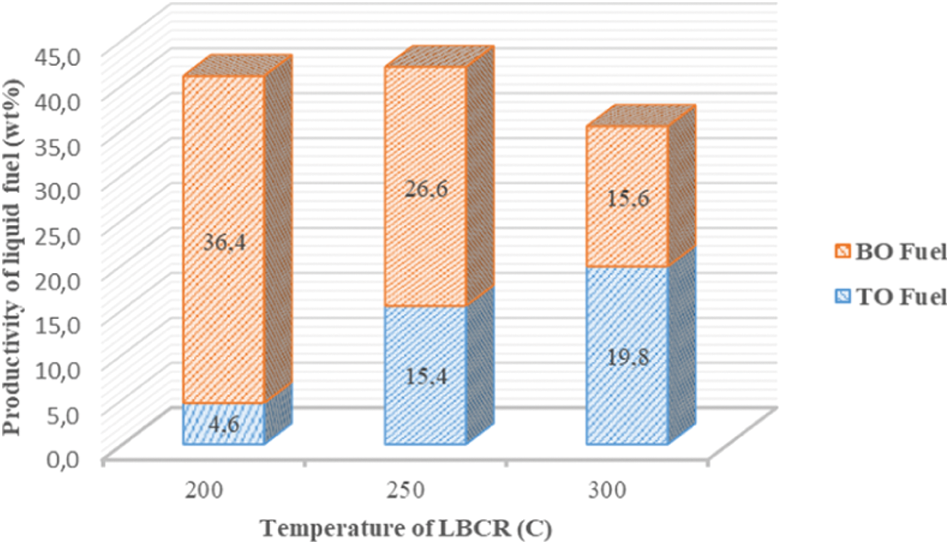
Figure 5: Productivity of liquid fuel based on both of TO and BO liquid fuel
3.2 Selectivity of Liquid Fuel
In this study, the selectivity was regarding to amount of gasoline-rich (C5–C12) liquid fuel product derived from mixed MMSW for one period of pyrolysis process. Fig. 6 illustrates the distribution of hydrocarbons range in liquid fuels produced from various experiments and various LBCR temperatures (200∘C, 250∘C, 300∘C) and then compared with the conventional liquid fuel including gasoline-88 and diesel-48 that usually used in Indonesia. Six types of liquid fuels from the experimental results were obtained that involve BO-250, BO-300, TO-250, TO-300, direct catalyst and without catalyst. Among them, the liquid fuel TO-250 has the highest fraction in the gasoline fraction (C5–C12) and a small amount of the diesel fraction (C13–C20).
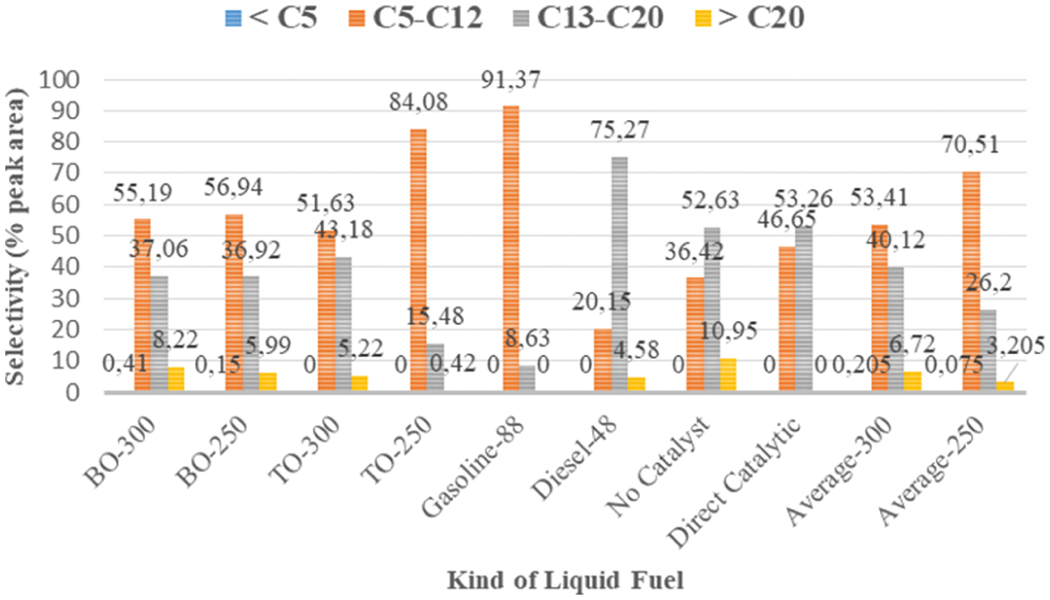
Figure 6: Distribution of hydrocarbon range in liquid fuel
This showed the presence of LBCR has promoted the secondary cracking of gaseous fraction to produce more gasoline products. In addition, bed temperature significantly affected the selectivity behavior. However, an increase in temperature results in a increased of diesel fraction. This showed the small portion of the hydrocarbon chain of the diesel fraction was not condensed at 300°C. This is due to the diesel melting point at 230°C–260°C and gasoline at 100°C–150°C [29]. Furthermore, the presence of dolomite catalyst in pyrolysis facilitated the process at low temperature and the product result is more uniform in the distribution of hydrocarbon chains. This is characterized by increasing short hydrocarbon chains in the liquid fuel such as gasoline fraction.
Moreover, the significant increase of gasoline fraction at 250°C was achieved compared to the mass yield from the pyrolysis process under direct catalyst and without catalyst. When average value of gasoline grade (TO and BO) at 250°C (average-250) was considered, it was found t increase by around 93.6% and 51.14% of gasoline when compared to the without catalyst and direct catalytic pyrolysis, respectively. Also, an increase of 32.01% was obtained when compared with the average-300 of liquid fuel. This improvement of gasoline fraction has proven that LBCR and catalyst have an important role in the upgrading process due to multi secondary cracking along the LBCR line.
3.3 Physical Properties of Liquid Fuel
Fig. 7 shows some physical properties of liquid fuels produced by mixed MMSW including viscosity, density, flash point and solid content. These properties are very influential in application especially to internal combustion engines [30]. Kinematic viscosity is an important fluid parameter that must be determined prior to applied in an engine. If the viscosity is too high, excessive friction and pumping problem will take place in the engine and combustion process problems can be encountered. The kinematic viscosity (ASTM D-445) of pyrolytic liquid fuel of TO-250 and BO-250 were measured of 0.79 and 2.98 cSt, respectively. Whereas direct catalytic and without catalyst have viscosity of 1.995 and 2.662 cSt, respectively. It seen, the kinematic viscosity of TO-250 liquid fuel was comparably with the conventional gasoline-88 fuel (0.8 cSt) and BO-250 liquid fuel was comparably better than the conventional diesel-48 fuel (5.0 cSt) and lower than the gasoline-88 fuel. However, the kinematic viscosity of B-250 liquid fuel was slightly less than the both of direct catalytic and without catalyst of viscosity.
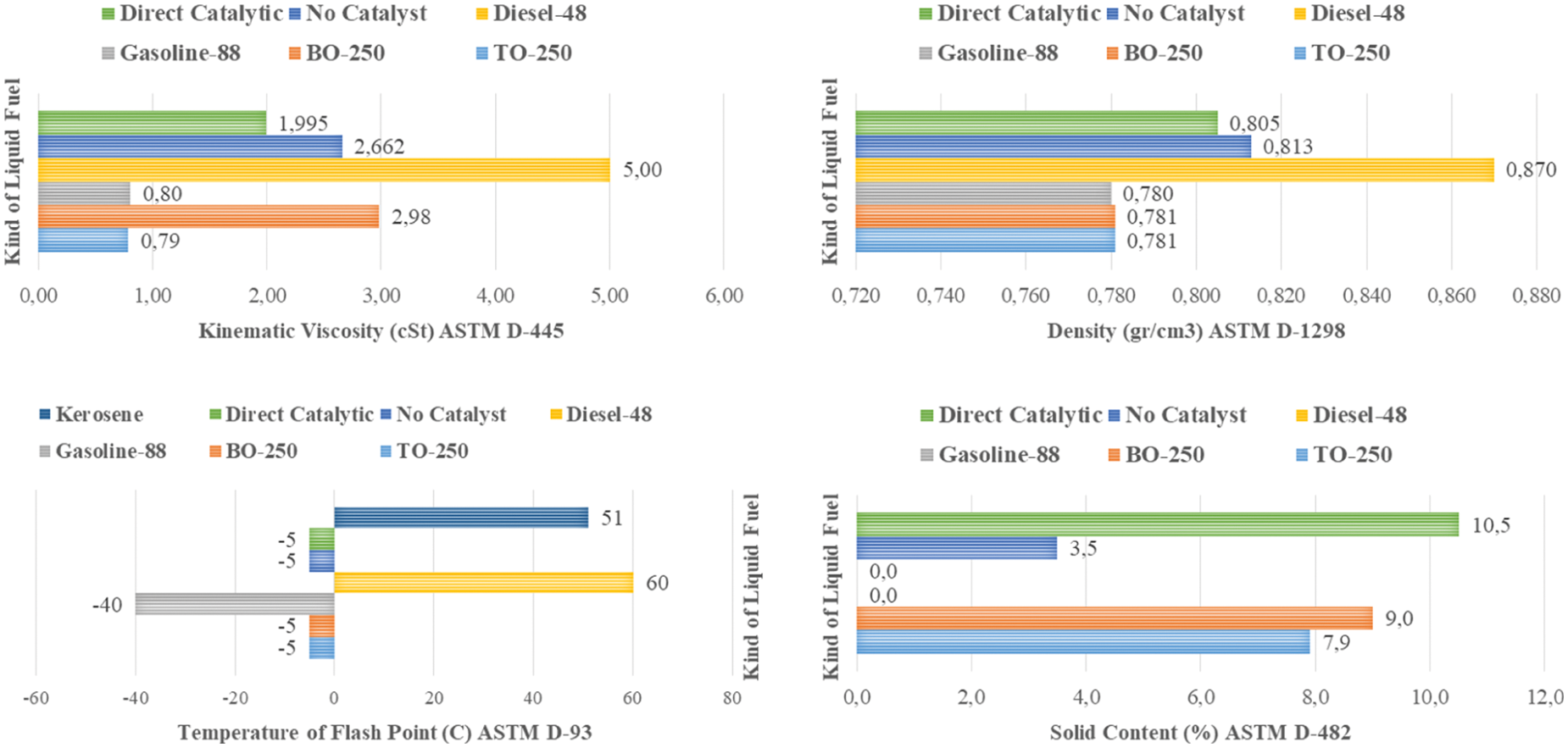
Figure 7: Physical properties of liquid fuel based on ASTM Standard
Density is also an important property of liquid fuels, therefore when the density is high, fuel supply will be slightly lacking in the engine. Otherwise, when liquid fuel has a low density, the consumption will be very high which can cause damage to the engine. From Fig. 6 the density (ASTM D-1298) of TO-250 and BO-250 are 0.781 g/cm3, respectively, and this density value is lower than diesel fuel and similar with the gasoline fuel. Gasoline fraction improvement was significant contribution in the increase of liquid fuel density from mixed MMSW. This indicates that liquid fuels from MMSW can substitute conventional liquid fuels. Another important property of liquid fuels is the flash point which represents the lowest temperature conditions. These liquid fuels can evaporate to form a combustible mixture in the air. This temperature is regarding to safety problems in storage and transportation. The TO-250 and BO-250 liquid fuel have a flash point −5°C (ASTM D-93) which indicates that they were contain a lot of light flammable fractions. The serious handling in storage and transportation were necessary, but they are easier to handle compared to gasoline fuel. In addition, however the TO-250 and BO-250 liquid fuel were still containing some solid residue regarding to use catalyst and unwashed mixed MMSW. The purification process was still necessary to be engaged.
Gasoline-rich liquid fuel produced from waste hydrocarbon materials is the main requirement of a pyrolysis process. The use of LBCR with natural dolomite catalyst in a stepwise pyrolysis process can increase the gasoline fraction in the product of liquid fuels from the MMSW and it was describe that an improvement in liquid fuel quality was achieved by LBCR. The temperature of 250°C of LBCR is the appropriate temperature to be employed for high quality of liquid fuel. Thus, this method is strong recommended to be applied in the great cities around the world in MMSW management practice regarding to low cost of MMSW management and following by the economic advantages. However, the use of LBCR will require a lot of thermal energy for long reactor heated. In the future it is necessary to study the LBCR equipment is placed in the first reactor due to improve the thermal efficiency of the system. And it is being carried out in our laboratory with the varied in raw material included several kind of coal, MMSW and biomaterial such jatropha and nyampung crude oil.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Ouda, O. K. M., Raza, S. A., Nizami, A. S., Rehan, M., Al-Waked, R. et al. (2016). Waste to energy potential: A case study of Saudi Arabia. Renewable and Sustainable Energy Reviews, 61, 328–340. DOI 10.1016/j.rser.2016.04.005. [Google Scholar] [CrossRef]
2. Hwansoo, J., Divine, D. S., Godfred, O., Dae, S. L., Seung, H. W. (2019). Characterization and adsorption performance evaluation of waste char by-product from industrial gasification of solid refuse fuel from municipal solid waste. Waste Management, 91, 33–41. DOI 10.1016/j.wasman.2019.04.053. [Google Scholar] [CrossRef]
3. Ayeleru, O. O., Okonta, F. N., Ntuli, F. (2018). Municipal solid waste generation and characterization in the city of Johannesburg: A pathway for the implementation of zero waste. Waste Management, 79, 87–97. DOI 10.1016/j.wasman.2018.07.026. [Google Scholar] [CrossRef]
4. Ball, A. S., Shahsavari, E., Aburto-Medina, A., Kadali, K. K., Shaiban, A. A. J. et al. (2018). Biostabilization of municipal solid waste fractionsfrom an advanced waste treatment plant. Journal of King Saud University–Science, 29, 145–150. DOI 10.1016/j.jksus.2016.10.005. [Google Scholar] [CrossRef]
5. Moya, D., Aldás, C., López, G., Kaparaju, P. (2017). Municipal solid waste as a valuable renewable energy resource: A worldwide opportunity of energy recovery by using waste-to-energy technologies. Energy Procedia, 134, 286–295. DOI 10.1016/j.egypro.2017.09.618. [Google Scholar] [CrossRef]
6. Fivga, A., Domitriou, I. (2018). Pyrolysis of plastic waste for production of heavy fuel substitute: A techno-economic assessment. Energy, 149, 865–874. DOI 10.1016/j.energy.2018.02.094. [Google Scholar] [CrossRef]
7. Vasudevan, P., Sharma, S., Kumar, A. (2005). Liquid fuel from biomass: An overview. Journal of Scientific & Industrial Research, 64, 822–831. [Google Scholar]
8. Alexander, K. (2002). Gasification: An alternative process for energy recovery and disposal of municipal solid wastes (Thesis). Department of Earth and Environmental Engineering, Fu Foundation School of Engineering and Applied Science, Columbia University. [Google Scholar]
9. Agung, N. P., Gandidi, I. M. (2012). Emission factor of single pellet cake seed jatropha curcas in a fix bed reactor. Journal of the Brazilian Society of Mechanical Sciences and Engineering, 34, 179–183. DOI 10.1590/S1678-58782012000200009. [Google Scholar] [CrossRef]
10. Louis, M., Mark, L., Valérie, O., Caroline, C. (2013). Biomass gasification and syngas combustion for greenhouse CO2 enrichment. BioResources, 8(2). [Google Scholar]
11. Demirbas, A. (2009). Pyrolysis mechanisms of biomass materials. Energy Sources, Part A: Recovery, Utilization, and Environmental Effects, 31(13). DOI 10.1080/15567030801952268. [Google Scholar] [CrossRef]
12. Ayhan, D. (2004). Effects of temperature and particle size on bio-char yield from pyrolysis of agricultural residues. Journal of Analytical and Applied Pyrolysis, 72(2), 243–248. DOI 10.1016/j.jaap.2004.07.003. [Google Scholar] [CrossRef]
13. Fredy, S., Saptoadi, H., Sulistyo, H. H. H. H., Rohmat, T. A. (2017). Effect of heating rate on the slow pyrolysis behaviour and Its kinetic parameters of oil-palm shell. International Journal of Renewable Energy Research, 7, 3. DOI 10.20508/ijrer.v7i3.5906.g7144. [Google Scholar] [CrossRef]
14. Mastral, F. J., Esperanza, E., Garcı́a, P., Juste, M. (2002). Pyrolysis of high-density polyethylene in a fluidised bed reactor: Influence of the temperature and residence time. Journal of Analytical and Applied Pyrolysis, 63, 1–15. DOI 10.1016/S0165-2370(01)00137-1. [Google Scholar] [CrossRef]
15. Punkkinen, H., Oasmaa, A., Laatikainen-Luntama, J., Nieminen, M., Laine-Ylijoki, J. (2017). Thermal conversion of plastic containing waste: A review. CLIC Innovation Research Report, pp. 1–77. ARVI, Helsinki. [Google Scholar]
16. Obid, T. (2014). A comparison of catalysts zeolite and calcined dolomite for gas production from pyrolysis of municipal solid waste (MSW). Ecological Engineering, 69, 237–243. DOI 10.1016/j.ecoleng.2014.04.004. [Google Scholar] [CrossRef]
17. Novie, P., Transmissia, N. S., Asep, B. D. N. (2016). Review: Agricultural wastes as a source of silica material. Indonesian Journal of Science and Technology, 1(1), 82–106. DOI 10.17509/ijost.v1i1.8619. [Google Scholar] [CrossRef]
18. Abu Bakar, M. S., Titiloye, J. O. (2013). Catalytic pyrolysis of rice husk for bio-oil production. Journal of Analytical and Applied Pyrolysis, 103, 362–368. DOI 10.1016/j.jaap.2012.09.005. [Google Scholar] [CrossRef]
19. Funda, A., Ates, F., Borsodi, N. (2013). Comparision of real waste (MSW and MPW) pyrolysis in batch reactor over different catalysts. Part I: Product yields, gas and pyrolysis oil properties. Bioresource Technology, 133, 443–454. DOI 10.1016/j.biortech.2013.01.112. [Google Scholar] [CrossRef]
20. Muhammad, C., Onwudili, J. A., Williams, P. T. (2015). Catalytic pyrolysis of waste plastic from electrical and electronic equipment. Journal of Analytical and Applied Pyrolysis, 113, 332–339. DOI 10.1016/j.jaap.2015.02.016. [Google Scholar] [CrossRef]
21. Ratnasari, D. K., Nahil, M. A., Williams, P. T. (2017). Catalytic pyrolysis of waste plastics using staged catalysis for production of gasoline range hydrocarbon oils. Journal of Analytical and Applied Pyrolysis, 124, 631–637. DOI 10.1016/j.jaap.2016.12.027. [Google Scholar] [CrossRef]
22. Onwudili, J. A., Muhammad, C., Williams, P. T. (2018). Influence of catalyst bed temperature and properties of zeolite catalysts on pyrolysis-catalysis of a simulated mixed plastics sample for the production of upgraded fuels and chemicals. Journal of the Energy Institute, 92(5), 1337–1347. DOI 10.1016/j.joei.2018.10.001. [Google Scholar] [CrossRef]
23. Aguado, J., Serrano, D. P., San Miguel, G., Castro, M. C., Madrid, S. (2007). Feedstock recycling of polyethylene in a two-step thermo-catalytic reaction system. Journal of Analytical and Applied Pyrolysis, 79(1–2), 415–423. DOI 10.1016/j.jaap.2006.11.008. [Google Scholar] [CrossRef]
24. Saha, S., Kumar, J., Kumar, S., Pant, K. K. (2019). Thermal pyrolysis of polyolefins in two-step process: Role of secondary ractions. Indian Journal of Chemical Technology, 26, 396–403. DOI 123456789/50673. [Google Scholar]
25. Hmwe, C. S. S., Kyaw, K. T. (2015). Effect of various catalysts on fuel oil pyrolysis process of mixed plastic wastes. International Journal of Advances in Engineering & Technology, 8(5), 794–802. [Google Scholar]
26. Gandidi, M. I., Susila, M. D., Pambudi, A. N. (2017). Production of valuable pyrolytic oils from mixed municipal solid waste (MMSW) in Indonesia using non-isothermal and isothermal experimental. Case Studies in Thermal Engineering, 10, 357–361. DOI 10.1016/j.csite.2017.08.003. [Google Scholar] [CrossRef]
27. Riazi, M. R. (2005). Characterization and properties of petroleum fraction. Book of ASTM Manual Standard (First Edition), pp. 10–84. [Google Scholar]
28. Muhammad, C., Onwudili, J. A., Williams, P. T. (2015). Thermal degradation of real-world waste plastics and simulated mixed plastics in a two-stage pyrolysis–catalysis reactor for fuel production. Energy Fuels, 29, 2601–2609. DOI 10.1021/ef502749h. [Google Scholar] [CrossRef]
29. ICCT (2011). An introduction to petroleum refining and the production of ultra low sulfur gasoline and diesel fuel. Energy Economics Applied Optimization, pp. 1–35. Mathpro. [Google Scholar]
30. Setiawati, E., Edwar, F. (2012). Biodiesel processing technology from used cooking oil with microfiltration and transesterification techniques as alternative diesel engine fuels. Journal of Industry Research, 2, 1–11. [Google Scholar]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |