

 | Energy Engineering |  |
DOI: 10.32604/ee.2022.019172
REVIEW
An Updated Review on Low-Temperature Nanocomposites with a Special Focus on Thermal Management in Buildings
1Faculty of Mechanical & Automotive Engineering Technology, Universiti Malaysia Pahang, Pekan, 26600, Malaysia
2College of Engineering, Universiti Malaysia Pahang, Pekan, 26600, Malaysia
3Research Centre for Nanomaterials and Energy Technology, School of Engineering and Technology, Sunway University, Petaling Jaya, 47500, Malaysia
4Joint School of Nanoscience & Nanoengineering, North Carolina A&T State University, North Carolina, Greensboro, 27401, USA
*Corresponding Author: M. Samykano. Email: mahendran@ump.edu.my
Received: 07 September 2021; Accepted: 13 December 2021
Abstract: Buildings contribute to 33% of total global energy consumption, which corresponds to 38% of greenhouse gas emissions. Enhancing building’s energy efficiency remains predominant in mitigating global warming. Advancements in thermal energy storage (TES) techniques using phase change material (PCM) have gained much attention among researchers, primarily to minimize energy consumption and to promote the use of renewable energy sources. PCM technology stays as the most promising technology for developing high-performance and energy-efficient buildings. The major drawback of PCM is its poor thermal conductivity which limits its potential use which could be resolved by dispersing conductive nanofillers. The acquired database on synthesis routes, properties, and performance of nano-dispersed phase change materials (NDPCMs) with various techniques presented in the paper should deliver useful information in the production of NDPCMs with desirable characteristics mainly for building construction applications. An outline of contemporary developments and use of NDPCMs as TES medium is delivered. Finally, a brief discussion on challenges and the outlook was also made. In-depth research is needed to explore the fundamental mechanisms behind the enhanced thermal conductivity of NDPCM with nanofillers dispersion and also a thorough investigation on how these mechanisms drive improvement in building performance.
Keywords: Thermal conductivity; latent heat; building applications; energy savings
Abbreviations
| 3D | Three Dimensional |
| Al2O3 | Aluminium Oxide |
| BAPV | Building Attached Photovolatic |
| BIPV | Building Integrated Photovolatic |
| CA | Capric Acid |
| CsxWO3 | Cesium Tungsten Bronze |
| CuO | Copper (II) Oxide |
| CuS | Copper (II) Sulfide |
| ESS | Energy Storage Systems |
| GHG | Green House Gas |
| GNP | Graphene Nanoplatelets |
| GO | Graphene Oxide |
| h-BN | Hexagonal Boron Nitride |
| HNT | Halloysite Nanotube |
| HPAC | Highly Porous Activated Carbon |
| HVAC | Heating, Ventilation, and Air Conditioning |
| LA | Lauric Acid |
| LH | Latent Heat |
| MA | Myristic Acid |
| MPCM | Microencapsulated Phase Change Material |
| MWCNTs | Multi-Walled Carbon Nanotubes |
| NDPCMs | Nano-Dispersed Phase Change Materials |
| PAHXs | PCM-to-Air Heat Exchangers |
| PCM | Phase Change Material |
| PV | Photovoltaic |
| rGO | Reduced Graphene Oxide |
| SA | Stearic Acid |
| SiO2 | Silicon Dioxide |
| SWCNTs | Single-Walled Carbon Nanotubes |
| TC | Thermal Conductivity |
| TES | Thermal Energy Storage |
| TiO2 | Titanium Dioxide |
| xGnP | Exfoliated Graphite Nanoplatelets |
Buildings account for one-third of global energy consumption and remain a key contributor to the carbon footprint. Heating Ventilation & Air Conditioning (HVAC) systems hold a massive 50% share in building energy consumption [1]. Air conditioners hold a significant role in mitigating human vulnerability to adapt to changing climate because of uncertain climatic events such as heatwaves. It is expected that by the year 2100, the percentage of buildings that have air-conditioning units installed will be closer to 99.9%, from its present 2% position [2]. The UN member states are obliged to curtail the fossil fuel by 20% to attain reduction in greenhouse gas emission as per the Paris Agreement made in 2015 [3]. Increased energy consumption of buildings and associated GHG emissions makes energy-efficient building materials and energy storage technologies the predominant requisite of the hour [4]. The European Union, in its energy road map for 2050 proposed that 66% of energy must be availed from zero carbon sources [3]. According to Eurostat data in 2015, a staggering 39% of the total energy consumption was dedicated to building-related structures (a detailed pattern is shown in Fig. 1) [5]. In addition to peak load aversion, energy storage systems (ESS) mostly improve mobility, reliability, and even financial merit. ESS can effectively support both energy security and reduction in global warming thereby offering an invaluable service to the world [6]. Normalizing the energy demand curve (transferring the energy demand from peak hours and reducing the total peak demand) is a potential solution. Innovative techniques like the usage of thermal energy storage may deliver thermal inertia and avert peak energy demands [7].
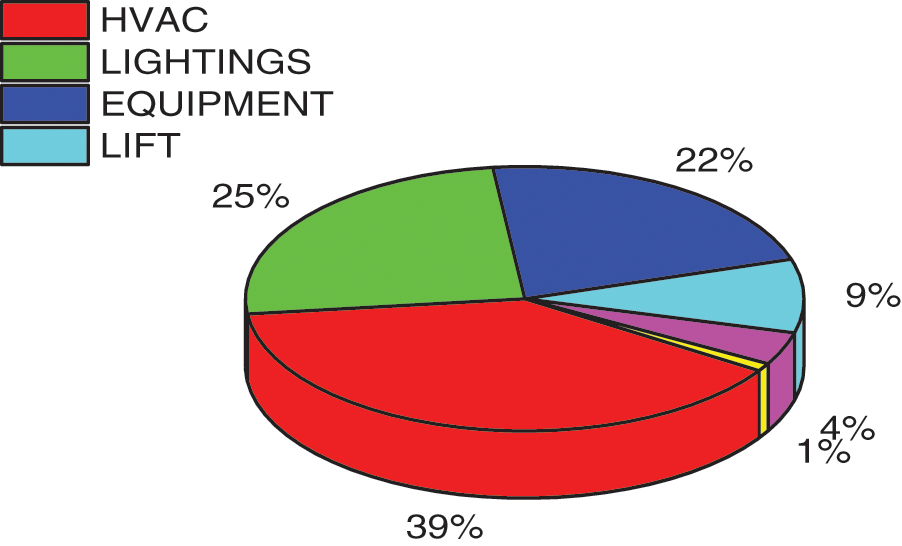
Figure 1: Energy consumption pattern in residential building sector [8]
Energy conversion systems rely heavily on thermal energy storage (TES) as it remains one of the vital systems for storing and retrieving energy. A reduction in fossil fuel usage and energy conservation could be attained with latent heat energy storage technology based on TES [9]. A paradigm shift towards green energy sources is of paramount importance as nonrenewable fuels attributes to 75% of greenhouse gas emissions [3]. Sensible heat storage and latent heat storage comes under TES [9,10]. TES permits a logical use of stored heat energy and delivers significant advantages like; cutting the running costs, swapping the peak load, reducing running costs, and even lowering GHG emissions [10]. TES system could be integrated with HVAC applications to deliver a shift in the thermal load from higher to lower conditions [11]. Phase change materials (phase transition shown in Fig. 2) inherent high energy storage density. Thermal conductivity, energy storage density, and melting temperature characterize the thermal performance of a PCM (classification of PCM is given in Fig. 3) [12]. It is highly advisable to have a PCM with swift melting and solidification rates for the proper functioning of a PCM-based thermal energy storage system. However, the thermal conductivity of commonly used PCMs remains comparatively low (paraffin-0.205 W/m.K [13]) which is the primary drawback that hampers their utilization [14].
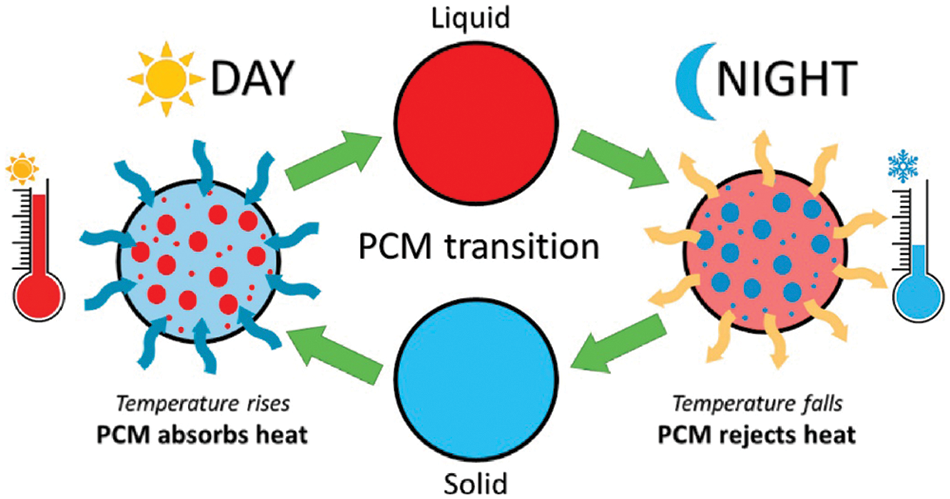
Figure 2: Phase transition of PCM in hot climate [5]
The inherent mass of the structure remains a major stakeholder in Thermal energy storage associated with it. Contemporary buildings maintain a heavier mass in comparison with current high-rise buildings, which thereby results in higher temperature fluctuations for indoor air temperature [7]. The Phase Change Materials (PCMs) integrated with structures could be used for improving the heat storage density of buildings [15]. The phase transition temperature of the PCM primarily helps in the building’s thermal management. PCMs could be deployed either as active systems (HVAC systems) or passive systems (heat dissipation methods) [1] and a lot of parameters (cost, availability, energy price) depends on their implementation [7]. Passive design delivered maximal energy efficiency as it relies on an abundant, clean, and reliable energy source, the Sun [16].
To outrun the problem of poor thermal conductivity in PCMs various thermal energy transport enhancement techniques have been attempted. Dispersion of fins, heat pipes, foams, micro, macro, and even nanoencapsulation, application of multiple PCMs, and adding high thermally conductive materials. Among these dispersing, highly conductive nano-sized materials in PCMs significantly enhances its thermal conductivity
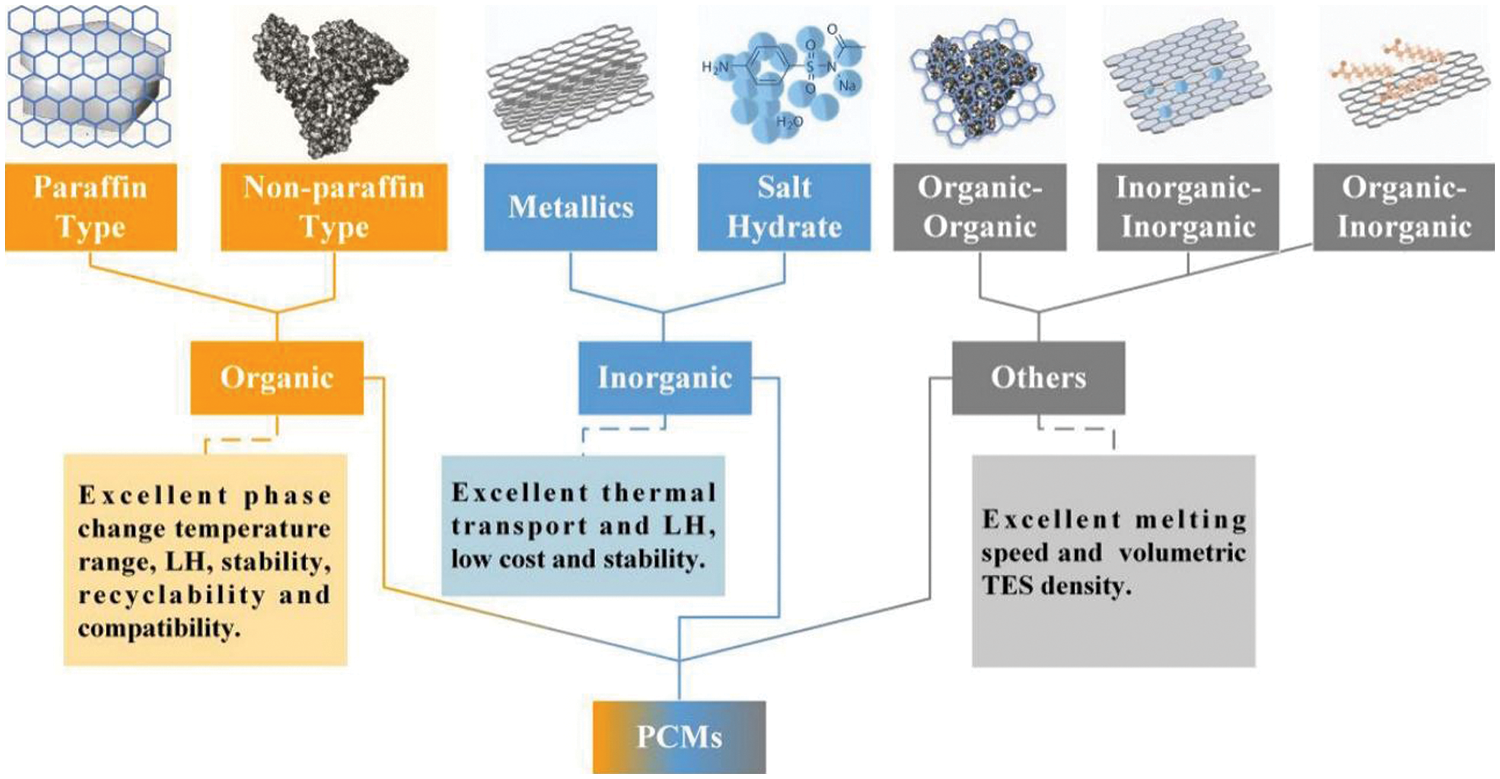
Figure 3: Classification of PCM [21]
Ma et al. [22] made the lone attempt to review the building applications of NDPCMs. An outline of contemporary development and application of NDPCMs, when used as TES media, was provided. Following are prior review attempts on PCM usage on the building. Raquel et al. [23] made a comprehensive review to explore the relation between phase change materials, energy deficiency, and energy efficacy. Applications of PCM in glazed zones, ceiling areas, walls, and floors of the buildings were detailed and mentioned about PCM selection criteria. Kumar et al. [24] primarily analyzed micro-encapsulated PCM-based research works and observed an improvement in thermal efficiency with MPCM. Real-time testing with mortar, brick, cement, wallboard, gypsum, and MPCM-based simulation works were reviewed. Li et al. [25] condensed the research articles discussing the optical and thermal efficiency of PCM-based glazing units. Experimental and simulation works with glazing units containing PCM were analyzed. Challenges and upcoming works in the area were also presented. Dardir et al. [26] summarized the publications made mainly on free cooling in buildings using PCM incorporated air heat exchangers. Merits and demerits of PCM containing air heat exchangers (PAHXs) for the desert climate were discussed in detail. The effect of phase change temperature, inlet temperature, and air flow rates on PAHXs free cooling in buildings were also explained. Berardi et al. [27] attempted to review the usage of PCM in concrete for building applications. Microencapsulation and macro encapsulation methods for avoiding PCM leakage were also explained in the concrete-PCM perspective. Jimenez et al. [28] reviewed studies related to numerical thermal modeling of conventional solar chimneys and solar chimneys with phase change materials (PCM).The tools for analysis were Computational fluid dynamics (CFD) and Global energy balance (GEB), of which CFD was found to be a powerful tool to investigate numerous aspects concerning the heat transfer mechanisms in a solar chimney. Rathore et al. [29] made a comprehensive review on energy-efficient microencapsulated PCM for buildings and Materials for encapsulation. The potential of PCM in indoor thermal management of buildings was also analyzed. Shah et al. [30] condensed research works on NDPCMs that could be employed in building applications. Kasaeian et al. [31] summarized and critically analyzed research articles on integrating PCMS and NDPCMs onto buildings.
This review article is trying to focus on research works that had reported variations in thermophysical properties of PCM when dispersed with nanoparticles. Numerous research papers were published on PCMs and NDPCMs. Among these published ones, only a handful of them were focusing on low temperature PCMs. Moreover, a few of them have highlighted low temperature thermal energy storage applications of NDPCM. This review paper tries to highlight the research works where NDPCMs have delivered a competitive edge over PCMs in thermal management of buildings. It also provides a deep insight into the thermophysical characteristics of NDPCMs. A detailed discussion on the impact of nanoparticle dispersion on thermal conductivity and latent heat along with its reasons for variations are also briefed. As per authors knowledge the different methods adopted for thermal management in buildings using nano composite phase change materials are summarized for the first time. A comprehensive and in-depth coverage on thermophysical properties of low temperature NDPCMs makes this article substantial and different when compared with other available reviews in the same domain.
This review paper attempts to summarize the relevant research articles published where the usage of NDPCMs had made a profound impact on the thermal management of buildings. The generic problems and key issues related to the use of this new class of materials in buildings for improved indoor thermal performance and enhanced energy efficiency are discussed. The review article is structured in the following pattern. A brief overview of energy usage patterns in buildings and methods to mitigate them are provided in Section 1. A synopsis on different synthesis routes for low temperature is given in Section 2 with a detailed focus on two-step method. Section 3 provides a discussion of reasons for variations in thermal conductivity and latent heat. Thermal management applications of NDPCMs are systematically condensed in Section 4. Section 5 delivers the challenges, and a conclusion is given at the end.
2 Synthesis Routes for Nano-Dispersed Phase Change Materials
The synthesis of nanocomposites does not include the mere incorporation of nanofillers into a matrix. For obtaining consistent dispersion, appropriate mixing and stabilization approaches are necessary. NDPCMs can be synthesized either by one-step or two-step methods. In one-step method, nanomaterial production and dispersion occur simultaneously in the base PCM, and in the two-step method the nanofillers are synthesized first, and then added into the base. The two-step method remains further economical, and it is widely preferred in NDPCM synthesis [14,32].
Nano additives were synthesized and dispersed simultaneously in one-step method. Drying, storage, transportation, and mixing of nanoparticles in PCM are not adopted in this method. It can reduce the particle agglomeration issue [32]. This synthesis pattern is preferred for small-scale production as it is costly in comparison with two-step method. Xu et al. [33] adopted one-step method (shown in the Fig. 4), to synthesize CuS decorated MWCNT dispersed in Paraffin. Calculated amounts of CuO, sublimation sulfur, oleic acid, paraffin, and MWCNT were added to a three-distillation flask of 50-ml capacity. The flask was stirred continuously for 3.5 h at 180°C, and the resultant was a NDPCM of Cus-MWCNT/paraffin. Several researchers [34,35] had followed similar synthesis routes.
First nano additives are collected in dry powder form using various mechanical and chemical methods (chemical reduction, sol-gel, and ball milling) in two-step method. The nano additives are then added to the base matrix for getting a consistent dispersion (ultrasonic bath magnetic stirring and high shear mixing). The main advantage of this method is that it can synthesis NDPCMs on a large scale. Particle agglomeration stays as a major setback with this method [32].
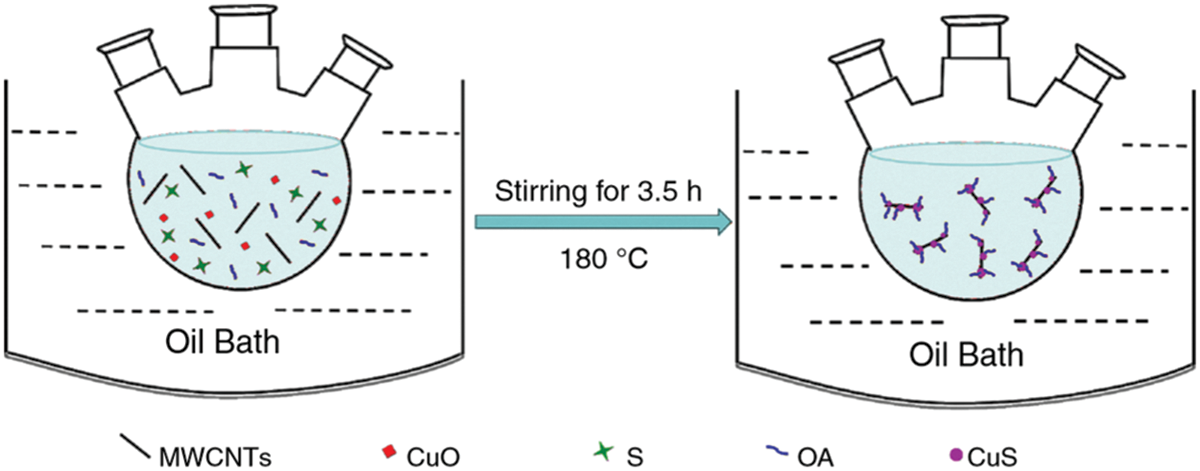
Figure 4: One-step synthesis
The consistency of nanocomposite relies on the nature of nanofillers and base PCM. Nanofillers can be hydrophobic/hydrophilic and base matrix could be polar/non-polar. Hydrophilic nanofillers (oxides) can be easily dispersed into polar base fluids (water) and hydrophobic nanofillers (MWCNTs) can be dispersed in non-polar base fluids (oils) with no surfactant. However, when hydrophobic nanoparticles had to be dispersed into polar base fluids and hydrophilic in non-polar base fluids, then surfactants are needed to stabilize the nanocomposite (in liquid state) [36,37]. Surfactants stay as a bridge between nanofillers and PCMs and create continuity between both [38]. Surfactants contain hydrophilic and lipophilic groups. They modify the surface properties of the particles and prevents cluster formation [39]. Fig. 5 shows the classification of surfactants. Surfactants generate a barrier and minimize surface tension between liquid PCM and dispersed nano additives. The repulsive forces and zeta potential generated due to surfactant stabilize the composite in the liquid state [36]. Surfactants are divided into anionic, cationic, non-ionic, and amphoteric [40]. At high temperatures, surfactants might exhibit altered properties or even generate foam [41]. They might cause a decline in thermal conductivity values with the thermal resistance created among nanofillers and PCM [42]. The different synthesis methods of low-temperature NDPCMs using surfactants are discussed in Table 1.
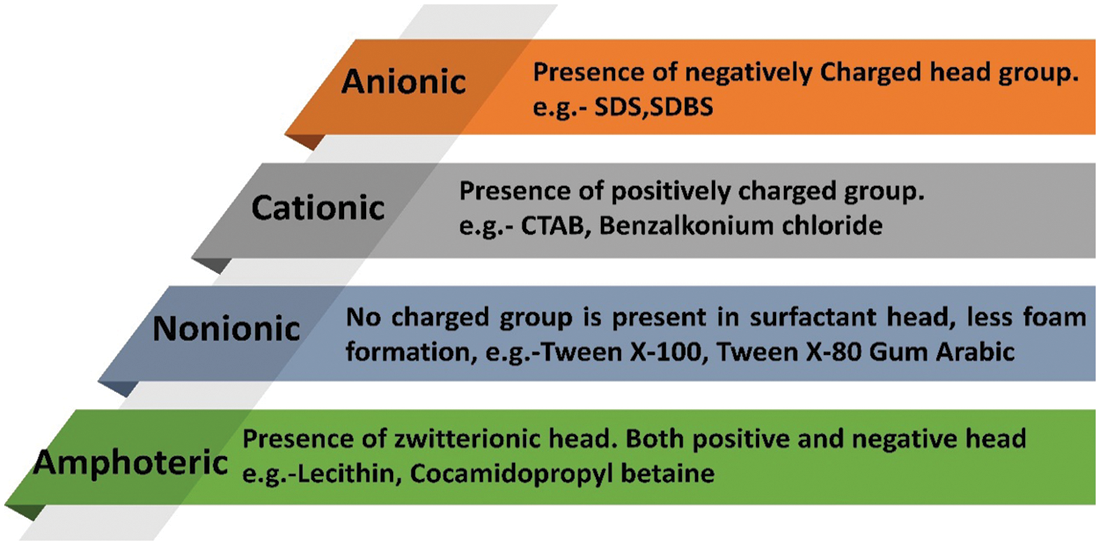
Figure 5: Classification of surfactants

The various two-step methods widely adopted for synthesizing low-temperature nanocomposites are briefed below. Karaipekli et al. [49] initially transferred expanded pearlite to a flask integrated with a vacuum system. N-eicosane was gradually added to the flask in the presence of air for half an hour to facilitate infiltration of n-eicosane. The composite was then allowed to cool for a day to solidify the n-eicosane entirely within expanded perlite pores. The mass fractions of n-eicosane were varied from 30%–70% until a leakage-free sample was obtained. A composite obtained with 60% of eicosane was found to be form stable. A calculated quantity of CNT was dispersed in acetone using ultrasonication during the second step. To the previously prepared form stable PCM, CNT/acetone was added and stirred continuously for 180 min with a magnetic stirrer. Finally, using oven (60°C for 360 min) acetone was removed. The same synthesis method was also used in the following study [50]. Li et al. [51] dissolved anhydrous calcium chloride in pristine water with a molar ratio of 1:6. Strontium chloride hexahydrate (2 wt%.) was added as a nucleating agent. The mixture was put to a temperature of 50°C and stirred (magnetic stirrer at 300 rpm) for 15 min. To promote crystallization, the mixture was cooled down to 40°C. For adsorption of CaCl2·6H2O onto porous SiO2 nanoparticles, the former was melted down entirely at 50°C, and SiO2 nanoparticles were gradually added to the PCM matrix. To ensure uniform dispersion of nanoparticles in PCM, the mixture was again put to magnetic stirring for half an hour at 300 rpm. Finally, the mixture was cooled down to obtain the Form stable NDPCM. Li et al. [52] mixed CNT(nitric acid-treated)/ethanol suspensions in stearic acid/ethanol (shown in Fig. 6). The mass of CNTs was varied from 50%, 33%, and 25%, then subjected to ultrasonic treatment for half an hour. Then the mixture was stirred for 4 h under 70°C in a water bath. Finally, the mixture was made ethanol-free by vacuum drying at 80°C for 24 h. Researchers [53,54] had also followed a similar synthesis method. Kazemi et al. [44] melted pure paraffin thoroughly, and MWCNT with parameters OD < 8 (nm), length 30 (nm) were added to PCM. The suspension was put to an ultrasonic bath for 30 minutes to prevent agglomeration. Gum Arabic (surfactant) was added to ensure proper dispersion of nano additives. The mixture was then sonicated to 1.5 h which yields the NDPCM. This is the most widely preferred two-step synthesis route [48,55]. Su et al. [56] prepared a eutectic mixture of octadecane and stearic acid based on the lowest eutectic point theory. The optimum ratio for the eutectic of SA:ODE was estimated as 2:98 at a phase transition temperature of 25°C. The mixture was heated up to 70°C followed by intense stirring for 5 min until the entire mixture was melted. The mixtures were cooled down to promote crystallization at room temperature. Estimated quantities of hBN were added to the liquid eutectic mixtures and stirred vigorously for 10 min at 70°C for uniform dispersion of nanoparticles into the eutectic mixture. A similar route was adopted in this study [57].
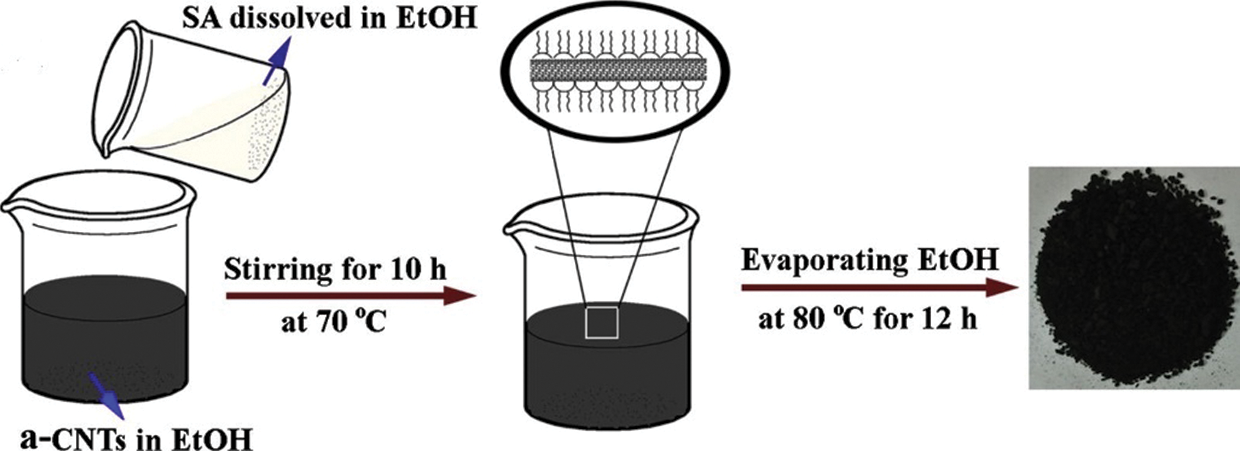
Figure 6: NDPCM synthesis by Direct Impregnation method [52]
The various two-step methods widely adopted for synthesizing low-temperature nanocomposites are briefed below. Karaipekli et al. [49] initially transferred expanded pearlite to a flask integrated with a vacuum system. N-eicosane was gradually added to the flask in the presence of air for half an hour to facilitate infiltration of n-eicosane. The composite was then allowed to cool for a day to solidify the n-eicosane entirely within expanded perlite pores. The mass fractions of n-eicosane were varied from 30%–70% until a leakage-free sample was obtained. A composite obtained with 60% of eicosane was found to be form stable. A calculated quantity of CNT was dispersed in acetone using ultrasonication during the second step. To the previously prepared form stable PCM, CNT/acetone was added and stirred continuously for 180 min with a magnetic stirrer. Finally, using oven (60°C for 360 min) acetone was removed. The same synthesis method was also used in the following study [50]. Li et al. [51] dissolved anhydrous calcium chloride in pristine water with a molar ratio of 1:6. Strontium chloride hexahydrate (2wt.%) was added as a nucleating agent. The mixture was put to a temperature of 50°C and stirred (magnetic stirrer at 300 rpm) for 15 min. To promote crystallization, the mixture was cooled down to 40°C. For adsorption of CaCl2·6H2O onto porous SiO2 nanoparticles, the former was melted down entirely at 50°C, and SiO2 nanoparticles were gradually added to the PCM matrix. To ensure uniform dispersion of nanoparticles in PCM, the mixture was again put to magnetic stirring for half an hour at 300 rpm. Finally, the mixture was cooled down to obtain the Form stable NDPCM. Li et al. [52] mixed CNT(nitric acid-treated)/ethanol suspensions in stearic acid/ethanol (shown in Fig. 6). The mass of CNTs was varied from 50%, 33%, and 25%, then subjected to ultrasonic treatment for half an hour. Then the mixture was stirred for 4 h under 70°C in a water bath. Finally, the mixture was made ethanol-free by vacuum drying at 80°C for 24 h. Researchers [53,54] had also followed a similar synthesis method. Kazemi et al. [44] melted pure paraffin thoroughly, and MWCNT with parameters OD < 8 (nm), length 30 (nm) were added to PCM. The suspension was put to an ultrasonic bath for 30 min to prevent agglomeration. Gum Arabic (surfactant) was added to ensure proper dispersion of nano additives. The mixture was then sonicated to 1.5 h which yields the NDPCM. This is the most widely preferred two-step synthesis route [48,55]. Su et al. [56] prepared a eutectic mixture of octadecane and stearic acid based on the lowest eutectic point theory. The optimum ratio for the eutectic of SA:ODE was estimated as 2:98 at a phase transition temperature of 25°C. The mixture was heated up to 70°C followed by intense stirring for 5 min until the entire mixture was melted. The mixtures were cooled down to promote crystallization at room temperature. Estimated quantities of hBN were added to the liquid eutectic mixtures and stirred vigorously for 10 min at 70°C for uniform dispersion of nanoparticles into the eutectic mixture. A similar route was adopted in this study [57].
Researchers are also going on in the direction of employing functionalized nanoparticles to enhance dispersion stability. Sonication is the most widely preferred two-step synthesis route. Generally, for form stable NDPCMs vacuum impregnation is widely preferred as it delivers maximum adsorption.
3 Thermophysical Characterization
The characteristics (shape, size, loading concentration) of nano additives alter the thermophysical properties of dispersing PCM matrix. Hence the evaluation and determination of thermophysical properties is a crucial factor [14,22]. The major thermophysical properties of low-temperature nanocomposite phase change materials are summarized in Table 2.

3.1 Impact of Nanoparticle Dispersion on Thermal Conductivity
Nanotechnology along with its attained advancements had enabled particles synthesis even at a nanoscale (10−9). These nanoparticles mainly comprise of metallic, metallic oxide, carbon nanotube (SWCNT, MWCNT), graphene, graphite, and even hybrid ones [14]. The classification of nanoparticles is given in Fig. 7. Nanoparticles possess some signature properties in contrast to solids due to their exorbitant surface area-volume ratio [66]. The dispersion of nanofillers with extremely high thermal conductivity (monolayer graphene 5300 W/m.K [67]) is anticipated to enhance the thermal energy transport in PCM. The amount of nanofillers dispersed must be good enough to generate thermally conducting pathways [68]. The formed thermally conducting paths facilitate the heat transfer process within the composite and this was seconded by Zhang et al. [69]. Dispersing highly conductive nanofillers does not guarantee a nanocomposite with superior conductivity. Factors like the homogenous distribution of conductive fillers in the PCM should be taken into consideration. Particle accumulation causes improved Van der Waal forces among nanofillers and this force eventually attracts the nanofillers and they remain clustered [70].
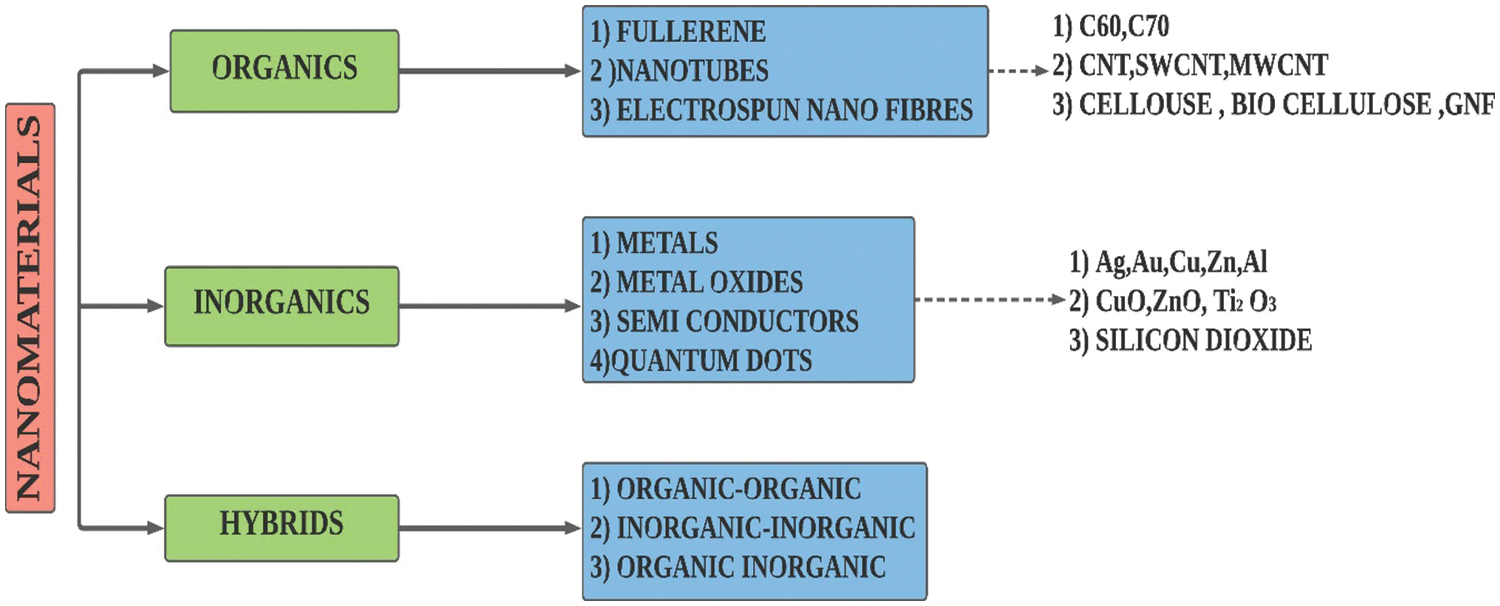
Figure 7: Classification of nanoparticles
Ashiq et al. [59] synthesized NG/paraffin composites with 146% improvement in thermal cond-uctivity and they provided thermal comfort for more than 4 h and 2 h at ambient temperatures of 35°C and 40°C. Arshad et al. [43] reported a downward drift in the λ value of paraffin and nanocomposites along with rising temperatures. The phase transition turns the organized microstructured pure paraffin (solid-phase) into a disorganized microstructured (liquid-phase) paraffin. The thermal transport in solids is mainly attributed due to lattice vibration when the molecules vibrate inside their lattice structure. Even at a lower temperature the particle inside a crystal vibrates near the equilibrium position. So, the PCM lattice could be considered as a coupled mass and spring system. When the crystal is subjected to heat the vibrations will deviate from equilibrium position. The generated high energy phonons diffuse, and it forms the temperature gradient. Thus, in the presence of a temperature gradient heat will drift from hot end to cold end [71,72]. The free-electron motion and lattice vibration are prominent in solids than liquids. Hence solids normally have a greater λ value than liquids. Arshad et al. [62] dispersed different carbon nanoparticles in paraffin to form nanocomposite and obtained thermal conductivity increment in the range of 180%. Numerous factors account for this increment and are concentration, interfacial thermal resistance high aspect ratio, clustering, Brownian motion, purity, and intermolecular interaction [73]. A longer phase transition time may result in bigger micro-scale grains formation; which shortens thermal resistance between layers leading to an increment in λ value [74]. According to Zhang et al. [50], the addition of multi-layer graphene (MLG) accounts for the 282% increment in λ value in the composite. Due to the planar morphology of graphene nanosheets, the thermal interface resistance got reduced in n-eicosane/graphene PCM composites, which introduces an increment in heat transfer of samples during heating and cooling [75,76]. The improved thermal conductivity accounts for a reduction of 45.7% and 67.1% in thermal storage and release times of C20/MLG45 when compared with pure PCM.
Li et al. [51] used nano-silica of different pore sizes to synthesize form stable composites. The λ value of form stable PCM got reduced with a reduction in pore diameter despite an increase in nanoparticles loading rate. It could be due to phonon-phonon scattering at the interface of nanoparticles and composites. This produces thermal resistance in non-metallic materials and gets intense along with a large specific area [77].
According to Motahar et al. [64], the thermal conductivity of both PCM and the nanocomposite (octadecane/TiO2) counts on temperature and it becomes intense at a temperature range of 25°C–30°C where the crystalline structure of PCM is unsteady. The λ value varied from 0.375 W/m.K–0.394 W/m.K and 0.144 W/m.K–0.150 W/m.K when the sample temperatures were 5°C and 55°C. The λ value was 0.852 W/m.K near the melting point. Arshad et al. [43] accounted for this abrupt rise. A similar phenomenon was previously reported by Wang et al. [78] with palmitic acid/CNT composite. Higher thermal conductivity of the composite close to phase transition temperature is highly desirable in thermal energy storage applications using PCMs. Xie et al. [48] reported a 30% reduction in thermal conductivity with calcium chloride hexahydrate/cesium tungsten bronze nanocomposite. Strontium chloride hexahydrate was used as the nucleating agent. The probable causes for reduced thermal conductivity could be the interface resistance among components hinder the rise in conductivity with low nanoparticle concentration and the elevated viscosity caused by surfactant dampens the heat transfer with 1.5 wt.% SiO2 CA–MA. Martin et al. [55] had an outstanding 142% rise in thermal conductivity. The thermal conductivity varies linearly along with nanoparticles content (0–1.5 wt.%) This could be detailed by phenomena like phonon interaction, clustering of nanoparticles (proved by SEM results) Brownian motion, and surface morphology effects which all could be the reasons for this enhancement [79,75].
Arshad et al. [43] analyzed the
3.1.4 Discussion on Thermal Conductivity
The
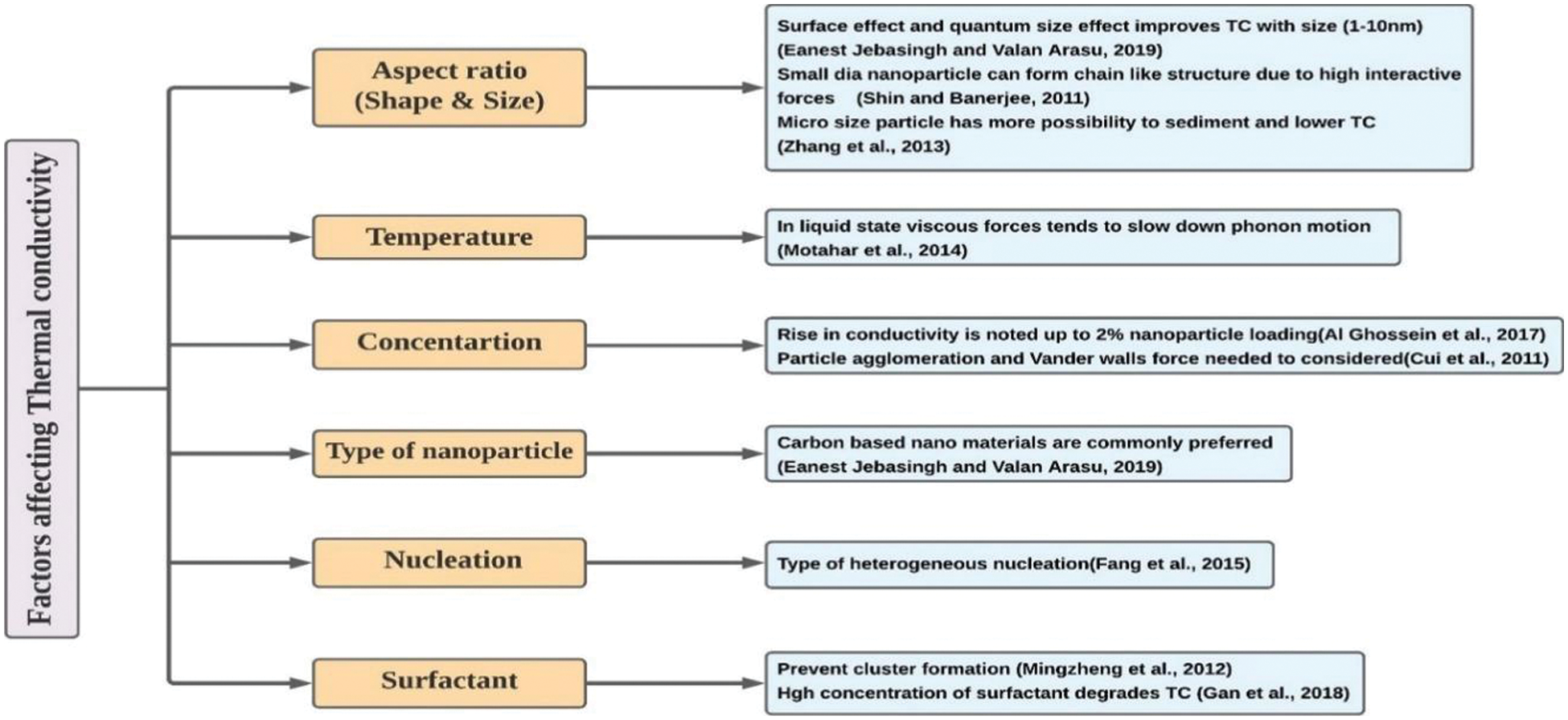
Figure 8: Significant factors influencing the thermal conductivity of nanocomposites
3.2 Impact of Nanoparticle Dispersion on Latent Heat
The predominant property of PCMs is their comparatively higher latent heat capacity and this privilege of PCMs could be availed when it undergoes a phase transition process. However, the degraded
Putra et al. [58] used theories like interfacial liquid layering, particle clustering, and Brownian motion to justify the decline in melting enthalpy and specific heat capacity of the nanocomposites. The interfacial liquid layering theory explains that the dispersion of nano additives could affect the molecular structure of base fluid. A solid layer was created by base fluid molecules. The stretching of molecular bonds in nearby molecules causes the solid layer to get exposed to low energy to separate molecular bonds in melting and freezing. The Brownian motion explains that the rapid movement of nano additives in the base fluid weakens the bonding in base fluid, so it takes less energy to melt and freeze [14]. The theory of particle clustering was related to the decline in melting enthalpy and specific heat capacity of the composite and the clustering strengthens the thermal conductivity. However, nanographene particles attract paraffin to form particle groups that could minimize the energy for melting and freezing because of weak molecular bonds [74]. Thermophysical properties were stable even after 1000 cycles. Jeon et al. [60] synthesized nano-composites with hexadecane xGnP. The Latent heat of hexadecane decreased slightly with xGnP loading. This is because of the good dispersion of xGnP in hexadecane with a high surface area and nanoparticle size. With Hussain et al. [61] the latent heat of nanocomposites got declined around 5% and was due to the physio-chemical changes in nano-composites. The stability of the composite was evaluated even after 200 cycles and the latent heat remained constant. Dispersion of the highly porous activated carbon (HPAC) suppresses the sub-cooling effect in eutectic PCM and improves the
With nano-silica/CaCl2·6H2O nano-composite, Li et al. [51] had a 31% decline in latent heat. The relative error among experimental and calculated latent heat tend to follow a linear pattern along with the pore diameter of nano-SiO2. The potential reason behind this phenomenon could be; the restricting effect of SiO2 tends to get weak, as pore diameter rises [84]. Arshad et al. [46] accounted for a decline in latent heat during melting and solidification process. The TiO2 nano additives take over the paraffin molecules resulting in a reduction in melting enthalpy of NDPCM. As the interaction between TiO2 and paraffin gets stronger, the higher will be the latent heat capacity of NDPCM. Using advanced wet impregnation technique, Saxena et al. [53] synthesized nonadecane/rutile TiO2 nano-composites. Along with nanoparticle dispersion, a reduction of phase transition temperature was noted which, was due to enhanced thermal conductivity (37%). A 7.6% fall in latent heat capacity for NDPCMs was attributed due to the addition of nanofillers which were solid and does not undergo a phase transition in the melting range of PCM. Li et al. [47] added γ-Al2O3 nanoparticles and SrCl2·6H2O (nucleating agent) in CaCl2·6H2O to form nanocomposites. Latent heat got declined by 12.7%. The dispersion of γ-Al2O3 nanofillers caused a fall in the degree of supercooling, and a maximum enhancement in thermal conductivity. The degree of supercooling of CaCl2·6H2O/γ-Al2O3 nanocomposite lies in the range of 0°C–2°C, which indicates that surplus latent heat was liberated from the nanocomposites.
Arshad et al. [43] reported a decrease in melting enthalpy for nanocomposites and was due to the increment in
3.2.4 Discussion on Latent Heat
In general, nanofiller dispersion in salt hydrates minimizes the stored enthalpy whereas with organic PCMs this decrease remains relatively low. Thus, the energy storage performance of the salt hydrate remains untouched as the latent heat values remain over 100 kJ/kg. The ratio of effective PCM in a nanocomposite that can successfully store heat by completing solidification is provided by the term crystallization factor Fc. When a part of PCMs got confined in the porous structure of the matrix, (disorder PCMs) and cannot complete the crystallization, it leads to a decrease in Fc value [85]. A lower shift in crystallinity values of the composite means that the incorporated PCM can still work efficiently even though the content of nanofillers in the composite was raised to a higher level. Furthermore, thermal properties got enhanced, as the thermal conductivity got improved and the subcooling degree was reduced or eliminated.
4 Thermal Management in Buildings with Nano Composite Phase Change Materials
As per International Energy Agency reports, the global energy consumption trends are expecting a rise of 44% for 1.5 decades from 2016 [86]. Buildings incur almost 30%–40% of energy usage in the European Union which accounts for 30% of greenhouse gas emissions. In buildings, 60% of the energy is designated for HVAC systems [30,87]. The ultimate necessity of low energy consumption paved the way for new methods of temperature management in buildings [87]. To store and release excess thermal energy PCMs could be deployed. High performance together with the energy-efficient building was made possible with PCMs. They primarily store energy as latent heat and possess quite compatible energy storage density [88]. However, the conventional PCMs possess a low heat transfer rate and it can be improved by dispersing thermally conductive nanoparticles [89].
Carneiro et al. [90] evaluated the heat transfer performance of a building wall model under the influence of TiO2 coated plastering mortar. When compared with the standard wall model the TiO2 coated mortar degrades the steady-state thermal behavior of the wall by 10% (for conduction thermal resistance) and 13% (for thermal transmission coefficient). Moreover, the exterior wall with TiO2 nanofillers on the façade enhances the water evaporation rate by 136%. A 136% increment in the water evaporation rate was observed with TiO2 nanofiller coated exterior façade
Parameshwaran et al. [91] synthesized a hybrid nanocomposite (Ethyl trans-cinnamate/silver–titania). The composite exhibited improved thermophysical properties. The NDPCM exhibited enhanced thermal stability than pure PCM. When compared with pure PCM freezing and melting duration of NDPCM got reduced by 23.9% and 8.5%. Energy efficiency and savings were obtained with freezing rate reduction. The viscosity got enhanced to 3.89% with a rise in hybrid nanoparticle loading This suggests that there exists a relative dependency between thermal properties and viscosity of the NDPCM.
According to Lechner active cooling methods are termed as “mechanical equipment to satisfy the needs (of cooling within a building) not provided by nature” [92]. Active methods mainly rely on electric power and heat as energy sources [92]. The heat energy could be the exhaust heat derived from other processes if available [93] or even from district heating systems. Moreover, renewable energy systems could be deployed to run the active devices [94]. Nonetheless, apart from fans, all active methods have significant energy demand [95]. Active thermal management applications for cooling in buildings are categorized as: free cooling, active solar façade, ventilated Trombe wall, thermally activated building structures with PCM (PCM-TABS), evaporative and radiative systems, geo cooling, and ice storage [5]. Said et al. [96] introduced a novel technique to improve the productivity of an AC unit by integrating a PCM heat exchanger with the condenser of the AC unit. Nano additives (Al2O3, CuO, and Cu) were dispersed into PCM. The cold ambient air at night was used to charge. The cold energy storage inside the NDPCM was charged by using cold ambient air at night. The hot air at daytime was cooled down by passing it through the NDPCM heat exchanger before it flows to the AC condenser. The AC unit delivered a maximum power saving of about 7.41% (NDPCM-Cu nanoparticles 5%) for an inlet temperature of 45°C. The best conditions for this method could be achieved with optimization studies. Parameshwaran et al. [97] used a MATLAB simulation environment to study the effect of silver nanoparticles dispersed in water on a variable air volume air conditioning system. For year-round operation, LHTES air conditioner with silver nanoparticles delivered an energy saving of 36%–58% on–peak and 24%–51% average per day. An improvement in the overall thermal performance of the system was noted. SVAV-AC gave energy saving of 7.5%–18.6% on-peak and 7.9%–17.8% average per day. In total, the combined air conditioning system was found to be beneficial in attaining good thermal comfort, acceptable indoor air quality, and energy redistribution needs of the building. Parameshwaran et al. [98] used simulations to investigate the performance of silver-titania/Dimethyl-adipate HiTES (hybrid nanocomposites based CTES) system integrated on a building HVAC system for year-round weather conditions. Thermal conductivity enhancement lies in the range (7.3%–58.4%). The complete freezing time of the hybrid nanocomposites got reduced by 15%. A 46.3% decline in chiller cooling capacity (under part load) and 39.6% (on-peak load) was noted. The novel system gained 27.3% and 32.5% reduction on-peak energy savings potential in summer and winter respectively. Reported an yearly energy savings of 12.2% in summer and 10.2% in winter.
4.2 Passive Thermal Management
Passive thermal management mention technologies/design features devised to reduce building temperature with zero or even with minimal energy consumption [99] to improve the structure’s energy efficiency [100]. The amount of energy consumption is small in comparison to active methods [99,100]. Moreover, renewable energy sources power them [100]. The use of PCM for passive thermal management comprises of integrating them in: free cooling, passive solar façade, building envelope components (floors, ceilings, walls, windows, shutters, and blinds) Trombe wall, solar chimney [5].
4.2.1 Building Impregnated Systems
Ashok et al. [101] used shape stable bio NDPCM to analyze the thermal energy penetration performance of NDPCM integrated buildings. The green buildings improve thermal comfort inside the building and conserve energy. A novel NDPCM integrated composite was introduced to a hollow solid concrete block. An experimental building of size 1 m3 was constructed to analyze the thermal effects of NDPCM (paraffin/Graphene) integration. Chitosan Shell was used as a shell to prevent leakage. The building integrated with NDPCM showed a maximum reduction of 5.3°C in room temperature when compared with building without NDPCM. The indoor temperature of the NDPCM integrated building increased by 1.8°C when the outdoor ambient temperature dropped below 23.6°C. The NDPCM integration delivered an improved thermal comfort in buildings for all seasons. Thus, NDPCM integration in buildings provides passive cooling of buildings along with energy savings. Ranjbar et al. [54] used the impregnation technique to synthesize heptadecane/SiO2 nanocomposite. The thermal performance of nanocomposite mixed with gypsum was analyzed by examining the effect on the indoor temperatures of the test room. The nanocomposite had excellent thermal cycling reliability and thermal conductivity. When the mass % of nanocomposite in the gypsum board was increased, the rate of temperature rise got reduced. In the test room, indoor temperature fluctuation curves declined from 41.2°C to 40.2°C, 37.4°C, and 35.5°C when the top gypsum board had 0, 1, 4, and 8 wt.%, of nanocomposites. These results showed that the A5 nanocomposite-gypsum composite had an excellent thermal performance. They could be deployed for TES and building thermal management. Sarrafha et al. [102] used the finite difference method to measure the effectiveness of nano encapsulated NDPCM (octadecane/MWCNT) integration in building walls. During the summer, autumn, and winter days, a 50.1%, 18.5%, and 39.7% increment in latent heat activation with NDPCM integration was observed. Moreover, a rise in the aspect ratio of MWCNT’s had a noticeable incremental effect on thermal conductivity, up to a certain limit for NDPCM loading rate. Li et al. [51] analyzed the thermal management potential of CaCl2·6H2O dispersed in silica for a laboratory-scale test chamber. When compared with pure PCM, NS1 needed the longest hysteresis time (about 7 min) before peak temperature was observed. During the cooling, the temperature curve of nanocomposites had a smooth decline. The form stable NDPCMs were able to reduce the indoor peak temperature greatly during the melting process and extended the duration of heat preservation during the cooling. FSNDPCMs could maintain suitable indoor temperatures when the external temperature is lower than the required internal temperature during winter. Hence the heating load could be drastically minimized. From these results it is evident that NS1 had relatively good thermal properties, making them efficient thermal management materials. Xie et al. [48] successfully reduced the room temperature using a NDPCM (CaCl2·6H2O/CsxWO3) kept between doubled layered windowpane of the test chamber. The loading rate of nanoparticles was varied from 0.25%–1.0% with an increment of 0.25%. The non-freezing liquid layer [103] (between PCM and nano-particles) accounts for the difference between calculated and experimental values of latent heat. There was a slight deviation in melting point with nanocomposites which can be neglected. The degree of supercooling was slightly reduced for nanocomposite which is primarily because nanoparticles acted as nucleating agents and facilitate heterogeneous nucleation and thereby increases the crystallization rate [104]. Too much loading of nanoparticles hinders crystal growth, and this is visible from the results. The thermal reliability was established by thermal cycler testing. After 100 cycles the latent heat was reduced by 4.1% and melting temperature by 1.75% which establishes the thermal reliability of the nanocomposite. The thermal conductivity got degraded from 0.732 W/m.K (pure) to 0.584 W/m.K (NDPCM). The degradation could be due to interface resistance between components (for low loading of nanoparticles). The elevated viscosity of CTAB also lowers the heat transfer rate. The glass window with NDPCMs delivered a 90%NIR radiation shielding. The indoor temperature was maintained between 22°C–28°C for a longer duration. Temperature fluctuations were minimized and exhibited thermal inertia. Li et al. [105] conducted both simulation and experimental studies to analyze the indoor performance of a double glazed window pane filled with NDPCM. The key findings were, (1) indoor temperature depends on nanoparticle size and concentration. (2) The impact is more significant in winter. (3) The energy consumption could be minimized to 1.5% in summer, 2% in autumn, and 4% in winter (based on NP volume fraction & size). The energy consumption rises with the volume fraction of nanoparticles and a fall in energy consumption was noted with large particle diameter. The lowest energy consumption was recorded with a nanoparticle concentration of 1% as well as a particle diameter of 100 nm with all seasons. The simulated results were found to agree with measured values. The temperature difference between the interior surface of glazed windows and the indoor environment was mainly in the range of 1°C–4°C for summer, 5°C–10°C for autumn, and 14°C–16°C for winter seasons. Venkitraj et al. [106] studied the performance of a building model integrated with macro-packed NDPCM (Neo Pentyl Glycol/0.1% CuO). The thermal conductivity got enhanced by 12.7% along with a reduction in charging and discharging time. An 11% decline in latent heat was observed with 100 thermal cyclings of the nanocomposite. The NDPCM integration was able to deliver a reduction of 3.5°C in room temperature. Li et al. [52] observed that the maximum temperature gained by SA/CNT composite was almost 133% higher when compared with pure stearic acid. The exposure duration in the solar simulator was 1250 s. The NDPCM got cooled down to 20°C by 2500 s. However, the final temperature of SA was 125% higher. This enhancement was due to enhanced photon capture and molecular heating by optical absorption of CNTs. The composite had high thermal management abilities, especially for buildings. Sayyar et al. [107] incorporated NDPCM (capric acid/palmitic acid- graphite nanosheets) into gypsum wallboard using a sandwich structure. Under simulated day and night conditions the thermal performance of gypsum wallboards incorporated with NDPCM was evaluated. The energy efficiency of NDPCM integrated tests cells (representing a scaled building). was estimated using a numerical model. The use of NDPCM in wallboards enabled (i) reduced fluctuations in interior temperature and (ii) shifted (delayed) the time for peak temperature attainment. With NDPCMs the energy demand for cooling was 544 J and for heating was 332 J. Energy demands for cooling (2224 J) and heating(1967 J) were comparatively high in the absence of NDPCM. A 79% reduction in indoor temperature and improvement in energy savings was noted. The indoor temperature was maintained within the comfort zone. Biswas et al. [108] used both numerical simulation and experiments to evaluate the performance of a prototype of shape stable NDPCM enhanced wallboard. The efficiency gained by the system was found to rise with a rise in temperature (19°C–23°C). The integration showed a reduction in cooling electricity consumption.
4.2.2 Building Integrated Systems
Photovoltaic (PV) systems used on buildings can be classified into two Building attached PVs (BAPVs) and Building Integrated PV(BIPV) [109]. BAPVs are simply attached to the buildings and do not have any structural functions. Conversely, BIPVs are PV modules, which can be integrated into building envelopes (roofs/façade) by swapping traditional construction materials [110]. Therefore, BIPVs have a significant impact on building functionality and could be recognized as an indispensable part of the building energy system. The electrical efficiency of BIPV declines with a rise in working temperature. The operating temperature must be maintained at standard test conditions to outrun this drawback. PCMs were found to be most appropriate and perfect for cooling PV cells when compared with other technologies as they possess good energy storage density, zero operating and maintenance cost, and zero external power required to operate the system [111]. The integration of the PCM with the PV panel delivers additional thermal comfort along with power savings [112]. The operating temperature of BIPV can be maintained steady and an improved efficiency was attained with PCM usage. However, the heat transfer rate of conventional PCMs were low and can be enhanced by dispersing thermally conductive nanomaterials [89].
Nada et al. [113] installed three PV panel configurations, a normal PV panel, a PV panel with PCM, and a PV panel with NDPCM(paraffin/Al2O3) mounted on a building wall. The results showed that in stand-alone PV, PV with PCM, and PV with NDPCM, the panel temperatures got minimized by 1.8°C and 10.6°C, respectively. Their efficiency was improved by 5.7% and 13.2% when compared with PV panels. Sharma et al. [114] attempted to deliver passive thermal management in Building-Integrated Concentrated Photovoltaics using micro fins, PCM, and NDPCMs (paraffin/CuO). A 12.5°C reduction in temperature at the center of the systems was noted using micro-fins with NDPCM as compared to the micro-fins and PCMs had a reduction of 10.7°C. The NDPCM with the un-finned surface had a temperature reduction of 11.2°C whereas with the un-finned PCM the reduction was 9.6°C. Ma et al. [22] dispersed a 10% volume fraction of Cu nanoparticles in paraffin. A lumped enthalpy model was used for PCM modeling. Mathematical models were used in estimating the thermal conductivity (Maxwell model) and latent heat of NDPCM. The building system simulation of the building system was done using TRNSYS. The amount of heat absorbed showed an 8.3% increment with NDPCMs (23.85 kWh) when compared with pure PCM (22.03 kWh). Similarly, the heat discharged was also higher with NDPCMs. NDPCMs (10.66 kWh) discharged 25.1% more heat when compared with PCMs (8.52 kwh). The tests were performed in winter for a duration of three days. Kant et al. [89] used computational fluid dynamics to analyze the impact of various nanofillers on the working temperature of a BIPV. Octadecane dispersed in Al2O3, Cu, CuO, and TiO2 were used as nanocomposites. The result established that the dispersion of nanofillers in an optimum quantity greatly minimized the working temperature of BIPV for a prolonged time. The maximal temperature fall was noted with Cu and the minimal with TiO2. The panel temperature was kept below 40°C for 1 h with 5% Cu nanoparticles. NDPCM minimized the working temperature of the PV panel when compared with pure PCM. A maximal operating temperature difference of 1.65°C, 1.19°C, and 1.15°C was noted for 3 days at around 12.50 PM.
Most studies implied that the thermal conductivity of NDPCM stays comparatively higher than the base material [115]. A high-grade thermal conductivity can compensate for negative effects like sub-cooling. The most common synthesis route is sonication in which the power used, and duration remains different in almost all cases. A uniform protocol for synthesis must be devised. The latent heat behavior of NDPCM must be well studied. Most of the studies indicated that nanoparticle dispersion degrades the latent heat and vice versa. In-depth, studies must be done to address the inconsistency in the findings. The reliability of thermophysical properties must be done with required cycling tests. The optimum nanofiller concentration which can deliver both maximum thermal conductivity and minimal degradation of latent heat must be identified. Thermal cycling may affect nanoparticles dispersion where the crystallization structure of NDPCM could be altered and may eventually affect entire thermophysical properties. The leakage of NDPCMs during phase transfer was averted with form stable NDPCMs. The supporting skeleton used lowers the thermal conductivity. Particle agglomeration in NDPCM relies on repulsive and Van der Waals forces. During heating, Van der Waals forces (attractive force) in nanofillers becomes weak and the repulsive force becomes stronger. Consequently, it reduces the agglomeration tendency of nanofillers. The repulsive force varies linearly along with the temperature. During cooling, the nanofillers tend to agglomerate and sediment, which could be averted by shortening the solidification time. Under a short solidification time, nanofillers are restrained from moving close to each other or even falling due to gravitational force. Further study on this aspect should be done. Moreover, researchers should also focus on the safety aspect of NDPCM towards human health and the environment since the fillers being used are at the nano level. Also, a Techno-economic analysis, life cycle analysis (LCA), and life cycle cost analysis (LCCA) must be done to justify the application of nanocomposites. The simulation and porotype studies conducted in applications must be integrated to a larger level to prove their viability.
During the selection of PCM/NDPCM researchers should consider factors like
Funding Statement: The authors would like to acknowledge Universiti Malaysia Pahang (UMP) for the financial assistance given under RDU 213308 and DRS, Sunway University through Sunway University’s International Research Network Grant Scheme (IRNGS) 2021 (STR-IRNGS-SET-RCNMET-01-2021) for carrying out this work.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Bhamare, D. K., Rathod, M. K., Banerjee, J. (2020). Numerical model for evaluating thermal performance of residential building roof integrated with inclined phase change material (PCM) layer. Journal of Building Engineering, 28, 101018. DOI 10.1016/j.jobe.2019.101018. [Google Scholar] [CrossRef]
2. Akpinar-Ferrand, E., Singh, A. (2010). Modeling increased demand of energy for air conditioners and consequent CO2 emissions to minimize health risks due to climate change in India. Environmental Science and Policy, 13(8), 702–712. DOI 10.1016/j.envsci.2010.09.009. [Google Scholar] [CrossRef]
3. Jacob, J., Pandey, A. K., Rahim, N. A., Selvaraj, J., Samykano, M. et al. (2021). Concentrated Photovoltaic Thermal (CPVT) systems: Recent advancements in clean energy applications, thermal management and storage. Journal of Energy Storage, 45(5), 103369. DOI 10.1016/j.est.2021.103369. [Google Scholar] [CrossRef]
4. Khan, R. J., Bhuiyan, M. Z. H., Ahmed, D. H. (2020). Investigation of heat transfer of a building wall in the presence of phase change material (PCM). Energy and Built Environment, 1(2), 199–206. DOI 10.1016/j.enbenv.2020.01.002. [Google Scholar] [CrossRef]
5. Faraj, K., Khaled, M., Faraj, J., Hachem, F., Castelain, C. (2020). Phase change material thermal energy storage systems for cooling applications in buildings: A review. Renewable and Sustainable Energy Reviews, 119, 109579. DOI 10.1016/j.rser.2019.109579. [Google Scholar] [CrossRef]
6. Alizadeh, M., Sadrameli, S. M. (2016). Development of free cooling based ventilation technology for buildings: Thermal energy storage (TES) unit, performance enhancement techniques and design considerations--A review. Renewable and Sustainable Energy Reviews, 58(4), 619–645. DOI 10.1016/j.rser.2015.12.168. [Google Scholar] [CrossRef]
7. Wang, H., Lu, W., Wu, Z., Zhang, G. (2020). Parametric analysis of applying PCM wallboards for energy saving in high-rise lightweight buildings in Shanghai. Renewable Energy, 145(1), 52–64. DOI 10.1016/j.renene.2019.05.124. [Google Scholar] [CrossRef]
8. Department of Environment and Energy (2013). HVAC energy breakdown. HVAC Hess, 36–37. [Google Scholar]
9. Oró, E., de Gracia, A., Castell, A., Farid, M. M., Cabeza, L. F. (2012). Review on phase change materials (PCMs) for cold thermal energy storage applications. Applied Energy, 99, 513–533. DOI 10.1016/j.apenergy.2012.03.058. [Google Scholar] [CrossRef]
10. Heier, J., Bales, C., Martin, V. (2015). Combining thermal energy storage with buildings--A review. Renewable and Sustainable Energy Reviews, 42(2), 1305–1325. DOI 10.1016/j.rser.2014.11.031. [Google Scholar] [CrossRef]
11. Hamada, Y., Fukai, J. (2005). Latent heat thermal energy storage tanks for space heating of buildings: Comparison between calculations and experiments. Energy Conversion and Management, 46(20), 3221–3235. DOI 10.1016/j.enconman.2005.03.009. [Google Scholar] [CrossRef]
12. Ji, H., Sellan, D. P., Pettes, M. T., Kong, X., Ji, J. et al. (2014). Enhanced thermal conductivity of phase change materials with ultrathin-graphite foams for thermal energy storage. Energy and Environmental Science, 7(3), 1185–1192. DOI 10.1039/C3EE42573H. [Google Scholar] [CrossRef]
13. Paul, J., Pandey, A. K., Mishra, Y. N., Said, Z., Mishra, Y. K. et al. (2022). Nano-enhanced organic form stable PCMs for medium temperature solar thermal energy harvesting: Recent progresses, challenges, and opportunities. Renewable and Sustainable Energy Reviews, 161, 112321. DOI 10.1016/j.rser.2022.112321. [Google Scholar] [CrossRef]
14. Paul, J., Kadirgama, K., Samykano, M., Pandey, A. K., Tyagi, V. V. (2022). A comprehensive review on thermophysical properties and solar thermal applications of organic nano composite phase change materials. Journal of Energy Storage, 45(5), 103415. DOI 10.1016/j.est.2021.103415. [Google Scholar] [CrossRef]
15. Jebasingh, E. B., Arasu, V. A. (2020). Characterisation and stability analysis of eutectic fatty acid as a low cost cold energy storage phase change material. Journal of Energy Storage, 31, 101708. DOI 10.1016/j.est.2020.101708. [Google Scholar] [CrossRef]
16. Navarro, L., de Gracia, A., Niall, D., Castell, A., Browne, M. et al. (2016). Thermal energy storage in building integrated thermal systems: A review. Part 2. Integration as passive system. Renewable Energy, 85, 1334–1356. DOI 10.1016/j.renene.2015.06.064. [Google Scholar] [CrossRef]
17. Laraib Tariq, S., Muhammad Ali, H., Ammar Akram, M., Mansoor Janjua, M., Ahmadlouydarab, M. (2020). Nanoparticles enhanced phase change materials (NePCMs)–A recent review. Applied Thermal Engineering, 176, 115305. DOI 10.1016/j.applthermaleng.2020.115305. [Google Scholar] [CrossRef]
18. Rosen, M. A., Tang, R., Dincer, I. (2004). Effect of stratification on energy and exergy capacities in thermal storage systems. International Journal of Energy Research, 28(2), 177–193. DOI 10.1002/(ISSN)1099-114X. [Google Scholar] [CrossRef]
19. Elgafy, A., Lafdi, K. (2005). Effect of carbon nanofiber additives on thermal behavior of phase change materials. Carbon, 43(15), 3067–3074. DOI 10.1016/j.carbon.2005.06.042. [Google Scholar] [CrossRef]
20. Jacob, J., Pandey, A. K., Rahim, N. A., Selvaraj, J., Samykano, M. et al. (2021). Investigation on thermophysical properties of metallic oxide nanoparticle dispersed in fatty acid. Materials Today: Proceedings, 47, 2864–2868. DOI 10.1016/j.matpr.2021.03.639. [Google Scholar] [CrossRef]
21. Qiu, L., Ouyang, Y., Feng, Y., Zhang, X. (2019). Review on micro/nano phase change materials for solar thermal applications. Renewable Energy, 140(1), 513–538. DOI 10.1016/j.renene.2019.03.088. [Google Scholar] [CrossRef]
22. Ma, Z., Lin, W., Sohel, M. I. (2016). Nano-enhanced phase change materials for improved building performance. Renewable and Sustainable Energy Reviews, 58(15006), 1256–1268. DOI 10.1016/j.rser.2015.12.234. [Google Scholar] [CrossRef]
23. Raquel Leite da Cunha, S., Luís Barroso de Aguiar, J. (2020). Phase change materials and energy efficiency of buildings: A review of knowledge. Journal of Energy Storage, 27, 101083. DOI 10.1016/j.est.2019.101083. [Google Scholar] [CrossRef]
24. Kumar, P., Rathore, S., Kumar, S., Kumar, N. (2020). Potential of microencapsulated PCM for energy savings in buildings: A critical review. Sustainable Cities and Society, 53, 101884. DOI 10.1016/j.scs.2019.101884. [Google Scholar] [CrossRef]
25. Li, D., Wu, Y., Wang, B., Liu, C., Arıcı, M. (2020). Optical and thermal performance of glazing units containing PCM in buildings: A review. Construction and Building Materials, 233(9), 117327. DOI 10.1016/j.conbuildmat.2019.117327. [Google Scholar] [CrossRef]
26. Dardir, M., Panchabikesan, K., Haghighat, F., El, M. (2019). Opportunities and challenges of PCM-to-air heat exchangers (PAHXs) for building free cooling applications—A comprehensive review. Journal of Energy Storage, 22, 157–175. DOI 10.1016/j.est.2019.02.011. [Google Scholar] [CrossRef]
27. Berardi, U., Gallardo, A. A. (2019). Properties of concretes enhanced with phase change materials for building applications. Energy & Buildings, 199(10), 402–414. DOI 10.1016/j.enbuild.2019.07.014. [Google Scholar] [CrossRef]
28. Jiménez-xamán, C., Xamán, J., Moraga, N. O., Hernández-pérez, I., Zavala-guillén, I. et al. (2019). Solar chimneys with a phase change material for buildings: An overview using CFD and global energy balance. Energy & Buildings, 186, 384–404. DOI 10.1016/j.enbuild.2019.01.014. [Google Scholar] [CrossRef]
29. Rathore, P. K. S., Shukla, S. K. (2019). Potential of macroencapsulated pcm for thermal energy storage in buildings: A comprehensive review. Construction and Building Materials, 225(2), 723–744. DOI 10.1016/j.conbuildmat.2019.07.221. [Google Scholar] [CrossRef]
30. Shah, K. W. (2018). PT US CR. Energy & Buildings, 175. DOI 10.1016/j.est.2020.101708. [Google Scholar] [CrossRef]
31. Kasaeian, A., bahrami, L., Pourfayaz, F., Khodabandeh, E., Yan, W. M. (2017). Experimental studies on the applications of PCMs and nano-PCMs in buildings: A critical review. Energy and Buildings, 154(6), 96–112. DOI 10.1016/j.enbuild.2017.08.037. [Google Scholar] [CrossRef]
32. Ali, H. M., Babar, H., Shah, T. R., Sajid, M. U., Qasim, M. A. et al. (2018). Preparation techniques of TiO2 nanofluids and challenges: A review. Applied Sciences, 8(4), 587. DOI 10.3390/app8040587. [Google Scholar] [CrossRef]
33. Xu, B., Zhang, C., Chen, C., Zhou, J., Lu, C. et al. (2018). One-step synthesis of CuS-decorated MWCNTs/paraffin composite phase change materials and their light-heat conversion performance. Journal of Thermal Analysis and Calorimetry, 133(3), 1417–1428. DOI 10.1007/s10973-018-7192-0. [Google Scholar] [CrossRef]
34. Xu, B., Wang, B., Zhang, C., Zhou, J. (2017). Synthesis and light-heat conversion performance of hybrid particles decorated MWCNTs/paraffin phase change materials. Thermochimica Acta, 652, 77–84. DOI 10.1016/j.tca.2017.03.003. [Google Scholar] [CrossRef]
35. Liang, W., Wang, L., Zhu, Z., Qian, C., Sun, H. et al. (2017). In situ preparation of polyethylene glycol/ silver nanoparticles composite phase change materials with enhanced thermal conductivity. ChemistrySelect, 2(12), 3428–3436. DOI 10.1002/slct.201700381. [Google Scholar] [CrossRef]
36. Hwang, Y., Lee, J. K., Lee, C. H., Jung, Y. M., Cheong, S. I. et al. (2007). Stability and thermal conductivity characteristics of nanofluids. Thermochimica Acta, 455(1–2), 70–74. DOI 10.1016/j.tca.2006.11.036. [Google Scholar] [CrossRef]
37. Sharaf, O. Z., Taylor, R. A., Abu-Nada, E. (2020). On the colloidal and chemical stability of solar nanofluids: From nanoscale interactions to recent advances. Physics Reports, 867(4), 1–84. DOI 10.1016/j.physrep.2020.04.005. [Google Scholar] [CrossRef]
38. Yu, H., Hermann, S., Schulz, S. E., Gessner, T., Dong, Z. et al. (2012). Optimizing sonication parameters for dispersion of single-walled carbon nanotubes. Chemical Physics, 408, 11–16. DOI 10.1016/j.chemphys.2012.08.020. [Google Scholar] [CrossRef]
39. Zhou, M. Z., Xia, G. D., Li, J., Chai, L., Zhou, L. J. (2012). Analysis of factors influencing thermal conductivity and viscosity in different kinds of surfactant solutions. Experimental Thermal and Fluid Science, 36, 22–29. DOI 10.1016/j.expthermflusci.2011.07.014. [Google Scholar] [CrossRef]
40. Babita, Sharma, S. K., Gupta, S. M. (2016). Preparation and evaluation of stable nanofluids for heat transfer application: A review. Experimental Thermal and Fluid Science, 79(1), 202–212. DOI 10.1016/j.expthermflusci.2016.06.029. [Google Scholar] [CrossRef]
41. Nasiri, A., Shariaty-Niasar, M., Rashidi, A., Amrollahi, A., Khodafarin, R. (2011). Effect of dispersion method on thermal conductivity and stability of nanofluid. Experimental Thermal and Fluid Science, 35(4), 717–723. DOI 10.1016/j.expthermflusci.2011.01.006. [Google Scholar] [CrossRef]
42. Wang, J. J., Zheng, R. T., Gao, J. W., Chen, G. (2012). Heat conduction mechanisms in nanofluids and suspensions. Nano Today, 7(2), 124–136. DOI 10.1016/j.nantod.2012.02.007. [Google Scholar] [CrossRef]
43. Arshad, A., Jabbal, M., Yan, Y. (2020). Preparation and characteristics evaluation of mono and hybrid nano-enhanced phase change materials (NePCMs) for thermal management of microelectronics. Energy Conversion and Management, 205, 112444. DOI 10.1016/j.enconman.2019.112444. [Google Scholar] [CrossRef]
44. Kazemi, M., Kianifar, A., Niazmand, H. (2019). Nanoparticle loading effect on the performance of the paraffin thermal energy storage material for building applications. Journal of Thermal Analysis and Calorimetry, 139, 3769–3775. DOI 10.1007/s10973-019-08647-1. [Google Scholar] [CrossRef]
45. Harikrishnan, S., Deenadhayalan, M., Kalaiselvam, S. (2014). Experimental investigation of solidification and melting characteristics of composite PCMs for building heating application. Energy Conversion and Management, 86(45), 864–872. DOI 10.1016/j.enconman.2014.06.042. [Google Scholar] [CrossRef]
46. Arshad, A., Jabbal, M., Shi, L., Darkwa, J., Weston, N. J. et al. (2021). Development of TiO2/RT-35HC based nanocomposite phase change materials (NCPCMs) for thermal management applications. Sustainable Energy Technologies and Assessments, 43, 100865. DOI 10.1016/j.seta.2020.100865. [Google Scholar] [CrossRef]
47. Li, X., Zhou, Y., Nian, H., Zhang, X., Dong, O. et al. (2017). Advanced nanocomposite phase change material based on calcium chloride hexahydrate with aluminum oxide nanoparticles for thermal energy storage. Energy and Fuels, 31(6), 6560–6567. DOI 10.1021/acs.energyfuels.7b00851. [Google Scholar] [CrossRef]
48. Xie, N., Niu, J., Wu, T., Gao, X., Fang, Y. et al. (2019). Fabrication and characterization of CaCl2·6H2O composite phase change material in the presence of CsxWO3 nanoparticles. Solar Energy Materials and Solar Cells, 200, 110034. DOI 10.1016/j.solmat.2019.110034. [Google Scholar] [CrossRef]
49. Karaipekli, A., Biçer, A., Sarı, A., Veer, V. (2017). Thermal characteristics of expanded perlite/paraffin composite phase change material with enhanced thermal conductivity using carbon nanotubes. Energy Conversion and Management, 134, 373–381. DOI 10.1016/j.enconman.2016.12.053. [Google Scholar] [CrossRef]
50. Zhang, B., Li, C., Liu, Q. (2021). N-eicosane/multilayer graphene composite phase change materials for electro-thermal conversion and storage. Thermal Science and Engineering Progress, 25, 101039. DOI 10.1016/j.tsep.2021.101039. [Google Scholar] [CrossRef]
51. Li, Y., Liu, Q., Liu, Y., Wang, D., Song, W. et al. (2020). Calcium chloride hexahydrate/nano-SiO2 composites as form-stable phase change materials for building energy conversation: The influence of pore size of nano-SiO2. Energy & Buildings, 208, 109672. DOI 10.1016/j.enbuild.2019.109672. [Google Scholar] [CrossRef]
52. Li, B., Nie, S., Hao, Y., Liu, T., Zhu, J. et al. (2015). Stearic-acid/carbon-nanotube composites with tailored shape-stabilized phase transitions and light-heat conversion for thermal energy storage. Energy Conversion and Management, 98, 314–321. DOI 10.1016/j.enconman.2015.04.002. [Google Scholar] [CrossRef]
53. Saxena, R., Dwivedi, C., Dutta, V., Kaushik, S. C., Rakshit, D. (2021). Nano-enhanced PCMs for low-temperature thermal energy storage systems and passive conditioning applications. Clean Technologies and Environmental Policy, 23(4), 1161–1168. DOI 10.1007/s10098-020-01854-7. [Google Scholar] [CrossRef]
54. Ranjbar, S. G., Roudini, G., Barahuie, F. (2020). Fabrication and characterization of phase change material-SiO2 nanocomposite for thermal energy storage in buildings. Journal of Energy Storage, 27, 101168. DOI 10.1016/j.est.2019.101168. [Google Scholar] [CrossRef]
55. Martín, M., Villalba, A., Fernández, A. I., Barreneche, C. (2019). Development of new nano-enhanced phase change materials (NEPCM) to improve energy efficiency in buildings: Lab-scale characterization. Energy & Buildings, 192, 75–83. DOI 10.1016/j.enbuild.2019.03.029. [Google Scholar] [CrossRef]
56. Su, D., Jia, Y., Alva, G., Tang, F., Fang, G. (2016). Preparation and thermal properties of n-octadecan/stearic acid eutectic mixtures with hexagonal boron nitride as phase change materials for thermal energy storage. Energy and Buildings, 131, 35–41. DOI 10.1016/j.enbuild.2016.09.022. [Google Scholar] [CrossRef]
57. Luo, Z., Zhang, H., Gao, X., Xu, T., Fang, Y. et al. (2017). Fabrication and characterization of form-stable capric-palmitic-stearic acid ternary eutectic mixture/nano-SiO2 composite phase change material. Energy and Buildings, 147(9), 41–46. DOI 10.1016/j.enbuild.2017.04.005. [Google Scholar] [CrossRef]
58. Putra, N., Amin, M., Kosasih, E. A., Luanto, R. A., Abdullah, N. A. (2017). Characterization of the thermal stability of RT 22 HC/graphene using a thermal cycle method based on thermoelectric methods. Applied Thermal Engineering, 124, 62–70. DOI 10.1016/j.applthermaleng.2017.06.009. [Google Scholar] [CrossRef]
59. Ashiq, M., Francisco, R., Kumar, M. B. S. (2019). Enhancement of heat transfer in para ffi n wax PCM using nano graphene composite for industrial helmets. Journal of Energy Storage, 26, 100982. DOI 10.1016/j.est.2019.100982. [Google Scholar] [CrossRef]
60. Jeon, J., Jeong, S. G., Lee, J. H., Seo, J., Kim, S. (2012). High thermal performance composite PCMs loading xGnP for application to building using radiant floor heating system. Solar Energy Materials and Solar Cells, 101, 51–56. DOI 10.1016/j.solmat.2012.02.028. [Google Scholar] [CrossRef]
61. Hussain, S. I., Dinesh, R., Roseline, A. A., Dhivya, S., Kalaiselvam, S. (2017). Enhanced thermal performance and study the influence of sub cooling on activated carbon dispersed eutectic PCM for cold storage applications. Energy and Buildings, 143(6), 17–24. DOI 10.1016/j.enbuild.2017.03.011. [Google Scholar] [CrossRef]
62. Arshad, A., Jabbal, M., Shi, L., Yan, Y. (2021). Thermophysical characteristics and enhancement analysis of carbon-additives phase change mono and hybrid materials for thermal management of electronic devices. Journal of Energy Storage, 34, 102231. DOI 10.1016/j.est.2020.102231. [Google Scholar] [CrossRef]
63. Barreneche, C., Martín, M., Calvo-de la Rosa, J.,, Majó, M., Fernández, A. I. (2019). Own-synthetize nanoparticles to develop nano-enhanced phase change materials (NEPCM) to improve the energy efficiency in buildings. Molecules, 24(7), 1232. DOI 10.3390/molecules24071232. [Google Scholar] [CrossRef]
64. Motahar, S., Nikkam, N., Alemrajabi, A. A., Khodabandeh, R., Toprak, M. S. et al. (2014). Experimental investigation on thermal and rheological properties of n-octadecane with dispersed TiO2 nanoparticles. International Communications in Heat and Mass Transfer, 59(4), 68–74. DOI 10.1016/j.icheatmasstransfer.2014.10.016. [Google Scholar] [CrossRef]
65. Song, S., Qiu, F., Zhu, W., Guo, Y., Zhang, Y. et al. (2019). Polyethylene glycol/halloysite@Ag nanocomposite PCM for thermal energy storage: Simultaneously high latent heat and enhanced thermal conductivity. Solar Energy Materials and Solar Cells, 193, 237–245. DOI 10.1016/j.solmat.2019.01.023. [Google Scholar] [CrossRef]
66. Choi, S. (1998). Nanofluid technology: Current status and future research. Korea-U.S. Technical Conference on Strategic Technologies, Vienna, VA (USA). https://www.osti.gov/biblio/11048. [Google Scholar]
67. Balandin, A. A., Ghosh, S., Bao, W., Calizo, I., Teweldebrhan, D. et al. (2008). Superior thermal conductivity of single-layer graphene. Nano Letters, 8(3), 902–907. DOI 10.1021/nl0731872. [Google Scholar] [CrossRef]
68. Zeng, J. L., Cao, Z., Yang, D. W., Xu, F., Sun, L. X. et al. (2009). Effects of MWNTs on phase change enthalpy and thermal conductivity of a solid-liquid organic PCM. Journal of Thermal Analysis and Calorimetry, 95(2), 507–512. DOI 10.1007/s10973-008-9275-9. [Google Scholar] [CrossRef]
69. Zhang, Q., Luo, Z., Guo, Q., Wu, G. (2017). Preparation and thermal properties of short carbon fibers/erythritol phase change materials. Energy Conversion and Management, 136, 220–228. DOI 10.1016/j.enconman.2017.01.023. [Google Scholar] [CrossRef]
70. Cui, Y., Liu, C., Hu, S., Yu, X. (2011). The experimental exploration of carbon nanofiber and carbon nanotube additives on thermal behavior of phase change materials. Solar Energy Materials and Solar Cells, 95(4), 1208–1212. DOI 10.1016/j.solmat.2011.01.021. [Google Scholar] [CrossRef]
71. Maire, J., Anufriev, R., Yanagisawa, R., Ramiere, A., Volz, S. et al. (2017). Heat conduction tuning by wave nature of phonons. Science Advances, 3(8), 1–7. DOI 10.1126/sciadv.1700027. [Google Scholar] [CrossRef]
72. Wu, S., Yan, T., Kuai, Z., Pan, W. (2020). Thermal conductivity enhancement on phase change materials for thermal energy storage: A review. Energy Storage Materials, 25, 251–295. DOI 10.1016/j.ensm.2019.10.010. [Google Scholar] [CrossRef]
73. Eanest Jebasingh, B., Valan Arasu, A. (2019). A comprehensive review on latent heat and thermal conductivity of nanoparticle dispersed phase change material for low-temperature applications. Energy Storage Materials, 24, 52–74. DOI 10.1016/j.ensm.2019.07.031. [Google Scholar] [CrossRef]
74. Zabalegui, A., Lokapur, D., Lee, H. (2014). Nanofluid PCMs for thermal energy storage: Latent heat reduction mechanisms and a numerical study of effective thermal storage performance. International Journal of Heat and Mass Transfer, 78(1–2), 1145–1154. DOI 10.1016/j.ijheatmasstransfer.2014.07.051. [Google Scholar] [CrossRef]
75. Kim, D., Kwon, Y., Cho, Y., Li, C., Cheong, S. et al. (2009). Convective heat transfer characteristics of nanofluids under laminar and turbulent flow conditions. Current Applied Physics, 9(2), e119–e123. DOI 10.1016/j.cap.2008.12.047. [Google Scholar] [CrossRef]
76. Mahian, O., Kolsi, L., Amani, M., Estellé, P., Ahmadi, G. et al. (2019). Recent advances in modeling and simulation of nanofluid flows--Part I: Fundamentals and theory. Physics Reports, 790, 1–48. DOI 10.1016/j.physrep.2018.11.004. [Google Scholar] [CrossRef]
77. Lv, P., Liu, C., Rao, Z. (2016). Experiment study on the thermal properties of paraffin/kaolin thermal energy storage form-stable phase change materials. Applied Energy, 182(9–10), 475–487. DOI 10.1016/j.apenergy.2016.08.147. [Google Scholar] [CrossRef]
78. Wang, J., Xie, H., Xin, Z., Li, Y., Chen, L. (2010). Enhancing thermal conductivity of palmitic acid based phase change materials with carbon nanotubes as fillers. Solar Energy, 84(2), 339–344. DOI 10.1016/j.solener.2009.12.004. [Google Scholar] [CrossRef]
79. Gao, J. W., Zheng, R. T., Ohtani, H., Zhu, D. S., Chen, G. (2009). Experimental investigation of heat conduction mechanisms in nanofluids. Clue on clustering. Nano Letters, 9(12), 4128–4132. DOI 10.1021/nl902358m. [Google Scholar] [CrossRef]
80. Babapoor, A., Karimi, G. (2015). Thermal properties measurement and heat storage analysis of paraffinnanoparticles composites phase change material: Comparison and optimization. Applied Thermal Engineering, 90, 945–951. DOI 10.1016/j.applthermaleng.2015.07.083. [Google Scholar] [CrossRef]
81. Zou, D., Ma, X., Liu, X., Zheng, P., Hu, Y. (2018). Thermal performance enhancement of composite phase change materials (PCM) using graphene and carbon nanotubes as additives for the potential application in lithium-ion power battery. International Journal of Heat and Mass Transfer, 120, 33–41. DOI 10.1016/j.ijheatmasstransfer.2017.12.024. [Google Scholar] [CrossRef]
82. Al Ghossein, R. M., Hossain, M. S., Khodadadi, J. M. (2017). Experimental determination of temperature-dependent thermal conductivity of solid eicosane-based silver nanostructure-enhanced phase change materials for thermal energy storage. International Journal of Heat and Mass Transfer, 107(10), 697–711. DOI 10.1016/j.ijheatmasstransfer.2016.11.059. [Google Scholar] [CrossRef]
83. Yavari, F., Fard, H. R., Pashayi, K., Rafiee, M. A., Zamiri, A. et al. (2011). Enhanced thermal conductivity in a nanostructured phase change composite due to low concentration graphene additives. Journal of Physical Chemistry C, 115(17), 8753–8758. DOI 10.1021/jp200838s. [Google Scholar] [CrossRef]
84. Zhang, D., Tian, S., Xiao, D. (2007). Experimental study on the phase change behavior of phase change material confined in pores. Solar Energy, 81(5), 653–660. DOI 10.1016/j.solener.2006.08.010. [Google Scholar] [CrossRef]
85. Li, C., Xie, B., Chen, D., Chen, J., Li, W. et al. (2019). Ultrathin graphite sheets stabilized stearic acid as a composite phase change material for thermal energy storage. Energy, 166(9), 246–255. DOI 10.1016/j.energy.2018.10.082. [Google Scholar] [CrossRef]
86. Administration, U. S. E. I. (2019). International energy outlook 2019 with projections to 2050. Choice Reviews Online, 85. https://www.eia.gov/ieo. [Google Scholar]
87. Iten, M., Liu, S., Shukla, A. (2016). A review on the air-PCM-TES application for free cooling and heating in the buildings. Renewable and Sustainable Energy Reviews, 61, 175–186. DOI 10.1016/j.rser.2016.03.007. [Google Scholar] [CrossRef]
88. Zalba, B., Marín, J. M., Cabeza, L. F., Mehling, H. (2004). Free-cooling of buildings with phase change materials. International Journal of Refrigeration, 27(8), 839–849. DOI 10.1016/j.ijrefrig.2004.03.015. [Google Scholar] [CrossRef]
89. Kant, K., Anand, A., Shukla, A., Sharma, A. (2020). Heat transfer study of building integrated photovoltaic (BIPV) with nano-enhanced phase change materials. Journal of Energy Storage, 30, 101563. DOI 10.1016/j.est.2020.101563. [Google Scholar] [CrossRef]
90. Carneiro, J. O., Vasconcelos, G., Azevedo, S., Jesus, C., Palha, C. et al. (2014). The evaluation of the thermal behaviour of a mortar based brick masonry wall coated with TiO2 nanoparticles: An experimental assessment towards energy efficient buildings. Energy and Buildings, 81, 1–8. DOI 10.1016/j.enbuild.2014.06.006. [Google Scholar] [CrossRef]
91. Parameshwaran, R., Deepak, K., Saravanan, R., Kalaiselvam, S. (2014). Preparation, thermal and rheological properties of hybrid nanocomposite phase change material for thermal energy storage. Applied Energy, 115(14304), 320–330. DOI 10.1016/j.apenergy.2013.11.029. [Google Scholar] [CrossRef]
92. Lechner, N. (2015). Heating, cooling, lighting; sustainable design methods for architects. 4th edition, pp. 148–162. Hoboken: Wiley. [Google Scholar]
93. Etheridge, D. (2016). Natural ventilation of buildings, pp. 375–411. Hoboken: Wiley. [Google Scholar]
94. Hien, W. N., Liping, W., Chandra, A. N., Pandey, A. R., Xiaolin, W. (2005). Effects of double glazed facade on energy consumption, thermal comfort and condensation for a typical office building in Singapore. Energy and Buildings, 37(6), 563–572. DOI 10.1016/j.enbuild.2004.08.004. [Google Scholar] [CrossRef]
95. Oropeza-Perez, I., Østergaard, P. A. (2018). Active and passive cooling methods for dwellings: A review. Renewable and Sustainable Energy Reviews, 82(1), 531–544. DOI 10.1016/j.rser.2017.09.059. [Google Scholar] [CrossRef]
96. Said, M. A., Hassan, H. (2018). Effect of using nanoparticles on the performance of thermal energy storage of phase change material coupled with air-conditioning unit. Energy Conversion and Management, 171, 903–916. DOI 10.1016/j.enconman.2018.06.051. [Google Scholar] [CrossRef]
97. Parameshwaran, R., Kalaiselvam, S. (2014). Energy conservative air conditioning system using silver nano-based PCM thermal storage for modern buildings. Energy and Buildings, 69, 202–212. DOI 10.1016/j.enbuild.2013.09.052. [Google Scholar] [CrossRef]
98. Parameshwaran, R., Kalaiselvam, S. (2013). Energy efficient hybrid nanocomposite-based cool thermal storage air conditioning system for sustainable buildings. Energy, 59, 194–214. DOI 10.1016/j.energy.2013.06.064. [Google Scholar] [CrossRef]
99. Geetha, N. B., Velraj, R. (2012). Passive cooling methods for energy efficient buildings with and without thermal energy storage-a review. Energy Education Science and Technology Part A: Energy Science and Research, 29(2), 913–946. [Google Scholar]
100. Pacheco, R., Ordóñez, J., Martínez, G. (2012). Energy efficient design of building: A review. Renewable and Sustainable Energy Reviews, 16(6), 3559–3573. DOI 10.1016/j.rser.2012.03.045. [Google Scholar] [CrossRef]
101. Ashok, V., Geetha, N. B., Rajkumar, S., Pauline, T. (2021). Experimental investigations for thermal energy management by encapsulation of nano -enhanced bio phase change material in buildings. Energy Sources, Part A: Recovery, Utilization, and Environmental Effects, 1–19. DOI 10.1080/15567036.2021.1967517. [Google Scholar] [CrossRef]
102. Sarrafha, H., Kasaeian, A., Jahangir, M. H., Taylor, R. A. (2021). Transient thermal response of multi-walled carbon nanotube phase change materials in building walls. Energy, 224, 120120. DOI 10.1016/j.energy.2021.120120. [Google Scholar] [CrossRef]
103. Sagara, A., Nomura, T., Tsubota, M., Okinaka, N., Akiyama, T. (2014). Improvement in thermal endurance of D-mannitol as phase-change material by impregnation into nanosized pores. Materials Chemistry and Physics, 146(3), 253–260. DOI 10.1016/j.matchemphys.2014.03.009. [Google Scholar] [CrossRef]
104. Li, X., Zhou, Y., Nian, H., Ren, X., Dong, O. et al. (2016). Phase change behavior of latent heat storage media based on calcium chloride hexahydrate composites containing strontium chloride hexahydrate and oxidation expandable graphite. Applied Thermal Engineering, 102, 38–44. DOI 10.1016/j.applthermaleng.2016.03.098. [Google Scholar] [CrossRef]
105. Li, D., Wu, Y., Liu, C., Zhang, G., Arıcı, M. (2018). Energy investigation of glazed windows containing Nano-PCM in different seasons. Energy Conversion and Management, 172, 119–128. DOI 10.1016/j.enconman.2018.07.015. [Google Scholar] [CrossRef]
106. Venkitaraj, K. P., Suresh, S., Praveen, B., Nair, S. C. (2018). Experimental heat transfer analysis of macro packed neopentylglycol with CuO nano additives for building cooling applications. Journal of Energy Storage, 17(1), 1–10. DOI 10.1016/j.est.2018.02.005. [Google Scholar] [CrossRef]
107. Sayyar, M., Weerasiri, R. R., Soroushian, P., Lu, J. (2014). Experimental and numerical study of shape-stable phase-change nanocomposite toward energy-efficient building constructions. Energy and Buildings, 75(3), 249–255. DOI 10.1016/j.enbuild.2014.02.018. [Google Scholar] [CrossRef]
108. Biswas, K., Lu, J., Soroushian, P., Shrestha, S. (2014). Combined experimental and numerical evaluation of a prototype nano-PCM enhanced wallboard. Applied Energy, 131(5), 517–529. DOI 10.1016/j.apenergy.2014.02.047. [Google Scholar] [CrossRef]
109. Biyik, E., Araz, M., Hepbasli, A., Shahrestani, M., Yao, R. et al. (2017). A key review of building integrated photovoltaic (BIPV) systems. Engineering Science and Technology, an International Journal, 20(3), 833–858. DOI 10.1016/j.jestch.2017.01.009. [Google Scholar] [CrossRef]
110. Henemann, A. (2008). BIPV: Built-in solar energy. Renewable Energy Focus, 9(6), 14–19. DOI 10.1016/S1471-0846(08)70179-3. [Google Scholar] [CrossRef]
111. Anand, A., Shukla, A., Sharma, A. (2020). Recapitulation on latent heat hybrid buildings. International Journal of Energy Research, 44(3), 1370–1407. DOI 10.1002/er.4920. [Google Scholar] [CrossRef]
112. Hu, W., Song, M., Jiang, Y., Yao, Y., Gao, Y. (2019). A modeling study on the heat storage and release characteristics of a phase change material based double-spiral coiled heat exchanger in an air source heat pump for defrosting. Applied Energy, 236(7), 877–892. DOI 10.1016/j.apenergy.2018.12.057. [Google Scholar] [CrossRef]
113. Yang, L., Huang, J., Zhou, F. (2020). Thermophysical properties and applications of nano-enhanced PCMs: An update review. Energy Conversion and Management, 214(8), 112876. DOI 10.1016/j.enconman.2020.112876. [Google Scholar] [CrossRef]
114. Sharma, S., Micheli, L., Chang, W., Tahir, A. A., Reddy, K. S. et al. (2020). Nano-enhanced Phase Change Material for thermal management of BICPV. Applied Energy, 208, 1–15. DOI 10.1016/j.apenergy.2017.09.076. [Google Scholar] [CrossRef]
115. Maghrabie, H. M., Elsaid, K., Sayed, E. T., Radwan, A., Abo-Khalil, A. G. et al. (2022). Phase change materials based on nanoparticles for enhancing the performance of solar photovoltaic panels: A review. Journal of Energy Storage, 48, 103937. DOI 10.1016/j.est.2021.103937. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |