

 | Fluid Dynamics & Materials Processing |  |
DOI: 10.32604/fdmp.2022.020059
ARTICLE
Assessment of the Performances of Carboxylic Acid Monomers as Fluid Loss Additives for Oil-Well Cement
1Zhanjiang Branch of CNOOC (China) Co., Ltd., Zhanjiang, 524057, China
2School of Petroleum Engineering, China University of Petroleum (East China), Qingdao, 266580, China
*Corresponding Author: Mengran Xu. Email: xumengran19970105@163.com
Received: 01 November 2021; Accepted: 18 January 2022
Abstract: The application of polycarboxylic acid as a fluid loss additive for cement (i.e., a substance specifically designed to lower the volume of filtrate that passes through the cement) can prolong the thickening time of cement slurries. Given the lack of data about the effects of carboxylic acid monomers as possible components for the additives traditionally used for oil-well cement, in this study different cases are experimentally investigated considering different types of these substances, concentrations, temperatures, and magnesium ion contamination. The results demonstrate that itaconic acid has a strong retarding side effect, while maleic and acrylic acids have similar influences on the thickening time of the cement slurry. The rheological properties of the cement slurry tend to deteriorate when the carboxylic acid monomer content in the fluid loss additive is increased to 40%. If the temperature exceeds 80°C, there is a significant decrease in the related impact on the thickening duration. With an increase in the intrusion of magnesium ions to >0.5%, both the rheological properties of the cement slurry and the thickening time are affected in a negative way.
Keywords: Fluid-loss control additive; cement slurry; carboxylic acid monomer; thickening performance
The addition of fluid loss additives to cement slurries is necessary to control the filtration of the slurries for the successful completion of cementing operations as well as to improve cementing quality [1,2]. Such additives include inorganic fine particles and polymer fluid loss additives [3,4]. These additives include polyvinyl alcohol film-forming fluid loss additives and polycarboxylic acid fluid loss additives; currently, the latter is most widely used [5,6]. Although carboxylic acids serve as key functional groups for water loss during take-off and landing, previous research shows that this group of fluid loss additives induces strong thickening and retarding side effects [7,8]. In some cases, these effects may lead to fluctuations in cement slurry consistency and threaten the safety of cement-based construction. Thus, selecting appropriate carboxylic acid monomers is essential to the development of fluid loss additives, particularly for oil well cement.
Researchers have studied the relationship between the thickening duration of the cement slurry and the presence of the carboxyl group in fluid loss additives, clearly demonstrating that this group imparts a strong retarding side effect. However, the effects of carboxylic acid monomer types at different concentrations and under environmental conditions (i.e., temperature and ion concentration) on the properties of fluid loss additives have not been investigated in detail. Therefore, it is difficult to rationally select carboxylic acid monomers for the synthesis of fluid loss additives [9,10]. To develop a fluid loss additive that exhibits good control of water loss from cement slurries, it is necessary to investigate the influence of carboxylic acid monomers in fluid loss additives under different conditions.
In this study, acrylic acid (AA), maleic acid (MA), and itaconic acid (IA) were the selected carboxylic acid monomers used in the synthesis of fluid loss additives. The study revealed the effects of the type and content of carboxylic acid monomers on the performance of the fluid loss additives (in terms of amount of water lost, rheological properties, and thickening duration for cement slurries) under different temperature and magnesium-ion-concentration conditions. This study facilitates the development of novel, effective fluid loss additives.
Acrylamide (AM), IA, MA, AA, ammonium persulfate (APS), sodium bisulfite (SOB), 2-acrylamide-2-methyl propionic sulfonic acid (AMPS), and magnesium chloride hexahydrate were purchased from Sinopharm Chemical Reagent Co., Ltd. (China) Class G oil well cement (OPC) was obtained from Jiahua Special Cement Co., Ltd. (China), distilled water.
2.2.1 Determination of Monomer Ratio
Based on preliminary experiments and the literatures [11–14], a monocarboxylic acid (AA) and dicarboxylic acids (MA and IA) were initially selected as different source monomers of carboxylic acid groups to synthesize fluid loss additives. To maintain an equal molar ratio of the carboxylic acid groups, the molar ratio of each reaction monomer for AMPS:AM:AA was 45:45:10, for AMPS:AM:MA was 45:45:5, and for AMPS:AM:IA was 45:45:5. Based on this monomer ratio, three types of fluid loss additives were synthesized. The fluid loss additives synthesized from the AA, MA, and IA monomers were named types AA, MA, and IA fluid loss additives, respectively.
2.2.2 Other Reaction Conditions
The total mass percentage of monomers was 9%, the mass percentage of the initiator was 0.2% (i.e., 0.1% SOB, 0.1% APS), the reaction temperature was set at 60°C, and the reaction time was 4 h.
2.2.3 Synthesis of Fluid Loss Additives
Specific quantities of distilled water, AMPS, AM, and the other carboxylic acid monomers were weighed in large and small beakers. These materials were added to the large beaker filled with distilled water, and constantly stirred for complete dissolution. Then, specific quantities of SOB and APS were successively added to the small beaker and continuously stirred for complete dissolution. The solution in the small beaker was gradually mixed with the solution in the large beaker, while the solution in the small beaker was constantly stirred using a glass rod to fully dissolve the mixture. The mixed solution in the large beaker was sealed with a plastic wrap after stirring, and placed in a 60°C water bath for 4 h. The resultant reaction product was dried and crushed to obtain a solid fluid loss additive powder.
2.2.4 Cement Slurry Preparation and Performance Test Method
The water-solid ratio of the system was 0.44. The preparation, rheological performance, thickening duration, and water loss tests for the cement slurries were carried out in accordance with the American Petroleum Institute requirements (10B-2-2013).
3.1 Influence of Carboxylic Acid Group Source Monomer Type
3.1.1 Influence of Three Fluid Loss Additives on Water Loss
The water loss of the cement slurries was tested at 75°C with different concentrations of the AA, MA, and IA fluid loss additives with a 10% carboxyl group mole fraction (Fig. 1).

Figure 1: Relationship between different concentrations of the fluid loss additives and their water loss controllability
Increasing the concentration of the fluid loss additives gradually decreased the water loss of the cement slurries (Fig. 1). When the concentration increased from 0% to 1%, the water loss of the cement slurries significantly decreased to <100 mL, meeting the water loss requirements of cement slurries in cementing operations. When the concentration was increased to 1.5%, the water loss of the cement slurries decreased to <50 mL. This indicates that AA, MA, and IA have the ability to control water loss.
3.1.2 Influence of Three Fluid Loss Additives on Cement Slurry Fluidity
The mole fraction of the carboxyl group of AA, MA, and IA was 10%, and the rheological properties of the cement slurries with 0.5% of the fluid loss additives at room temperature were determined (Table 1).
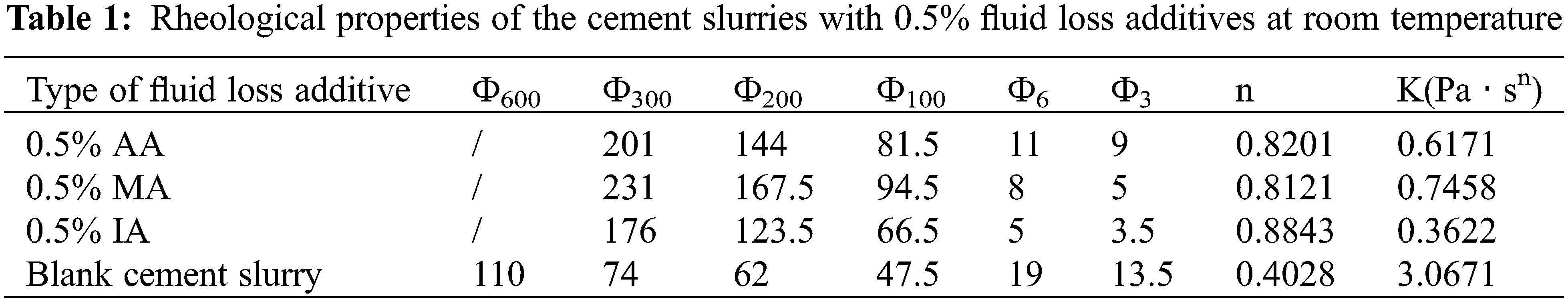
The rheological properties of the cement slurries containing the fluid loss additives were higher than those of the blank cement slurry (Table 1). The cement slurry containing the IA fluid loss additive exhibited the highest rheological properties, while the properties for the cement slurry containing the MA fluid loss additive were similar to those of the slurry containing the AA fluid loss additive.
The mole fraction of the carboxyl group of AA, MA, and IA was 10%, and the rheological properties of the cement slurries with different concentrations of the AA, MA, and IA were determined at 75°C. Figs. 2 and 3 present the variation in the fluidity index (n) and the consistency coefficient (K) with an increase in the concentration of fluid loss additives.
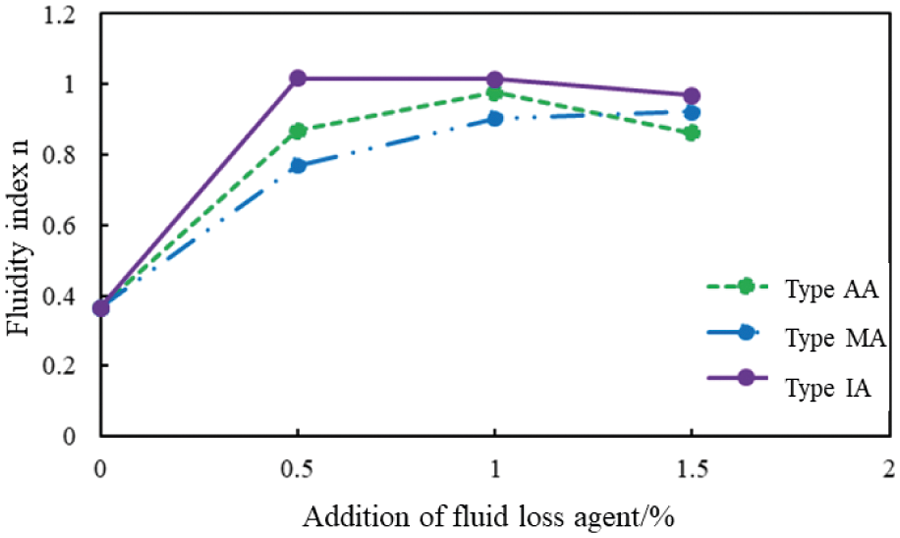
Figure 2: Fluidity index (n) of cement slurries with different fluid loss additive concentrations at 75°C
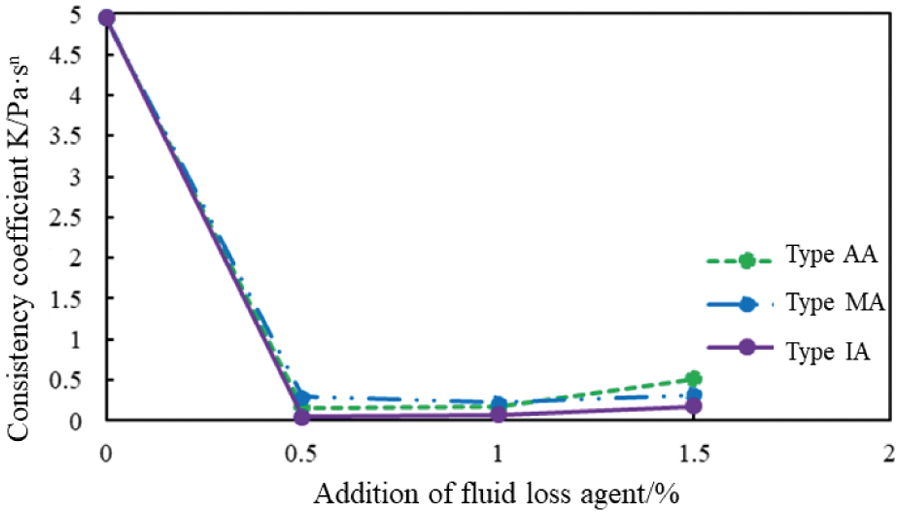
Figure 3: Consistency coefficient (K) of cement slurries with different fluid loss additive concentrations at 75°C
At 75°C, the rheological properties of the cement slurries containing AA and IA had improved (Figs. 2 and 3). However, the rheological properties of the cement slurry containing the MA and the blank cement slurry had deteriorated, which is not conducive to cementing operations. The cement slurries containing AA and IA also exhibited high rheological properties when the fluid loss additive concentration was 1%. The rheological properties of the cement slurry containing MA improved with an increase in the fluid loss additive concentration.
The amount of water lost from the cement slurries was within 100 mL when the fluid loss additive concentration was 1%, whereas it was low when the concentration was 1.5%; however, a dispersant was added in the latter slurries as they were thick. To prevent the dispersant from interfering in the experimental results, the fluid loss additive concentration in the subsequent experiments was set to 1%.
3.1.3 Influence of Fluid Loss Additives on Cement Slurry Thickening Duration
The mole fraction of the carboxyl group of AA, MA, and IA was 10%. At 75°C, the thickening duration of the cement slurries, with a concentration of 1% of the fluid loss additives, was tested (Fig. 4).
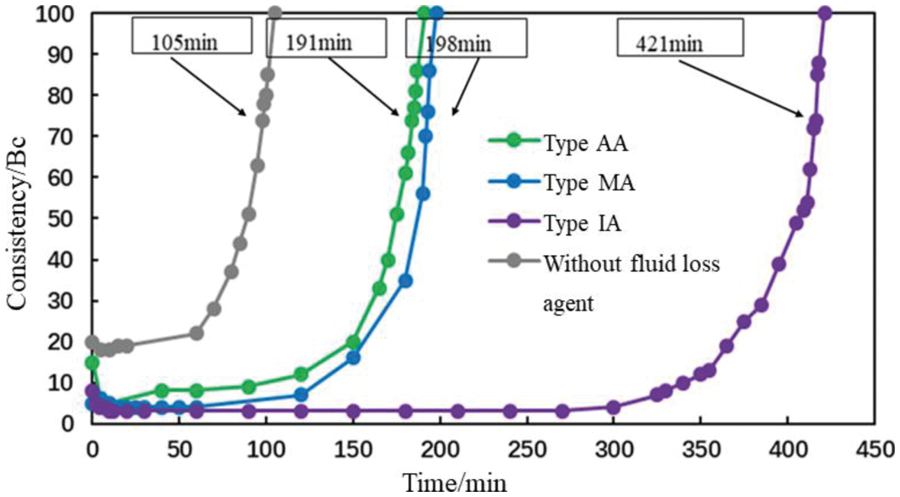
Figure 4: Normal pressure thickening curve of the cement slurries with 1% fluid loss additives (conditions: carboxyl content = 10%, temperature = 75°C)
The thickening curves of the cement slurries with 1% fluid loss additives were initially stable, then increased rapidly with no “bulge” or other phenomena (Fig. 4). The initial consistency was below 15 Bc, meeting the requirements for cementing operations. AA and MA had the same effect on cement slurry thickening. The thickening duration of the cement slurry containing the IA fluid loss additive was up to 7 h; this was 316 min longer than that of the blank cement slurry.
Thus, AA and MA possessed good water loss controllability and induced a moderate retarding side effect, making them advantageous in adjusting the cement slurry formula for in-field applications. IA demonstrated a strong retarding side effect and, thus, was not conducive to cementing operations. Therefore, AA and MA were selected for further analysis, and the effects of their carboxyl groups on cement slurry injection performance were examined.
3.2 Influence of Carboxylic Acid Monomer Content
3.2.1 Adjustment of Monomer Ratio of Type AA and Type MA Fluid Loss Additives
The relationship between the carboxyl content of the fluid loss additive and the thickening duration of the cement slurry was further investigated by synthesizing fluid loss additives with different carboxyl contents. Table 2 lists the monomer ratio of the type AA and type MA fluid loss additives with different carboxyl contents.
3.2.2 Influence of Carboxyl Group Mole Fraction on the Water Loss Controllability of the Fluid Loss Additive
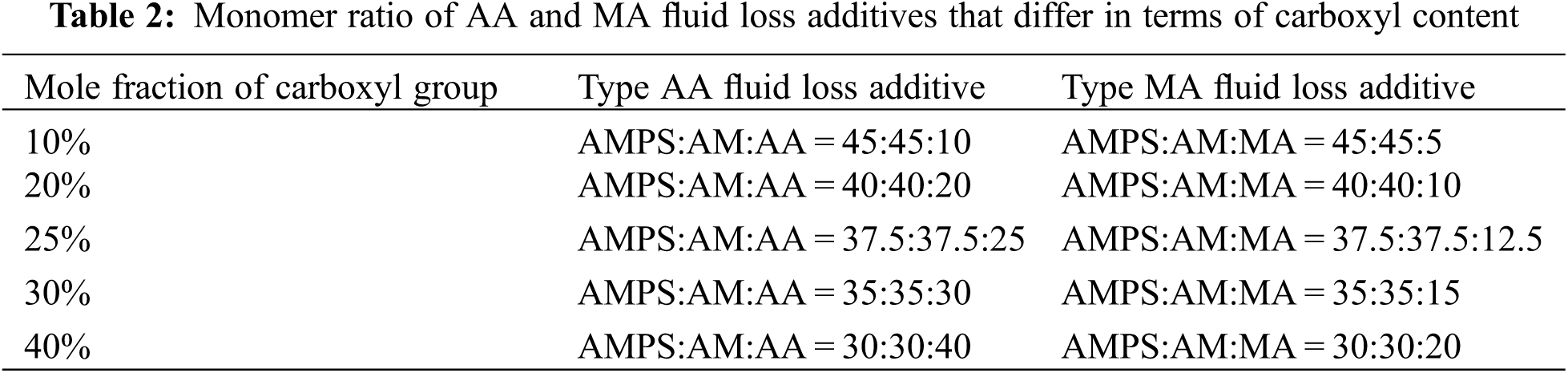
To further explore the relationship between the carboxyl group mole fraction of fluid loss additives and the water loss controllability, the amount of water lost from the cement slurry was determined at 75°C (Fig. 5).
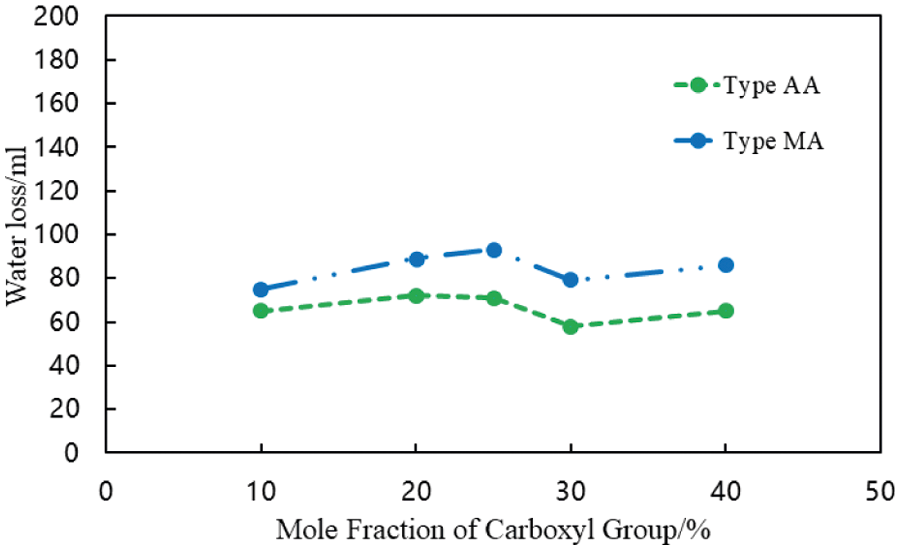
Figure 5: Water loss controllability of fluid loss additives with different the carboxyl contents (conditions: fluid loss additive concentration = 1%; temperature = 75°C)
There was no substantial change in the water loss of the cement slurry with an increase in the carboxyl group mole fraction for the type AA and type MA fluid loss additives (Fig. 5). Thus, the water loss controllability of the fluid loss additive did not improve with an increase in the carboxyl group mole fraction of the fluid loss additive.
3.2.3 Influence of Carboxyl Group Mole Fraction on the Rheological Properties of the Cement Slurry
The rheological properties of the AA and MA containing cement slurries with different carboxyl group mole fractions (1% fluid loss additive concentration) were tested at room temperature. Figs. 6–9 illustrate variations in the fluidity index and consistency coefficient with an increase to the carboxyl mole percentage.
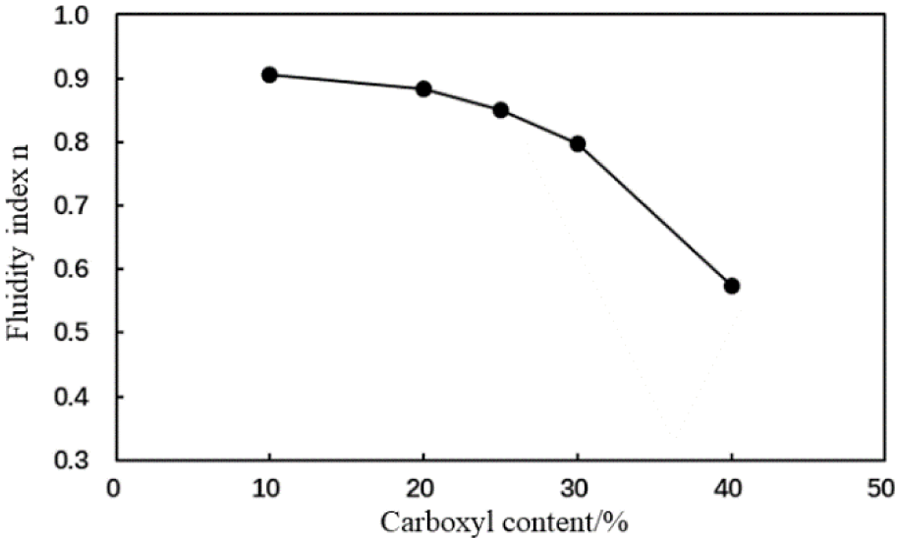
Figure 6: Fluidity index (n) of cement slurry containing the AA fluid loss additive with different carboxyl group mole fractions (conditions: fluid loss additive concentration = 1%; temperature = room temperature)
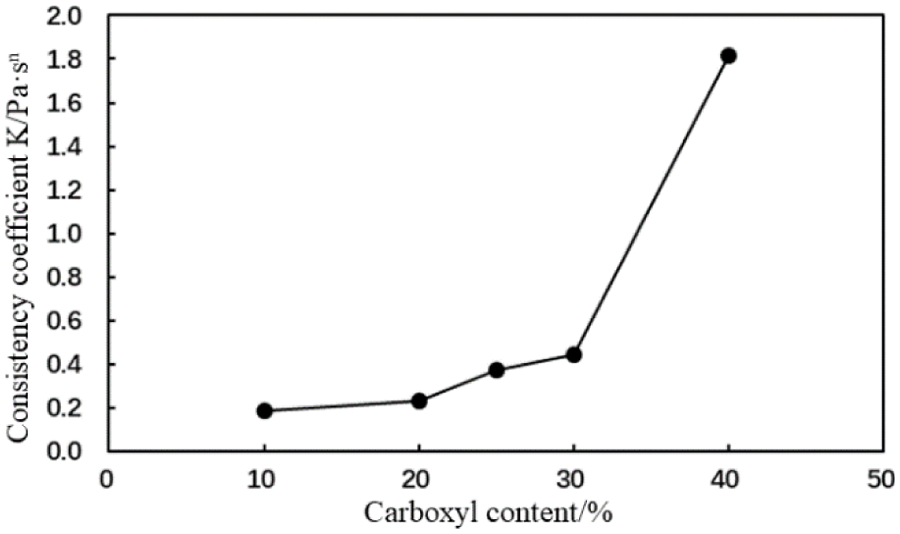
Figure 7: Consistency coefficient (K) of the cement slurry containing the AA fluid loss additive with different carboxyl contents (conditions: fluid loss additive concentration = 1%; temperature = room temperature)
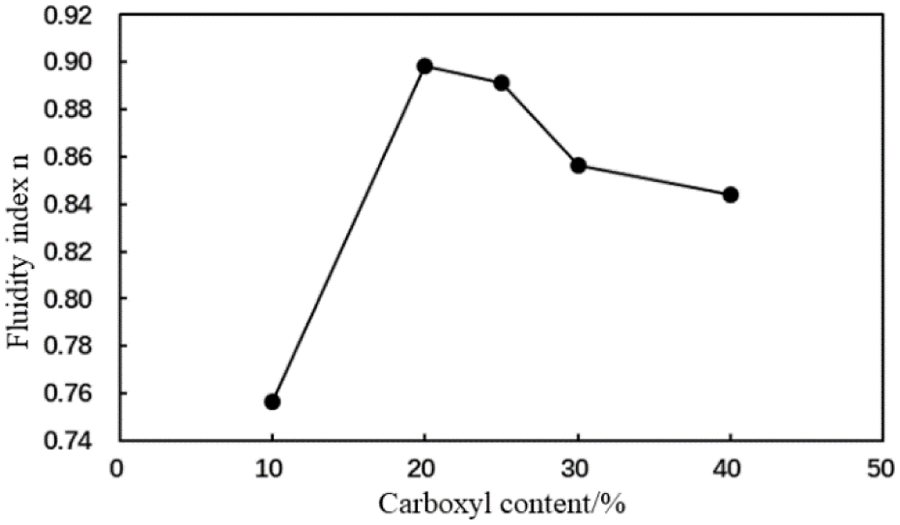
Figure 8: Fluidity index (n) of the cement slurry containing the MA fluid loss additive with different carboxyl contents (conditions: fluid loss additive concentration = 1%; temperature = room temperature)
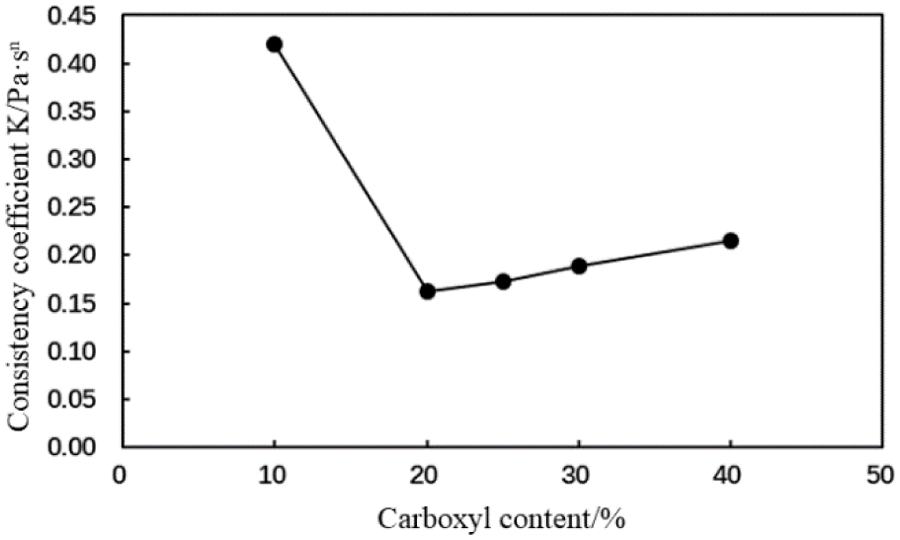
Figure 9: Consistency coefficient (K) of the cement slurry containing the MA fluid loss additive with different carboxyl contents (conditions: fluid loss additive concentration = 1%; temperature = room temperature)
The fluidity index of the cement slurry decreased and the consistency coefficient increased with an increase in the carboxyl group mole fraction of the type AA fluid loss additive (Figs. 6 and 7). The rheological properties of the cement slurry deteriorated, which is not conducive to cement slurry pumping during cementing operations.
The fluidity index of the cement slurry initially increased and subsequently decreased, and the consistency coefficient of the cement slurry initially decreased then with an increase in the carboxyl group mole fraction of the MA fluid loss additive (Figs. 8 and 9). When the carboxyl group mole fraction was 20%, the consistency coefficient was the lowest, which indicated a reduced viscosity. Therefore, the rheological properties of the type MA fluid loss additive improve when the carboxyl group mole fraction is 20%.
3.2.4 Influence of Carboxyl Group Mole Fraction on Cement Slurry Thickening Duration
An atmospheric pressure thickener was used to determine the thickening duration for the cement slurries (with 1% fluid loss additive) containing the type AA and type MA fluid loss additives with different carboxyl group mole fractions. The thickening curves of each cement slurry are shown in Figs. 10 and 11, and the initial consistency of the cement slurry is shown in Figs. 12 and 13.
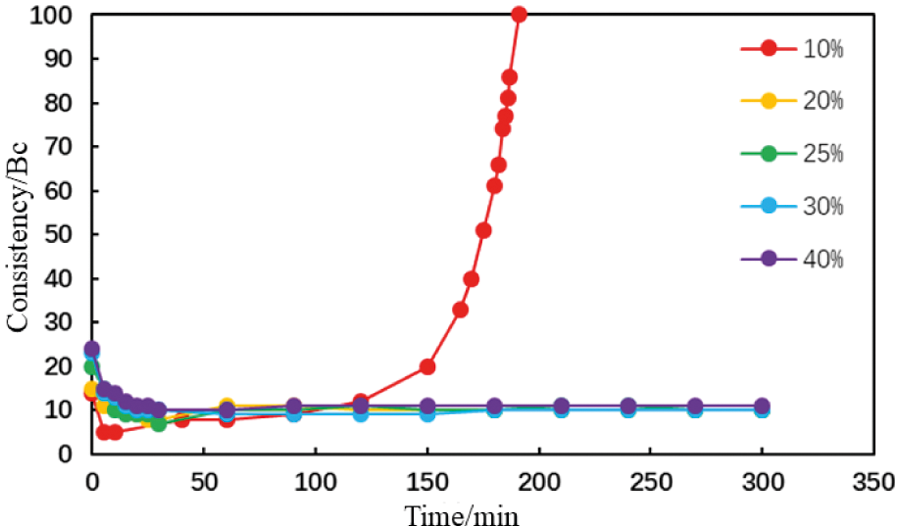
Figure 10: Thickening curve of the type AA fluid loss additive cement slurry with different carboxyl contents (conditions: fluid loss additive concentration = 1%; temperature = 75°C)
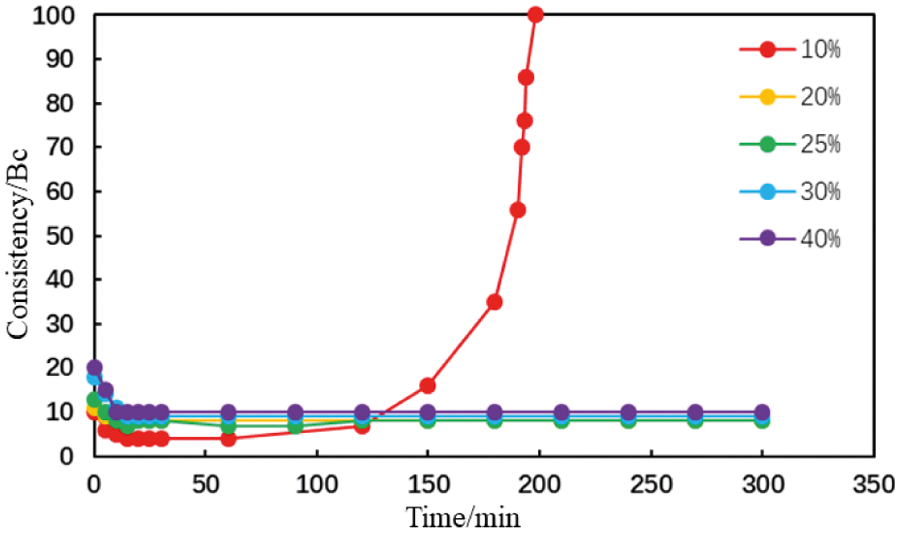
Figure 11: Thickening curve of the type MA fluid loss additive cement slurry with different carboxyl contents (conditions: fluid loss additive concentration = 1%; temperature = 75°C)
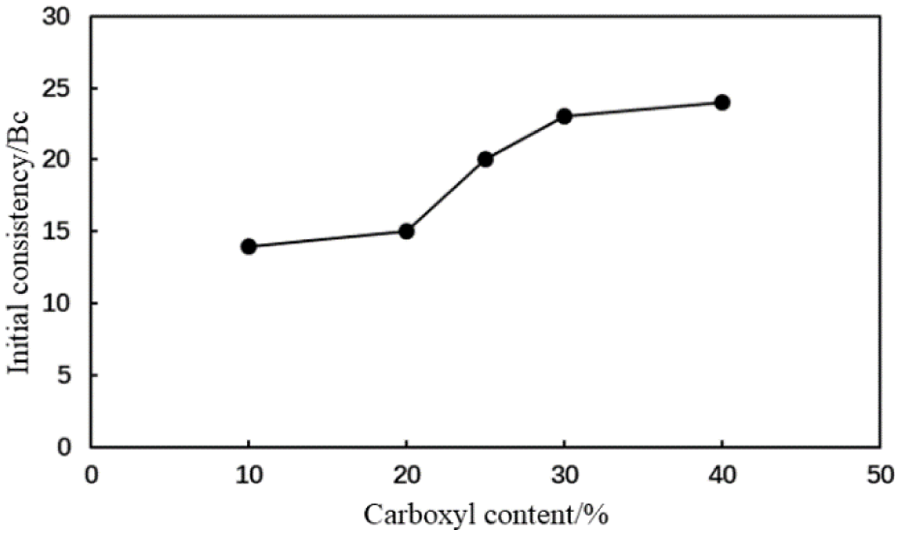
Figure 12: Initial consistency of the type AA fluid loss additive cement slurry with different carboxyl contents
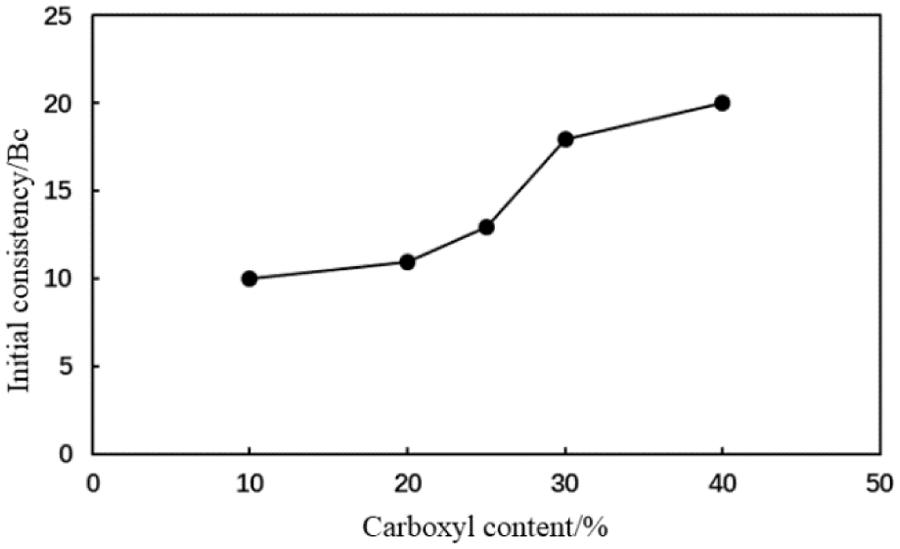
Figure 13: Initial consistency of the type AA fluid loss additive cement slurry with different carboxyl contents
When the mole fraction of the carboxyl group in the AA fluid loss additive was 10%, the thickening curve of the cement slurry initially decreased, stabilized, and finally increased rapidly at a thickening duration of 191 min (Fig. 10). When the mole fraction of the carboxyl group increased to >20%, the thickening curve of the cement slurry initially decreased then stabilized at a low value. The initial consistency of the cement slurry gradually increased to a small extent with the carboxyl group mole fraction in the type AA fluid loss additive (Fig. 11). The initial consistency was <30 Bc, meeting the requirements of cementing operations. Moreover, when the mole fraction of the carboxyl group in the MA fluid loss additive was 10%, the thickening curve of the cement slurry initially decreased, stabilized, and then increased rapidly at a thickening duration of 198 min (Fig. 12). When the mole fraction of the carboxyl group increased to >20%, the thickening curve of the cement slurry initially decreased, then stabilized at a low value. The initial consistency of the cement slurry was <20 Bc, and gradually increased with the carboxyl group mole fraction in the MA fluid loss additive (Fig. 13). These results show that increasing the mole fraction of the carboxyl group in the AA and MA fluid loss additives will increase the initial consistency of the cement slurries and will induce a very strong retarding side effect.
These results offer preliminary insights into the influence of the carboxylic acid monomer content on the thickening performance of the cement slurry. With an increase in the carboxylic acid monomer content in fluid loss additives, the rheological properties of the cement slurry gradually deteriorate, there is a slight increase in the initial consistency of the slurry, and the thickening duration increases rapidly. The increase in the carboxyl monomer content induces a strong retarding side effect in the cement slurry, which is not conducive to in-field cementing operations.
3.3 Influence of Carboxylic Acid Monomer at Different Temperatures
3.3.1 Influence of Temperature on the Water Loss Controllability of Each Fluid Loss Additive
The water loss controllability of different fluid loss additives in the cement slurries at different temperatures was tested; Fig. 14 presents these results.
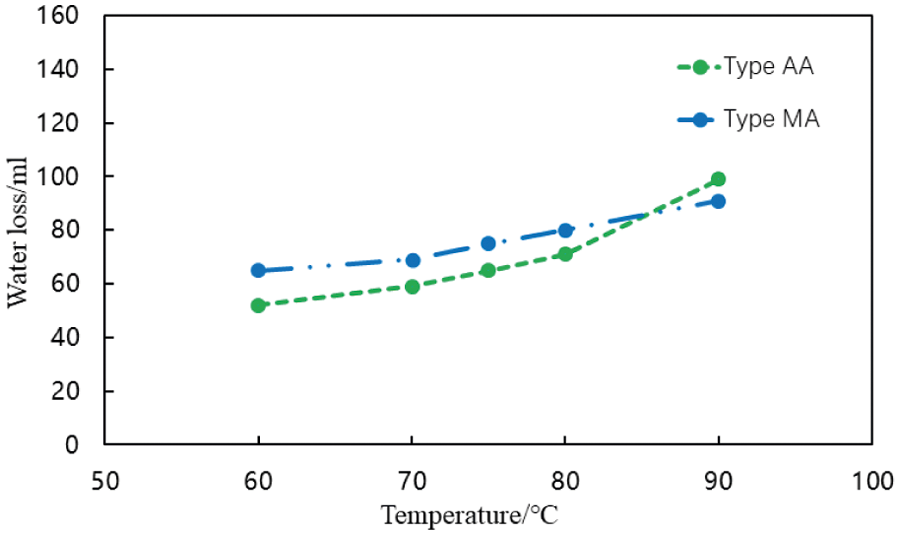
Figure 14: Water loss controllability of different fluid loss additives in cement slurries at various temperatures (conditions: fluid loss additive concentration = 1%, carboxyl group mole fraction = 10%)
Fig. 14 shows that the water loss from each cement slurry gradually increases with temperature. When the temperature exceeded 80°C, the water loss of each cement slurry increased significantly although it still met the requirements of cementing operations. The amount of water lost from the cement slurry containing the AA fluid loss additive was increased to a greater extent with increasing temperature than that from the cement slurry containing the MA fluid loss additive.
3.3.2 Influence of Temperature on Thickening Duration of Cement
The thickening duration of two cement slurries containing 1% fluid loss additive at 60, 70, 75, 80, and 90°C was tested; Fig. 15 presents the results of this test.
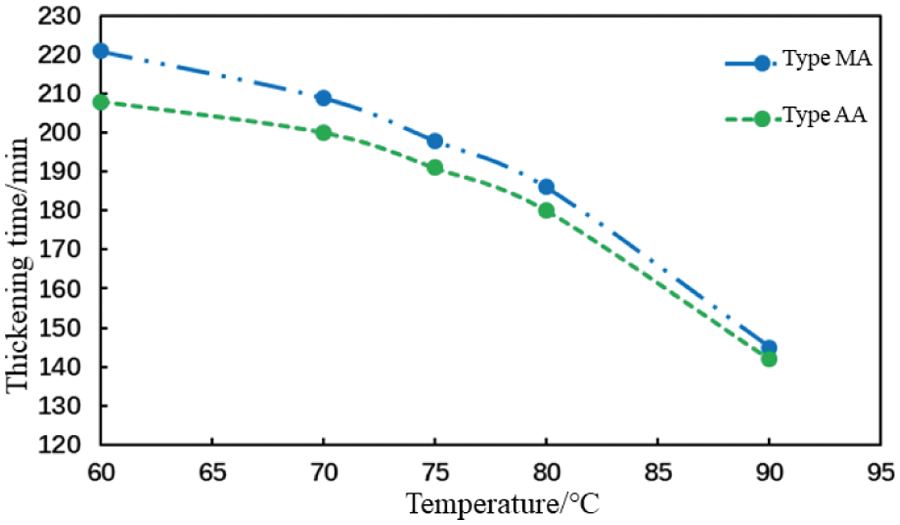
Figure 15: Thickening duration of the cement slurry with different fluid loss additives at varying temperatures (conditions: fluid loss additive concentration = 1%; carboxyl group mole fraction = 10%)
Fig. 15 indicates that a temperature increase will reduce the thickening duration of cement slurries with various fluid loss additives. The influence of different fluid loss additives on the thickening duration of the cement slurries tended to be the same with an increase in temperature.
3.4 Influence of Carboxylic Acid Monomers after Magnesium Ion Intrusion
To explore the influence of invasive underground magnesium ions on fluid loss additives, MgCl2 was added to the cement slurry containing fluid loss additive to carry out the cement slurry performance evaluation experiment. The proportion of added magnesium ions was 0%, 0.1%, 0.3%, 0.5%, and 0.7%; these values were calculated on the basis of the cement quality.
3.4.1 Influence of Magnesium Ion Intrusion on the Water Loss Controllability of Various Fluid Loss Additives
The water loss controllability of different fluid loss additives in cement slurries with added magnesium ion concentrations was assessed (Fig. 16).
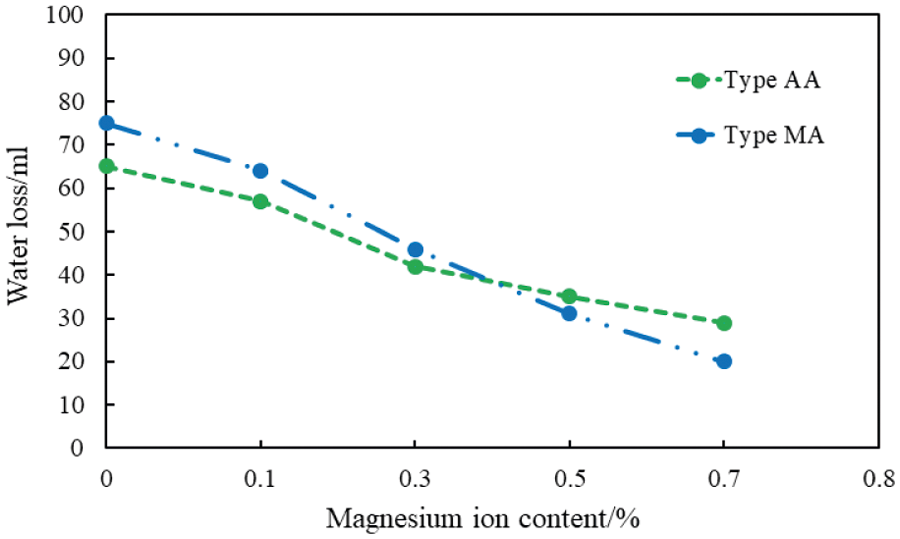
Figure 16: Water loss of the cement slurry with different magnesium ion concentrations (conditions: fluid loss additive concentration = 1%; carboxyl content group mole fraction = 10%; temperature = 75°C)
The water loss of the cement slurry decreases with the intrusion of magnesium ions (Fig. 16). The water loss of the cement slurry containing the AA fluid loss additive decreased with an increase in the magnesium ion concentration, although the reduction range was narrower than that of the cement slurry containing the MA fluid loss additive. The reduction range of the water loss of each cement slurry gradually narrowed with increasing magnesium ion content.
3.4.2 Influence of Magnesium Ion on the Rheological Properties of Cement Slurries with Fluid Loss Additives
The rheological properties of the cement slurries with different magnesium ion concentrations (1% fluid loss additive concentration, 10% carboxyl group mole fraction) were tested at room temperature. Figs. 17–20 present the variation in the fluidity index and consistency coefficient for each cement slurry.
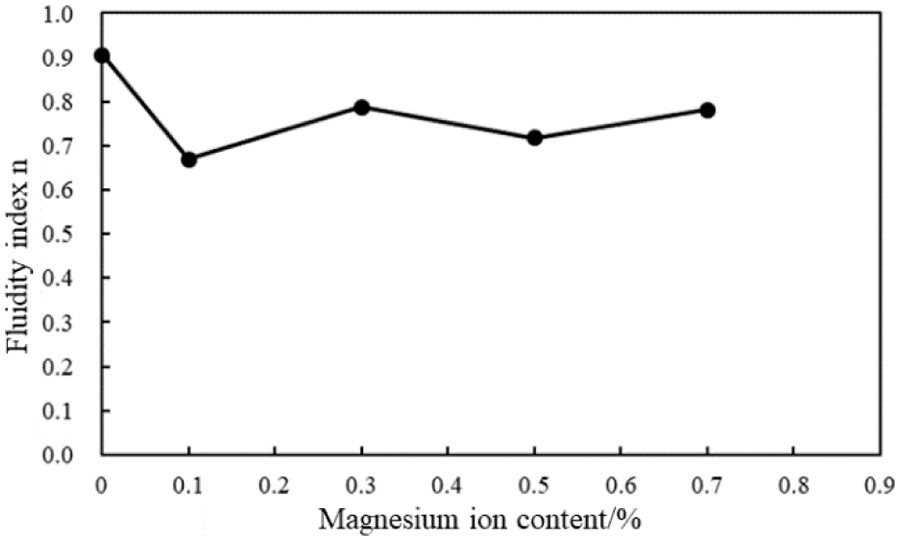
Figure 17: Fluidity index (n) of the cement slurries with different magnesium ion concentrations (conditions: AA fluid loss additive concentration = 1%; carboxyl group mole fraction = 10%)
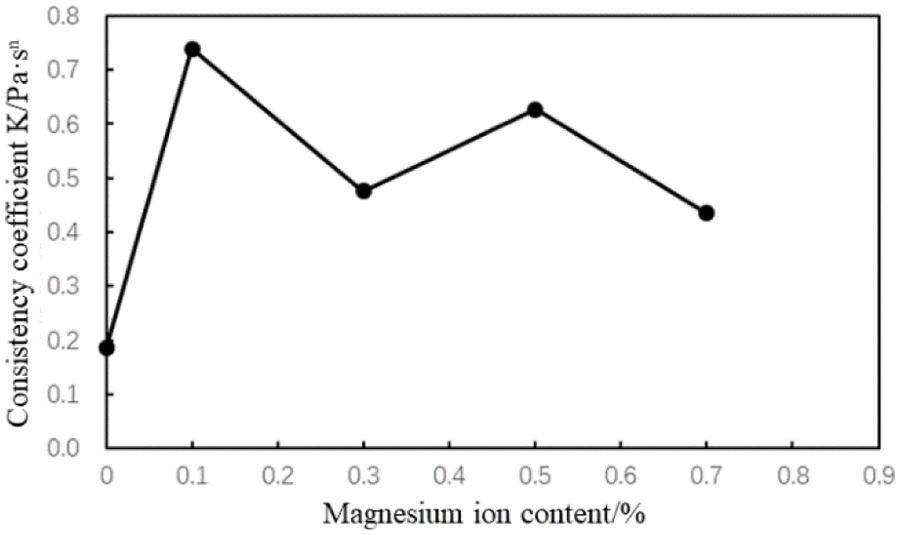
Figure 18: Consistency coefficient (K) of the cement slurries with different magnesium ion concentrations (conditions: AA fluid loss additive concentration = 1%; carboxyl group mole fraction = 10%)
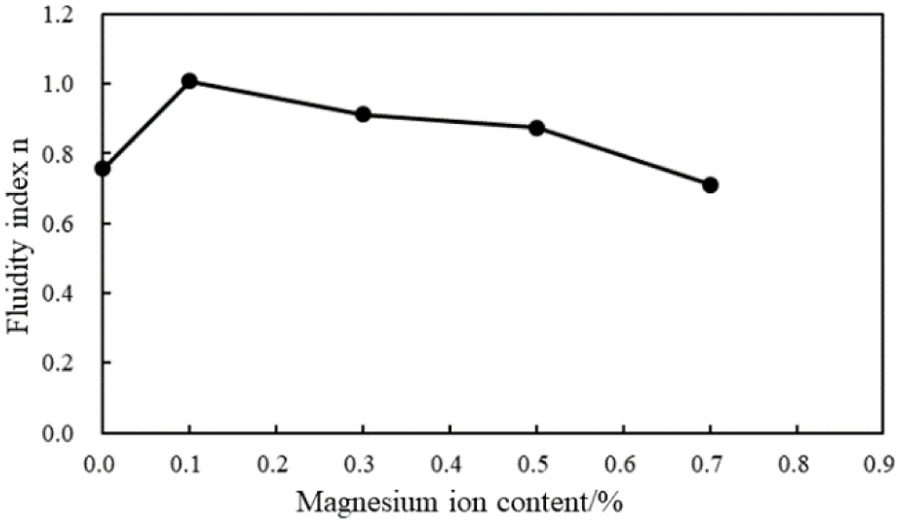
Figure 19: Fluidity index (n) of the cement slurries with different magnesium ion concentrations (conditions: MA fluid loss additive concentration = 1%; carboxyl group mole fraction = 10%)
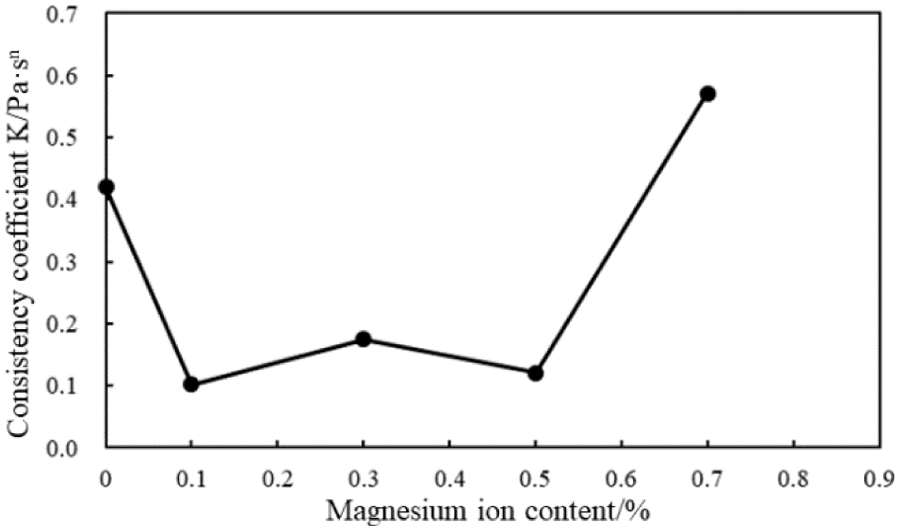
Figure 20: Consistency coefficient (K) of the cement slurries with different magnesium ion concentrations (conditions: MA fluid loss additive concentration = 1%; carboxyl group mole fraction = 10%)
The fluidity index of the cement slurries decreased while the consistency coefficient increases with the intrusion of magnesium ions (Figs. 17 and 18). When the concentration of magnesium ions in the cement slurry is 0.1%–0.7%, the fluidity index and consistency coefficient fluctuate slightly. Thus, the rheological properties of the cement slurry containing the AA fluid loss additive were less affected by the magnesium ion concentration.
Fig. 19 shows that with the intrusion of the magnesium ions, the fluidity index of the cement slurry increases; however, the fluidity index of the cement slurries decreases with an increase in the magnesium ion concentration. Fig. 20 indicates that with the intrusion of the magnesium ions, the consistency coefficient of the cement slurries decreases; when the magnesium ion concentration was 0.1%–0.5%, the consistency coefficient fluctuates slightly. However, when the magnesium ion concentration was 0.7%, the consistency coefficient and, thus, viscosity of the cement slurries increase significantly. These results demonstrate that the rheological properties of the cement slurry containing the MA fluid loss additive gradually deteriorate with an increase in the magnesium ion concentration.
3.4.3 Influence of Magnesium Ions on the Thickening Duration of Cement Slurries Containing Fluid Loss Additives
The thickening curve for of the cement slurries containing the fluid loss additives with different magnesium ion concentrations was determined at 75°C by using the atmospheric pressure thickener; the concentration of the fluid loss additive was 1%, and the carboxyl group mole fraction was 10%. The results of this experiment are shown in Figs. 21 and 22.
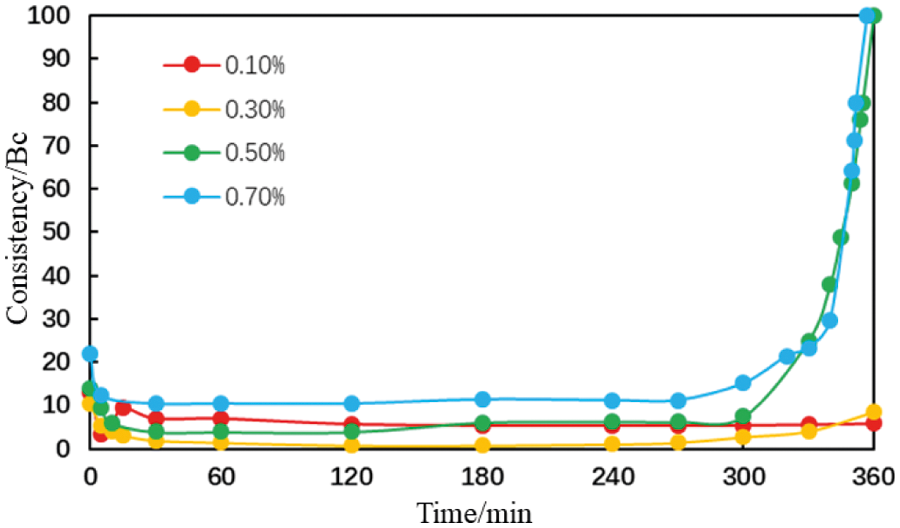
Figure 21: Thickening curve of the cement slurries with different magnesium ion concentrations (condition: AA fluid loss additive concentration = 1%; carboxyl group mole fraction = 10%; temperature = 75°C)
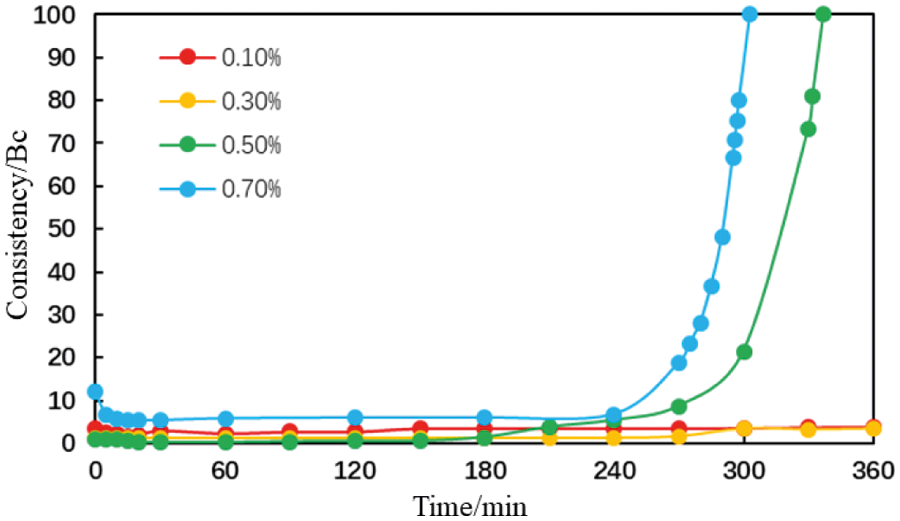
Figure 22: Thickening curve of the cement slurries with different magnesium ion concentrations (conditions: MA fluid loss additive concentration = 1%; carboxyl group mole fraction = 10%; temperature = 75°C)
When the magnesium ion concentrations was 0.1% and 0.3%, the consistency of the cement slurries was maintained at a low level within 6 h (Figs. 21 and 22). When the magnesium ion concentration was 0.5%, the thickening curve of the cement slurries initially decreased, stabilized, and then increased rapidly. The thickening duration further reduced when the magnesium ion concentration was increased to 0.7%. This shows that the thickening duration for the cement slurry containing the AA fluid loss additive decreases gradually with an increase in the magnesium ion concentration although this change is small. With an increase in the magnesium ion concentration, the thickening duration for the cement slurry containing the MA fluid loss additive decreases gradually; this change was more significant.
These results show that the intrusion of magnesium ions has a negligible effect on the thickening duration for the cement slurry containing the AA fluid loss additive. With an increase in the magnesium ion concentration, the rheological properties of the cement slurries fluctuates slightly and there is a small reduction in the thickening duration. The intrusion of magnesium ions had a considerable impact on the thickening duration for the cement slurry containing the MA fluid loss additive. With an increase in the magnesium ion concentration, the rheological properties of the cement slurries gradually deteriorated, and there was a significant reduction in the thickening duration.
(1) Carboxyl groups in IA have a stronger retarding side effect on cement slurries; these effects were relatively moderate and similar to those induced by the AA and MA fluid loss additive counterparts. The AA and MA fluid loss additives possessed better water loss controllability.
(2) With an increase in the carboxylic acid monomer concentration in the synthetic fluid loss additive, the rheological properties of the cement slurry gradually deteriorate, a slight increase in the initial consistency is observed, and the thickening time increases rapidly. These changes causes the cement slurry to exhibit a strong retarding side effect. When the mole fraction of the carboxyl group in the fluid loss additive increases, the fluid loss additive does not possess water loss controllability.
(3) With an increase in temperature, the thickening duration for the cement slurry containing various fluid loss additives decreases, while the influence of different fluid loss additives on the thickening duration was the same.
(4) The intrusion of magnesium ions has a great influence on the thickening duration for the cement slurry containing the MA fluid loss additive. With an increase in the magnesium ion concentration, the rheological properties of the cement slurry deteriorate and there is a significant reduction in the thickening duration. The intrusion of magnesium ions has a negligible influence on the thickening duration of the cement slurry containing the AA fluid loss additive; however, increases in the magnesium ion concentration caused slight fluctuations in the rheological properties of the cement slurry, whose thickening duration decreases slightly.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Xiao, Q., Rao, P. H., Xiao, W. F., Liu, X. X., Zhang, W. Q. (2014). Preparation of a novel for polymer as fluid loss additive for high temperature oil well cementing. Russian Journal of Applied Chemistry, 87(9), 1377–1381. DOI 10.1134/S1070427214090328. [Google Scholar] [CrossRef]
2. Oyewole, T. S., Johann, P. (2013). Preparation and properties of a dispersing fluid loss additive based on humic acid graft copolymer suitable for cementing high temperature (200°C) oil wells. Journal of Applied Polymer Science, 129(5), 2544–2553. DOI 10.1002/app.38980. [Google Scholar] [CrossRef]
3. Ma, J. Y., Zheng, H. L., Tian, M. Z., Liu, L. W., Chen, W. et al. (2013). Synthesis, characterization, and flocculation performance of anionic polyacrylamide P (AM-AA-AMPS). Journal of Applied Polymer Science, 129(4), 1984–1991. DOI 10.1002/app.38900. [Google Scholar] [CrossRef]
4. Daniel, B., Johann, P. (2012). Role of colloidal polymer associates for the effectiveness of hydroxyethyl cellulose as a fluid loss control additive in oil well cement. Journal of Applied Polymer Science, 126(S1), E25–E34. DOI 10.1002/app.36529. [Google Scholar] [CrossRef]
5. Constantin, T., Johann, P. (2012). Working mechanism of a high temperature (200°C) synthetic cement retarder and its interaction with an AMPS®-based fluid loss polymer in oil well cement. Journal of Applied Polymer Science, 124(6), 4772–4781. DOI 10.1002/app.35535. [Google Scholar] [CrossRef]
6. Han, H. F., Zhang, J. H. (2013). Lightly branched poly(vinyl alcohol) for fluid loss additive. Journal of Applied Polymer Science, 130(6), 4608–4612. DOI 10.1002/app.39741. [Google Scholar] [CrossRef]
7. Johann, P., Andreas, B., Nils, R. L. (2007). Effect of different anchor groups on adsorption behavior and effectiveness of poly(N, N-dimethyl acrylamide-co-Ca 2-acrylamido-2-methylpropanesulfonate) as cement fluid loss additive in presence of acetone–formaldehyde–sulfite dispersant. Journal of Applied Polymer Science, 106(6), 3889–3894. DOI 10.1002/app.26897. [Google Scholar] [CrossRef]
8. Guo, S. L., Lu, Y., Bu, Y. H., Li, B. L. (2018). Effect of carboxylic group on the compatibility with retarder and the retarding side effect of the fluid loss control additive used in oil well cement. Royal Society Open Science, 5(9), 180490. DOI 10.1098/rsos.180490. [Google Scholar] [CrossRef]
9. Daniel, B., Johann, P. (2012). Mechanistic study on carboxymethyl hydroxyethyl cellulose as fluid loss control additive in oil well cement. Journal of Applied Polymer Science, 124(3), 2340–2347. DOI 10.1002/app.35278. [Google Scholar] [CrossRef]
10. Ma, C., Bu, Y. H., Chen, B. (2014). Preparation and performance of a lignosulfonate–AMPS–itaconic acid graft copolymer as retarder for modified phosphoaluminate cement. Construction and Building Materials, 60, 25–32. DOI 10.1016/j.conbuildmat.2014.02.064. [Google Scholar] [CrossRef]
11. Oyewole, T. S., Johann, P. (2016). Influence of electrolytes on the performance of a graft copolymer used as fluid loss additive in oil well cement. Journal of Petroleum Science and Engineering, 143, 86–94. DOI 10.1016/j.petrol.2016.02.021. [Google Scholar] [CrossRef]
12. Zhang, R., Huo, J. H., Peng, Z. G., Feng, Q., Chen, D. J. et al. (2016). Investigation of poly(AM/AMPS/MA) on the retarding performance of oil well cement. Applied Magnetic Resonance, 47(9), 987–1001. DOI 10.1007/s00723-016-0814-4. [Google Scholar] [CrossRef]
13. Huang, Y. M., Zhang, D. Y., Zheng, W. L. (2019). Synthetic copolymer (AM/AMPS/DMDAAC/SSS) as rheology modifier and fluid loss additive at HTHP for water-based drilling fluids. Journal of Applied Polymer Science, 136(30), 47813. DOI 10.1002/app.47813. [Google Scholar] [CrossRef]
14. Johann, P., Nils, R. L., Fatima, D. (2010). Competitive adsorption between an AMPS®-based fluid loss polymer and Welan gum biopolymer in oil well cement. Journal of Applied Polymer Science, 116(5), 2913–2919. DOI 10.1002/app.31865. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |