 | Energy Engineering |  |
DOI: 10.32604/ee.2022.020984
REVIEW
Progress on Platinum-Based Catalyst Layer Materials for H2-PEMFC
Department of Mechanical Engineering, Laboratory of Soft Energy Applications & Environmental Protection, University of West Attica, Athens, Greece
*Corresponding Author: Stefanos Tzelepis. Email: stzelepis@uniwa.gr
Received: 22 December 2021; Accepted: 14 April 2022
Abstract: The constant increase in energy demand and related environmental issues have made fuel cells an attractive technology as an alternative to conventional energy technologies. Like any technology, fuel cells face drawbacks that scientific society has been focused on to improve and optimize the overall technology. Thus, the cost is the main inhibitor for this technology due to the significantly high cost of the materials used in catalyst layers. The current discussion mainly focuses on the fundamental electrochemical half-cell reaction of hydrogen oxidation reaction (HOR) and oxygen reduction reaction (ORR) that are taking place in the catalyst layers consisting of Platinum-based and Platinum-non noble metals. For this purpose, studies from the literature are presented and analyzed by highlighting and comparing the variations on the catalytic activity within the experimental catalyst layers and the conventional ones. Furthermore, an economic analysis of the main platinum group metals (PGMs) such as Platinum, Palladium and Ruthenium is introduced by presenting the economic trends for the last decade.
Keywords: PEMFC; catalyst layer materials; platinum; noble metals; non-noble metals
Abbreviations
| AFC | Alkaline Fuel Cell |
| AOR | Alcohol Oxidation Reaction |
| CA | Chrono Amperommetry |
| CV | Cyclic Voltammetry |
| DRIFT | Diffuse Reflectance Infrared Fourier Transform |
| ECSA | Electrochemical Surface Area |
| EIS | Electrical Impendence Spectroscopy |
| EDX | Energy Dispersive X-Ray |
| FCEV | Fuel Cell Electric Vehicle |
| HOR | Hydrogen Oxidation Reaction |
| LSV | Linear Sweep Voltammetry |
| MCFC | Molten Carbonate Fuel Cell |
| MOR | Methanol Oxidation Reaction |
| ORR | Oxygen Reduction Reaction |
| PAFC | Phosphoric Acid Fuel Cell |
| PEMFC | Proton Exchange Membrane Fuel Cell |
| PGM | Platinum Group Metal |
| RDE | Rotating Disk Electrode |
| SOFC | Solid Oxide Fuel Cell |
| SEM | Scanning Electron Microscopy |
| TEM | Transmission Electron Microscopy |
| XRD | X-Ray Diffraction |
| XPS | X-Ray Photoelectron Spectroscop |
The need for clean power in modern societies is constantly increasing as energy requirements increase. Many countries seek new policies, strategies, and technologies to compensate for the constantly growing emissions rate. To this extent, the emissions adversely affect both nature (climate change, thermal pollution, and water pollution) and human health, issues that have been discussed over the last two decades. In addition to environmental and human problems, the depletion of fossil fuels has led researchers, scientists, and policymakers to discover alternative ways to utilize energy sources such as renewables, nuclear, and hydrogen.
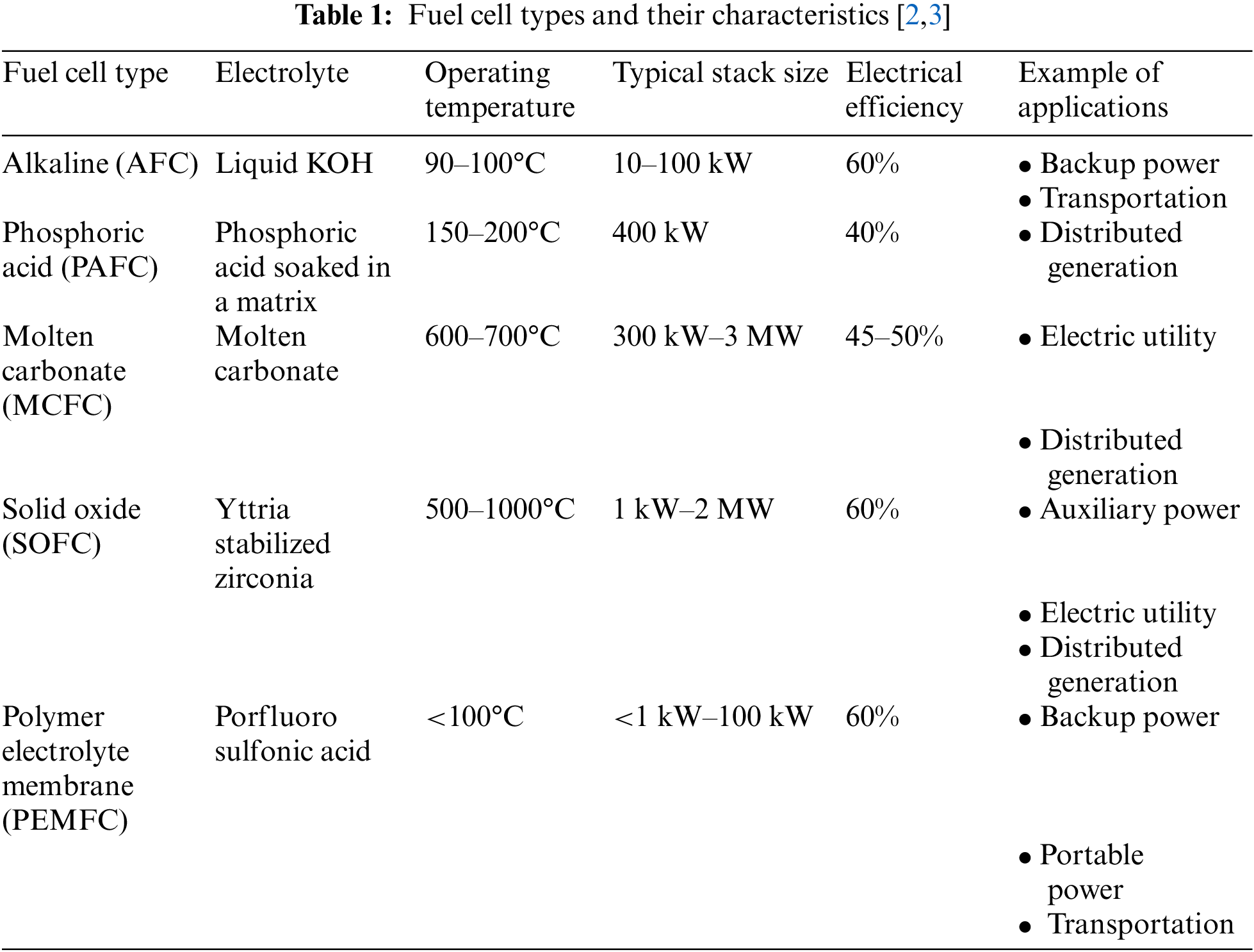
Fuel cells are a promising technology that converts the chemical energy of hydrogen into electricity without emitting any greenhouse gases. The low emissions, zero noise, and pollution have made them one of the cleanest power sources. Fuel cells can be categorized according to the type of electrolyte and fuel used. Thus, various types of fuel cells have been developed throughout the years, such as Alkaline Fuel Cells (AFC), Phosphoric Acid Fuel Cells (PAFC), Molten Carbonate Fuel Cells (MCFC), Solid Oxide Fuel Cells (SOFC), and Proton Exchange Membrane Fuel Cells (PEMFC), with the latter being one of the most reliable and ready to deploy fuel cell types from small to large applications. The main advantages of PEMFCs are the high-power densities, significantly low operating temperatures, fast start-up times, and easy stacking, allowing them to be a proper solution for mobile and stationary applications. In Table 1, the various types of fuel cells, their main components, and operating conditions are listed [1].
In terms of PEMFC’s operation, the anode side (fuel electrode) is supplied with hydrogen while simultaneously the cathode side is fed with pure air or oxygen. Hydrogen in the anode is oxidized through an electrochemical half-cell reaction to protons (H+) and deliberates electrons–Hydrogen Oxidation Reaction (HOR). The electrons flow through an external electric circuit to the cathode side. At the same time, protons are conducted by the polymer electrolyte and transferred to the cathode side, where they recombine with oxygen, protons, and electrons, forming H2O–Oxygen Reduction Reaction (ORR) as is depicted in Fig. 1. Eqs. (1)–(3) describe the electrochemical reactions that are taking place [4]:
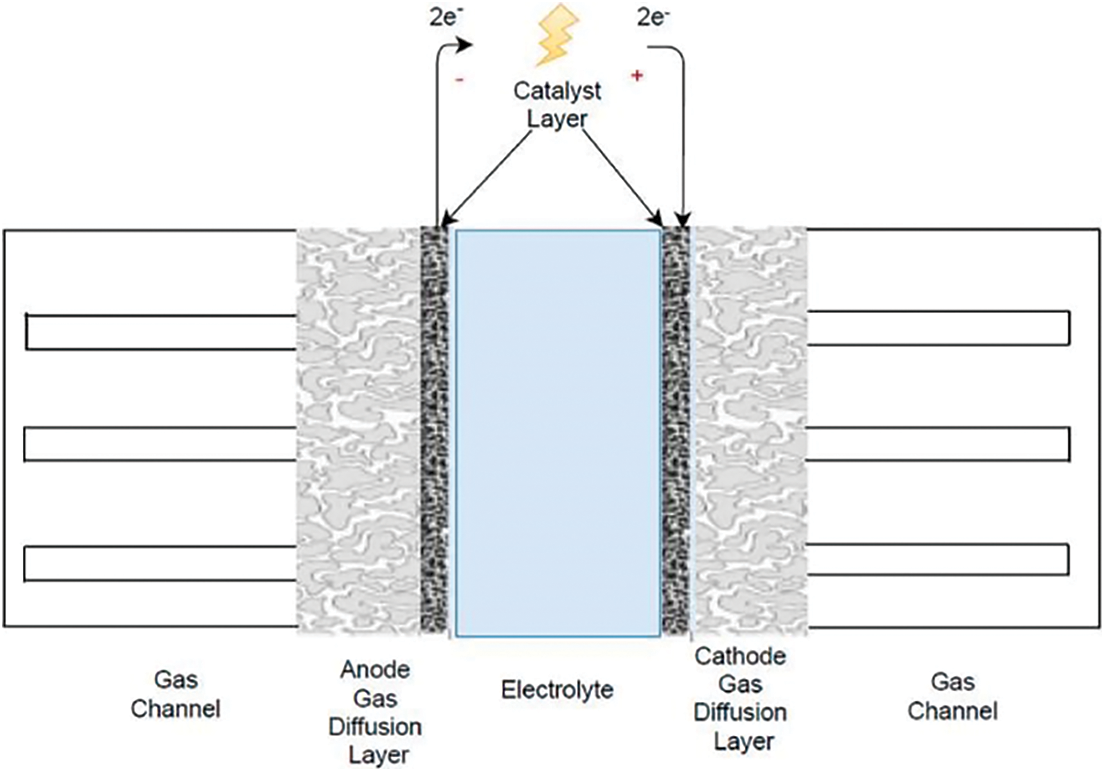
Figure 1: Schematic representation of PEMFC [5]
The aforementioned electrochemical procedures and the corresponding transport phenomena of the reactants are taking place in the main components from which the PEMFC is structured. The components of a PEMFC are the ion exchange membrane with a solid structure, the porous gas diffusion layers (GDL) in both anode and cathode sides, which are electrically conductive. The catalyst layers (CL) are located at the interface between the gas diffusion layer and the membrane in which the electrochemical half-cell reactions of HOR and ORR occur. The cell interconnects the flow plates that feed the fuel (H2) and the oxidant to the reactive sites through flow channels. More specifically, the GDL, CL, and the electrolyte are constructed together, forming the so-called Membrane Electrode Assembly (MEA), which can be characterized as the heart of the PEMFC. In general terms, PEMFCs are prepared as single cells to cover small power requirements or stacks where many cells are connected in series for achieving the voltage and power output according to the application.
However, PEMFCs still face some essential issues in terms of cost, durability, and performance. In this respect, researchers have conducted significant work to examine these issues to provide solutions and turn the PEMFCs into a more reliable and efficient technology. In this context, the main interest of research has been focused on challenges regarding (1) the anode and cathode catalyst layer poisoning with impurities which can lead to the decrease of PEMFC’s performance, (2) the geometry of gas flow channel for achieving the optimum conditions of mass and heat transfer phenomena on both sides, (3) minimization of platinum loading on catalyst layers and (4) the electrolyte crossover. At this point, relative studies regarding the issues mentioned above will be discussed to provide a general view of the work done in each field.
Shabani et al. [6] studied the durability and performance degradation of PEMFCs considering the most critical associated with the current technology. The air contaminants, fuel impurities cleansers, and some materials, from which PEMFC is composed, have been proved to impact PEMFC’s performance negatively. Their work highlighted the solutions for increasing the tolerance of PEMFC catalyst layers to deal with poisonous impurities. Yang et al. [7] studied the performance of PEMFC using a new type of gas channel, the so-called waved flow channel. The main parameters investigated were velocity, concentration, and electrical performance. The results showed that the waved channel has better benefits regarding the transport phenomena in porous layers. That relies on the fact that the waved channel improves the forced convection and causes a higher amount of reactant gases to flow through the GDL. Francia et al. [8] highlighted that hydrogen crossover could cause undesired diffusion of the gas from the anode to the cathode through the membrane, which usually can lead to deterioration of perfluorinated ionomer membranes causing gradual degradation on PEMFC’s performance. Thus, in their research developed a mathematical model to directly determine hydrogen crossover permeation rate. The results showed that hydrogen crossover current densities increased from 0.12 to 0.32 mA/cm2 by decreasing the membrane’s thickness and the operating temperature.
As discussed previously, catalyst layers are the main part of PEMFCs where half-cell reactions of HOR and ORR are initiated. In PEMFCs, platinum is commonly used because it is the most active noble metal. In general, platinum-based catalysts are preferred for PEMFC with comparatively clean reactants. Nonetheless, the major challenge of the platinum-based catalysts arises when the supplied fuel contains residual carbon monoxide (CO), which can gradually degrade PEMFC’s performance. The catalytic activity on the cathode side (ORR) by applying other metal surfaces such as Au, Ir, Rh has been investigated. On the other hand, the electro-catalytic activity of the anode side (HOR) has also been examined using various platinum-group metals, including Pt, Ru, Pd, Ir, Os, and Rh. The overall improvement of PEMFCs’ performance by applying different noble metals will be extensively discussed in the following chapters. The current work will be focused on bimetallic CLs consisting of Pt alloyed with noble metals and Pt combined with non-noble metals for HOR and ORR in PEMFCs. Worth mentioning that significant efforts have been conducted regarding the investigation of CLs in Alcohol oxidation reaction (AOR) on both noble and non-non noble metals [9–11] as well as in the trimetallic CLs for Methanol oxidation reaction (MOR) [12]. However, the scope of the current work relies on the materials applied in the CLs of PEMFCs for HOR and ORR.
2 Existing Solutions on Applied CL Materials
As mentioned in the previous section, electrocatalysts are the heart of PEMFCs in which electrochemical reactions are taking place and consequently affect the fuel cell’s overall performance and life. In this respect, tremendous efforts have been reported further to optimize the existing solutions applied on CLs in terms of (1) optimizing the platinum loading, (2) applying additional noble or non-noble metals for enhancing the performance of the electrochemical reaction, and minimizing the construction cost of CLs. Usually, platinum (Pt) nanoparticles are loaded in carbon support (Pt/C), making the most widely used and reported case of CL. The main benefits of Pt/C CLs rely on their high catalytic activity and better stability than other noble metals. However, the application of Pt/C is impeded with various challenges, mainly due to the significantly increased cost of Pt and the fact that it can easily be dissolved and aggregated when the operating conditions are significantly poor. In addition, the standard carbon supports materials for Pt and Pt-alloy CLs experience corrosion due to electrochemical oxidation on the surface, leading to the dissolution of the Pt nanoparticles [13].
On the other hand, considerable efforts were made on developing non-noble metal-based CLs. The Platinum group metal-free (PGM-free) CLs can be described as metal-nitrogen-carbon (M-N-C) structure in which M is an earth-abundant transitional metal (M = Co, Fe, Ni, Mn, etc.). These metals have gained attention due to the high catalytic activity presented on ORR, simultaneously with the utilization of abundant and inexpensive materials [14]. Considering the anode and cathode PEMFCs, Pt/C electrocatalyst cannot be characterized as a state-of-the-art material. At the same time, they cannot provide significantly high catalytic performance at feasible platinum loading accounting due to the high cost. One of the main disadvantages of applying Pt as a single catalytic unit is the CO tolerance of the CL when the supplied reactant gas is not 100% pure. This can lead to the occupation of the active catalytic sites and cause a reduction in the efficiency of the CL. Alloying Pt with a second element has been ascertained, enhancing the primary element’s electric activity [15]. However, even with reduced Pt loading, the catalytic performance has not met the requirements for broad commercialization in fuel cells. Thus, the production of cheap CLs is one of the main challenges. In this context, the binary alloyed CLs can be separated into the Pt/noble metals and Pt/non-noble metals CLs.
3 ORR and HOR on Platinum Groups Metal
This section describes the ORR on both Pt alloys and other noble metals. Initially, the catalytic activity of Pt is strongly dependent on its O2 absorption energy, the dissociation energy of O-O bond, and the corresponding binding energy of OH on the Pt surface. Moreover, theoretical calculations on O2 binding energy on various metals have been conducted and have shown that Pt has the highest catalytic activity. In more detail, the catalytic activity among other noble metals follows this order: Pt > Pd > Ir > Rh. When Pt is alloyed with other metals, enhanced catalytic activity is presented. This is explained by the change in the electronic structure and the corresponding geometric effect in terms of Pt-Pt interatomic distance. More precisely, Pt alloying presents changes in lattice contraction, which leads to those as mentioned above favourable Pt-Pt distance, causing better O2 adsorption.
As discussed previously, ORR in other noble metals such as Au, Ir, Rh, etc., has also been investigated. These metals show lower catalytic activity than Pt, while they are not electrochemically stable. Fig. 2 shows the trend in ORR activity for various noble metals, indicating that Pt has the highest activity compared to other materials.
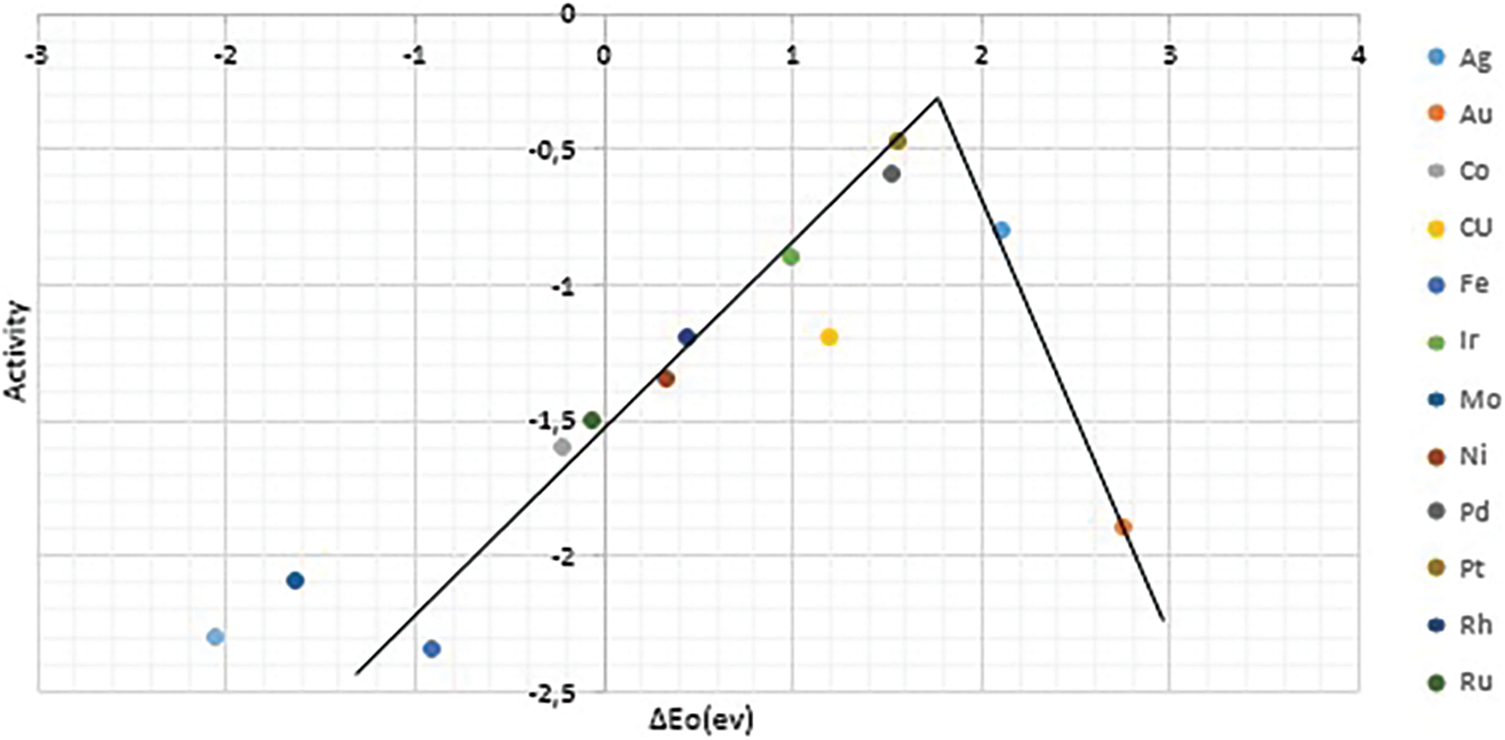
Figure 2: Trends in oxygen reduction activity plotted as a function of the oxygen binding energy [16]
On the other hand, HOR is one of the most important areas of fuel cells where the interaction with the catalyst surface activates the reactants. Since the catalyzed electrochemical reaction occurs at the catalyzed electrode/electrolyte interface, the electrochemical reaction strongly depends on the potential difference between the catalyst, the electrolyte, and the catalyst’s surface morphology. Indicatively, the exchange current density can vary from 10−3 A·cm−2 at a Pt electrode to 10−12 at a Mercury (Hg) electrode for the HOR, and from 10−10 A·cm−2 at a Pt electrode to 10−8 A·cm−2 at a Copper (Cu) electrode for ORR. Worth mentioning that HOR on Pt is approximately 5 to 7 magnitudes more rapid than the ORR.
Thus, the adsorption and desorption of reactants on the catalyst surface can directly affect the catalytic ability. In this respect, the exchange current density of the HOR in relation to the enthalpy of H adsorption is correlated. If the enthalpy is too small, a slow adsorption kinetic will occur. If the enthalpy is too high, the desorption of H becomes difficult. Hence, an intermediate value in the enthalpy of H adsorption is required for the CL to be active [15].
Although HOR has fast kinetics on pure Pt catalyst surfaces, poisoning should be addressed when the supplied hydrogen contains impurities such as carbon monoxide. As a result, CO is absorbed from the active areas, affecting the reactivity of the electrode surface by blocking H2 adsorption. Another disadvantage relies on the significantly high cost of Pt. Therefore, research has been conducted in the last decades focusing on the development of catalysts by alloying Pt with other metals that are CO tolerant for reducing the use of Pt. Nonetheless, alloying Pt with a second element has been proved to enhance the first material’s electrocatalytic performance, achieved by (1) bifunctional effects whereby the second component provides one of the necessary reactants, (2) electronic effect in which the second component alters the electronic properties of the catalytically active metal, and (3) morphological effects, in which the dilution of the active component (Pt) with catalytically inert metal changes the distribution of the active sites which can lead to opening new pathways of reaction [15].
Noble metal based catalyst layers (NMBCs) have been a hotspot with significant interest for tackling several issues presented in PEMFCs due to the high utilization of noble metals, cost efficiency, and the promoted catalytic properties. Materials alloyed with Pt include Ruthenium (Ru), Palladium (Pd), and Gold (Au). For this purpose, major studies are reported regarding the materials that have been applied with Pt for examining the performance of CL. Additional studies will be presented regarding the investigation of various compositions on common Pt/C CLs and the effects on PEMFC’s performance. The studies reported in the following sub-chapters concern the overall operation and performance of PEMFCs considering both anode and cathode electrochemical reactions.
Ruthenium (Ru) is a noble metal usually applied on ORR electrocatalysts because of its improved tolerance on CO [15]. Albers et al. [17] examined a series of Pt-Ru/C fuel cell catalysts combining several techniques such as transmission electron microscopy (TEM), scanning transmission electron microscopy (SEM), energy dispersive X-ray microanalysis, X-ray diffraction (XRD), and inelastic incoherent neutron scattering. The local composition of the precious metal particles with different sizes and compositions indicated a proportional increase between particles and the Pt/Ru ratio. At the same time, Ruthenium is shown to prevent the agglomeration of the platinum particles from retaining smaller particle sizes. Saeed et al. [18] evaluated the performance of a PEMFC considering various parameters such as the flow pattern, flow rate, and the degradation of Pt-Ru/C CL. In their study, scanning electron microscopy (SEM) images for the topography of the electrode were used and showed degradation in the elements of CL due to the corrosion phenomenon as a direct result of the electrochemical reaction among Pt-Ru. The authors concluded that the metals alloyed presented an increased current at less negative values while others showed a decrease in the current to less positive values. Brkovic et al. [19] issued the durability in terms of poisoning of CLs and cost for the commercialization of PEMFCs. The authors developed a tungsten-carbide-oxide as a new noncarbon based catalyst support for Pt-Ru (Pt-Ru/WxCyOz) based anode PEMFC CL. The performance of the developed CL was investigated via cyclic voltammetry, linear can voltammetry, and rotating disk electrode voltammetry. The developed CL was tested as an anode CL in a PEMFC. A synthetic reformate was used as a fuel in PEMFC, which presented a significant power drop of 35.3% for a commercial Pt-Ru/C CL, while for the developed CL anode catalyst, this drop was around 16%. Henry et al. [20] studied carbon supported Pt-Ru/C CLs used in the anode side of the PEMFC. In this respect, the evolution of MEA after 1000 h of operation for ageing was investigated. Their analyses showed the dissolution of Ru from the PtRu/C within the micro-porous layer and the membrane. Overall, the results indicated that within the membrane, the Ru reduction is catalyzed by Pt and hydrogen. In addition, the localization of the precipitation band near the cathode showed that Pt came from the dissolution of cathodic Pt/C and that both Pt and Ru ions were reduced by the hydrogen crossover. Chen et al. [21] studied the performance of a PEMFC with an active area of 112.85 cm2 and with anode CL consisting of Pt-Ru. The PEMFC stack was examined under various reformate gases of different concentrations of CO. The results showed that the increase of CO concentrations or decrease of H2 concentration of the CO-contained reformate gas has a negative impact on the performance of the PEMFC. The study showed that the PEMFC stack consisting of a Pt-Ru anode CL could tolerate a CO concentration above 50 ppm under non-diluted H2, whereas in the case of diluted H2, the CL could tolerate a CO concentration of 10 ppm.
Another possible candidate for alloying Pt is the palladium (Pd), which presents identical facets and lattice constants and similar outer electronic configuration as Pt [22]. Zhou et al. [23] compared two types of CLs, Pt-Pd/C, and Pt/C, evaluated as ORR CLs. For both types of CLs, physical and electrochemical techniques were applied to investigate the structure, performance, and durability. Overall, the electrochemically active surface area, particle size distribution, polarization behaviour, and electrochemistry impedance spectroscopy suggested that Pt-Pd/C has better durability than Pt/C. Cho et al. [24] presented a study proposing a new structure of bimetallic electrocatalysts to investigate ORR performance relative to a CL based on monometallic Pt nanoparticles. The examined CL was platinum on non-spherical Pd-Pt bimetallic supported on functionalized graphene (Pt-on-PdPt/fG). This CL presented enhanced ORR activity and durability in comparison to commercial Pt/C. More specifically, a specific activity of 1.89 mA/cm2 at 0.9 V was achieved from the Pt-on-PdPt/fG. At the same time, the commercial CL presented a specific activity around 0.23 mA/cm2 at 0.9 V. Carlos Calderón et al. [25] studied catalysts consisting of Pt-Pd (35 wt% and atomic ratio around 1:2) supported on carbon black, carbon nanofibers and carbon xerogels for evaluating their activity towards the ORR. Both XRD and TEM analyses showed low particle and crystallite sizes with good dispersion on carbon supports. Regarding the ORR, the Pt-Pd nanoparticles supported on black appeared to have the best performances in terms of high current densities, high specific and mass activities, and the number of moles participating in ORR and water formation. Thus, the improved performance of the examined CL was explained due to the modification of Pt-Pt interatomic distance by Pd and the holding of the hydrogen peroxide formed during the ORR process. Brouzgou et al. [26] investigated Pt-Pd on carbon supports in terms of CO tolerance and durability as both anode and cathode CLs on PEMFCs. The durability and electrocatalytic activity were investigated via electrochemical impedance spectroscopy (EIS), cyclic voltammetry (CV), linear sweep voltammetry (LSV), chronoamperometry (CA), and rotating disk electrode (RDE). The authors found that Pt-Pd3/C presented exhibit performance compared to Pt/C in terms of CO tolerance. Moreover, Pt-Pd/C and Pt-Pd3/C presented the highest mass activities, estimated at 339.4 and 410 mA/mgPt at 0.9 V, which was 4 and 5 times higher ascertained in commercial Pt/C (82.75 mA/mgPt). Zhang et al. [27] investigated the synthesis of Pd-Pt-based core-shell nanoparticles. Pd-Pt/C CL was compared with Pd-PtNi/C, and the latter presented enhanced ORR mass activity, estimated at 1.29 A/mgPt. Worth mentioning that by incorporating a third metal into the shell layer, the optimized mass activity of Pd@PtNiFe/C and Pd@PtNiCu/C CL was 1.1 and 1.4 times higher than Pd@PtNi/C. Bharti et al. [28] examined a CL consisting of Pt-Pd/CNT (Carbon NanoTubes) and prepared via microwave assisted, polyol route in the presence of sodium dodecyl sulfate (SDS) Pt-Pd/CNT-S or cetyltrimethylammonium bromide (CTAB) Pt-Pd/CNT-C, and their performance was compared with Pt-Pd/CNT, which was prepared identically without using any surfactant. The in-situ electrochemical characterization improved the catalytic activity Pt-Pd/CNT-S compared to Pt-Pd/CNT-C and Pt-Pd/CNT. Moreover, high fuel cell performance achieved by Pt-Pd/CNT-S having less Pt loading around (−12 wt%) than commercial pure Pt or Pt/C (20 wt%) CLs.
Another noble metal studied is gold (Au). The incorporation with Pt can restrain the dissolution and migration of CO poisoning, resulting in improved performance of the electrochemical reaction of ORR [29]. Deng et al. [30] studied the performance of Pt-Au/TiO2 CL for the ORR by examining various Pt/Au/Ti ratios. The scope of the study was to enhance the electrocatalytic activity of platinum-based CLs for ORR in PEMFCs. The results presented an improved performance above 4.19 times in mass activity compared to commercial Pt/C CLs with a ratio of 20% Pt/C. Jiang et al. [31] examined the performance of bimetallic Pt-Au nanoalloys. The in-situ diffuse reflectance infrared fourier transform (DRIFT) and X-ray photoelectron spectroscopy (XPS) proved that the electronic synergy effect between Au and Pt reduced the agglomeration of the noble metal and thus reduced CO poisoning. Furthermore, due to the synergy effect, the catalytic activity of the alloy increased by 20% compared to the single metal Pt catalysts. Lin et al. [32] investigated carbon supported Au-Pt core shell nano structured CLs. The developed CL was tested in terms of ORR activity via LSV and RDE. According to the authors, Au-Pt with atomic ratio 2:4 had the best catalytic performance towards ORR. Additionally, the stability of Au-Pt/C was examined via CV for 500 cycles. The performance of the MEA prepared by Au-Pt/C as cathode CL produced a maximum power density of 479 mW/cm2 with the operating conditions being at 0.431 V using H2 and O2 at 80°C. Jeong et al. [33] examined Pt-Au on carbon catalysts at various Pt/Au ratios for ORR in PEMFC. The maximum power was increased with Au loading reaching a maximum value at 370.3 mW/cm2 with Pt/Au ratio at 90/10. On the other hand, when more than 10% gold was added, the maximum power was decreased, while the CL with 20% Au loading presented lower maximum power compared to the Pt/C. The authors concluded that the CL with 10% Au loading had the highest performance by exhibiting a 30% higher power density than Pt/C (370.3 mW/cm2 compared to 293.3 mW/cm2). Beltran-Gastelum et al. [34] examined CL consisting of Pt-Au supported on reduced graphene oxide (Pt-Au/rGO). The nanomaterials were characterized by Raman, XRD, thermogravimetric analysis, and TEM. The developed CL was evaluated as an electrocatalyst for ORR. Furthermore, Pt-Au/rGO was compared to Pt/rGO to study the performance of the PEMFC. Thus, it concluded that the maximum power density was 20 mW/cm2 and 70 mW/cm2 for Pt/rGO and Pt-Au/rGO, respectively. Finally, Dorjgotov et al. [35] designed an Au-Pt core-shell nanocatalyst with high activity and stability. Using density functional theory, the authors validated their experimental results. In this sense, a power density above 2 W/cm2 was achieved at low Pt loading (around 0.1 mg/cm2) using a bilayer Au-Pt core-shell CL, which was highly durable.
PEMFCs usually incorporate Pt catalysts since they provide a large surface area and large accessible pores for hydrogen and oxygen to reach the CLs. In this respect, numerous efforts have been conducted for optimizing and extracting the maximum performance from these types of CLs. Chen et al. [36] examined the utilization of Pt for reducing mass transfer losses and improving the performance of PEMFC under low humidity and high current densities. Polarization curves, CV, and EIS were employed to characterize and compare the effects of Pt/C ratio and Nafion content gradient on the performance of PEMFCs under different humidification conditions. Their results indicated that the performance of the MEA could be significantly improved by allocating more Nafion and Pt/C in the sublayer near the membrane in the cathode catalyst layer. The MEA with optimal gradient cathode catalyst layer resulted in improved catalysts utilization, compared to MEA with a single cathode catalyst layer (0.403 gPt kW-1rated and 0.711 gPt kW-1rated under 80 RH% and 20 RH%, respectively). van Dao et al. [37] introduced a study to fabricate an efficient structural catalyst electrode of Pt/C consisting of double catalyst layers. The prepared Pt/C double catalyst layer electrode with Pt-dispersed and Pt-concentrated catalyst layers demonstrated better electrochemical properties than the individual Pt/C single catalyst layer electrode. The improved electrochemical performance of double catalyst layer electrodes was due to the increase of the Pt catalytic active area to the electrolyte when separating Pt catalysts into two layers, compared to single catalyst layer electrodes. Fan et al. [38] studied the degradation characteristics of PEMFCs with Pt black and Pt/C catalyst after 100 h of operation. The CL was investigated via electrochemical techniques and morphological characterization methods. Their experiments found that the degradation of Pt black CL was more severe than that of Pt/C CL. According to SEM, TEM, and XPS results, the decay of Pt black catalyst was mainly caused by Pt agglomeration and oxidation, causing a higher ohmic resistance, higher mass transfer resistance, and severer degradation of performance. The degradation of Pt/C catalyst was mainly due to reducing electrochemical surface area and carbon corrosion. The larger carbon corrosion made micropores and thicker supporting structures, resulting in performance degradation. Ostraverkh et al. [39] investigated the case of thin-film Pt with ultra-low metal loading ranging within 1 to 200 μg/cm2. The role of platinum loading and membrane thickness was investigated considering ionomer free carbon substrates. The results were compared with the commercial electrodes applied in PEMFCs and showed that thin-film Pt electrodes could provide platinum utilization 2 orders of magnitude higher than the standard Pt/C catalysts by achieving simultaneously similar power efficiency and long-term stability. Samad et al. [40] emphasized on different cases of supported CLs and separated them into two main categories (1) carbon-based and (2) non-carbon-based. The study compared the various CL supports in terms of morphology, electro-catalytic activity and structural characteristics. To this extent, all the discussed catalyst supports, presented high potentials as possible catalyst support candidates.
5 Pt and Non-Noble Metals in CLs
As already mentioned, the cost reduction of CL is one of the main challenges. The combination of Pt with other metals can increase the catalytic efficiency with simultaneous reduction of the Pt loading. Some of the non-nobles that have been already investigated are Sc, Ti, V, Cr, Mn, Fe, Co, Ni, etc. [41]. The Pt-Ni, Pt-Cu, Pt-Co, and Pt-Fe are some of the cases that have been examined as solutions for lowering the cost and enhancing catalytic activity compared to other monometallic materials [13]. In recent studies, Nickel (Ni) has been used to form Pt-Ni, due to the improved electronic characteristics it provides to Pt by shifting the electron transfer from the first material to the second via the synergistic effect presented when Pt and Ni are combined. To this extent, Beermann et al. [42] studied the properties of Pt-Ni alloy nanoparticles and reported remarkable high activities for ORR, providing exceptional potential on the fuel cell’s cathode. More specifically, ~25 times higher ORR was observed (2.7 A mg-1Pt at 0.9 V) compared to commercially available Pt/C CLs. Nigel et al. [43] investigated Pt-Ni CLs by controlling the ratio between Pt and Ni. The analysis showed~6 times higher mass activity for ORR about Pt/C. The high catalytic activity was attributed to the enhanced Ni loading near the surface. Hennining et al. [44] investigated unsupported PtNi alloy aerogel CL in PEMFC. The results showed that the optimized CL had 2.5 larger surface specific ORR activity than Pt/C and maintained 90% of the initial electrocatalytic activity. Kim et al. [45] introduced a Pt-Cu alloy thin film CL, which could be prepared by electrodeposition. The test was conducted using a single cell. The experiment revealed that a porous thin Pt-Cu alloy catalyst layer was successfully formed on the micro-porous layer/carbon paper. The synthesized Pt-Cu alloy catalyst was more durable than a conventional Pt/C in single cell tests. Ohyagi et al. [46] examined the durability of Pt-based Co alloy catalyst supported on carbon. In their study, the catalyst layer was studied via cyclic voltammetry to evaluate the electrochemical surface area (ECSA) and they investigated the polarization properties for assessing the activity of Pt-Co/C CL. Their results showed that the ECSA for Pt-Co/C was slowly decreased compared to the Pt/C CL during testing, with an initial ECSA at 62% and 37% for Pt-Co/C and Pt/C, respectively.
6 Economic Trends of the Materials Used in CLs
Platinum is a widely used material in the electronics sector, forecasted to be increased by over 40% in 2021, also considering the fuel cells’ demand. Worth mentioning that the transport sector is expected to be the largest contributor to fuel cell demand growth. Toyota alone announced to produce around 30,000 fuel cell electric vehicles (FCEVs). It should also be considered that heavy duty vehicles will start to incorporate fuel cells, leading to additional growth of fuel cells [47]. From a technical perspective, batteries in heavy duty vehicles are less suitable for long distances considering the significantly high weight of tracks comparing to fuel cells [47]. The total share of platinum used in fuel cells different sectors is presented in Fig. 3 for the 2016–2021 period. Road vehicles and stationary power applications are the sectors that fuel cells are widely used. Furthermore, in the transportation sector (including road and other vehicles), fuel cell applications are constantly increased, indicating the transition into greener solutions. The evolution of Pt price is presented in Fig. 4. The price has a downward trend ranging between 1500–1800 $ in 2011 and reaching 950 $ in 2021. However, on a short term, Pt price presents many fluctuations, which affect the fuel cells’ construction cost.
On the other hand, Palladium (Pd) is used in fuel cell catalysts to reduce platinum loading. Pd has been tested in fuel cells as Pt co-catalyst [48]. However, the Palladium market still remains on deficit on 2021 with industrial and autocatalyst demand exceeding the needs on primary and secondary supplies [47]. High Palladium prices have fueled active thrifting programs at virtually all global auto markets, starting to rise. Moreover, there is still some scope for near-term growth in PGM loadings in several major markets.
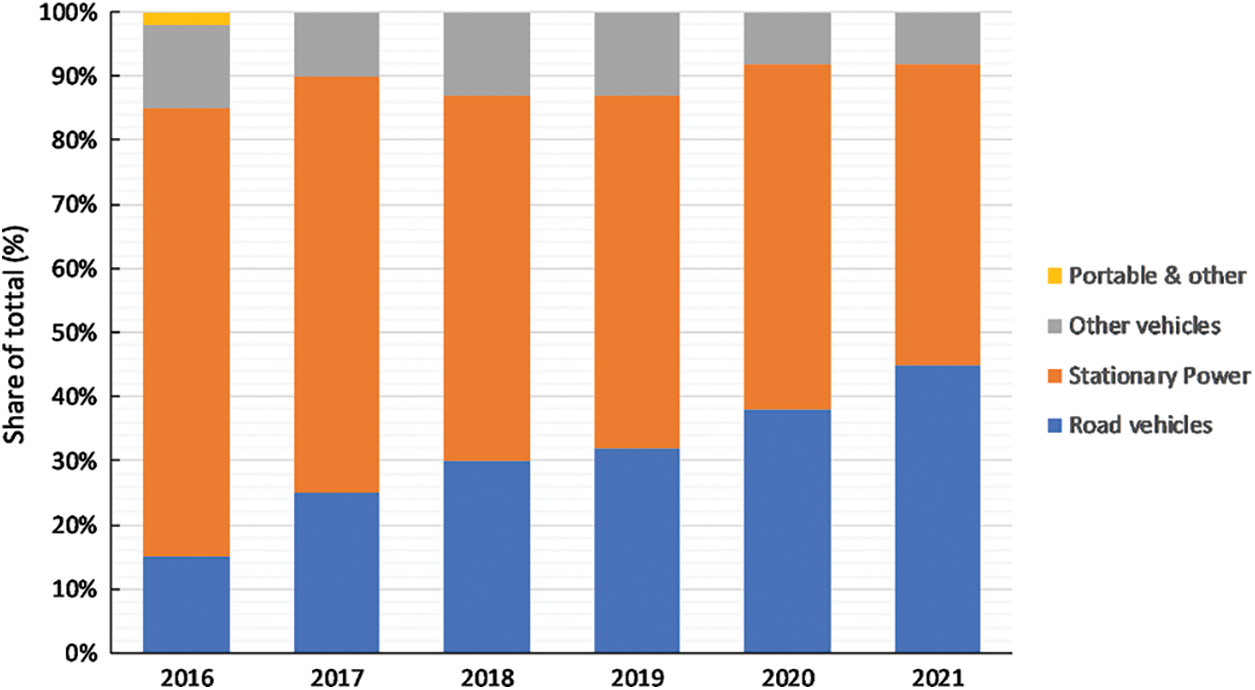
Figure 3: Platinum share in fuel cells demand [47]
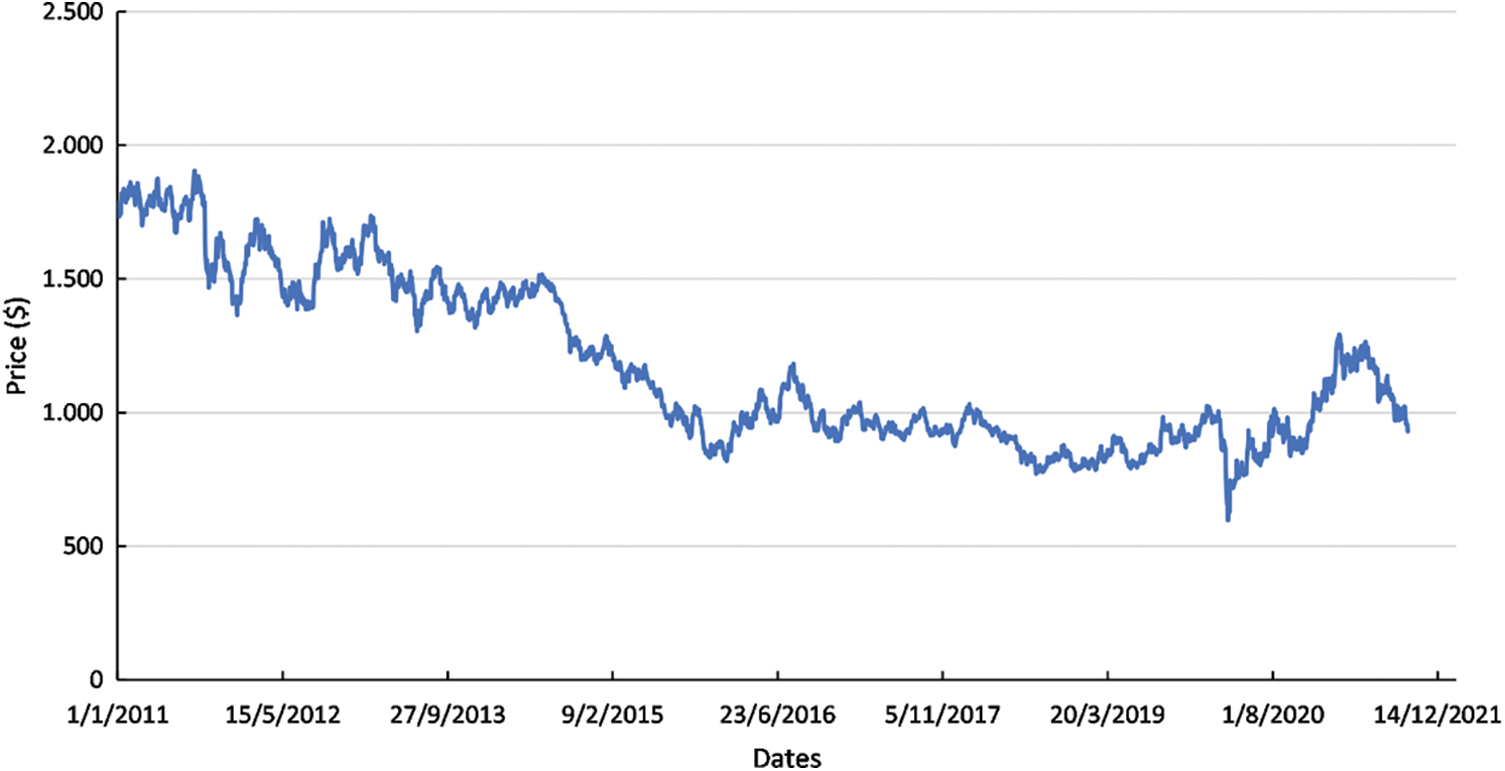
Figure 4: Platinum range price per ounce [49]
Palladium’s share of the PGM mix is expected to fall as platinum-containing formulations find wider acceptance. In this respect, the palladium market remained in deficit in 2021, with industrial and autocatalyst demand greatly exceeding combined primary and secondary supplies. In Fig. 5, Palladium’s price is depicted, showing that the overall price of Pd is lower in comparison to Pt at the beginning of the previous decade. Worth mentioning that a constant increase is presented in the Pd’s price in the last decade, reaching prices higher than Platinum. However, a price drop is highlighted during the second semester of 2021. Considering Fig. 6, some of the industrial applications present significant gains this year, while others have shown a long downward trend. To this extent, electronics sector have decreased the use of Palladium replacing it with Nickel, while the application on chemicals’ sector, the utilization of Pd has been increased.
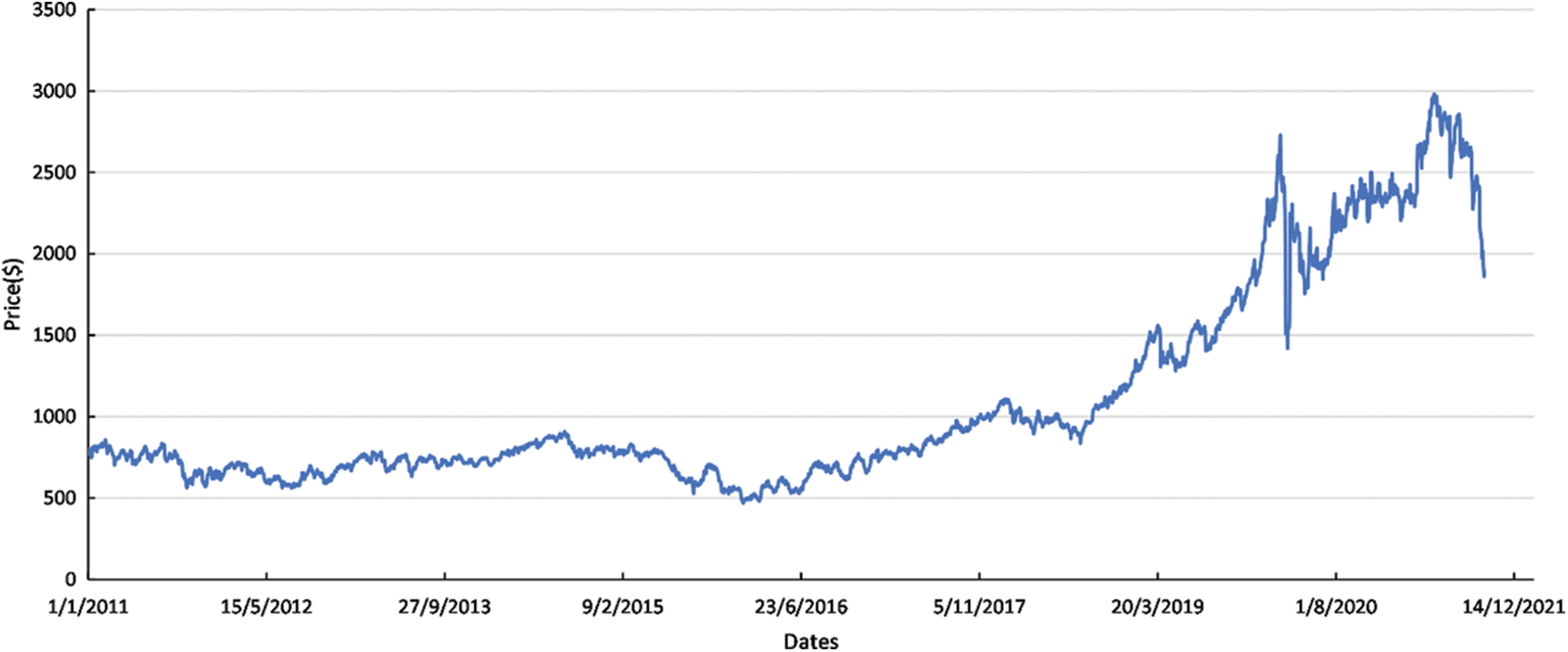
Figure 5: Palladium range price per ounce [49]

Figure 6: Palladium share in industrial applications [47]
Ruthenium is also one of the most promising materials for reducing the Pt loading on PEMFCs and being a co-catalyst. From a supply point of view, Ru price is at significant low levels considering the high levels of other PGMs such as rhodium (Rh) and iridium (Ir) which may it leads to a future demand for Ru as a suitable replacement for PGMs. In this context in Fig. 7, the Ru price is depicted for the last ~10 years, indicating significantly lower prices compared to Pt and Pd. More specifically, a considerable increase in the Ru price has been highlighted in the last three years but still at a lower levels comparing to Pt and Pd. In Fig. 8, the share of Ru in various industrial applications is depicted, concluding that the overall share of Ru has remain constant the last five years while individual sectors are presenting similar share through the years.
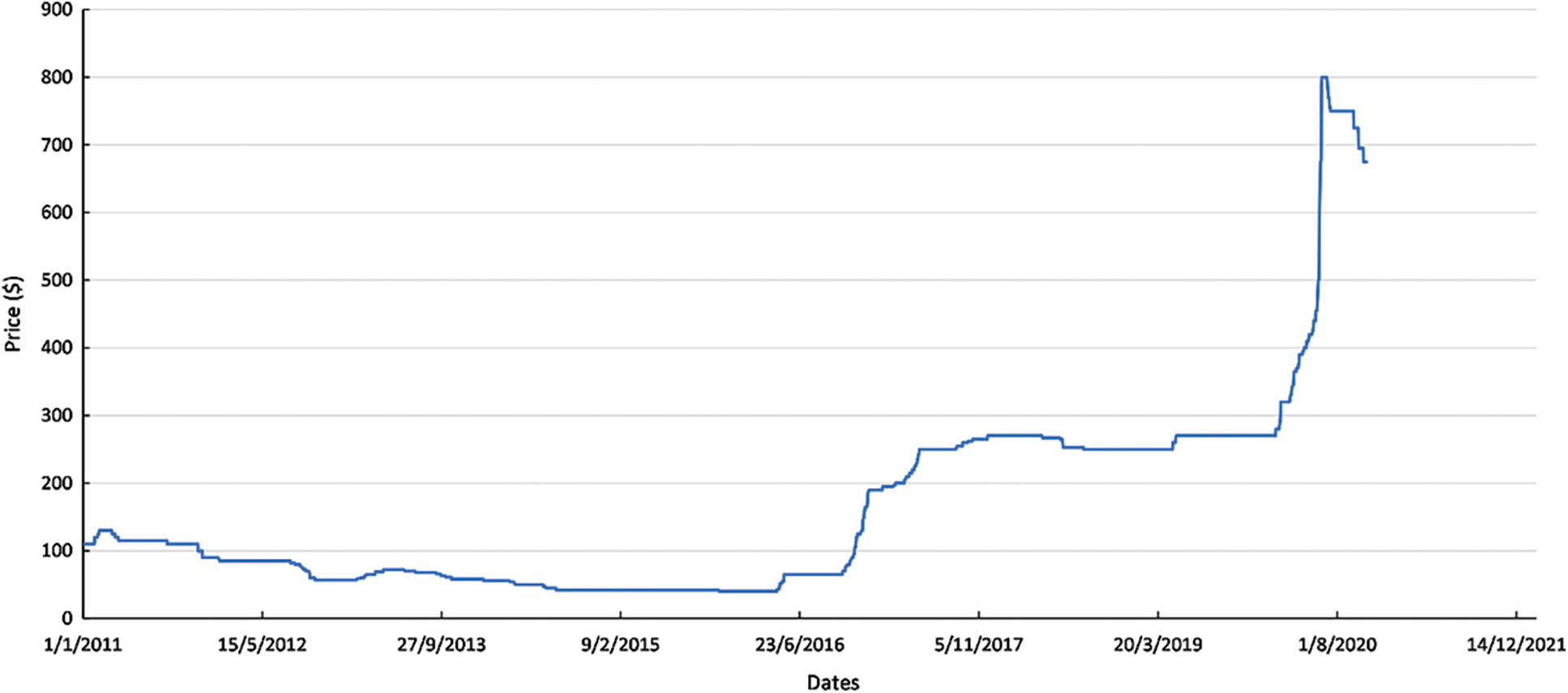
Figure 7: Ruthenium range price per ounce [49]

Figure 8: Ruthenium share in industrial applications [47]
In the current work, various studies have been examined to present a clear view of the work done in the specific field towards minimizing the Pt loading and establishing CO tolerant catalyst layers. Thus, the studies are separated into two main categories. The first category concerns those examining the properties of CLs consisting of Pt and Pt alloyed with a noble metal and the second on CLs consisting of Pt alloyed with non-noble metals. The first category is subdivided into the CLs alloyed with Ru, Pd, and Au. For each category, tables with information such as the scope and the conclusion of each study is introduced. The same structure is following for the cases of Pt alloyed with non-noble metals for a comprehensive summary which helps the reader to compare the differences between each case and identify the promising solutions.
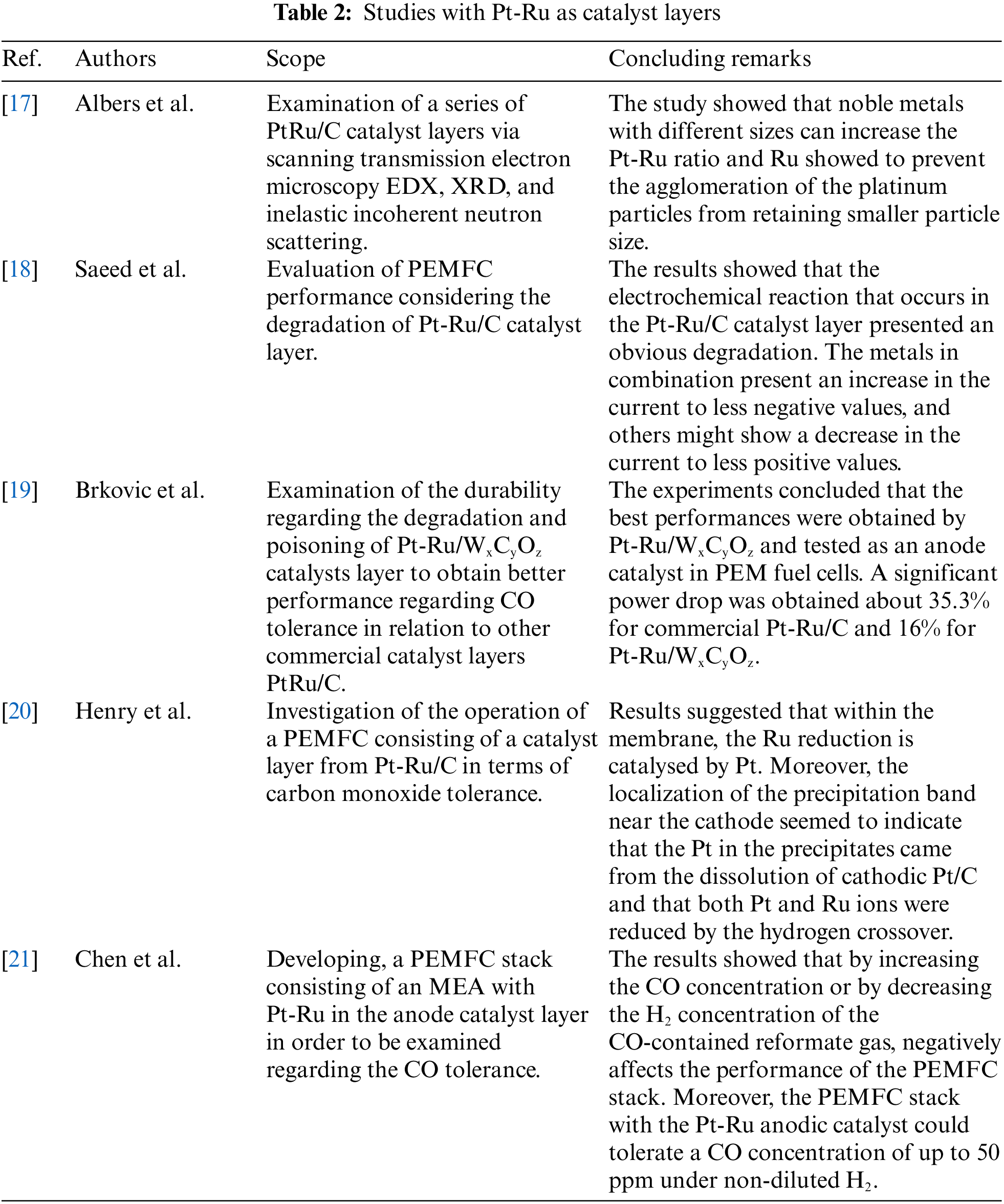
In Table 2, the studies concerning PEMFCs with CLs consisting of Pt-Ru are presented. Worth mentioning that a significant effort has been made in the last years to comprehend the benefits of Ru. In all examined cases, Pt-Ru CLs were investigated to evaluate the performance of PEMFC using a different type of CL to minimize the cost and enhance the durability (CO tolerance). Ru can be determined as a poor catalyst being several orders of magnitude less active (~0.002 A/cm2 while pure Pt has ≥ 0.3 A/cm2). Due to the significant high catalytic activity of Pt, CLs alloyed with Pt can sustain HOR at a decent level in terms of current density reaching ~500 mA/cm2 [50]. Overall, Pt-Ru CLs are shown to improve the operation of PEMFCs and can be considered as a viable and promising solution.
In Table 3 studies in which Pt-Pd was applied as the main CL in PEMFCs are presented. In all examined cases, the proposed CLs were investigated in terms of durability in CO tolerance and electric activity to verify if the specific type of CL can be a promising solution. Pt-Pd was examined as both anode and cathode CLs for HOR and ORR. The results presented an optimized electrochemical surface area compared to the conventional Pt/C. The electric activity was also enhanced in all examined cases, confirming that Pt-Pd can provide significant benefits. In general, Pd could partially replace Pt in CLs.

In Table 4 research work done on CLs consisting of Pt-Au is included. As in the previous cases, PtAu were investigated in terms of CO tolerance and the overall enhancement of PEMFCs performance for both anode and cathode CLs. The characterization of the CL was obtained via XPS and TEM techniques. The data provided by the experimental studies were compared with commercially available Pt/C CLs and showed significant improvement on PEMFC’s performance. Generally, by taking into consideration the results from the reviewed studies on Tables 2–4, it is obtained that bimetallic CLs are a promising solution solving many issues that are presented in the technology of PEMFCs. Nonetheless, further investigation should be made on a larger scale to identify the performance of PEMFC stacks to achieve a more detailed view of the operation when bimetallic CLs are applied and the drawbacks that could be arise.

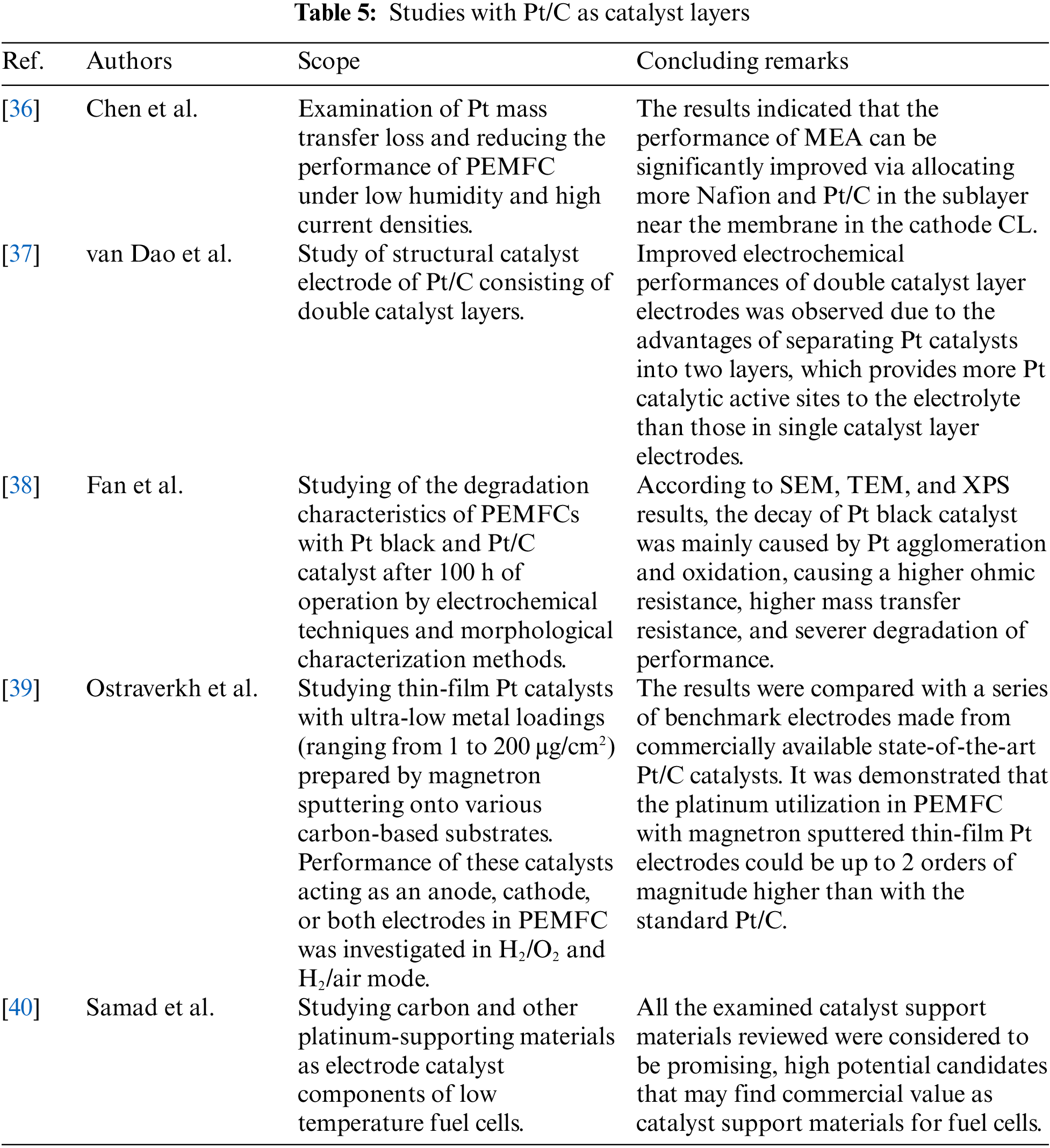
Tables 2–5 present studies investigating CLs composed with Pt and additional noble metals such as Ru, Au, Pd, considering also the conventional cases of Pt/C CLs. For each case, the catalyst composition and the corresponding results regarding the experimentally measured performance are presented commercially available electrodes, with typical composition of Pt/C. Concluding remarks are briefly described in each case and also summarized below. Study [21] showed that increasing the CO concentration or decreasing the H2 concentration of the CO-contained reformate gas can negatively affect the PEMFC stack’s performance. Moreover, the PEMFC stack with the Pt-Ru anodic catalyst can tolerate a CO concentration of up to 50 ppm under non-diluted. Furthermore, study [24] examined the case of Pt-Pd and compared it with Pt/C electrodes. Auspicious results were presented, indicating a catalytic enhancement activity around 8.2 times to Pt/C catalysts. The new electrode composition delivered 1.89 mA cmPt−2 while the conventional electrode produced 0.23 mA cmPt−2, measured at 0.9 V. In addition, study [30] examined the use of Au compared to a conventional catalyst having a composition of 20% Pt/C. Significant good results were presented, indicating an enhancement in mass activity around 4.19 times and 2.82 times regarding the specific activity. However, should also be considered, except for the significantly improved catalytic properties, is the high cost of Au. Study [39] examined Pt/C catalysts with ultra-low loading to settle the optimum point in terms of loading in both anode and cathode catalyst layer sides. Their results showed that loading range between 2–8 μg Pt per cm2 on the anode catalyst and 30–50 μg Pt per cm2 on the cathode catalyst proved to enhance the overall performance of the PEMFC.
The application of non-noble metal as CLs in PEMFCs could help the sustainable commercialization of the technology. Regardless of the various challenges, such as the catalytic activity and the poor stability, non-noble metals are an attractive and promising solution with a lot of interest being focused on the last years. Non-noble metals are in abundance and significantly low cost. To this extent, in Table 6 the case of Pt alloyed with non-noble metals is presented, describing the scope of each study and the corresponding results.
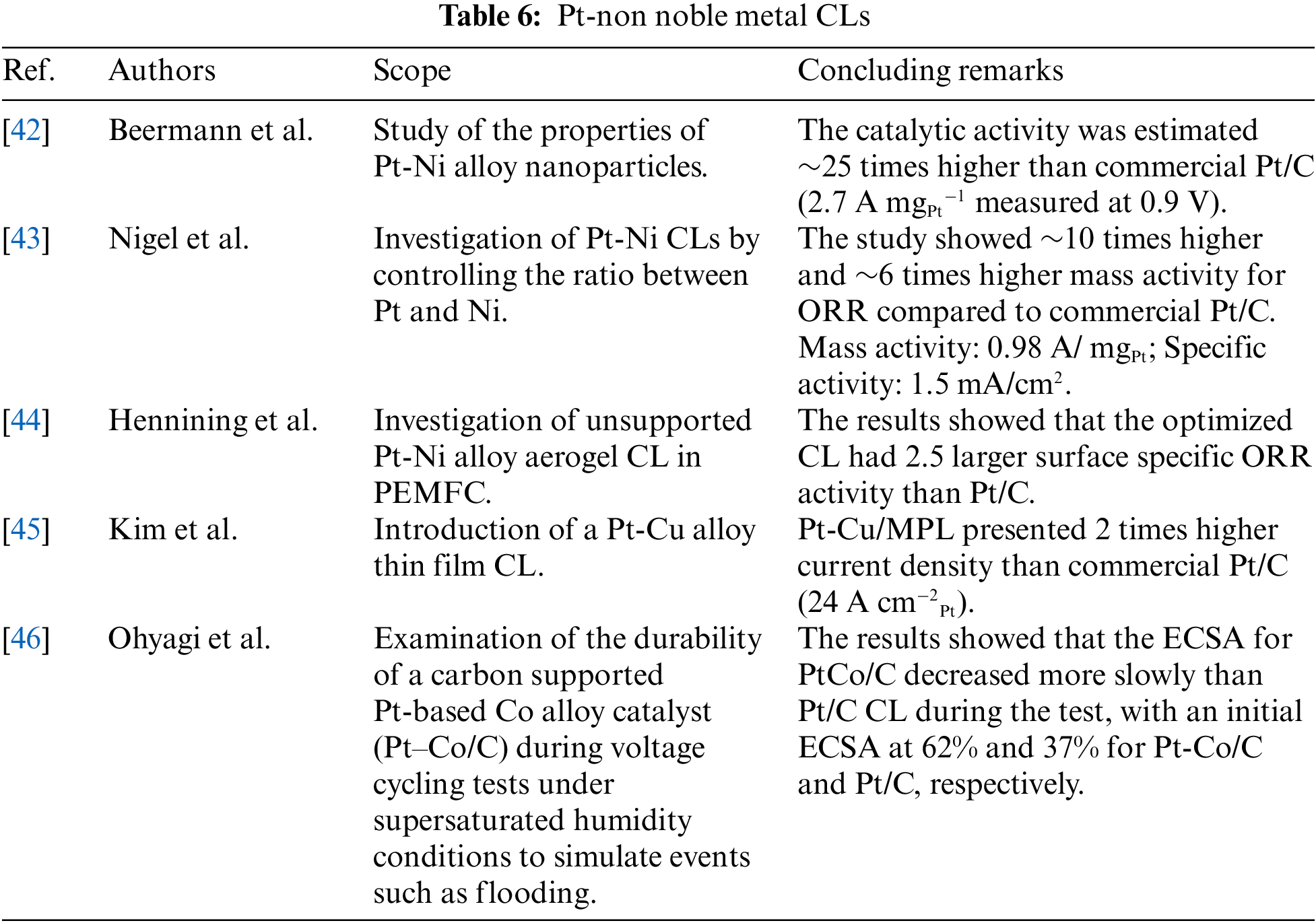
Studies [42–44] investigated Pt-Ni catalysts. In all three cases, Pt-Ni catalysts showed significantly enhanced results compared to conventional Pt/C catalysts. Moreover, these catalysts can be more efficient for ORR, as presented in Table 6, showing significantly improved mass and specific electrochemical activity. In more detail, study [42] concluded that Pt-Ni catalysts could provide an enhanced catalytic activity around 25 times in relation to common Pt/C electrodes. Study [43] focused on ORR’s performance in Pt-Ni catalysts. The results showed that specific activity was ~10 times higher and ~6 times higher mass activity for ORR compared to commercial Pt/C. Additionally, study [44] investigated Pt-Ni aerogel CL in PEMFC. The results showed an increased specific surface by 2.5 for ORR compared to conventional Pt/C. Study [45] investigated Pt-Cu catalysts which presents 2 times better catalytic activity in relation to Pt/C electrodes. The under-examination electrode produced a current density of 59 A cm−2 at 0.6 V, while the Pt/C electrode produced 24 A cm−2. Study [46] examined Pt-Co/C catalysts in terms of durability. The results showed that the electrochemical surface area was decreasing at a lower rate than the Pt/C catalysts.
Last but not least, it should be mentioned that additional research should be conducted in all the aforementioned CL cases to examine the performance of PEMFCs as an integrated system and not only the performance of specific catalyst layer compositions. This could lead to additional challenges that should be resolved while, at the same time, more conclusions on the behaviour of PEMFC in terms of both electrochemical and transport phenomena will be drawn. Nonetheless, the cost of producing state-of-the art CLs was presented. In Chapter 6 the economic trends of the most studied noble metals is showed by presenting the price fluctuations during the last decade. Pt remains at a higher prices compared to Ru and Pd. Thus, the need to turn into more economically viable materials is a priority for the technology of fuel cells. Worth mentioning that in the studies above, the cost of construction of CLs hasn’t been considered. To this extent, the cost of constructing new electrode compositions should be further examined to enable commercial feasibility.
To sum up, an increased interest has been raised for PEMFCs in the last few years, leading to novel approaches and significant breakthroughs in material properties. The CL compositions examined in the present work seem to be ambitious. Numerous studies have investigated Pt with noble metals and Pt-free CLs. Most of them consider carbon support, with fewer studies on non-carbon materials. Furthermore, the significantly high cost of Pt has been a major limitation for years. The reduction of Pt loading on cathode loading without decreasing the durability and the overall performance of PEMFC remaining a demanding task. Another major problem in PEMFCs is the activation losses presented on ORR. Several studies have been focused on optimizing the ORR catalytic activity by alloying Pt with other noble metals, presenting encouraging results. In this respect, they reduce the Pt loading using transition metals such as Ni, Co, Ru, Au, etc. Moreover, the reduction of Pt loading can lower the overall performance of fuel cells, but the presence of modified carbon support can create stronger metal support and thus could maintain the durability and the corresponding catalytic activity of PEMFC, even under reduced Pt loading. Future work that should be done can be separated into four levels: (1) minimizing the cost of synthesis of state-of-the-art CLs which can help to accelerate fuel cell commercialization, (2) develop an efficient synthesis that can lead to a high electrochemical surface area, emphasizing on parameters such as metal weight ratio and temperature should be highlighted, (3) research on molecular modelling provides an insight view of the phenomena and the interactions between the metal catalyst and its support and investigates the morphology of the CL, the active sites, and the corresponding electronic structure and conduction, (4) enhance the research on macroscopic modelling of PEMFC including all the information from the molecular modelling to examine PEMFCs as an integrated system and identify how the changes in CL affect the overall operation.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Kavadias, K. A., Kosmas, V., Tzelepis, S. (2022). Sizing, optimization, and financial analysis of a Green Hydrogen Refueling station in remote regions. Energies, 15(2), 547. DOI 10.3390/en15020547. [Google Scholar] [CrossRef]
2. Comparison of Fuel Cell Technologies. Energy.gov. https://www.energy.gov/eere/fuelcells/comparison-fuel-cell-technologies. [Google Scholar]
3. Behling, N. H. (2013). Fuel cells and the challenges ahead. Fuel Cells, 7–36. [Google Scholar]
4. Kavadias, K. A., Tzelepis, S. (2020). A simplified optimization model for sizing proton-exchange membrane fuel cells. Journal of Energy and Power Technology, 2(3), 24. DOI 10.21926/jept.2003011. [Google Scholar] [CrossRef]
5. Tzelepis, S., Kavadias, K. A., Marnellos, G. E., Xydis, G. (2021). A review study on proton exchange membrane fuel cell electrochemical performance focusing on anode and cathode catalyst layer modelling at macroscopic level. Renewable and Sustainable Energy Reviews, 151(35), 111543. DOI 10.1016/j.rser.2021.111543. [Google Scholar] [CrossRef]
6. Shabani, B., Hafttananian, M., Khamani, S., Ramiar, A., Ranjbar, A. A. (2019). Poisoning of proton exchange membrane fuel cells by contaminants and impurities: Review of mechanisms, effects, and mitigation strategies. Journal of Power Sources, 427(5), 21–48. DOI 10.1016/j.jpowsour.2019.03.097. [Google Scholar] [CrossRef]
7. Yan, Y. T., Tsai, K. T., Chen, C. K. (2013). The effects of the PEM fuel cell performance with the waved flow channels. Journal of Applied Mathematic, 1–14. [Google Scholar]
8. Francia, C., Ijeri, V. S., Specchia, S., Spinelli, P. (2011). Estimation of hydrogen crossover through Nafion® membranes in PEMFCs. Journal of Power Sources, 196(4), 1833–1839. DOI 10.1016/j.jpowsour.2010.09.058. [Google Scholar] [CrossRef]
9. Gao, F., Zhang, Y., Ren, F., Shiraishi, Y., Du, Y. (2020). Universal surfactant-free strategy for self-standing 3D tremella-like Pd-M (M = Ag, Pb, and Au) nanosheets for superior alcohols electrocatalysis. Advanced Functional Materials, 30(16), 2000255. DOI 10.1002/adfm.202000255. [Google Scholar] [CrossRef]
10. Zhang, Y., Gao, F., You, H., Li, Z. L., Zou, B. et al. (2022). Recent advances in one-dimensional noble-metal-based catalysts with multiple structures for efficient fuel-cell electrocatalysis. Coordination Chemistry Reviews, 450, 214244. DOI 10.1016/j.ccr.2021.214244. [Google Scholar] [CrossRef]
11. Gao, F., Zhang, Y., Song, P. J., Wang, B. Y., Sun, Q. et al. (2019). Shape-control of one-dimensional PtNi nanostructures as efficient electrocatalysts for alcohol electrooxidation. Nanoscale, 11(11), 4831–4836. DOI 10.1039/C8NR09892A. [Google Scholar] [CrossRef]
12. Liao, W., Zhou, S., Wang, Z., Liu, F., Cao, J. et al. (2022). Composition-controlled effects of Pb content in PtPbRu trimetallic nanoparticles on the electrocatalytic oxidation performance of methanol. Fuel, 308(14), 122073. DOI 10.1016/j.fuel.2021.122073. [Google Scholar] [CrossRef]
13. Ren, X., Lv, Q. Y., Liu, L., Liu, B., Wang, Y. et al. (2020). Current progress of Pt and Pt-based electrocatalysts used for fuel cells. Sustainable Energy Fuels, 4(1), 15–30. DOI 10.1039/C9SE00460B. [Google Scholar] [CrossRef]
14. Nie, Y., Li, L., Wei, Z. (2015). Recent advancements in Pt and Pt-free catalysts for oxygen reduction reaction. Royal Society of Chemistry, 44(8), 2168–2201. DOI 10.1039/C4CS00484A. [Google Scholar] [CrossRef]
15. Zhang, J. (2008). PEM fuel cell electrocatalysts and catalyst layers. London: Springer London. [Google Scholar]
16. Nørskov, J. K., Rossmeisl, J., Logadottir, A., Lindqvist, L. (2004). Origin of the overpotential for oxygen reduction at a fuel-cell cathode. The Journal of Physical Chemistry B, 108(46), 17886–17892. DOI 10.1021/jp047349j. [Google Scholar] [CrossRef]
17. Albers, P. W., Weber, W., Kunzmann, K., Lopez, M., Parker, S. F. (2008). Characterisation of carbon supported platinumruthenium fuel cell catalysts of different degree of alloying. Surface Science, 602(23), 3611–3616. DOI 10.1016/j.susc.2008.10.006. [Google Scholar] [CrossRef]
18. Saeed, F., Saidan, M., Said, A., Mustafa, M., Abdelhadi, A. et al. (2013). Effect of flow rate, flow direction, and silica addition on the performance of membrane and the corrosion behavior of Pt-Ru/C catalyst in PEMFC. Energy Conversion and Management, 75(6), 36–43. DOI 10.1016/j.enconman.2013.05.045. [Google Scholar] [CrossRef]
19. Brkovic, S. M., Marceta Kaninski, M. P., Lausevic, P. Z., Saponjic, A. B., Radulovic, A. M. et al. (2020). Non-stoichiometric tungsten-carbideoxide-supported Pt-Ru anode catalysts for PEM fuel cells–from basic electrochemistry to fuel cell performance. International Journal of Hydrogen Energy, 45(27), 13929–13938. DOI 10.1016/j.ijhydene.2020.03.086. [Google Scholar] [CrossRef]
20. Henry, P. A., Guétaz, L., Pélissier, N., Jacques, P. A., Escribano, S. (2015). Structural and chemical analysis by transmission electron microscopy of Pt-Ru membrane precipitates in proton exchange membrane fuel cell aged under reformate. Journal of Power Sources, 275(1), 312–321. DOI 10.1016/j.jpowsour.2014.10.167. [Google Scholar] [CrossRef]
21. Chen, C. Y., Huang, K. P. (2017). Performance and transient behavior of the kW-grade PEMFC stack with the Pt Ru catalyst under CO-contained diluted hydrogen. International Journal of Hydrogen Energy, 42(34), 22250–22258. DOI 10.1016/j.ijhydene.2017.06.037. [Google Scholar] [CrossRef]
22. Hu, C. C. (1994). Voltammetric investigation of Hydrogen Sorption/Desorption at/within Oxide-Derived Pd Electrodesin NaOH and H2S04. Journal of Electrochem, 141(11), 2996. [Google Scholar]
23. Zhou, Z. M., Shao, Z. G., Qin, X. P., Chen, X. G., Wei, Z. D. et al. (2010). Durability study of Pt-Pd/C as PEMFC cathode catalyst. International Journal of Hydrogen Energy, 35(4), 1719–1726. DOI 10.1016/j.ijhydene.2009.12.056. [Google Scholar] [CrossRef]
24. Cho, K. Y., Yeom, Y. S., Seo, H. Y., Leeb, A. S., Do, X. H. et al. (2017). Fine-sized Pt nanoparticles dispersed on PdPt bimetallic nanocrystals with non-covalently functionalized graphene toward synergistic effects on the oxygen reduction reaction. Electrochimica Acta, 257, 412–422. DOI 10.1016/j.electacta.2017.10.075. [Google Scholar] [CrossRef]
25. Carlos Calderón, J., Ndzuzo, L., Bladergroen, B. J., Pasupathi, S. (2018). Catalytic activity of carbon supported-Pt-Pd nanoparticles toward the oxygen reduction reaction. Materials Today: Proceedings, 5, 10551–10560. [Google Scholar]
26. Brouzgou, A., Seretis, A., Song, S., Shen, P. K., Tsiakaras, P. (2021). CO tolerance and durability study of PtMe(Me = Ir or Pd) electrocatalysts for H2-PEMFC application. International Journal of Hydrogen Energy, 46(26), 13865–13877. DOI 10.1016/j.ijhydene.2020.07.224. [Google Scholar] [CrossRef]
27. Zhang, Y., Ye, K., Gu, Q., Jiang, Q., Qin, J. et al. (2021). Optimized oxygen reduction activity by tuning shell component in Pd@Pt-based core-shell electrocatalysts. Journal of Colloid and Interface Science, 604, 301–309. DOI 10.1016/j.jcis.2021.06.136. [Google Scholar] [CrossRef]
28. Bharti, A., Cheruvally, G. (2018). Surfactant assisted synthesis of Pt-Pd/MWCNT and evaluation as cathode catalyst for proton exchange membrane fuel cell. International Journal of Hydrogen Energy, 43(31), 14729–14741. DOI 10.1016/j.ijhydene.2018.06.009. [Google Scholar] [CrossRef]
29. Zhong, X., Yu, H., Wang, X., Liu, L., Jiang, Y. et al. (2014). Pt@Au Nanorods uniformly decorated on pyridyne cycloaddition graphene as a highly effective electrocatalyst for oxygen reduction. ACS Applied Materials & Interfaces, 6(16), 13448–13454. DOI 10.1021/am5020452. [Google Scholar] [CrossRef]
30. Deng, X., Yin, S., Wu, X., Sun, M., Xie, Z. et al. (2018). Synthesis of PtAu/TiO2 nanowires with carbon skin as highly active and highly stable electrocatalyst for oxygen reduction reaction. Electrochimica Acta, 283, 987–996. DOI 10.1016/j.electacta.2018.06.139. [Google Scholar] [CrossRef]
31. Jiang, J., Lei, J., Hu, Y., Bia, W., Xu, N. et al. (2020). Electron transfer effect from Au to Pt in Au-Pt/TiO2 towards efficient catalytic activity in CO oxidation at low temperature. Applied Surface Science, 521, 146447. DOI 10.1016/j.apsusc.2020.146447. [Google Scholar] [CrossRef]
32. Lin, R., Zhang, H., Zhao, T., Caoa, C., Yanga, D. et al. (2012). Investigation of Au@Pt/C electro-catalysts for oxygen reduction reaction. Electrochimica Acta, 62, 263–268. DOI 10.1016/j.electacta.2011.12.018. [Google Scholar] [CrossRef]
33. Jeong, S. M., Kim, M. K., Kim, G. P., Kim, T. Y., Baeck, S. H. (2012). Preparation of Pt-Au/carbon catalysts by a reduction method and their electrocatalytic activities for oxygen reduction reactions. Chemical Engineering Journal, 198–199(2), 435–439. DOI 10.1016/j.cej.2012.04.002. [Google Scholar] [CrossRef]
34. Beltrán-Gastélum, M., Salazar-Gastélum, M. I., Flores-Hernández, J. R., Botte, G. G., Sicairos, S. P. et al. (2019). Pt-Au nanoparticles on graphene for oxygen reduction reaction: Stability and performance on proton exchange membrane fuel cell. Energy, 181(1), 1225–1234. DOI 10.1016/j.energy.2019.06.033. [Google Scholar] [CrossRef]
35. Dorjgotov, A., Jeon, Y., Hwang, J., Ulziidelgera, B., Kim, H. S. et al. (2017). Synthesis of durable small-sized bilayer Au@Pt nanoparticles for high performance PEMFC catalysts. Electrochimica Acta, 228(46), 389–397. DOI 10.1016/j.electacta.2017.01.083. [Google Scholar] [CrossRef]
36. Chen, G. Y., Wang, C., Lei, Y. J., Zhang, J., Mao, Z. et al. (2017). Gradient design of Pt/C ratio and Nafion content in cathode catalyst layer of PEMFCs. International Journal of Hydrogen Energy, 42(50), 29960–29965. DOI 10.1016/j.ijhydene.2017.06.229. [Google Scholar] [CrossRef]
37. van Dao, D., Adilbish, G., Lee, I. H., Yu, Y. T. (2019). Enhanced electrocatalytic property of Pt/C electrode with double catalyst layers for PEMFC. International Journal of Hydrogen Energy, 44(45), 24580–24590. DOI 10.1016/j.ijhydene.2019.07.156. [Google Scholar] [CrossRef]
38. Fan, L., Zhao, J., Luo, X., Tu, Z. (2022). Comparison of the performance and degradation mechanism of PEMFC with Pt/C and Pt black catalyst. International Journal of Hydrogen Energy, 47(8), 5418–5428. DOI 10.1016/j.ijhydene.2021.11.135. [Google Scholar] [CrossRef]
39. Ostroverkh, A., Johánek, V., Dubau, M., Kus, P., Khalakhan, I. et al. (2019). Optimization of ionomer-free ultra-low loading Pt catalyst for anode/cathode of PEMFC via magnetron sputtering. International Journal of Hydrogen Energy, 44(35), 19344–19356. DOI 10.1016/j.ijhydene.2018.12.206. [Google Scholar] [CrossRef]
40. Samad, S., Loh, K. S., Wong, W. Y., Lee, T. K., Sunarso, J. et al. (2018). Carbon and non-carbon support materials for platinumbased catalysts in fuel cells. International Journal of Hydrogen Energy, 43(16), 7823–7854. DOI 10.1016/j.ijhydene.2018.02.154. [Google Scholar] [CrossRef]
41. Zhang, L., Wilkinson, D. P., Liu, Y., Zhang, J. (2018). Progress in nanostructured (Fe or Co)/N/C non-noble metal electrocatalysts for fuel cell oxygen reduction reaction. Electrochimica Acta, 262(15), 326–336. DOI 10.1016/j.electacta.2018.01.046. [Google Scholar] [CrossRef]
42. Beermann, V., Gocyla, M., Kühl, S., Padgett, El, Schmies, H. et al. (2017). Tuning the electrocatalytic oxygen reduction reaction activity and stability of shape-controlled Pt-Ni nanoparticles by thermal annealing−elucidating the surface atomic structural and compositional changes. Journal of the American Chemical Society, 139(46), 16536–16547. DOI 10.1021/jacs.7b06846. [Google Scholar] [CrossRef]
43. Nigel, B., Yoonkook, S., Dohyung, K., Li, D., Yu, Y. et al. (2017). Control of architecture in rhombic dodecahedral Pt-Ni nanoframe. Journal of the American Chemical Society, 139(34), 11678–11681. DOI 10.1021/jacs.7b05584. [Google Scholar] [CrossRef]
44. Henning, S., Ishikawa, H., Kühn, L., Herranz, J., Muller, E. et al. (2017). Unsupported Pt-Ni aerogels with enhanced high current performance and durability in fuel cell cathodes. Angewandte Chemie, 56(36), 10707–10710. DOI 10.1002/anie.201704253. [Google Scholar] [CrossRef]
45. Kim, Y. (2017). Non-conventional Pt-Cu alloy/carbon paper electrochemical catalyst formed by electrodeposition using hydrogen bubble as template. Journal of Power Sources, 364(2473), 16–22. DOI 10.1016/j.jpowsour.2017.08.016. [Google Scholar] [CrossRef]
46. Ohyagi, S., Sasaki, T. (2013). Durability of a PEMFC Pt-Co cathode catalyst layer during voltage cycling tests under supersaturated humidity conditions. Electrochimica Acta, 102, 336–341. DOI 10.1016/j.electacta.2013.04.060. [Google Scholar] [CrossRef]
47. Johnsos Matthey (2021). PGM market report. https://matthey.com/-/media/files/pgm-market-report/jm-pgm-market-report-may-2021.pdf. [Google Scholar]
48. Antolini, E. (2009). Palladium in fuel cell catalysis. Energy & Environment Science, 2(9), 915. DOI 10.1039/b820837a. [Google Scholar]
49. Price charts–PMM. http://www.platinum.matthey.com/prices/price-charts. [Google Scholar] [CrossRef]
50. Zhang, J. (2008). PEM fuel cell electrocatalysts and catalyst layers. London: Springer London. [Google Scholar]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |